The Effects of Oxytocin on Appetite Regulation, Food Intake and Metabolism in Humans
Abstract
:1. Introduction
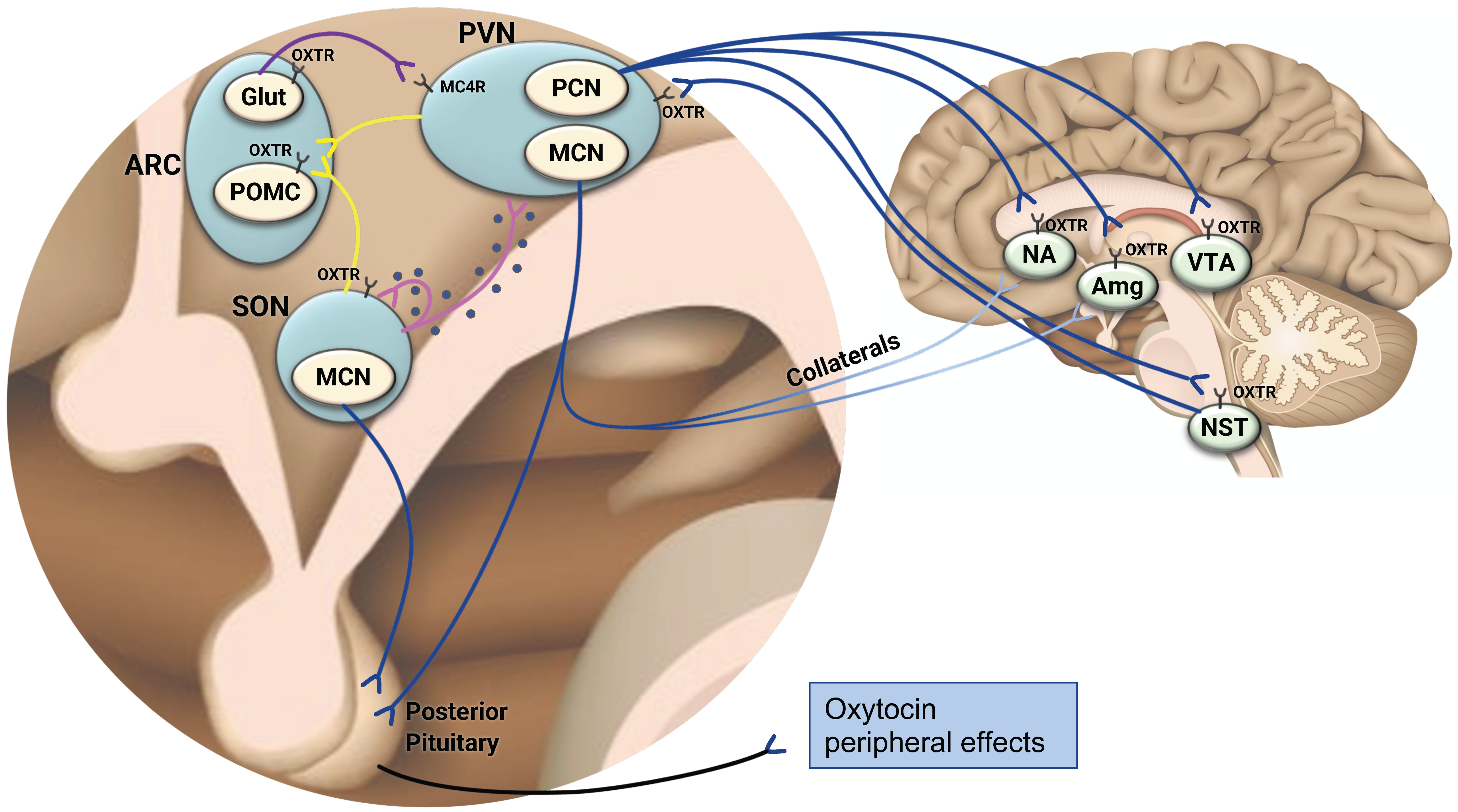
2. The Central Oxytocinergic System—A Framework to Understand the Role of Oxytocin in the Coordination of Energy Balance
3. The Distribution of the Oxytocin Receptor in the Brain—Further Evidence Linking Oxytocin and Appetite Regulation
4. The Oxytocin Receptor in the Periphery—Expression in Key Metabolic Organs
5. Data from Animal Models-Paving the Path to Oxytocin Translational Research
5.1. Oxytocin Administration in Animal Studies
5.2. Oxytocin and Oxytocin Receptor Gene Knockout Experiments
6. Observational Studies in Humans-Relation of Oxytocin to Food Intake, Weight Status and Metabolism
6.1. Characterization of Endogenous Oxytocin Levels in Humans—Important Technical Aspects and Challenges
6.2. Oxytocin Measurement in Humans
6.3. Sex Differences in the Oxytocin System
6.4. The Relation between Peripheral Oxytocin Levels and Central Oxytocin Activity
6.5. Alterations of Endogenous Oxytocin Levels in the Setting of Obesity and Metabolic Syndrome
6.6. The Relation of Peripheral Oxytocin Levels to Bone Health
7. Interventional Studies of Oxytocin Administration in Humans
7.1. Effects of Oxytocin Administration on Eating Behavior, Related Neurocircuitry, and Metabolism
7.2. Oxytocin in Individuals with Obesity Associated with Hypothalamic and Pituitary Impairment
8. Oxytocin as a Therapeutic Agent in Obesity and Metabolic Syndrome—Promise, Fundamental Challenges and Future Directions
Author Contributions
Funding
Conflicts of Interest
References
- Dale, H.H. On some physiological actions of ergot. J. Physiol. 1906, 34, 163–206. [Google Scholar] [CrossRef] [PubMed]
- Ott, I.; Scott, J.C. The action of infundibulin upon the mammary secretion. Proc. Soc. Exp. Biol. Med. 1910, 8, 48–49. [Google Scholar] [CrossRef] [Green Version]
- Lee, H.J.; Macbeth, A.H.; Pagani, J.H.; Young, W.S., 3rd. Oxytocin: The great facilitator of life. Prog. Neurobiol. 2009, 88, 127–151. [Google Scholar] [CrossRef] [Green Version]
- Carter, C.S.; Kenkel, W.M.; MacLean, E.L.; Wilson, S.R.; Perkeybile, A.M.; Yee, J.R.; Ferris, C.F.; Nazarloo, H.P.; Porges, S.W.; Davis, J.M.; et al. Is Oxytocin “Nature’s Medicine”? Pharmacol. Rev. 2020, 72, 829–861. [Google Scholar] [CrossRef]
- McKay, E.C.; Counts, S.E. Oxytocin Receptor Signaling in Vascular Function and Stroke. Front. Neurosci. 2020, 14, 574499. [Google Scholar] [CrossRef]
- Jurek, B.; Neumann, I.D. The Oxytocin Receptor: From Intracellular Signaling to Behavior. Physiol. Rev. 2018, 98, 1805–1908. [Google Scholar] [CrossRef]
- Grinevich, V.; Neumann, I.D. Brain oxytocin: How puzzle stones from animal studies translate into psychiatry. Mol. Psychiatry 2021, 26, 265–279. [Google Scholar] [CrossRef]
- Churchland, P.S.; Winkielman, P. Modulating social behavior with oxytocin: How does it work? What does it mean? Horm. Behav. 2012, 61, 392–399. [Google Scholar] [CrossRef] [Green Version]
- McCormack, S.E.; Blevins, J.E.; Lawson, E.A. Metabolic Effects of Oxytocin. Endocr. Rev. 2020, 41. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ding, C.; Leow, M.K.; Magkos, F. Oxytocin in metabolic homeostasis: Implications for obesity and diabetes management. Obes. Rev. 2019, 20, 22–40. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Romano, A.; Friuli, M.; Cifani, C.; Gaetani, S. Oxytocin in the neural control of eating: At the crossroad between homeostatic and non-homeostatic signals. Neuropharmacology 2020, 171, 108082. [Google Scholar] [CrossRef] [PubMed]
- Lawson, E.A.; Olszewski, P.K.; Weller, A.; Blevins, J.E. The role of oxytocin in regulation of appetitive behaviour, body weight and glucose homeostasis. J. Neuroendocr. 2020, 32, e12805. [Google Scholar] [CrossRef] [PubMed]
- Blevins, J.E.; Ho, J.M. Role of oxytocin signaling in the regulation of body weight. Rev. Endocr. Metab. Disord. 2013, 14, 311–329. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bluher, M. Obesity: Global epidemiology and pathogenesis. Nat. Rev. Endocrinol. 2019, 15, 288–298. [Google Scholar] [CrossRef]
- Maejima, Y.; Yokota, S.; Nishimori, K.; Shimomura, K. The Anorexigenic Neural Pathways of Oxytocin and Their Clinical Implication. Neuroendocrinology 2018, 107, 91–104. [Google Scholar] [CrossRef]
- Gainer, H. Cell-type specific expression of oxytocin and vasopressin genes: An experimental odyssey. J. Neuroendocr. 2012, 24, 528–538. [Google Scholar] [CrossRef] [Green Version]
- Schwartz, M.W.; Woods, S.C.; Porte, D., Jr.; Seeley, R.J.; Baskin, D.G. Central nervous system control of food intake. Nature 2000, 404, 661–671. [Google Scholar] [CrossRef]
- Cornejo, M.P.; Hentges, S.T.; Maliqueo, M.; Coirini, H.; Becu-Villalobos, D.; Elias, C.F. Neuroendocrine Regulation of Metabolism. J. Neuroendocr. 2016, 28. [Google Scholar] [CrossRef] [Green Version]
- Ludwig, M.; Leng, G. Dendritic peptide release and peptide-dependent behaviours. Nat. Rev. Neurosci. 2006, 7, 126–136. [Google Scholar] [CrossRef]
- Rosen, G.J.; de Vries, G.J.; Goldman, S.L.; Goldman, B.D.; Forger, N.G. Distribution of oxytocin in the brain of a eusocial rodent. Neuroscience 2008, 155, 809–817. [Google Scholar] [CrossRef] [Green Version]
- Brown, C.H.; Ludwig, M.; Tasker, J.G.; Stern, J.E. Somato-dendritic vasopressin and oxytocin secretion in endocrine and autonomic regulation. J. Neuroendocr. 2020, 32, e12856. [Google Scholar] [CrossRef]
- Pow, D.V.; Morris, J.F. Dendrites of hypothalamic magnocellular neurons release neurohypophysial peptides by exocytosis. Neuroscience 1989, 32, 435–439. [Google Scholar] [CrossRef]
- Ludwig, M.; Stern, J. Multiple signalling modalities mediated by dendritic exocytosis of oxytocin and vasopressin. Philos. Trans. R. Soc. B Biol. Sci. 2015, 370, 20140182. [Google Scholar] [CrossRef] [Green Version]
- Bealer, S.L.; Armstrong, W.E.; Crowley, W.R. Oxytocin release in magnocellular nuclei: Neurochemical mediators and functional significance during gestation. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2010, 299, R452–R458. [Google Scholar] [CrossRef]
- Meyer-Lindenberg, A.; Domes, G.; Kirsch, P.; Heinrichs, M. Oxytocin and vasopressin in the human brain: Social neuropeptides for translational medicine. Nat. Rev. Neurosci. 2011, 12, 524–538. [Google Scholar] [CrossRef]
- Zhang, B.; Qiu, L.; Xiao, W.; Ni, H.; Chen, L.; Wang, F.; Mai, W.; Wu, J.; Bao, A.; Hu, H.; et al. Reconstruction of the Hypothalamo-Neurohypophysial System and Functional Dissection of Magnocellular Oxytocin Neurons in the Brain. Neuron 2021, 109, 331–346.e7. [Google Scholar] [CrossRef] [PubMed]
- Maejima, Y.; Sakuma, K.; Santoso, P.; Gantulga, D.; Katsurada, K.; Ueta, Y.; Hiraoka, Y.; Nishimori, K.; Tanaka, S.; Shimomura, K.; et al. Oxytocinergic circuit from paraventricular and supraoptic nuclei to arcuate POMC neurons in hypothalamus. FEBS Lett. 2014, 588, 4404–4412. [Google Scholar] [CrossRef] [Green Version]
- Roh, E.; Song, D.K.; Kim, M.S. Emerging role of the brain in the homeostatic regulation of energy and glucose metabolism. Exp. Mol. Med. 2016, 48, e216. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Miller, G.D. Appetite Regulation: Hormones, Peptides, and Neurotransmitters and Their Role in Obesity. Am. J. Lifestyle Med. 2019, 13, 586–601. [Google Scholar] [CrossRef] [PubMed]
- Grinevich, V.; Knobloch-Bollmann, H.S.; Eliava, M.; Busnelli, M.; Chini, B. Assembling the Puzzle: Pathways of Oxytocin Signaling in the Brain. Biol. Psychiatry 2016, 79, 155–164. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ross, H.E.; Cole, C.D.; Smith, Y.; Neumann, I.D.; Landgraf, R.; Murphy, A.Z.; Young, L.J. Characterization of the oxytocin system regulating affiliative behavior in female prairie voles. Neuroscience 2009, 162, 892–903. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Meye, F.J.; Adan, R.A. Feelings about food: The ventral tegmental area in food reward and emotional eating. Trends Pharmacol. Sci. 2014, 35, 31–40. [Google Scholar] [CrossRef] [PubMed]
- Scofield, M.D.; Heinsbroek, J.A.; Gipson, C.D.; Kupchik, Y.M.; Spencer, S.; Smith, A.C.; Roberts-Wolfe, D.; Kalivas, P.W. The Nucleus Accumbens: Mechanisms of Addiction across Drug Classes Reflect the Importance of Glutamate Homeostasis. Pharmacol. Rev. 2016, 68, 816–871. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Raam, T. Oxytocin-Sensitive Neurons in Prefrontal Cortex Gate Social Recognition Memory. J. Neurosci. 2020, 40, 1194–1196. [Google Scholar] [CrossRef]
- Olivera-Pasilio, V.; Dabrowska, J. Oxytocin Promotes Accurate Fear Discrimination and Adaptive Defensive Behaviors. Front. Neurosci. 2020, 14, 583878. [Google Scholar] [CrossRef]
- Douglass, A.M.; Kucukdereli, H.; Ponserre, M.; Markovic, M.; Grundemann, J.; Strobel, C.; Alcala Morales, P.L.; Conzelmann, K.K.; Luthi, A.; Klein, R. Central amygdala circuits modulate food consumption through a positive-valence mechanism. Nat. Neurosci. 2017, 20, 1384–1394. [Google Scholar] [CrossRef]
- Rinaman, L. Oxytocinergic inputs to the nucleus of the solitary tract and dorsal motor nucleus of the vagus in neonatal rats. J. Comp. Neurol. 1998, 399, 101–109. [Google Scholar] [CrossRef]
- Grill, H.J.; Hayes, M.R. Hindbrain neurons as an essential hub in the neuroanatomically distributed control of energy balance. Cell Metab. 2012, 16, 296–309. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xiao, L.; Priest, M.F.; Nasenbeny, J.; Lu, T.; Kozorovitskiy, Y. Biased Oxytocinergic Modulation of Midbrain Dopamine Systems. Neuron 2017, 95, 368–384.e5. [Google Scholar] [CrossRef] [Green Version]
- Hasan, M.T.; Althammer, F.; Silva da Gouveia, M.; Goyon, S.; Eliava, M.; Lefevre, A.; Kerspern, D.; Schimmer, J.; Raftogianni, A.; Wahis, J.; et al. A Fear Memory Engram and Its Plasticity in the Hypothalamic Oxytocin System. Neuron 2019, 103, 133–146.e8. [Google Scholar] [CrossRef] [PubMed]
- Fenselau, H.; Campbell, J.N.; Verstegen, A.M.; Madara, J.C.; Xu, J.; Shah, B.P.; Resch, J.M.; Yang, Z.; Mandelblat-Cerf, Y.; Livneh, Y.; et al. A rapidly acting glutamatergic ARC-->PVH satiety circuit postsynaptically regulated by alpha-MSH. Nat. Neurosci. 2017, 20, 42–51. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brown, C.H.; Bains, J.S.; Ludwig, M.; Stern, J.E. Physiological regulation of magnocellular neurosecretory cell activity: Integration of intrinsic, local and afferent mechanisms. J. Neuroendocr. 2013, 25, 678–710. [Google Scholar] [CrossRef] [Green Version]
- Ohlsson, B.; Truedsson, M.; Djerf, P.; Sundler, F. Oxytocin is expressed throughout the human gastrointestinal tract. Regul. Pept. 2006, 135, 7–11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Colaianni, G.; Sun, L.; Di Benedetto, A.; Tamma, R.; Zhu, L.L.; Cao, J.; Grano, M.; Yuen, T.; Colucci, S.; Cuscito, C.; et al. Bone marrow oxytocin mediates the anabolic action of estrogen on the skeleton. J. Biol. Chem. 2012, 287, 29159–29167. [Google Scholar] [CrossRef] [Green Version]
- Leng, G.; Sabatier, N. Oxytocin-The Sweet Hormone? Trends Endocrinol. Metab. 2017, 28, 365–376. [Google Scholar] [CrossRef] [Green Version]
- Kingsbury, M.A.; Bilbo, S.D. The inflammatory event of birth: How oxytocin signaling may guide the development of the brain and gastrointestinal system. Front. Neuroendocr. 2019, 55, 100794. [Google Scholar] [CrossRef] [PubMed]
- Dumais, K.M.; Veenema, A.H. Presence and Absence of Sex Differences in Structure and Function of the Brain Oxytocin System: Implications for Understanding the Regulation of Social Behavior. In Sex Differences in the Central Nervous System; Shansky, R.M., Ed.; Academic Press: Cambridge, MA, USA, 2016; pp. 247–295. [Google Scholar]
- Grund, T.; Tang, Y.; Benusiglio, D.; Althammer, F.; Probst, S.; Oppenlander, L.; Neumann, I.D.; Grinevich, V. Chemogenetic activation of oxytocin neurons: Temporal dynamics, hormonal release, and behavioral consequences. Psychoneuroendocrinology 2019, 106, 77–84. [Google Scholar] [CrossRef] [PubMed]
- Winterton, A.; Westlye, L.T.; Steen, N.E.; Andreassen, O.A.; Quintana, D.S. Improving the precision of intranasal oxytocin research. Nat. Hum. Behav. 2021, 5, 9–18. [Google Scholar] [CrossRef]
- Gimpl, G.; Fahrenholz, F. The oxytocin receptor system: Structure, function, and regulation. Physiol. Rev. 2001, 81, 629–683. [Google Scholar] [CrossRef] [Green Version]
- Liu, C.M.; Davis, E.A.; Suarez, A.N.; Wood, R.I.; Noble, E.E.; Kanoski, S.E. Sex Differences and Estrous Influences on Oxytocin Control of Food Intake. Neuroscience 2020, 447, 63–73. [Google Scholar] [CrossRef]
- Boccia, M.L.; Petrusz, P.; Suzuki, K.; Marson, L.; Pedersen, C.A. Immunohistochemical localization of oxytocin receptors in human brain. Neuroscience 2013, 253, 155–164. [Google Scholar] [CrossRef]
- Quintana, D.S.; Rokicki, J.; van der Meer, D.; Alnaes, D.; Kaufmann, T.; Cordova-Palomera, A.; Dieset, I.; Andreassen, O.A.; Westlye, L.T. Oxytocin pathway gene networks in the human brain. Nat. Commun. 2019, 10, 668. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schroeder, L.E.; Leinninger, G.M. Role of central neurotensin in regulating feeding: Implications for the development and treatment of body weight disorders. Biochim. Biophys. Acta Mol. Basis Dis. 2018, 1864, 900–916. [Google Scholar] [CrossRef] [PubMed]
- Mathioudakis, L.; Bourbouli, M.; Daklada, E.; Kargatzi, S.; Michaelidou, K.; Zaganas, I. Localization of Human Glutamate Dehydrogenases Provides Insights into Their Metabolic Role and Their Involvement in Disease Processes. Neurochem. Res. 2019, 44, 170–187. [Google Scholar] [CrossRef] [PubMed]
- Frank, S.; Kullmann, S.; Veit, R. Food related processes in the insular cortex. Front. Hum. Neurosci. 2013, 7, 499. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lin, Y.T.; Chen, C.C.; Huang, C.C.; Nishimori, K.; Hsu, K.S. Oxytocin stimulates hippocampal neurogenesis via oxytocin receptor expressed in CA3 pyramidal neurons. Nat. Commun. 2017, 8, 537. [Google Scholar] [CrossRef]
- Rubin, R.D.; Watson, P.D.; Duff, M.C.; Cohen, N.J. The role of the hippocampus in flexible cognition and social behavior. Front. Hum. Neurosci. 2014, 8. [Google Scholar] [CrossRef] [Green Version]
- Allman, J.M.; Hakeem, A.; Erwin, J.M.; Nimchinsky, E.; Hof, P. The anterior cingulate cortex. The evolution of an interface between emotion and cognition. Ann. N. Y. Acad. Sci. 2001, 935, 107–117. [Google Scholar] [CrossRef]
- Yuan, P.; Raz, N. Prefrontal cortex and executive functions in healthy adults: A meta-analysis of structural neuroimaging studies. Neurosci. Biobehav. Rev. 2014, 42, 180–192. [Google Scholar] [CrossRef] [Green Version]
- Newman, L.A.; Creer, D.J.; McGaughy, J.A. Cognitive control and the anterior cingulate cortex: How conflicting stimuli affect attentional control in the rat. J. Physiol. Paris 2015, 109, 95–103. [Google Scholar] [CrossRef] [Green Version]
- Miller, E.K. The prefrontal cortex and cognitive control. Nat. Rev. Neurosci. 2000, 1, 59–65. [Google Scholar] [CrossRef] [PubMed]
- Kimura, T.; Saji, F.; Nishimori, K.; Ogita, K.; Nakamura, H.; Koyama, M.; Murata, Y. Molecular regulation of the oxytocin receptor in peripheral organs. J. Mol. Endocrinol. 2003, 30, 109–115. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Welch, M.G.; Margolis, K.G.; Li, Z.; Gershon, M.D. Oxytocin regulates gastrointestinal motility, inflammation, macromolecular permeability, and mucosal maintenance in mice. Am. J. Physiol. Gastrointest. Liver Physiol. 2014, 307, G848–G862. [Google Scholar] [CrossRef] [Green Version]
- Welch, M.G.; Tamir, H.; Gross, K.J.; Chen, J.; Anwar, M.; Gershon, M.D. Expression and developmental regulation of oxytocin (OT) and oxytocin receptors (OTR) in the enteric nervous system (ENS) and intestinal epithelium. J. Comp. Neurol. 2009, 512, 256–270. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Katsurada, K.; Maejima, Y.; Nakata, M.; Kodaira, M.; Suyama, S.; Iwasaki, Y.; Kario, K.; Yada, T. Endogenous GLP-1 acts on paraventricular nucleus to suppress feeding: Projection from nucleus tractus solitarius and activation of corticotropin-releasing hormone, nesfatin-1 and oxytocin neurons. Biochem. Biophys. Res. Commun. 2014, 451, 276–281. [Google Scholar] [CrossRef]
- Sabatier, N.; Leng, G.; Menzies, J. Oxytocin, feeding, and satiety. Front. Endocrinol. 2013, 4, 35. [Google Scholar] [CrossRef] [Green Version]
- Everett, N.A.; Turner, A.J.; Costa, P.A.; Baracz, S.J.; Cornish, J.L. The vagus nerve mediates the suppressing effects of peripherally administered oxytocin on methamphetamine self-administration and seeking in rats. Neuropsychopharmacology 2021, 46, 297–304. [Google Scholar] [CrossRef]
- Colaianni, G.; Sun, L.; Zaidi, M.; Zallone, A. The “love hormone” oxytocin regulates the loss and gain of the fat-bone relationship. Front. Endocrinol. 2015, 6, 79. [Google Scholar] [CrossRef] [Green Version]
- Breton, C.; Haenggeli, C.; Barberis, C.; Heitz, F.; Bader, C.R.; Bernheim, L.; Tribollet, E. Presence of functional oxytocin receptors in cultured human myoblasts. J. Clin. Endocrinol. Metab. 2002, 87, 1415–1418. [Google Scholar] [CrossRef]
- Elabd, C.; Basillais, A.; Beaupied, H.; Breuil, V.; Wagner, N.; Scheideler, M.; Zaragosi, L.E.; Massiera, F.; Lemichez, E.; Trajanoski, Z.; et al. Oxytocin controls differentiation of human mesenchymal stem cells and reverses osteoporosis. Stem Cells 2008, 26, 2399–2407. [Google Scholar] [CrossRef] [Green Version]
- Kim, Y.S.; Ahn, Y.; Kwon, J.S.; Cho, Y.K.; Jeong, M.H.; Cho, J.G.; Park, J.C.; Kang, J.C. Priming of mesenchymal stem cells with oxytocin enhances the cardiac repair in ischemia/reperfusion injury. Cells Tissues Organs 2012, 195, 428–442. [Google Scholar] [CrossRef]
- Elabd, C.; Cousin, W.; Upadhyayula, P.; Chen, R.Y.; Chooljian, M.S.; Li, J.; Kung, S.; Jiang, K.P.; Conboy, I.M. Oxytocin is an age-specific circulating hormone that is necessary for muscle maintenance and regeneration. Nat. Commun. 2014, 5, 4082. [Google Scholar] [CrossRef] [Green Version]
- Kim, G.; Kim, J.H. Impact of Skeletal Muscle Mass on Metabolic Health. Endocrinol. Metab. 2020, 35, 1–6. [Google Scholar] [CrossRef]
- Colucci, S.; Colaianni, G.; Mori, G.; Grano, M.; Zallone, A. Human osteoclasts express oxytocin receptor. Biochem. Biophys. Res. Commun. 2002, 297, 442–445. [Google Scholar] [CrossRef]
- Colaianni, G.; Sun, L.; Zaidi, M.; Zallone, A. Oxytocin and bone. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2014, 307, R970–R977. [Google Scholar] [CrossRef]
- Tamma, R.; Colaianni, G.; Zhu, L.L.; DiBenedetto, A.; Greco, G.; Montemurro, G.; Patano, N.; Strippoli, M.; Vergari, R.; Mancini, L.; et al. Oxytocin is an anabolic bone hormone. Proc. Natl. Acad. Sci. USA 2009, 106, 7149–7154. [Google Scholar] [CrossRef] [Green Version]
- Sun, L.; Lizneva, D.; Ji, Y.; Colaianni, G.; Hadelia, E.; Gumerova, A.; Ievleva, K.; Kuo, T.C.; Korkmaz, F.; Ryu, V.; et al. Oxytocin regulates body composition. Proc. Natl. Acad. Sci. USA 2019. [Google Scholar] [CrossRef]
- Kovacs, C.S.; Fuleihan Gel, H. Calcium and bone disorders during pregnancy and lactation. Endocrinol. Metab. Clin. N. Am. 2006, 35, 21–51. [Google Scholar] [CrossRef]
- Yi, K.J.; So, K.H.; Hata, Y.; Suzuki, Y.; Kato, D.; Watanabe, K.; Aso, H.; Kasahara, Y.; Nishimori, K.; Chen, C.; et al. The regulation of oxytocin receptor gene expression during adipogenesis. J. Neuroendocr. 2015, 27, 335–342. [Google Scholar] [CrossRef]
- Yuan, J.; Zhang, R.; Wu, R.; Gu, Y.; Lu, Y. The effects of oxytocin to rectify metabolic dysfunction in obese mice are associated with increased thermogenesis. Mol. Cell Endocrinol. 2020, 514, 110903. [Google Scholar] [CrossRef]
- Roberts, Z.S.; Wolden-Hanson, T.; Matsen, M.E.; Ryu, V.; Vaughan, C.H.; Graham, J.L.; Havel, P.J.; Chukri, D.W.; Schwartz, M.W.; Morton, G.J.; et al. Chronic hindbrain administration of oxytocin is sufficient to elicit weight loss in diet-induced obese rats. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2017, 313, R357–R371. [Google Scholar] [CrossRef] [Green Version]
- Edwards, M.M.; Nguyen, H.K.; Herbertson, A.J.; Dodson, A.D.; Wietecha, T.; Wolden-Hanson, T.; Graham, J.L.; O’Brien, K.D.; Havel, P.J.; Blevins, J.E. Chronic Hindbrain Administration of Oxytocin Elicits Weight Loss in Male Diet-Induced Obese Mice. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2021. [Google Scholar] [CrossRef]
- Ricquier, D. Uncoupling protein 1 of brown adipocytes, the only uncoupler: A historical perspective. Front. Endocrinol. 2011, 2, 85. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rui, L. Brown and Beige Adipose Tissues in Health and Disease. Compr. Physiol. 2017, 7, 1281–1306. [Google Scholar] [CrossRef] [PubMed]
- Xi, D.; Long, C.; Lai, M.; Casella, A.; O’Lear, L.; Kublaoui, B.; Roizen, J.D. Ablation of Oxytocin Neurons Causes a Deficit in Cold Stress Response. J. Endocr. Soc. 2017, 1, 1041–1055. [Google Scholar] [CrossRef] [Green Version]
- Seale, P.; Bjork, B.; Yang, W.; Kajimura, S.; Chin, S.; Kuang, S.; Scime, A.; Devarakonda, S.; Conroe, H.M.; Erdjument-Bromage, H.; et al. PRDM16 controls a brown fat/skeletal muscle switch. Nature 2008, 454, 961–967. [Google Scholar] [CrossRef] [Green Version]
- Harms, M.J.; Ishibashi, J.; Wang, W.; Lim, H.W.; Goyama, S.; Sato, T.; Kurokawa, M.; Won, K.J.; Seale, P. Prdm16 is required for the maintenance of brown adipocyte identity and function in adult mice. Cell Metab. 2014, 19, 593–604. [Google Scholar] [CrossRef] [Green Version]
- Chen, Q.; Shou, P.; Zheng, C.; Jiang, M.; Cao, G.; Yang, Q.; Cao, J.; Xie, N.; Velletri, T.; Zhang, X.; et al. Fate decision of mesenchymal stem cells: Adipocytes or osteoblasts? Cell Death Differ. 2016, 23, 1128–1139. [Google Scholar] [CrossRef] [Green Version]
- Zebisch, K.; Voigt, V.; Wabitsch, M.; Brandsch, M. Protocol for effective differentiation of 3T3-L1 cells to adipocytes. Anal. Biochem. 2012, 425, 88–90. [Google Scholar] [CrossRef]
- Tang, Q.Q.; Otto, T.C.; Lane, M.D. Commitment of C3H10T1/2 pluripotent stem cells to the adipocyte lineage. Proc. Natl. Acad. Sci. USA 2004, 101, 9607–9611. [Google Scholar] [CrossRef] [Green Version]
- Sun, W.; Modica, S.; Dong, H.; Wolfrum, C. Plasticity and heterogeneity of thermogenic adipose tissue. Nat. Metab. 2021, 3, 751–761. [Google Scholar] [CrossRef] [PubMed]
- Plante, E.; Menaouar, A.; Danalache, B.A.; Yip, D.; Broderick, T.L.; Chiasson, J.L.; Jankowski, M.; Gutkowska, J. Oxytocin treatment prevents the cardiomyopathy observed in obese diabetic male db/db mice. Endocrinology 2015, 156, 1416–1428. [Google Scholar] [CrossRef] [PubMed]
- Gajdosechova, L.; Krskova, K.; Segarra, A.B.; Spolcova, A.; Suski, M.; Olszanecki, R.; Zorad, S. Hypooxytocinaemia in obese Zucker rats relates to oxytocin degradation in liver and adipose tissue. J. Endocrinol. 2014, 220, 333–343. [Google Scholar] [CrossRef] [Green Version]
- Kloting, N.; Bluher, M. Adipocyte dysfunction, inflammation and metabolic syndrome. Rev. Endocr. Metab. Disord. 2014, 15, 277–287. [Google Scholar] [CrossRef]
- Esser, N.; Legrand-Poels, S.; Piette, J.; Scheen, A.J.; Paquot, N. Inflammation as a link between obesity, metabolic syndrome and type 2 diabetes. Diabetes Res. Clin. Pract. 2014, 105, 141–150. [Google Scholar] [CrossRef] [Green Version]
- Szeto, A.; Cecati, M.; Ahmed, R.; McCabe, P.M.; Mendez, A.J. Oxytocin reduces adipose tissue inflammation in obese mice. Lipids Health Dis. 2020, 19, 188. [Google Scholar] [CrossRef] [PubMed]
- Deblon, N.; Veyrat-Durebex, C.; Bourgoin, L.; Caillon, A.; Bussier, A.L.; Petrosino, S.; Piscitelli, F.; Legros, J.J.; Geenen, V.; Foti, M.; et al. Mechanisms of the anti-obesity effects of oxytocin in diet-induced obese rats. PLoS ONE 2011, 6, e25565. [Google Scholar] [CrossRef] [Green Version]
- Suzuki, M.; Honda, Y.; Li, M.Z.; Masuko, S.; Murata, Y. The localization of oxytocin receptors in the islets of Langerhans in the rat pancreas. Regul. Pept. 2013, 183, 42–45. [Google Scholar] [CrossRef]
- Mohan, S.; Khan, D.; Moffett, R.C.; Irwin, N.; Flatt, P.R. Oxytocin is present in islets and plays a role in beta-cell function and survival. Peptides 2018, 100, 260–268. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, S.; Wei, F.Y.; Matsunaga, T.; Matsunaga, N.; Kaitsuka, T.; Tomizawa, K. Oxytocin Protects against Stress-Induced Cell Death in Murine Pancreatic beta-Cells. Sci. Rep. 2016, 6, 25185. [Google Scholar] [CrossRef]
- Snider, B.; Geiser, A.; Yu, X.P.; Beebe, E.C.; Willency, J.A.; Qing, K.; Guo, L.; Lu, J.; Wang, X.; Yang, Q.; et al. Long-Acting and Selective Oxytocin Peptide Analogs Show Antidiabetic and Antiobesity Effects in Male Mice. J. Endocr. Soc. 2019, 3, 1423–1444. [Google Scholar] [CrossRef] [Green Version]
- Maejima, Y.; Iwasaki, Y.; Yamahara, Y.; Kodaira, M.; Sedbazar, U.; Yada, T. Peripheral oxytocin treatment ameliorates obesity by reducing food intake and visceral fat mass. Aging 2011, 3, 1169–1177. [Google Scholar] [CrossRef]
- Altirriba, J.; Poher, A.L.; Caillon, A.; Arsenijevic, D.; Veyrat-Durebex, C.; Lyautey, J.; Dulloo, A.; Rohner-Jeanrenaud, F. Divergent effects of oxytocin treatment of obese diabetic mice on adiposity and diabetes. Endocrinology 2014, 155, 4189–4201. [Google Scholar] [CrossRef] [Green Version]
- Balazova, L.; Krskova, K.; Suski, M.; Sisovsky, V.; Hlavacova, N.; Olszanecki, R.; Jezova, D.; Zorad, S. Metabolic effects of subchronic peripheral oxytocin administration in lean and obese zucker rats. J. Physiol. Pharmacol. 2016, 67, 531–541. [Google Scholar]
- Jankowski, M.; Broderick, T.L.; Gutkowska, J. The Role of Oxytocin in Cardiovascular Protection. Front. Psychol. 2020, 11, 2139. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Wang, S.C.; Yang, H.; Lv, C.; Jia, S.; Liu, X.; Wang, X.; Meng, D.; Qin, D.; Zhu, H.; et al. Therapeutic Potential of Oxytocin in Atherosclerotic Cardiovascular Disease: Mechanisms and Signaling Pathways. Front. Neurosci. 2019, 13, 454. [Google Scholar] [CrossRef] [Green Version]
- Blevins, J.E.; Baskin, D.G. Translational and therapeutic potential of oxytocin as an anti-obesity strategy: Insights from rodents, nonhuman primates and humans. Physiol. Behav. 2015, 152, 438–449. [Google Scholar] [CrossRef]
- Arletti, R.; Benelli, A.; Bertolini, A. Influence of oxytocin on feeding behavior in the rat. Peptides 1989, 10, 89–93. [Google Scholar] [CrossRef]
- Arletti, R.; Benelli, A.; Bertolini, A. Oxytocin inhibits food and fluid intake in rats. Physiol. Behav. 1990, 48, 825–830. [Google Scholar] [CrossRef]
- Olson, B.R.; Drutarosky, M.D.; Chow, M.S.; Hruby, V.J.; Stricker, E.M.; Verbalis, J.G. Oxytocin and an oxytocin agonist administered centrally decrease food intake in rats. Peptides 1991, 12, 113–118. [Google Scholar] [CrossRef]
- Blouet, C.; Jo, Y.H.; Li, X.; Schwartz, G.J. Mediobasal hypothalamic leucine sensing regulates food intake through activation of a hypothalamus-brainstem circuit. J. Neurosci. 2009, 29, 8302–8311. [Google Scholar] [CrossRef]
- Baskin, D.G.; Kim, F.; Gelling, R.W.; Russell, B.J.; Schwartz, M.W.; Morton, G.J.; Simhan, H.N.; Moralejo, D.H.; Blevins, J.E. A new oxytocin-saporin cytotoxin for lesioning oxytocin-receptive neurons in the rat hindbrain. Endocrinology 2010, 151, 4207–4213. [Google Scholar] [CrossRef] [Green Version]
- Ong, Z.Y.; Alhadeff, A.L.; Grill, H.J. Medial nucleus tractus solitarius oxytocin receptor signaling and food intake control: The role of gastrointestinal satiation signal processing. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2015, 308, R800–R806. [Google Scholar] [CrossRef] [Green Version]
- Olszewski, P.K.; Klockars, A.; Levine, A.S. Oxytocin: A Conditional Anorexigen whose Effects on Appetite Depend on the Physiological, Behavioural and Social Contexts. J. Neuroendocr. 2016, 28. [Google Scholar] [CrossRef]
- Zhang, G.; Cai, D. Circadian intervention of obesity development via resting-stage feeding manipulation or oxytocin treatment. Am. J. Physiol. Endocrinol. Metab. 2011, 301, E1004–E1012. [Google Scholar] [CrossRef] [PubMed]
- Morton, G.J.; Thatcher, B.S.; Reidelberger, R.D.; Ogimoto, K.; Wolden-Hanson, T.; Baskin, D.G.; Schwartz, M.W.; Blevins, J.E. Peripheral oxytocin suppresses food intake and causes weight loss in diet-induced obese rats. Am. J. Physiol. Endocrinol. Metab. 2012, 302, E134–E144. [Google Scholar] [CrossRef]
- Blevins, J.E.; Graham, J.L.; Morton, G.J.; Bales, K.L.; Schwartz, M.W.; Baskin, D.G.; Havel, P.J. Chronic oxytocin administration inhibits food intake, increases energy expenditure, and produces weight loss in fructose-fed obese rhesus monkeys. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2015, 308, R431–R438. [Google Scholar] [CrossRef] [Green Version]
- Iwasaki, Y.; Maejima, Y.; Suyama, S.; Yoshida, M.; Arai, T.; Katsurada, K.; Kumari, P.; Nakabayashi, H.; Kakei, M.; Yada, T. Peripheral oxytocin activates vagal afferent neurons to suppress feeding in normal and leptin-resistant mice: A route for ameliorating hyperphagia and obesity. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2015, 308, R360–R369. [Google Scholar] [CrossRef] [Green Version]
- Maejima, Y.; Sedbazar, U.; Suyama, S.; Kohno, D.; Onaka, T.; Takano, E.; Yoshida, N.; Koike, M.; Uchiyama, Y.; Fujiwara, K.; et al. Nesfatin-1-regulated oxytocinergic signaling in the paraventricular nucleus causes anorexia through a leptin-independent melanocortin pathway. Cell Metab. 2009, 10, 355–365. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Horta, M.; Kaylor, K.; Feifel, D.; Ebner, N.C. Chronic oxytocin administration as a tool for investigation and treatment: A cross-disciplinary systematic review. Neurosci. Biobehav. Rev. 2020, 108, 1–23. [Google Scholar] [CrossRef] [PubMed]
- Blevins, J.E.; Thompson, B.W.; Anekonda, V.T.; Ho, J.M.; Graham, J.L.; Roberts, Z.S.; Hwang, B.H.; Ogimoto, K.; Wolden-Hanson, T.; Nelson, J.; et al. Chronic CNS oxytocin signaling preferentially induces fat loss in high-fat diet-fed rats by enhancing satiety responses and increasing lipid utilization. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2016, 310, R640–R658. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Leslie, M.; Silva, P.; Paloyelis, Y.; Blevins, J.; Treasure, J. A Systematic Review and Quantitative Meta-Analysis of Oxytocin’s Effects on Feeding. J. Neuroendocr. 2018, 30, e12584. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Noble, E.E.; Billington, C.J.; Kotz, C.M.; Wang, C. Oxytocin in the ventromedial hypothalamic nucleus reduces feeding and acutely increases energy expenditure. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2014, 307, R737–R745. [Google Scholar] [CrossRef] [Green Version]
- Camerino, C. Low sympathetic tone and obese phenotype in oxytocin-deficient mice. Obesity 2009, 17, 980–984. [Google Scholar] [CrossRef] [PubMed]
- Takayanagi, Y.; Kasahara, Y.; Onaka, T.; Takahashi, N.; Kawada, T.; Nishimori, K. Oxytocin receptor-deficient mice developed late-onset obesity. Neuroreport 2008, 19, 951–955. [Google Scholar] [CrossRef] [PubMed]
- Kasahara, Y.; Sato, K.; Takayanagi, Y.; Mizukami, H.; Ozawa, K.; Hidema, S.; So, K.H.; Kawada, T.; Inoue, N.; Ikeda, I.; et al. Oxytocin receptor in the hypothalamus is sufficient to rescue normal thermoregulatory function in male oxytocin receptor knockout mice. Endocrinology 2013, 154, 4305–4315. [Google Scholar] [CrossRef] [Green Version]
- Camerino, C. The New Frontier in Oxytocin Physiology: The Oxytonic Contraction. Int. J. Mol. Sci. 2020, 21, 5144. [Google Scholar] [CrossRef] [PubMed]
- Kasahara, Y.; Takayanagi, Y.; Kawada, T.; Itoi, K.; Nishimori, K. Impaired thermoregulatory ability of oxytocin-deficient mice during cold-exposure. Biosci. Biotechnol. Biochem. 2007, 71, 3122–3126. [Google Scholar] [CrossRef] [PubMed]
- Kublaoui, B.M.; Gemelli, T.; Tolson, K.P.; Wang, Y.; Zinn, A.R. Oxytocin deficiency mediates hyperphagic obesity of Sim1 haploinsufficient mice. Mol. Endocrinol. 2008, 22, 1723–1734. [Google Scholar] [CrossRef] [Green Version]
- Xi, D.; Gandhi, N.; Lai, M.; Kublaoui, B.M. Ablation of Sim1 neurons causes obesity through hyperphagia and reduced energy expenditure. PLoS ONE 2012, 7, e36453. [Google Scholar] [CrossRef]
- Swaab, D.F.; Purba, J.S.; Hofman, M.A. Alterations in the hypothalamic paraventricular nucleus and its oxytocin neurons (putative satiety cells) in Prader-Willi syndrome: A study of five cases. J. Clin. Endocrinol. Metab. 1995, 80, 573–579. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bittel, D.C.; Kibiryeva, N.; Sell, S.M.; Strong, T.V.; Butler, M.G. Whole genome microarray analysis of gene expression in Prader-Willi syndrome. Am. J. Med. Genet. A 2007, 143A, 430–442. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kabasakalian, A.; Ferretti, C.J.; Hollander, E. Oxytocin and Prader-Willi Syndrome. Curr. Top. Behav. Neurosci. 2018, 35, 529–557. [Google Scholar] [CrossRef] [PubMed]
- MacLean, E.L.; Wilson, S.R.; Martin, W.L.; Davis, J.M.; Nazarloo, H.P.; Carter, C.S. Challenges for measuring oxytocin: The blind men and the elephant? Psychoneuroendocrinology 2019, 107, 225–231. [Google Scholar] [CrossRef] [PubMed]
- Dumais, K.M.; Veenema, A.H. Vasopressin and oxytocin receptor systems in the brain: Sex differences and sex-specific regulation of social behavior. Front. Neuroendocr. 2016, 40, 1–23. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kagerbauer, S.M.; Debus, J.M.; Martin, J.; Gempt, J.; Jungwirth, B.; Hapfelmeier, A.; Podtschaske, A.H. Absence of a diurnal rhythm of oxytocin and arginine-vasopressin in human cerebrospinal fluid, blood and saliva. Neuropeptides 2019, 78, 101977. [Google Scholar] [CrossRef]
- Lefevre, A.; Mottolese, R.; Dirheimer, M.; Mottolese, C.; Duhamel, J.R.; Sirigu, A. A comparison of methods to measure central and peripheral oxytocin concentrations in human and non-human primates. Sci. Rep. 2017, 7, 17222. [Google Scholar] [CrossRef] [Green Version]
- Martin, J.; Kagerbauer, S.M.; Gempt, J.; Podtschaske, A.; Hapfelmeier, A.; Schneider, G. Oxytocin levels in saliva correlate better than plasma levels with concentrations in the cerebrospinal fluid of patients in neurocritical care. J. Neuroendocr. 2018, 30, e12596. [Google Scholar] [CrossRef]
- Mens, W.B.; Witter, A.; van Wimersma Greidanus, T.B. Penetration of neurohypophyseal hormones from plasma into cerebrospinal fluid (CSF): Half-times of disappearance of these neuropeptides from CSF. Brain Res. 1983, 262, 143–149. [Google Scholar] [CrossRef]
- Vankrieken, L.; Godart, A.; Thomas, K. Oxytocin determination by radioimmunoassay. Gynecol. Obstet. Investig. 1983, 16, 180–185. [Google Scholar] [CrossRef]
- Burbach, J.P.; De Kloet, E.R.; De Wied, D. Oxytocin biotransformation in the rat limbic brain: Characterization of peptidase activities and significance in the formation of oxytocin fragments. Brain Res. 1980, 202, 401–414. [Google Scholar] [CrossRef] [Green Version]
- Burbach, J.P.; Lebouille, J.L. Proteolytic conversion of arginine-vasopressin and oxytocin by brain synaptic membranes. Characterization of formed peptides and mechanisms of proteolysis. J. Biol. Chem. 1983, 258, 1487–1494. [Google Scholar] [CrossRef]
- Prieto, I.; Segarra, A.B.; de Gasparo, M.; Martinez-Canamero, M.; Ramirez-Sanchez, M. Divergent profile between hypothalamic and plasmatic aminopeptidase activities in WKY and SHR. Influence of beta-adrenergic blockade. Life Sci. 2018, 192, 9–17. [Google Scholar] [CrossRef]
- Franke, A.A.; Li, X.; Dabalos, C.; Lai, J.F. Improved oxytocin analysis from human serum and urine by orbitrap ESI-LC-HRAM-MS. Drug Test. Anal. 2020, 12, 846–852. [Google Scholar] [CrossRef] [PubMed]
- Amico, J.A.; Hempel, J. An oxytocin precursor intermediate circulates in the plasma of humans and rhesus monkeys administered estrogen. Neuroendocrinology 1990, 51, 437–443. [Google Scholar] [CrossRef] [PubMed]
- Leng, G.; Sabatier, N. Measuring Oxytocin and Vasopressin: Bioassays, Immunoassays and Random Numbers. J. Neuroendocr. 2016, 28. [Google Scholar] [CrossRef] [PubMed]
- Gutkowska, J.; Jankowski, M.; Antunes-Rodrigues, J. The role of oxytocin in cardiovascular regulation. Braz. J. Med. Biol. Res. 2014, 47, 206–214. [Google Scholar] [CrossRef] [Green Version]
- Kheterpal, I.; Kastin, A.J.; Mollah, S.; Yu, C.; Hsuchou, H.; Pan, W. Mass spectrometric quantification of MIF-1 in mouse brain by multiple reaction monitoring. Peptides 2009, 30, 1276–1281. [Google Scholar] [CrossRef] [Green Version]
- Khan, R.S.; Yu, C.; Kastin, A.J.; He, Y.; Ehrensing, R.H.; Hsuchou, H.; Stone, K.P.; Pan, W. Brain Activation by Peptide Pro-Leu-Gly-NH(2) (MIF-1). Int. J. Pept. 2010, 2010. [Google Scholar] [CrossRef] [Green Version]
- Uvnas Moberg, K.; Handlin, L.; Kendall-Tackett, K.; Petersson, M. Oxytocin is a principal hormone that exerts part of its effects by active fragments. Med. Hypotheses 2019, 133, 109394. [Google Scholar] [CrossRef] [PubMed]
- McCullough, M.E.; Churchland, P.S.; Mendez, A.J. Problems with measuring peripheral oxytocin: Can the data on oxytocin and human behavior be trusted? Neurosci. Biobehav. Rev. 2013, 37, 1485–1492. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Szeto, A.; McCabe, P.M.; Nation, D.A.; Tabak, B.A.; Rossetti, M.A.; McCullough, M.E.; Schneiderman, N.; Mendez, A.J. Evaluation of enzyme immunoassay and radioimmunoassay methods for the measurement of plasma oxytocin. Psychosom. Med. 2011, 73, 393–400. [Google Scholar] [CrossRef] [Green Version]
- Liu, D.; Han, X.; Liu, X.; Cheng, M.; He, M.; Rainer, G.; Gao, H.; Zhang, X. Measurement of ultra-trace level of intact oxytocin in plasma using SALLE combined with nano-LC-MS. J. Pharm. Biomed. Anal. 2019, 173, 62–67. [Google Scholar] [CrossRef] [Green Version]
- Franke, A.A.; Li, X.; Menden, A.; Lee, M.R.; Lai, J.F. Oxytocin analysis from human serum, urine, and saliva by orbitrap liquid chromatography-mass spectrometry. Drug Test Anal. 2019, 11, 119–128. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ishunina, T.A.; Swaab, D.F. Vasopressin and oxytocin neurons of the human supraoptic and paraventricular nucleus: Size changes in relation to age and sex. J. Clin. Endocrinol. Metab. 1999, 84, 4637–4644. [Google Scholar] [CrossRef] [PubMed]
- Wierda, M.; Goudsmit, E.; Van der Woude, P.F.; Purba, J.S.; Hofman, M.A.; Bogte, H.; Swaab, D.F. Oxytocin cell number in the human paraventricular nucleus remains constant with aging and in Alzheimer’s disease. Neurobiol. Aging 1991, 12, 511–516. [Google Scholar] [CrossRef] [Green Version]
- Fliers, E.; Swaab, D.F.; Pool, C.W.; Verwer, R.W. The vasopressin and oxytocin neurons in the human supraoptic and paraventricular nucleus; changes with aging and in senile dementia. Brain Res. 1985, 342, 45–53. [Google Scholar] [CrossRef] [Green Version]
- Altemus, M.; Jacobson, K.R.; Debellis, M.; Kling, M.; Pigott, T.; Murphy, D.L.; Gold, P.W. Normal CSF oxytocin and NPY levels in OCD. Biol. Psychiatry 1999, 45, 931–933. [Google Scholar] [CrossRef]
- Miller, M.; Bales, K.L.; Taylor, S.L.; Yoon, J.; Hostetler, C.M.; Carter, C.S.; Solomon, M. Oxytocin and vasopressin in children and adolescents with autism spectrum disorders: Sex differences and associations with symptoms. Autism Res. 2013, 6, 91–102. [Google Scholar] [CrossRef] [Green Version]
- Orihashi, R.; Mizoguchi, Y.; Imamura, Y.; Yamada, S.; Ueno, T.; Monji, A. Oxytocin and elderly MRI-based hippocampus and amygdala volume: A 7-year follow-up study. Brain Commun. 2020, 2, fcaa081. [Google Scholar] [CrossRef]
- Weisman, O.; Zagoory-Sharon, O.; Schneiderman, I.; Gordon, I.; Feldman, R. Plasma oxytocin distributions in a large cohort of women and men and their gender-specific associations with anxiety. Psychoneuroendocrinology 2013, 38, 694–701. [Google Scholar] [CrossRef] [PubMed]
- Zhong, S.; Monakhov, M.; Mok, H.P.; Tong, T.; Lai, P.S.; Chew, S.H.; Ebstein, R.P. U-shaped relation between plasma oxytocin levels and behavior in the trust game. PLoS ONE 2012, 7, e51095. [Google Scholar] [CrossRef] [Green Version]
- Feldman, R.; Zagoory-Sharon, O.; Weisman, O.; Schneiderman, I.; Gordon, I.; Maoz, R.; Shalev, I.; Ebstein, R.P. Sensitive parenting is associated with plasma oxytocin and polymorphisms in the OXTR and CD38 genes. Biol. Psychiatry 2012, 72, 175–181. [Google Scholar] [CrossRef]
- Taylor, S.E.; Saphire-Bernstein, S.; Seeman, T.E. Are plasma oxytocin in women and plasma vasopressin in men biomarkers of distressed pair-bond relationships? Psychol. Sci. 2010, 21, 3–7. [Google Scholar] [CrossRef] [Green Version]
- Weingarten, M.F.J.; Scholz, M.; Wohland, T.; Horn, K.; Stumvoll, M.; Kovacs, P.; Tonjes, A. Circulating Oxytocin Is Genetically Determined and Associated With Obesity and Impaired Glucose Tolerance. J. Clin. Endocrinol. Metab. 2019, 104, 5621–5632. [Google Scholar] [CrossRef]
- Huffmeijer, R.; van Ijzendoorn, M.H.; Bakermans-Kranenburg, M.J. Ageing and oxytocin: A call for extending human oxytocin research to ageing populations—A mini-review. Gerontology 2013, 59, 32–39. [Google Scholar] [CrossRef]
- Garforth, B.; Degnbol, H.; Terris, E.T.; Zak, P.J.; Winterdahl, M. Elevated plasma oxytocin levels and higher satisfaction with life in young oral contraceptive users. Sci. Rep. 2020, 10, 8208. [Google Scholar] [CrossRef]
- Roux, C.H.; Pisani, D.F.; Gillet, P.; Fontas, E.; Yahia, H.B.; Djedaini, M.; Ambrosetti, D.; Michiels, J.F.; Panaia-Ferrari, P.; Breuil, V.; et al. Oxytocin Controls Chondrogenesis and Correlates with Osteoarthritis. Int. J. Mol. Sci. 2020, 21, 3966. [Google Scholar] [CrossRef] [PubMed]
- Rilling, J.K.; DeMarco, A.C.; Hackett, P.D.; Chen, X.; Gautam, P.; Stair, S.; Haroon, E.; Thompson, R.; Ditzen, B.; Patel, R.; et al. Sex differences in the neural and behavioral response to intranasal oxytocin and vasopressin during human social interaction. Psychoneuroendocrinology 2014, 39, 237–248. [Google Scholar] [CrossRef] [Green Version]
- Lieberz, J.; Scheele, D.; Spengler, F.B.; Matheisen, T.; Schneider, L.; Stoffel-Wagner, B.; Kinfe, T.M.; Hurlemann, R. Kinetics of oxytocin effects on amygdala and striatal reactivity vary between women and men. Neuropsychopharmacology 2020, 45, 1134–1140. [Google Scholar] [CrossRef]
- Borland, J.M.; Rilling, J.K.; Frantz, K.J.; Albers, H.E. Sex-dependent regulation of social reward by oxytocin: An inverted U hypothesis. Neuropsychopharmacology 2019, 44, 97–110. [Google Scholar] [CrossRef] [Green Version]
- Hedges, V.L.; Heaton, E.C.; Amaral, C.; Benedetto, L.E.; Bodie, C.L.; D’Antonio, B.I.; Davila Portillo, D.R.; Lee, R.H.; Levine, M.T.; O’Sullivan, E.C.; et al. Estrogen Withdrawal Increases Postpartum Anxiety via Oxytocin Plasticity in the Paraventricular Hypothalamus and Dorsal Raphe Nucleus. Biol. Psychiatry 2020, 89, 929–938. [Google Scholar] [CrossRef]
- Acevedo-Rodriguez, A.; Mani, S.K.; Handa, R.J. Oxytocin and Estrogen Receptor beta in the Brain: An Overview. Front. Endocrinol. 2015, 6, 160. [Google Scholar] [CrossRef] [Green Version]
- Zuloaga, D.G.; Heck, A.L.; De Guzman, R.M.; Handa, R.J. Roles for androgens in mediating the sex differences of neuroendocrine and behavioral stress responses. Biol. Sex. Differ. 2020, 11, 44. [Google Scholar] [CrossRef]
- Dai, D.; Li, Q.C.; Zhu, Q.B.; Hu, S.H.; Balesar, R.; Swaab, D.; Bao, A.M. Direct Involvement of Androgen Receptor in Oxytocin Gene Expression: Possible Relevance for Mood Disorders. Neuropsychopharmacology 2017, 42, 2064–2071. [Google Scholar] [CrossRef]
- Wang, Y.L.; Yuan, Y.; Yang, J.; Wang, C.H.; Pan, Y.J.; Lu, L.; Wu, Y.Q.; Wang, D.X.; Lv, L.X.; Li, R.R.; et al. The interaction between the oxytocin and pain modulation in headache patients. Neuropeptides 2013, 47, 93–97. [Google Scholar] [CrossRef] [PubMed]
- Carson, D.S.; Berquist, S.W.; Trujillo, T.H.; Garner, J.P.; Hannah, S.L.; Hyde, S.A.; Sumiyoshi, R.D.; Jackson, L.P.; Moss, J.K.; Strehlow, M.C.; et al. Cerebrospinal fluid and plasma oxytocin concentrations are positively correlated and negatively predict anxiety in children. Mol. Psychiatry 2015, 20, 1085–1090. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kagerbauer, S.M.; Martin, J.; Schuster, T.; Blobner, M.; Kochs, E.F.; Landgraf, R. Plasma oxytocin and vasopressin do not predict neuropeptide concentrations in human cerebrospinal fluid. J. Neuroendocr. 2013, 25, 668–673. [Google Scholar] [CrossRef] [PubMed]
- Takagi, T.; Tanizawa, O.; Otsuki, Y.; Sugita, N.; Haruta, M.; Yamaji, K. Oxytocin in the cerebrospinal fluid and plasma of pregnant and nonpregnant subjects. Horm. Metab. Res. 1985, 17, 308–310. [Google Scholar] [CrossRef] [PubMed]
- Altemus, M.; Fong, J.; Yang, R.; Damast, S.; Luine, V.; Ferguson, D. Changes in cerebrospinal fluid neurochemistry during pregnancy. Biol. Psychiatry 2004, 56, 386–392. [Google Scholar] [CrossRef]
- Valstad, M.; Alvares, G.A.; Egknud, M.; Matziorinis, A.M.; Andreassen, O.A.; Westlye, L.T.; Quintana, D.S. The correlation between central and peripheral oxytocin concentrations: A systematic review and meta-analysis. Neurosci. Biobehav. Rev. 2017, 78, 117–124. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Martinez-Lorenzana, G.; Espinosa-Lopez, L.; Carranza, M.; Aramburo, C.; Paz-Tres, C.; Rojas-Piloni, G.; Condes-Lara, M. PVN electrical stimulation prolongs withdrawal latencies and releases oxytocin in cerebrospinal fluid, plasma, and spinal cord tissue in intact and neuropathic rats. Pain 2008, 140, 265–273. [Google Scholar] [CrossRef] [PubMed]
- Wotjak, C.T.; Ganster, J.; Kohl, G.; Holsboer, F.; Landgraf, R.; Engelmann, M. Dissociated central and peripheral release of vasopressin, but not oxytocin, in response to repeated swim stress: New insights into the secretory capacities of peptidergic neurons. Neuroscience 1998, 85, 1209–1222. [Google Scholar] [CrossRef]
- Schorr, M.; Marengi, D.A.; Pulumo, R.L.; Yu, E.; Eddy, K.T.; Klibanski, A.; Miller, K.K.; Lawson, E.A. Oxytocin and Its Relationship to Body Composition, Bone Mineral Density, and Hip Geometry Across the Weight Spectrum. J. Clin. Endocrinol. Metab. 2017, 102, 2814–2824. [Google Scholar] [CrossRef] [Green Version]
- Skinner, J.A.; Garg, M.L.; Dayas, C.V.; Burrows, T.L. Is weight status associated with peripheral levels of oxytocin? A pilot study in healthy women. Physiol. Behav. 2019, 212, 112684. [Google Scholar] [CrossRef]
- Moghaddam, S.A.P.; Amiri, P.; Saidpour, A.; Hosseinzadeh, N.; Abolhasani, M.; Ghorbani, A. The prevalence of food addiction and its associations with plasma oxytocin level and anthropometric and dietary measurements in Iranian women with obesity. Peptides 2019, 122, 170151. [Google Scholar] [CrossRef]
- Fu-Man, D.; Hong-Yu, K.; Bin-Hong, D.; Da-Na, L.; Xin-Yang, Y. Associations of oxytocin with metabolic parameters in obese women of childbearing age. Endokrynol. Pol. 2019, 70, 417–422. [Google Scholar] [CrossRef] [Green Version]
- Silber, M.; Almkvist, O.; Larsson, B.; Stock, S.; Uvnas-Moberg, K. The effect of oral contraceptive pills on levels of oxytocin in plasma and on cognitive functions. Contraception 1987, 36, 641–650. [Google Scholar] [CrossRef]
- Aulinas, A.; Pulumo, R.L.; Asanza, E.; Mancuso, C.J.; Slattery, M.; Tolley, C.; Plessow, F.; Thomas, J.J.; Eddy, K.T.; Miller, K.K.; et al. Endogenous Oxytocin Levels in Relation to Food Intake, Menstrual Phase, and Age in Females. J. Clin. Endocrinol. Metab. 2019, 104, 1348–1356. [Google Scholar] [CrossRef] [PubMed]
- Stefanska, A.; Bergmann, K.; Sypniewska, G. Metabolic Syndrome and Menopause: Pathophysiology, Clinical and Diagnostic Significance. Adv. Clin. Chem. 2015, 72, 1–75. [Google Scholar] [CrossRef] [PubMed]
- Maestrini, S.; Mele, C.; Mai, S.; Vietti, R.; Di Blasio, A.; Castello, L.; Surico, D.; Aimaretti, G.; Scacchi, M.; Marzullo, P. Plasma Oxytocin Concentration in Pre- and Postmenopausal Women: Its Relationship with Obesity, Body Composition and Metabolic Variables. Obes. Facts 2018, 11, 429–439. [Google Scholar] [CrossRef]
- Breuil, V.; Amri, E.Z.; Panaia-Ferrari, P.; Testa, J.; Elabd, C.; Albert-Sabonnadiere, C.; Roux, C.H.; Ailhaud, G.; Dani, C.; Carle, G.F.; et al. Oxytocin and bone remodelling: Relationships with neuropituitary hormones, bone status and body composition. Jt. Bone Spine 2011, 78, 611–615. [Google Scholar] [CrossRef] [PubMed]
- Breuil, V.; Panaia-Ferrari, P.; Fontas, E.; Roux, C.; Kolta, S.; Eastell, R.; Ben Yahia, H.; Faure, S.; Gossiel, F.; Benhamou, C.L.; et al. Oxytocin, a new determinant of bone mineral density in post-menopausal women: Analysis of the OPUS cohort. J. Clin. Endocrinol. Metab. 2014, 99, E634–E641. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chang, H.H.; Chang, W.H.; Chi, M.H.; Peng, Y.C.; Huang, C.C.; Yang, Y.K.; Chen, P.S. The OXTR Polymorphism Stratified the Correlation of Oxytocin and Glucose Homeostasis in Non-Diabetic Subjects. Diabetes Metab. Syndr. Obes. 2019, 12, 2707–2713. [Google Scholar] [CrossRef] [Green Version]
- Szulc, P.; Amri, E.Z.; Varennes, A.; Panaia-Ferrari, P.; Fontas, E.; Goudable, J.; Chapurlat, R.; Breuil, V. High serum oxytocin is associated with metabolic syndrome in older men-The MINOS study. Diabetes Res. Clin. Pract. 2016, 122, 17–27. [Google Scholar] [CrossRef]
- Qian, W.; Zhu, T.; Tang, B.; Yu, S.; Hu, H.; Sun, W.; Pan, R.; Wang, J.; Wang, D.; Yang, L.; et al. Decreased circulating levels of oxytocin in obesity and newly diagnosed type 2 diabetic patients. J. Clin. Endocrinol. Metab. 2014, 99, 4683–4689. [Google Scholar] [CrossRef] [Green Version]
- Al-Rawashdeh, A.; Kasabri, V.; Bulatova, N.; Akour, A.; Zayed, A.; Momani, M.; Khawaja, N.; Bustanji, H.; Hyasat, D. The correlation between plasma levels of oxytocin and betatrophin in non-diabetic and diabetic metabolic syndrome patients: A cross sectional study from Jordan. Diabetes Metab. Syndr. 2017, 11, 59–67. [Google Scholar] [CrossRef]
- Akour, A.; Kasabri, V.; Bulatova, N.; Al Muhaissen, S.; Naffa, R.; Fahmawi, H.; Momani, M.; Zayed, A.; Bustanji, Y. Association of Oxytocin with Glucose Intolerance and Inflammation Biomarkers in Metabolic Syndrome Patients with and without Prediabetes. Rev. Diabet. Stud. 2018, 14, 364–371. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Eisenberg, Y.; Dugas, L.R.; Akbar, A.; Reddivari, B.; Layden, B.T.; Barengolts, E. Oxytocin is lower in African American men with diabetes and associates with psycho-social and metabolic health factors. PLoS ONE 2018, 13, e0190301. [Google Scholar] [CrossRef] [Green Version]
- Coiro, V.; Passeri, M.; Davoli, C.; d’Amato, L.; Gelmini, G.; Fagnoni, F.; Schianchi, L.; Bentivoglio, M.; Volpi, R.; Chiodera, P. Oxytocin response to insulin-induced hypoglycemia in obese subjects before and after weight loss. J. Endocrinol. Investig. 1988, 11, 125–128. [Google Scholar] [CrossRef] [PubMed]
- Stock, S.; Granstrom, L.; Backman, L.; Matthiesen, A.S.; Uvnas-Moberg, K. Elevated plasma levels of oxytocin in obese subjects before and after gastric banding. Int. J. Obes. 1989, 13, 213–222. [Google Scholar]
- Pataky, Z.; Guessous, I.; Caillon, A.; Golay, A.; Rohner-Jeanrenaud, F.; Altirriba, J. Variable oxytocin levels in humans with different degrees of obesity and impact of gastric bypass surgery. Int. J. Obes. 2019, 43, 1120–1124. [Google Scholar] [CrossRef]
- Narmaki, E.; Borazjani, M.; Ataie-Jafari, A.; Hariri, N.; Doost, A.H.; Qorbani, M.; Saidpour, A. The combined effects of probiotics and restricted calorie diet on the anthropometric indices, eating behavior, and hormone levels of obese women with food addiction: A randomized clinical trial. Nutr. Neurosci. 2020, 1–13. [Google Scholar] [CrossRef]
- Erdman, S.E.; Poutahidis, T. Microbes and Oxytocin: Benefits for Host Physiology and Behavior. Int. Rev. Neurobiol. 2016, 131, 91–126. [Google Scholar] [CrossRef] [PubMed]
- Arakawa, R.; Febres, G.; Cheng, B.; Krikhely, A.; Bessler, M.; Korner, J. Prospective study of gut hormone and metabolic changes after laparoscopic sleeve gastrectomy and Roux-en-Y gastric bypass. PLoS ONE 2020, 15, e0236133. [Google Scholar] [CrossRef] [PubMed]
- Lawson, E.A.; Donoho, D.A.; Blum, J.I.; Meenaghan, E.M.; Misra, M.; Herzog, D.B.; Sluss, P.M.; Miller, K.K.; Klibanski, A. Decreased nocturnal oxytocin levels in anorexia nervosa are associated with low bone mineral density and fat mass. J. Clin. Psychiatry 2011, 72, 1546–1551. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lawson, E.A.; Ackerman, K.E.; Estella, N.M.; Guereca, G.; Pierce, L.; Sluss, P.M.; Bouxsein, M.L.; Klibanski, A.; Misra, M. Nocturnal oxytocin secretion is lower in amenorrheic athletes than nonathletes and associated with bone microarchitecture and finite element analysis parameters. Eur. J. Endocrinol. 2013, 168, 457–464. [Google Scholar] [CrossRef] [Green Version]
- Aulinas, A.; Guarda, F.J.; Yu, E.W.; Haines, M.S.; Asanza, E.; Silva, L.; Tritos, N.A.; Verbalis, J.; Miller, K.K.; Lawson, E.A. Lower Oxytocin Levels Are Associated with Lower Bone Mineral Density and Less Favorable Hip Geometry in Hypopituitary Men. Neuroendocrinology 2021, 111, 87–98. [Google Scholar] [CrossRef] [PubMed]
- Breuil, V.; Fontas, E.; Chapurlat, R.; Panaia-Ferrari, P.; Yahia, H.B.; Faure, S.; Euller-Ziegler, L.; Amri, E.Z.; Szulc, P. Oxytocin and bone status in men: Analysis of the MINOS cohort. Osteoporos. Int. 2015, 26, 2877–2882. [Google Scholar] [CrossRef] [PubMed]
- Quintana, D.S.; Lischke, A.; Grace, S.; Scheele, D.; Ma, Y.; Becker, B. Advances in the field of intranasal oxytocin research: Lessons learned and future directions for clinical research. Mol. Psychiatry 2021, 26, 80–91. [Google Scholar] [CrossRef]
- Ermisch, A.; Barth, T.; Ruhle, H.J.; Skopkova, J.; Hrbas, P.; Landgraf, R. On the blood-brain barrier to peptides: Accumulation of labelled vasopressin, DesGlyNH2-vasopressin and oxytocin by brain regions. Endocrinol. Exp. 1985, 19, 29–37. [Google Scholar] [PubMed]
- Lee, M.R.; Shnitko, T.A.; Blue, S.W.; Kaucher, A.V.; Winchell, A.J.; Erikson, D.W.; Grant, K.A.; Leggio, L. Labeled oxytocin administered via the intranasal route reaches the brain in rhesus macaques. Nat. Commun. 2020, 11, 2783. [Google Scholar] [CrossRef] [PubMed]
- Striepens, N.; Kendrick, K.M.; Hanking, V.; Landgraf, R.; Wullner, U.; Maier, W.; Hurlemann, R. Elevated cerebrospinal fluid and blood concentrations of oxytocin following its intranasal administration in humans. Sci. Rep. 2013, 3, 3440. [Google Scholar] [CrossRef] [PubMed]
- Dal Monte, O.; Noble, P.L.; Turchi, J.; Cummins, A.; Averbeck, B.B. CSF and blood oxytocin concentration changes following intranasal delivery in macaque. PLoS ONE 2014, 9, e103677. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, M.R.; Scheidweiler, K.B.; Diao, X.X.; Akhlaghi, F.; Cummins, A.; Huestis, M.A.; Leggio, L.; Averbeck, B.B. Oxytocin by intranasal and intravenous routes reaches the cerebrospinal fluid in rhesus macaques: Determination using a novel oxytocin assay. Mol. Psychiatry 2018, 23, 115–122. [Google Scholar] [CrossRef]
- Smith, A.S.; Korgan, A.C.; Young, W.S. Oxytocin delivered nasally or intraperitoneally reaches the brain and plasma of normal and oxytocin knockout mice. Pharmacol. Res. 2019, 146, 104324. [Google Scholar] [CrossRef]
- Neumann, I.D.; Maloumby, R.; Beiderbeck, D.I.; Lukas, M.; Landgraf, R. Increased brain and plasma oxytocin after nasal and peripheral administration in rats and mice. Psychoneuroendocrinology 2013, 38, 1985–1993. [Google Scholar] [CrossRef]
- Gossen, A.; Hahn, A.; Westphal, L.; Prinz, S.; Schultz, R.T.; Grunder, G.; Spreckelmeyer, K.N. Oxytocin plasma concentrations after single intranasal oxytocin administration-a study in healthy men. Neuropeptides 2012, 46, 211–215. [Google Scholar] [CrossRef] [PubMed]
- Spetter, M.S.; Feld, G.B.; Thienel, M.; Preissl, H.; Hege, M.A.; Hallschmid, M. Oxytocin curbs calorie intake via food-specific increases in the activity of brain areas that process reward and establish cognitive control. Sci. Rep. 2018, 8, 2736. [Google Scholar] [CrossRef]
- Lawson, E.A.; Marengi, D.A.; DeSanti, R.L.; Holmes, T.M.; Schoenfeld, D.A.; Tolley, C.J. Oxytocin reduces caloric intake in men. Obesity 2015, 23, 950–956. [Google Scholar] [CrossRef]
- Thienel, M.; Fritsche, A.; Heinrichs, M.; Peter, A.; Ewers, M.; Lehnert, H.; Born, J.; Hallschmid, M. Oxytocin’s inhibitory effect on food intake is stronger in obese than normal-weight men. Int. J. Obes. 2016, 40, 1707–1714. [Google Scholar] [CrossRef] [Green Version]
- Ott, V.; Finlayson, G.; Lehnert, H.; Heitmann, B.; Heinrichs, M.; Born, J.; Hallschmid, M. Oxytocin reduces reward-driven food intake in humans. Diabetes 2013, 62, 3418–3425. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- van der Klaauw, A.A.; Ziauddeen, H.; Keogh, J.M.; Henning, E.; Dachi, S.; Fletcher, P.C.; Farooqi, I.S. Oxytocin administration suppresses hypothalamic activation in response to visual food cues. Sci. Rep. 2017, 7, 4266. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Burmester, V.; Higgs, S.; Terry, P. Rapid-onset anorectic effects of intranasal oxytocin in young men. Appetite 2018, 130, 104–109. [Google Scholar] [CrossRef] [PubMed]
- Burmester, V.; Gibson, E.L.; Butler, G.; Bailey, A.; Terry, P. Oxytocin reduces post-stress sweet snack intake in women without attenuating salivary cortisol. Physiol. Behav. 2019, 212, 112704. [Google Scholar] [CrossRef] [PubMed]
- Plessow, F.; Marengi, D.A.; Perry, S.K.; Felicione, J.M.; Franklin, R.; Holmes, T.M.; Holsen, L.M.; Makris, N.; Deckersbach, T.; Lawson, E.A. Effects of Intranasal Oxytocin on the Blood Oxygenation Level-Dependent Signal in Food Motivation and Cognitive Control Pathways in Overweight and Obese Men. Neuropsychopharmacology 2018, 43, 638–645. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Morales, M.; Margolis, E.B. Ventral tegmental area: Cellular heterogeneity, connectivity and behaviour. Nat. Rev. Neurosci. 2017, 18, 73–85. [Google Scholar] [CrossRef] [PubMed]
- Kerem, L.; Hadjikhani, N.; Holsen, L.; Lawson, E.A.; Plessow, F. Oxytocin reduces the functional connectivity between brain regions involved in eating behavior in men with overweight and obesity. Int. J. Obes. 2020, 44, 980–989. [Google Scholar] [CrossRef]
- Striepens, N.; Schroter, F.; Stoffel-Wagner, B.; Maier, W.; Hurlemann, R.; Scheele, D. Oxytocin enhances cognitive control of food craving in women. Hum. Brain Mapp. 2016, 37, 4276–4285. [Google Scholar] [CrossRef]
- Plessow, F.; Marengi, D.A.; Perry, S.K.; Lawson, E.A. Oxytocin Administration Increases Proactive Control in Men with Overweight or Obesity: A Randomized, Double-Blind, Placebo-Controlled Crossover Study. Obesity 2021, 29, 56–61. [Google Scholar] [CrossRef] [PubMed]
- Mole, T.B.; Irvine, M.A.; Worbe, Y.; Collins, P.; Mitchell, S.P.; Bolton, S.; Harrison, N.A.; Robbins, T.W.; Voon, V. Impulsivity in disorders of food and drug misuse. Psychol. Med. 2015, 45, 771–782. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sellaro, R.; Colzato, L.S. High body mass index is associated with impaired cognitive control. Appetite 2017, 113, 301–309. [Google Scholar] [CrossRef]
- Devoto, F.; Zapparoli, L.; Bonandrini, R.; Berlingeri, M.; Ferrulli, A.; Luzi, L.; Banfi, G.; Paulesu, E. Hungry brains: A meta-analytical review of brain activation imaging studies on food perception and appetite in obese individuals. Neurosci. Biobehav. Rev. 2018, 94, 271–285. [Google Scholar] [CrossRef]
- Zhang, H.; Wu, C.; Chen, Q.; Chen, X.; Xu, Z.; Wu, J.; Cai, D. Treatment of obesity and diabetes using oxytocin or analogs in patients and mouse models. PLoS ONE 2013, 8, e61477. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Klement, J.; Ott, V.; Rapp, K.; Brede, S.; Piccinini, F.; Cobelli, C.; Lehnert, H.; Hallschmid, M. Oxytocin Improves beta-Cell Responsivity and Glucose Tolerance in Healthy Men. Diabetes 2017, 66, 264–271. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brede, S.; Fehr, S.; Dalla-Man, C.; Cobelli, C.; Lehnert, H.; Hallschmid, M.; Klement, J. Intranasal oxytocin fails to acutely improve glucose metabolism in obese men. Diabetes Obes. Metab. 2019, 21, 424–428. [Google Scholar] [CrossRef] [Green Version]
- Burt, R.L.; Leake, N.H.; Dannenburg, W.N. Effect of synthetic oxytocin on plasma nonesterified fatty acids, triglycerides, and blood glucose. Obstet. Gynecol. 1963, 21, 708–712. [Google Scholar] [PubMed]
- Kerem, L.; Lawson, E.A. Oxytocin, eating behavior and metabolism in humans. In The Human Hypothalamus, 1st ed.; Michael, J., Aminoff, F.B., Eds.; Elsevier: Amsterdam, The Netherlands, 2021; Volume 180. [Google Scholar]
- Atasoy, D.; Betley, J.N.; Su, H.H.; Sternson, S.M. Deconstruction of a neural circuit for hunger. Nature 2012, 488, 172–177. [Google Scholar] [CrossRef] [PubMed]
- Blevins, J.E.; Eakin, T.J.; Murphy, J.A.; Schwartz, M.W.; Baskin, D.G. Oxytocin innervation of caudal brainstem nuclei activated by cholecystokinin. Brain Res. 2003, 993, 30–41. [Google Scholar] [CrossRef] [PubMed]
- Rinaman, L.; Rothe, E.E. GLP-1 receptor signaling contributes to anorexigenic effect of centrally administered oxytocin in rats. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2002, 283, R99–R106. [Google Scholar] [CrossRef]
- Brierley, D.I.; Holt, M.K.; Singh, A.; de Araujo, A.; McDougle, M.; Vergara, M.; Afaghani, M.H.; Lee, S.J.; Scott, K.; Maske, C.; et al. Central and peripheral GLP-1 systems independently suppress eating. Nat. Metab. 2021, 3, 258–273. [Google Scholar] [CrossRef] [PubMed]
- Angulo, M.A.; Butler, M.G.; Cataletto, M.E. Prader-Willi syndrome: A review of clinical, genetic, and endocrine findings. J. Endocrinol. Investig. 2015, 38, 1249–1263. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Littleton, S.H.; Berkowitz, R.I.; Grant, S.F.A. Genetic Determinants of Childhood Obesity. Mol. Diagn. Ther. 2020, 24, 653–663. [Google Scholar] [CrossRef] [PubMed]
- Tauber, M.; Hoybye, C. Endocrine disorders in Prader-Willi syndrome: A model to understand and treat hypothalamic dysfunction. Lancet Diabetes Endocrinol. 2021, 9, 235–246. [Google Scholar] [CrossRef]
- Lukoshe, A.; van Dijk, S.E.; van den Bosch, G.E.; van der Lugt, A.; White, T.; Hokken-Koelega, A.C. Altered functional resting-state hypothalamic connectivity and abnormal pituitary morphology in children with Prader-Willi syndrome. J. Neurodev. Disord. 2017, 9, 12. [Google Scholar] [CrossRef] [Green Version]
- van Nieuwpoort, I.C.; Sinnema, M.; Castelijns, J.A.; Twisk, J.W.; Curfs, L.M.; Drent, M.L. The GH/IGF-I axis and pituitary function and size in adults with Prader-Willi syndrome. Horm. Res. Paediatr. 2011, 75, 403–411. [Google Scholar] [CrossRef]
- Fountain, M.D.; Aten, E.; Cho, M.T.; Juusola, J.; Walkiewicz, M.A.; Ray, J.W.; Xia, F.; Yang, Y.; Graham, B.H.; Bacino, C.A.; et al. The phenotypic spectrum of Schaaf-Yang syndrome: 18 new affected individuals from 14 families. Genet. Med. 2017, 19, 45–52. [Google Scholar] [CrossRef] [Green Version]
- Fountain, M.D., Jr.; Schaaf, C.P. MAGEL2 and Oxytocin-Implications in Prader-Willi Syndrome and Beyond. Biol. Psychiatry 2015, 78, 78–80. [Google Scholar] [CrossRef]
- Chen, H.; Victor, A.K.; Klein, J.; Tacer, K.F.; Tai, D.J.; de Esch, C.; Nuttle, A.; Temirov, J.; Burnett, L.C.; Rosenbaum, M.; et al. Loss of MAGEL2 in Prader-Willi syndrome leads to decreased secretory granule and neuropeptide production. JCI Insight 2020, 5. [Google Scholar] [CrossRef]
- Reichova, A.; Schaller, F.; Bukatova, S.; Bacova, Z.; Muscatelli, F.; Bakos, J. The impact of oxytocin on neurite outgrowth and synaptic proteins in Magel2-deficient mice. Dev. Neurobiol. 2021, 81, 366–388. [Google Scholar] [CrossRef] [PubMed]
- Ates, T.; Oncul, M.; Dilsiz, P.; Topcu, I.C.; Civas, C.C.; Alp, M.I.; Aklan, I.; Ates Oz, E.; Yavuz, Y.; Yilmaz, B.; et al. Inactivation of Magel2 suppresses oxytocin neurons through synaptic excitation-inhibition imbalance. Neurobiol. Dis. 2019, 121, 58–64. [Google Scholar] [CrossRef] [PubMed]
- Meziane, H.; Schaller, F.; Bauer, S.; Villard, C.; Matarazzo, V.; Riet, F.; Guillon, G.; Lafitte, D.; Desarmenien, M.G.; Tauber, M.; et al. An Early Postnatal Oxytocin Treatment Prevents Social and Learning Deficits in Adult Mice Deficient for Magel2, a Gene Involved in Prader-Willi Syndrome and Autism. Biol. Psychiatry 2015, 78, 85–94. [Google Scholar] [CrossRef] [PubMed]
- Maillard, J.; Park, S.; Croizier, S.; Vanacker, C.; Cook, J.H.; Prevot, V.; Tauber, M.; Bouret, S.G. Loss of Magel2 impairs the development of hypothalamic Anorexigenic circuits. Hum. Mol. Genet. 2016, 25, 3208–3215. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marbach, F.; Elgizouli, M.; Rech, M.; Beygo, J.; Erger, F.; Velmans, C.; Stumpel, C.; Stegmann, A.P.A.; Beck-Wodl, S.; Gillessen-Kaesbach, G.; et al. The adult phenotype of Schaaf-Yang syndrome. Orphanet. J. Rare Dis. 2020, 15, 294. [Google Scholar] [CrossRef] [PubMed]
- Poitou, C.; Mosbah, H.; Clement, K. Mechanisms in Endocrinology: Update on treatments for patients with genetic obesity. Eur. J. Endocrinol. 2020, 183, R149–R166. [Google Scholar] [CrossRef]
- Einfeld, S.L.; Smith, E.; McGregor, I.S.; Steinbeck, K.; Taffe, J.; Rice, L.J.; Horstead, S.K.; Rogers, N.; Hodge, M.A.; Guastella, A.J. A double-blind randomized controlled trial of oxytocin nasal spray in Prader Willi syndrome. Am. J. Med. Genet. A 2014, 164A, 2232–2239. [Google Scholar] [CrossRef]
- Kuppens, R.J.; Donze, S.H.; Hokken-Koelega, A.C. Promising effects of oxytocin on social and food-related behaviour in young children with Prader-Willi syndrome: A randomized, double-blind, controlled crossover trial. Clin. Endocrinol. 2016, 85, 979–987. [Google Scholar] [CrossRef]
- Dykens, E.M.; Miller, J.; Angulo, M.; Roof, E.; Reidy, M.; Hatoum, H.T.; Willey, R.; Bolton, G.; Korner, P. Intranasal carbetocin reduces hyperphagia in individuals with Prader-Willi syndrome. JCI Insight 2018, 3. [Google Scholar] [CrossRef] [PubMed]
- Rose, S.R.; Horne, V.E.; Bingham, N.; Jenkins, T.; Black, J.; Inge, T. Hypothalamic Obesity: 4 Years of the International Registry of Hypothalamic Obesity Disorders. Obesity 2018, 26, 1727–1732. [Google Scholar] [CrossRef] [Green Version]
- Muller, H.L. Craniopharyngioma and hypothalamic injury: Latest insights into consequent eating disorders and obesity. Curr. Opin. Endocrinol. Diabetes Obes. 2016, 23, 81–89. [Google Scholar] [CrossRef] [Green Version]
- Lustig, R.H. Hypothalamic obesity after craniopharyngioma: Mechanisms, diagnosis, and treatment. Front. Endocrinol. 2011, 2, 60. [Google Scholar] [CrossRef] [Green Version]
- Gebert, D.; Auer, M.K.; Stieg, M.R.; Freitag, M.T.; Lahne, M.; Fuss, J.; Schilbach, K.; Schopohl, J.; Stalla, G.K.; Kopczak, A. De-masking oxytocin-deficiency in craniopharyngioma and assessing its link with affective function. Psychoneuroendocrinology 2018, 88, 61–69. [Google Scholar] [CrossRef]
- Daubenbuchel, A.M.; Hoffmann, A.; Eveslage, M.; Ozyurt, J.; Lohle, K.; Reichel, J.; Thiel, C.M.; Martens, H.; Geenen, V.; Muller, H.L. Oxytocin in survivors of childhood-onset craniopharyngioma. Endocrine 2016, 54, 524–531. [Google Scholar] [CrossRef] [Green Version]
- Hsu, E.A.; Miller, J.L.; Perez, F.A.; Roth, C.L. Oxytocin and Naltrexone Successfully Treat Hypothalamic Obesity in a Boy Post-Craniopharyngioma Resection. J. Clin. Endocrinol. Metab. 2018, 103, 370–375. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aulinas, A.; Plessow, F.; Asanza, E.; Silva, L.; Marengi, D.A.; Fan, W.; Abedi, P.; Verbalis, J.; Tritos, N.A.; Nachtigall, L.; et al. Low Plasma Oxytocin Levels and Increased Psychopathology in Hypopituitary Men With Diabetes Insipidus. J. Clin. Endocrinol. Metab. 2019, 104, 3181–3191. [Google Scholar] [CrossRef] [PubMed]
- Daughters, K.; Manstead, A.S.R.; Rees, D.A. Hypopituitarism is associated with lower oxytocin concentrations and reduced empathic ability. Endocrine 2017, 57, 166–174. [Google Scholar] [CrossRef] [Green Version]
- Ward, Z.J.; Bleich, S.N.; Cradock, A.L.; Barrett, J.L.; Giles, C.M.; Flax, C.; Long, M.W.; Gortmaker, S.L. Projected U.S. State-Level Prevalence of Adult Obesity and Severe Obesity. N. Engl. J. Med. 2019, 381, 2440–2450. [Google Scholar] [CrossRef]
- Kerem, L.; Holsen, L.; Fazeli, P.; Bredella, M.A.; Mancuso, C.; Resulaj, M.; Holmes, T.M.; Klibanski, A.; Lawson, E.A. Modulation of neural fMRI responses to visual food cues by overeating and fasting interventions: A preliminary study. Physiol. Rep. 2021, 8, e14639. [Google Scholar] [CrossRef]
- Reppert, S.M.; Perlow, M.J.; Artman, H.G.; Ungerleider, L.G.; Fisher, D.A.; Klein, D.C. The circadian rhythm of oxytocin in primate cerebrospinal fluid: Effects of destruction of the suprachiasmatic nuclei. Brain Res. 1984, 307, 384–387. [Google Scholar] [CrossRef]
- Santoso, P.; Nakata, M.; Ueta, Y.; Yada, T. Suprachiasmatic vasopressin to paraventricular oxytocin neurocircuit in the hypothalamus relays light reception to inhibit feeding behavior. Am. J. Physiol. Endocrinol. Metab. 2018, 315, E478–E488. [Google Scholar] [CrossRef] [Green Version]
- Asarian, L.; Geary, N. Sex differences in the physiology of eating. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2013, 305, R1215–R1267. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sample, C.H.; Davidson, T.L. Considering sex differences in the cognitive controls of feeding. Physiol. Behav. 2018, 187, 97–107. [Google Scholar] [CrossRef] [PubMed]
- Pflimlin, E.; Zhou, Z.; Amso, Z.; Fu, Q.; Lee, C.; Muppiddi, A.; Joseph, S.B.; Nguyen-Tran, V.; Shen, W. Engineering a Potent, Long-Acting, and Periphery-Restricted Oxytocin Receptor Agonist with Anorexigenic and Body Weight Reducing Effects. J. Med. Chem. 2020, 63, 382–390. [Google Scholar] [CrossRef] [PubMed]
- Mohan, S.; McCloskey, A.G.; McKillop, A.M.; Flatt, P.R.; Irwin, N.; Moffett, R.C. Development and characterisation of novel, enzymatically stable oxytocin analogues with beneficial antidiabetic effects in high fat fed mice. Biochim. Biophys. Acta Gen. Subj. 2021, 1865, 129811. [Google Scholar] [CrossRef] [PubMed]
- Song, Z.; Albers, H.E. Cross-talk among oxytocin and arginine-vasopressin receptors: Relevance for basic and clinical studies of the brain and periphery. Front. Neuroendocrinol. 2018, 51, 14–24. [Google Scholar] [CrossRef] [PubMed]
- Pierce, M.L.; French, J.A.; Murray, T.F. Comparison of the pharmacological profiles of arginine vasopressin and oxytocin analogs at marmoset, macaque, and human vasopressin 1a receptor. Biomed. Pharmacother. 2020, 126, 110060. [Google Scholar] [CrossRef] [PubMed]
- Neumann, I.D.; Landgraf, R. Balance of brain oxytocin and vasopressin: Implications for anxiety, depression, and social behaviors. Trends Neurosci. 2012, 35, 649–659. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kerem, L.; Lawson, E.A. The Effects of Oxytocin on Appetite Regulation, Food Intake and Metabolism in Humans. Int. J. Mol. Sci. 2021, 22, 7737. https://doi.org/10.3390/ijms22147737
Kerem L, Lawson EA. The Effects of Oxytocin on Appetite Regulation, Food Intake and Metabolism in Humans. International Journal of Molecular Sciences. 2021; 22(14):7737. https://doi.org/10.3390/ijms22147737
Chicago/Turabian StyleKerem, Liya, and Elizabeth A. Lawson. 2021. "The Effects of Oxytocin on Appetite Regulation, Food Intake and Metabolism in Humans" International Journal of Molecular Sciences 22, no. 14: 7737. https://doi.org/10.3390/ijms22147737
APA StyleKerem, L., & Lawson, E. A. (2021). The Effects of Oxytocin on Appetite Regulation, Food Intake and Metabolism in Humans. International Journal of Molecular Sciences, 22(14), 7737. https://doi.org/10.3390/ijms22147737





