Modification of Threonine-825 of SlBRI1 Enlarges Cell Size to Enhance Fruit Yield by Regulating the Cooperation of BR-GA Signaling in Tomato
Abstract
:1. Introduction
2. Results
2.1. SlBRI1 Thr-825 Influences Autophosphorylation of SlBRI1
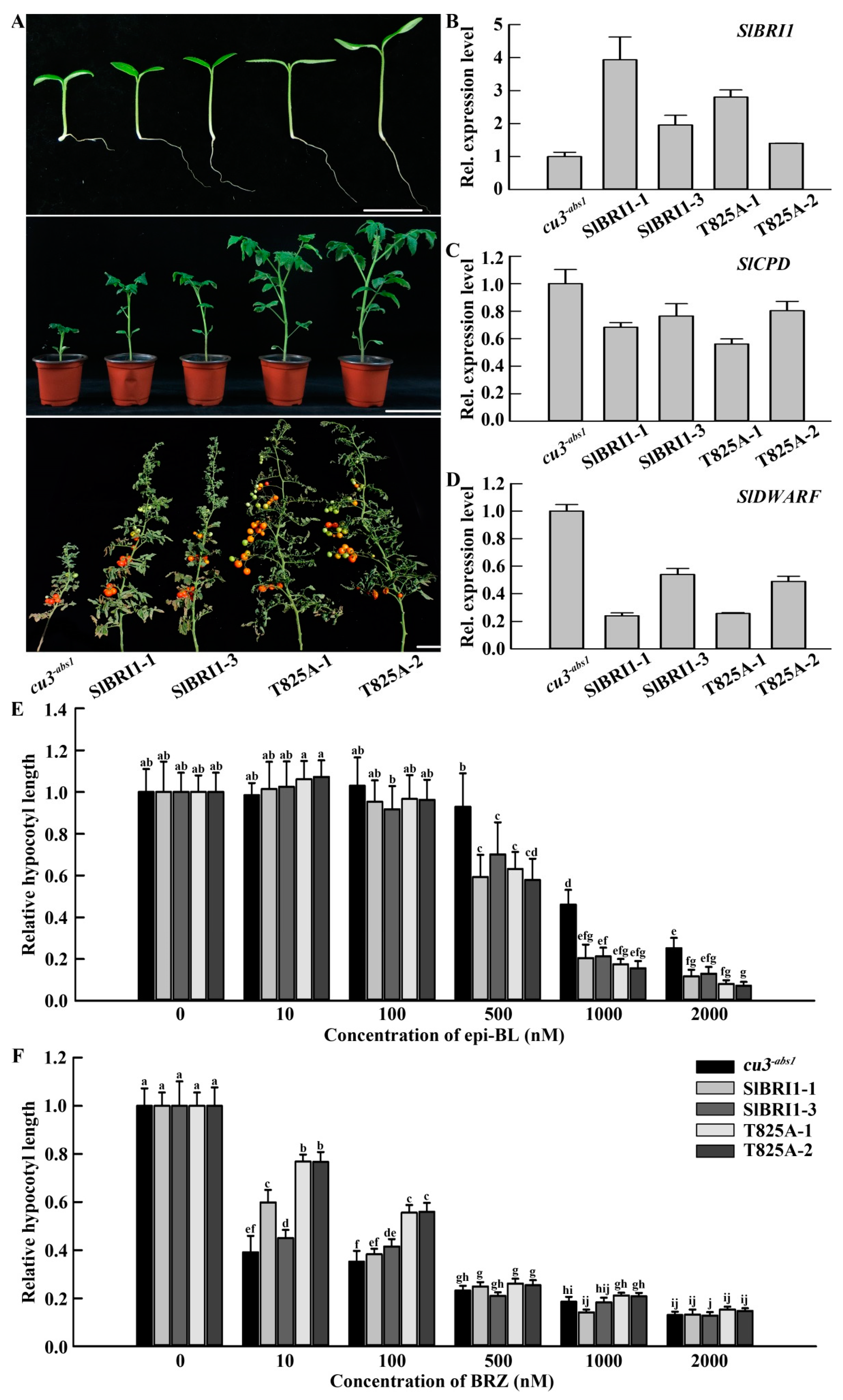
2.2. SlBRI1 T825A Affects BR Signaling
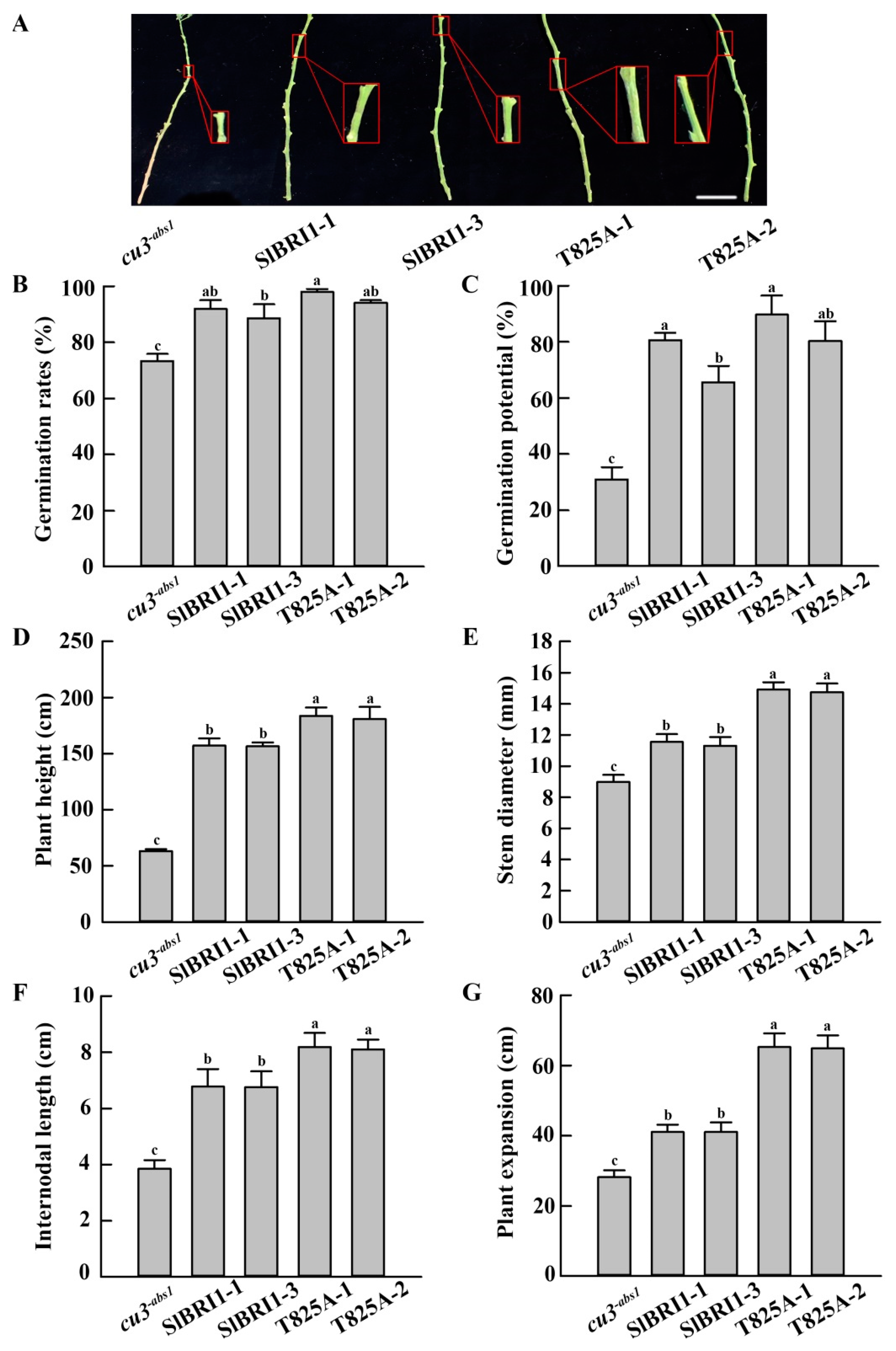
2.3. SlBRI1 T825A Promotes Plant Growth
2.4. SlBRI1 T825A Improves Tomato Yields
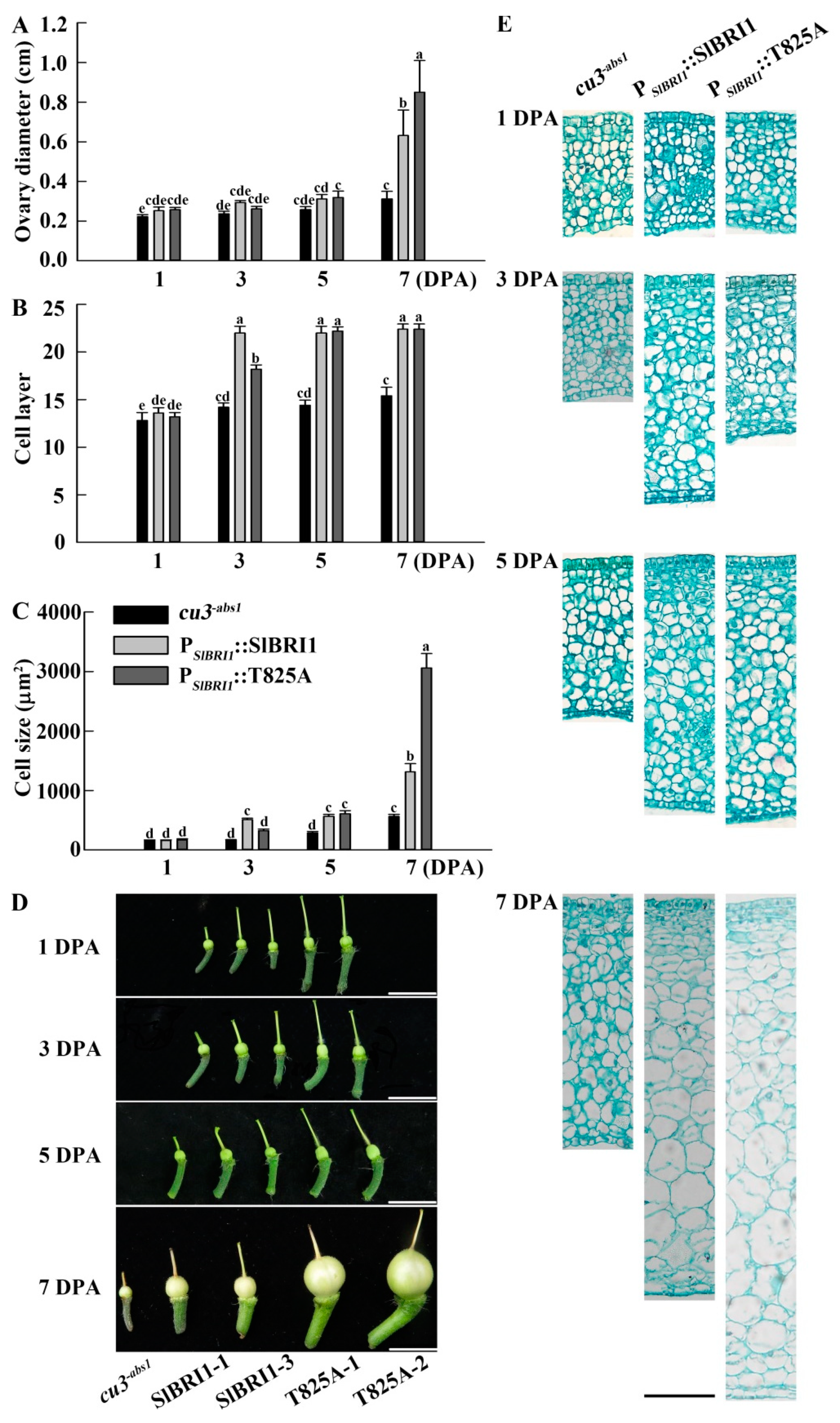
2.5. SlBRI1 T825A Influences Early Fruit Development
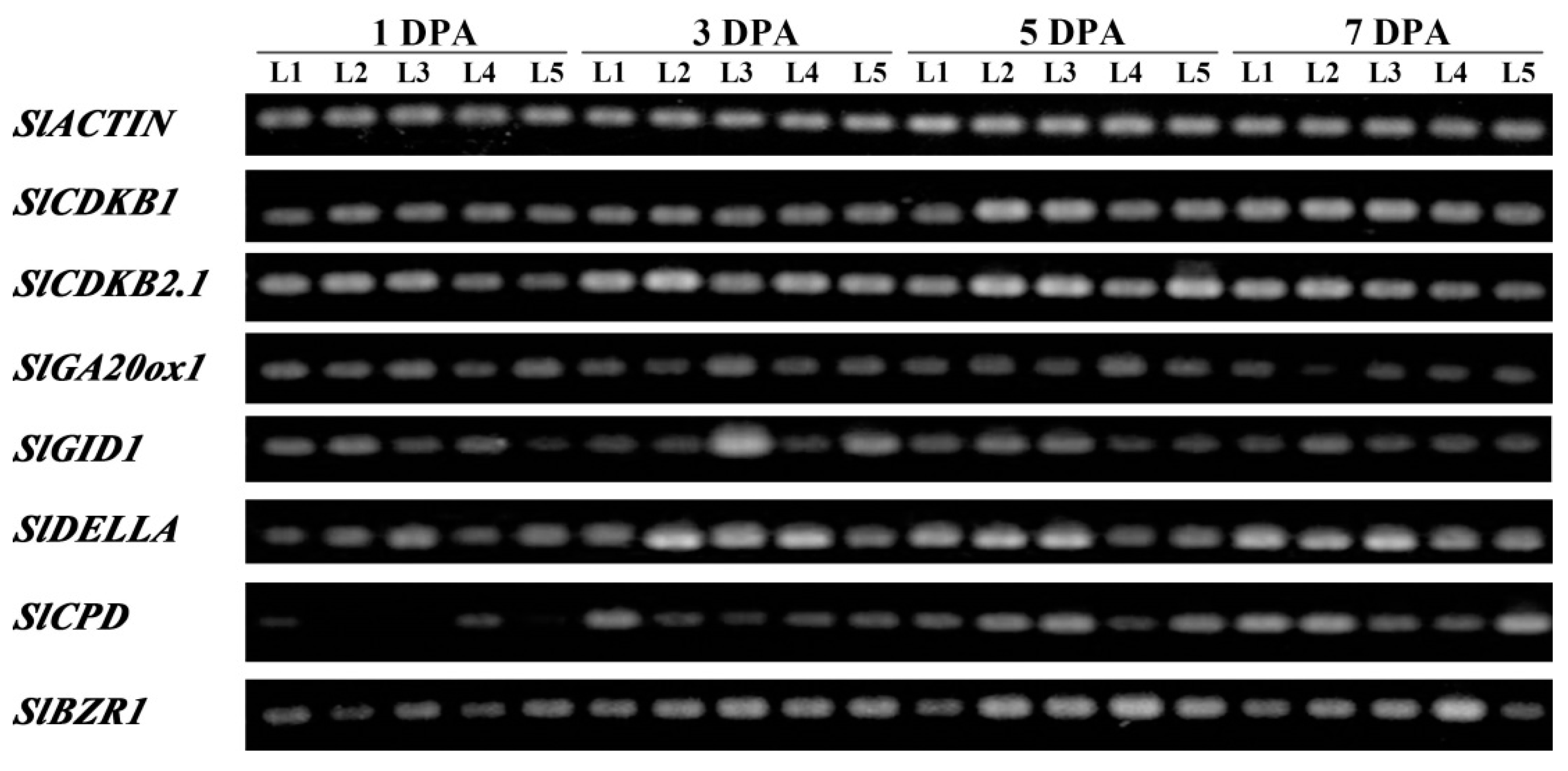
2.6. SlBRI1 T825A Affects Expression of Fruit Growth-Related Genes
2.7. SlBRI1 T825A, S1040A, and T1050A Differentially Affect BR and GA Signaling
3. Discussion
4. Materials and Methods
4.1. Amino Acid Sequence Alignment
4.2. Site-Directed Mutagenesis and Vector Construction
4.3. Tomato Transformation
4.4. Agronomic Trait Characterization
4.5. Paraffin Section Observation
4.6. Hypocotyl Elongation Response to Exogenous BL and BRZ
4.7. Relative Expression Analysis
4.8. Autophosphorylation Analysis In Vitro
4.9. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| BR | Brassinosteroid |
| BRI1 | Brassinosteroid Insensitive1 |
| IAA | Indole-3-acetic acid |
| GA | Gibberellic acid |
| Thr | Threonine |
| Ser | Serine |
| Tyr | Tyrosine |
| CDK | Cyclin-dependent kinase |
| LRR-RLK | Leucine-rich repeat receptor-like kinase |
| BZR1 | BRASSINAZOLERESISTANT1 |
| BES1 | BRI1-EMS SUPPRESSOR1 |
| T825A | Threonine-825-alanine |
| T825D | Threonine-825-aspartic acid |
| K916E | Lysine-916-Glutamic acid |
| S1040A | Serine-1040- alanine |
| T1050A | Threonine-1050-alanine |
| GFP | Green fluorescent protein |
| epi-BL | 24-Epibrassinolide |
| BRZ | Brassinazole |
| CPD | Constitutive Photomorphogenesis and Dwarf |
| DWARF | 6-DEOXOCASTASTERONE OXIDASE |
| GID1 | Gibberellin Insensitive Dwarf 1 |
| ARF2 | AUXIN RESPONSE FACTOR-2 |
| BIN2 | BRASSINOSTEROID INSENSITIVE 2 |
| qRT-PCR | Quantitative real-time PCR analysis |
| MS | Murashige and Skoog medium |
References
- Azzi, L.; Deluche, C.; Gevaudant, F.; Frangne, N.; Delmas, F.; Hernould, M.; Chevalier, C. Fruit growth-related genes in tomato. J. Exp. Bot. 2015, 66, 1075–1086. [Google Scholar] [CrossRef] [Green Version]
- Zhang, S.; Xu, M.; Qiu, Z.; Wang, K.; Du, Y.; Gu, L.; Cui, X. Spatiotemporal transcriptome provides insights into early fruit development of tomato (Solanum lycopersicum). Sci. Rep. 2016, 6, 23173. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pattison, R.J.; Csukasi, F.; Zheng, Y.; Fei, Z.; van der Knaap, E.; Catala, C. Comprehensive Tissue-Specific Transcriptome Analysis Reveals Distinct Regulatory Programs during Early Tomato Fruit Development. Plant Physiol. 2015, 168, 1684–1701. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Joubes, J.; Phan, T.H.; Just, D.; Rothan, C.; Bergounioux, C.; Raymond, P.; Chevalier, C. Molecular and biochemical characterization of the involvement of cyclin-dependent kinase A during the early development of tomato fruit. Plant Physiol. 1999, 121, 857–869. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dewitte, W.; Murray, J.A.H. The plant cell cycle. Annu. Rev. Plant Biol. 2003, 54, 235–264. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Enders, T.A.; Strader, L.C. Auxin Activity: Past, Present, and Future. Am. J. Bot. 2015, 102, 180–196. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Frick, E.M.; Strader, L.C. Roles for IBA-derived auxin in plant development. J. Exp. Bot. 2018, 69, 169–177. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oh, M.H.; Honey, S.H.; Tax, F.E. The Control of Cell Expansion, Cell Division, and Vascular Development by Brassinosteroids: A Historical Perspective. Int. J. Mol. Sci. 2020, 21, 1743. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, S.; Wang, X.J.; Tan, G.F.; Zhou, W.Q.; Wang, G.L. Gibberellin and the plant growth retardant Paclobutrazol altered fruit shape and ripening in tomato. Protoplasma 2020, 257, 853–861. [Google Scholar] [CrossRef]
- Li, Z.C.; He, Y.H. Roles of Brassinosteroids in Plant Reproduction. Int. J. Mol. Sci. 2020, 21, 872. [Google Scholar] [CrossRef] [Green Version]
- Liu, L.; Jia, C.; Zhang, M.; Chen, D.; Chen, S.; Guo, R.; Guo, D.; Wang, Q. Ectopic expression of a BZR1-1D transcription factor in brassinosteroid signalling enhances carotenoid accumulation and fruit quality attributes in tomato. Plant Biotechnol. J. 2014, 12, 105–115. [Google Scholar] [CrossRef] [PubMed]
- Nolan, T.; Chen, J.N.; Yin, Y.H. Cross-talk of Brassinosteroid signaling in controlling growth and stress responses. Biochem. J. 2017, 474, 2641–2661. [Google Scholar] [CrossRef] [PubMed]
- Zhiponova, M.K.; Vanhoutte, I.; Boudolf, V.; Betti, C.; Dhondt, S.; Coppens, F.; Mylle, E.; Maes, S.; Gonzalez-Garcia, M.P.; Cano-Delgado, A.I.; et al. Brassinosteroid production and signaling differentially control cell division and expansion in the leaf. New Phytol. 2013, 197, 490–502. [Google Scholar] [CrossRef]
- De Rybel, B.; Audenaert, D.; Vert, G.; Rozhon, W.; Mayerhofer, J.; Peelman, F.; Coutuer, S.; Denayer, T.; Jansen, L.; Nguyen, L.; et al. Chemical Inhibition of a Subset of Arabidopsis thaliana GSK3-like Kinases Activates Brassinosteroid Signaling. Chem. Biol. 2009, 16, 594–604. [Google Scholar] [CrossRef] [PubMed]
- Li, J.M.; Chory, J. A putative leucine-rich repeat receptor kinase involved in brassinosteroid signal transduction. Cell 1997, 90, 929–938. [Google Scholar] [CrossRef] [Green Version]
- Kim, T.W.; Guan, S.H.; Sun, Y.; Deng, Z.P.; Tang, W.Q.; Shang, J.X.; Sun, Y.; Burlingame, A.L.; Wang, Z.Y. Brassinosteroid signal transduction from cell-surface receptor kinases to nuclear transcription factors. Nat. Cell Biol. 2009, 11, 1254–1260. [Google Scholar] [CrossRef] [PubMed]
- Clouse, S.D. Brassinosteroid Signal Transduction: From Receptor Kinase Activation to Transcriptional Networks Regulating Plant Development. Plant Cell 2011, 23, 1219–1230. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, Z.Y.; Wang, Q.; Chong, K.; Wang, F.; Wang, L.; Bai, M.; Jia, C. The brassinosteroid signal transduction pathway. Cell Res. 2006, 16, 427–434. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yamamuro, C.; Ihara, Y.; Wu, X.; Noguchi, T.; Fujioka, S.; Takatsuto, S.; Ashikari, M.; Kitano, H.; Matsuoka, M. Loss of function of a rice brassinosteroid insensitive1 homolog prevents internode elongation and bending of the lamina joint. Plant Cell 2000, 12, 1591–1606. [Google Scholar] [CrossRef] [Green Version]
- Nakamura, A.; Fujioka, S.; Sunohara, H.; Kamiya, N.; Hong, Z.; Inukai, Y.; Miura, K.; Takatsuto, S.; Yoshida, S.; Ueguchi-Tanaka, M.; et al. The role of OsBRI1 and its homologous genes, OsBRL1 and OsBRL3, in rice. Plant Physiol. 2006, 140, 580–590. [Google Scholar] [CrossRef] [Green Version]
- Kir, G.; Ye, H.X.; Nelissen, H.; Neelakandan, A.K.; Kusnandar, A.S.; Luo, A.D.; Inze, D.; Sylvester, A.W.; Yin, Y.H.; Becraft, P.W. RNA Interference Knockdown of BRASSINOSTEROID INSENSITIVE1 in Maize Reveals Novel Functions for Brassinosteroid Signaling in Controlling Plant Architecture. Plant Physiol. 2015, 169, 826–839. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hou, Q.; Saima, S.; Ren, H.; Ali, K.; Bai, C.; Wu, G.; Li, G. Less Conserved LRRs Is Important for BRI1 Folding. Front. Plant Sci. 2019, 10, 634. [Google Scholar] [CrossRef] [PubMed]
- Oh, M.H.; Wang, X.F.; Kota, U.; Goshe, M.B.; Clouse, S.D.; Huber, S.C. Tyrosine phosphorylation of the BRI1 receptor kinase emerges as a component of brassinosteroid signaling in Arabidopsis. Proc. Natl. Acad. Sci. USA 2009, 106, 658–663. [Google Scholar] [CrossRef] [Green Version]
- Oh, M.H.; Sun, J.D.; Oh, D.H.; Zielinski, R.E.; Clouse, S.D.; Huber, S.C. Enhancing Arabidopsis Leaf Growth by Engineering the BRASSINOSTEROID INSENSITIVE1 Receptor Kinase. Plant Physiol. 2011, 157, 120–131. [Google Scholar] [CrossRef] [Green Version]
- Wang, Q.N.; Wang, S.F.; Gan, S.F.; Wang, X.; Liu, J.W.; Wang, X.F. Role of Specific Phosphorylation Sites of Arabidopsis Brassinosteroid-Insensitive 1 Receptor Kinase in Plant Growth and Development. J. Plant Growth Regul. 2016, 35, 755–769. [Google Scholar] [CrossRef]
- Bajwa, V.S.; Wang, X.F.; Blackburn, R.K.; Goshe, M.B.; Mitra, S.K.; Williams, E.L.; Bishop, G.J.; Krasnyanski, S.; Allen, G.; Huber, S.C.; et al. Identification and Functional Analysis of Tomato BRI1 and BAK1 Receptor Kinase Phosphorylation Sites. Plant Physiol. 2013, 163, 30–42. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nie, S.; Huang, S.; Wang, S.; Cheng, D.; Liu, J.; Lv, S.; Li, Q.; Wang, X. Enhancing Brassinosteroid Signaling via Overexpression of Tomato (Solanum lycopersicum) SlBRI1 Improves Major Agronomic Traits. Front. plant Sci. 2017, 8, 1386. [Google Scholar] [CrossRef] [Green Version]
- Wang, X.F.; Goshe, M.B.; Soderblom, E.J.; Phinney, B.S.; Kuchar, J.A.; Li, J.; Asami, T.; Yoshida, S.; Huber, S.C.; Clouse, S.D. Identification and functional analysis of in vivo phosphorylation sites of the Arabidopsis BRASSINOSTEROID-INSENSITIVE1 receptor kinase. Plant Cell 2005, 17, 1685–1703. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, S.; Liu, J.; Zhao, T.; Du, C.; Nie, S.; Zhang, Y.; Lv, S.; Huang, S.; Wang, X. Modification of Threonine-1050 of SlBRI1 regulates BR Signalling and increases fruit yield of tomato. BMC Plant Biol. 2019, 19, 256. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Hu, T.; Tian, A.; Luo, B.; Du, C.; Zhang, S.; Huang, S.; Zhang, F.; Wang, X. Modification of Serine 1040 of SIBRI1 Increases Fruit Yield by Enhancing Tolerance to Heat Stress in Tomato. Int. J. Mol. Sci. 2020, 21, 7681. [Google Scholar] [CrossRef] [PubMed]
- Giovannoni, J.J. Genetic regulation of fruit development and ripening. Plant Cell 2004, 16, S170–S180. [Google Scholar] [CrossRef] [Green Version]
- Morinaka, Y.; Sakamoto, T.; Inukai, Y.; Agetsuma, M.; Kitano, H.; Ashikari, M.; Matsuoka, M. Morphological alteration caused by brassinosteroid insensitivity increases the biomass and grain production of rice. Plant Physiol. 2006, 141, 924–931. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chono, M.; Honda, I.; Zeniya, H.; Yoneyama, K.; Saisho, D.; Takeda, K.; Takatsuto, S.; Hoshino, T.; Watanabe, Y. A semidwarf phenotype of barley uzu results from a nucleotide substitution in the gene encoding a putative brassinosteroid receptor. Plant Physiol. 2003, 133, 1209–1219. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Honda, I.; Zeniya, H.; Yoneyama, K.; Chono, M.; Kaneko, S.; Watanabe, Y. Uzu mutation in barley (Hordeum vulgare L.) reduces the leaf unrolling response to brassinolide. Biosci. Biotech. Biochem. 2003, 67, 1194–1197. [Google Scholar] [CrossRef] [Green Version]
- Gillaspy, G.; Bendavid, H.; Gruissem, W. Fruits—A Developmental Perspective. Plant Cell 1993, 5, 1439–1451. [Google Scholar] [CrossRef] [Green Version]
- Jiang, J.R.; Clouse, S.D. Expression of a plant gene with sequence similarity to animal TGF-beta receptor interacting protein is regulated by brassinosteroids and required for normal plant development. Plant J. 2001, 26, 35–45. [Google Scholar] [CrossRef]
- Joubes, J.; Walsh, D.; Raymond, P.; Chevalier, C. Molecular characterization of the expression of distinct classes of cyclins during the early development of tomato fruit. Planta 2000, 211, 430–439. [Google Scholar] [CrossRef] [PubMed]
- Baldet, P.; Hernould, M.; Laporte, F.; Mounet, F.; Just, D.; Mouras, A.; Chevalier, C.; Rothan, C. The expression of cell proliferation-related genes in early developing flowers is affected by a fruit load reduction in tomato plants. J. Exp. Bot. 2006, 57, 961–970. [Google Scholar] [CrossRef] [Green Version]
- Czerednik, A.; Busscher, M.; Bielen, B.A.M.; Wolters-Arts, M.; de Maagd, R.A.; Angenent, G.C. Regulation of tomato fruit pericarp development by an interplay between CDKB and CDKA1 cell cycle genes. J. Exp. Bot. 2012, 63, 2605–2617. [Google Scholar] [CrossRef] [Green Version]
- Andersen, S.U.; Buechel, S.; Zhao, Z.; Ljung, K.; Novak, O.; Busch, W.; Schuster, C.; Lohmann, J.U. Requirement of B2-type cyclin-dependent kinases for meristem integrity in Arabidopsis thaliana. Plant Cell 2008, 20, 88–100. [Google Scholar] [CrossRef] [Green Version]
- Gruszka, D. The Brassinosteroid Signaling Pathway-New Key Players and Interconnections with Other Signaling Networks Crucial for Plant Development and Stress Tolerance. Int. J. Mol. Sci. 2013, 14, 8740–8774. [Google Scholar] [CrossRef] [Green Version]
- Baghel, M.; Nagaraja, A.; Srivastav, M.; Meena, N.K.; Kumar, M.S.; Kumar, A.; Sharma, R.R. Pleiotropic influences of brassinosteroids on fruit crops: A review. Plant Growth Regul. 2019, 87, 375–388. [Google Scholar] [CrossRef]
- Clouse, S.D.; Sasse, J.M. Brassinosteroids: Essential regulators of plant growth and development. Annu. Rev. Plant Phys. 1998, 49, 427–451. [Google Scholar] [CrossRef] [Green Version]
- Clouse, S. Brassinosteroids. Curr. Biol. 2001, 11, R904. [Google Scholar] [CrossRef] [Green Version]
- Wang, F.R.; Bai, M.Y.; Deng, Z.P.; Oses-Prieto, J.A.; Burlingame, A.L.; Lu, T.G.; Chong, K.; Wang, Z.Y. Proteomic Study Identifies Proteins Involved in Brassinosteroid Regulation of Rice Growth. J. Integr. plant Biol. 2010, 52, 1075–1085. [Google Scholar] [CrossRef] [PubMed]
- Montoya, T.; Nomura, T.; Farrar, K.; Kaneta, T.; Yokota, T.; Bishop, G.J. Cloning the tomato curl3 gene highlights the putative dual role of the leucine-rich repeat receptor kinase tBRI1/SR160 in plant steroid hormone and peptide hormone signaling. Plant Cell 2002, 14, 3163–3176. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mathur, J.; Molnar, G.; Fujioka, S.; Takatsuto, S.; Sakurai, A.; Yokota, T.; Adam, G.; Voigt, B.; Nagy, F.; Maas, C.; et al. Transcription of the Arabidopsis CPD gene, encoding a steroidogenic cytochrome P450, is negatively controlled by brassinosteroids. Plant J. 1998, 14, 593–602. [Google Scholar] [CrossRef] [Green Version]
- Youn, J.H.; Kim, T.W.; Joo, S.H.; Son, S.H.; Roh, J.; Kim, S.; Kim, T.W.; Kim, S.K. Function and molecular regulation of DWARF1 as a C-24 reductase in brassinosteroid biosynthesis in Arabidopsis. J. Exp. Bot. 2018, 69, 1873–1886. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fenn, M.A.; Giovannoni, J.J. Phytohormones in fruit development and maturation. Plant J. 2021, 105, 446–458. [Google Scholar] [CrossRef]
- Depuydt, S.; Hardtke, C.S. Hormone Signalling Crosstalk in Plant Growth Regulation. Curr. Biol. 2011, 21, R365–R373. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, K.; Nakamura, Y.; Asami, T.; Yoshida, S.; Matsuo, T.; Okamoto, S. Physiological roles of brassinosteroids in early growth of Arabidopsis: Brassinosteroids have a synergistic relationship with gibberellin as well as auxin in light-grown hypocotyl elongation. J. Plant Growth Regul. 2003, 22, 259–271. [Google Scholar] [CrossRef]
- Hu, J.H.; Israeli, A.; Ori, N.; Sun, T.P. The Interaction between DELLA and ARF/IAA Mediates Crosstalk between Gibberellin and Auxin Signaling to Control Fruit Initiation in Tomato. Plant Cell 2018, 30, 1710–1728. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Lucas, M.; Daviere, J.M.; Rodriguez-Falcon, M.; Pontin, M.; Iglesias-Pedraz, J.M.; Lorrain, S.; Fankhauser, C.; Blazquez, M.A.; Titarenko, E.; Prat, S. A molecular framework for light and gibberellin control of cell elongation. Nature 2008, 451, 480–484. [Google Scholar] [CrossRef] [PubMed]
- Bai, M.Y.; Shang, J.X.; Oh, E.; Fan, M.; Bai, Y.; Zentella, R.; Sun, T.P.; Wang, Z.Y. Brassinosteroid, gibberellin and phytochrome impinge on a common transcription module in Arabidopsis. Nat. Cell Biol. 2012, 14, 810–817. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shinozaki, Y.; Ezura, K.; Hu, J.H.; Okabe, Y.; Benard, C.; Prodhomme, D.; Gibon, Y.; Sun, T.P.; Ezura, H.; Ariizumi, T. Identification and functional study of a mild allele of SlDELLA gene conferring the potential for improved yield in tomato. Sci. Rep. 2018, 8, 12043. [Google Scholar] [CrossRef] [PubMed]
- Carrera, E.; Ruiz-Rivero, O.; Peres, L.E.P.; Atares, A.; Garcia-Martinez, J.L. Characterization of the procera Tomato Mutant Shows Novel Functions of the SlDELLA Protein in the Control of Flower Morphology, Cell Division and Expansion, and the Auxin-Signaling Pathway during Fruit-Set and Development. Plant Physiol. 2012, 160, 1581–1596. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sun, T.P.; Gubler, F. Molecular mechanism of gibberellin signaling in plants. Annu. Rev. Plant Biol. 2004, 55, 197–223. [Google Scholar] [CrossRef] [Green Version]
- Griffiths, J.; Murase, K.; Rieu, I.; Zentella, R.; Zhang, Z.L.; Powers, S.J.; Gong, F.; Phillips, A.L.; Hedden, P.; Sun, T.P.; et al. Genetic characterization and functional analysis of the GID1 gibberellin receptors in Arabidopsis. Plant Cell 2006, 18, 3399–3414. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hirano, K.; Ueguchi-Tanaka, M.; Matsuoka, M. GID1-mediated gibberellin signaling in plants. Trends Plant Sci. 2008, 13, 192–199. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Yu, C.; Wu, H.; Luo, Z.; Ouyang, B.; Cui, L.; Zhang, J.; Ye, Z. Knockdown of a JmjC domain-containing gene JMJ524 confers altered gibberellin responses by transcriptional regulation of GRAS protein lacking the DELLA domain genes in tomato. J. Exp. Bot. 2015, 66, 1413–1426. [Google Scholar] [CrossRef] [Green Version]
- Li, J.; Sima, W.; Ouyang, B.; Wang, T.; Ziaf, K.; Luo, Z.; Liu, L.; Li, H.; Chen, M.; Huang, Y.; et al. Tomato SlDREB gene restricts leaf expansion and internode elongation by downregulating key genes for gibberellin biosynthesis. J. Exp. Bot. 2012, 63, 6407–6420. [Google Scholar] [CrossRef] [PubMed]
- Vert, G.; Walcher, C.L.; Chory, J.; Nemhauser, J.L. Integration of auxin and brassinosteroid pathways by Auxin Response Factor 2. Proc. Natl. Acad. Sci. USA 2008, 105, 9829–9834. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Frary, A.; Nesbitt, T.C.; Frary, A.; Grandillo, S.; van der Knaap, E.; Cong, B.; Liu, J.P.; Meller, J.; Elber, R.; Alpert, K.B.; et al. fw2.2: A quantitative trait locus key to the evolution of tomato fruit size. Science 2000, 289, 85–88. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Park, C.H.; Kim, T.W.; Son, S.H.; Hwang, J.Y.; Lee, S.C.; Chang, S.C.; Kim, S.H.; Kim, S.W.; Kim, S.K. Brassinosteroids control AtEXPA5 gene expression in Arabidopsis thaliana. Phytochemistry 2010, 71, 380–387. [Google Scholar] [CrossRef] [PubMed]
- Park, S.H.; Morris, J.L.; Park, J.E.; Hirschi, K.D.; Smith, R.H. Efficient and genotype-independent Agrobacterium-mediated tomato transformation. J. Plant Physiol. 2003, 160, 1253–1257. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Jong, M.; Wolters-Arts, M.; Garcia-Martinez, J.L.; Mariani, C.; Vriezen, W.H. The Solanum lycopersicum AUXIN RESPONSE FACTOR 7 (SlARF7) mediates cross-talk between auxin and gibberellin signalling during tomato fruit set and development. J. Exp. Bot. 2011, 62, 617–626. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, J.; Wen, J.Q.; Lease, K.A.; Doke, J.T.; Tax, F.E.; Walker, J.C. BAK1, an Arabidopsis LRR receptor-like protein kinase, interacts with BRI1 and modulates brassinosteroid signaling. Cell 2002, 110, 213–222. [Google Scholar] [CrossRef] [Green Version]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, S.; Lv, S.; Zhao, T.; Jiang, M.; Liu, D.; Fu, S.; Hu, M.; Huang, S.; Pei, Y.; Wang, X. Modification of Threonine-825 of SlBRI1 Enlarges Cell Size to Enhance Fruit Yield by Regulating the Cooperation of BR-GA Signaling in Tomato. Int. J. Mol. Sci. 2021, 22, 7673. https://doi.org/10.3390/ijms22147673
Wang S, Lv S, Zhao T, Jiang M, Liu D, Fu S, Hu M, Huang S, Pei Y, Wang X. Modification of Threonine-825 of SlBRI1 Enlarges Cell Size to Enhance Fruit Yield by Regulating the Cooperation of BR-GA Signaling in Tomato. International Journal of Molecular Sciences. 2021; 22(14):7673. https://doi.org/10.3390/ijms22147673
Chicago/Turabian StyleWang, Shufen, Siqi Lv, Tong Zhao, Meng Jiang, Dehai Liu, Shangtan Fu, Miaomiao Hu, Shuhua Huang, Yu Pei, and Xiaofeng Wang. 2021. "Modification of Threonine-825 of SlBRI1 Enlarges Cell Size to Enhance Fruit Yield by Regulating the Cooperation of BR-GA Signaling in Tomato" International Journal of Molecular Sciences 22, no. 14: 7673. https://doi.org/10.3390/ijms22147673
APA StyleWang, S., Lv, S., Zhao, T., Jiang, M., Liu, D., Fu, S., Hu, M., Huang, S., Pei, Y., & Wang, X. (2021). Modification of Threonine-825 of SlBRI1 Enlarges Cell Size to Enhance Fruit Yield by Regulating the Cooperation of BR-GA Signaling in Tomato. International Journal of Molecular Sciences, 22(14), 7673. https://doi.org/10.3390/ijms22147673








