Association of Dephosphorylated-Uncarboxylated Matrix Gla Protein and Risk of Major Bleeding in Patients Presenting with Acute Myocardial Infarction
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design and Ethical Considerations
2.2. Subjects and Inclusion/Exclusion Criteria
2.3. Clinical Evaluations
2.4. Laboratory Evaluations
2.5. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kumrić, M.; Tičinović Kurir, T.; Borovac, J.A.; Božić, J. The Role of Natural Killer (NK) Cells in Acute Coronary Syndrome: A Comprehensive Review. Biomolecules 2020, 10, 1514. [Google Scholar] [CrossRef]
- Perez-Quilis, C.; Leischik, R.; Lucia, A. Epidemiology of coronary heart disease and acute coronary syndrome. Ann. Transl. Med. 2016, 4, 256. [Google Scholar]
- Hamm, C.W.; Möllmann, H.; Bassand, J.P.; Van de Werf, F. Acute coronary syndrome. In The ESC Textbook of Cardiovascular Medicine, 2nd ed.; Oxford University Press: Oxford, UK, 2009. [Google Scholar]
- Amsterdam, E.A.; Wenger, N.K.; Brindis, R.G. 2014 AHA/ACC Guideline for the management of patients with non-ST-elevation acute coronary syndromes. A report of the American College of Cardiology/American Heart Association Task Force on practice guidelines. J. Am. Coll. Cardiol. 2014, 64, e139–e228. [Google Scholar] [CrossRef] [Green Version]
- Rao, S.V.; Eikelboom, J.A.; Granger, C.B.; Harrington, R.A.; Califf, R.M.; Bassand, J.P. Bleeding and blood transfusion issues in patients with non-ST-segment elevation acute coronary syndromes. Eur. Heart J. 2007, 28, 1193–1204. [Google Scholar] [CrossRef] [Green Version]
- Subherwal, S.; Bach, R.G.; Chen, A.Y. Baseline risk of major bleeding in non-ST-segment-elevation myocardial infarction: The CRUSADE (Can Rapid risk stratification of Unstable angina patients Suppress ADverse outcomes with Early implementation of the ACC/AHA Guidelines) bleeding score. Circulation 2009, 119, 1873–1882. [Google Scholar] [CrossRef] [Green Version]
- Flores-Ríos, X.; Couto-Mallón, D.; Rodríguez-Garrido, J. Comparison of the performance of the CRUSADE, ACUITY-HORIZONS, and ACTION bleeding risk scores in STEMI undergoing primary PCI: Insights from a cohort of 1391 patients. Eur. Heart J. Acute Cardiovasc. Care 2013, 2, 19–26. [Google Scholar] [CrossRef]
- Boden, H.; Velders, M.; van der Hoeven, B.L.; Schalij, M.J. TCT-557 the risk of in-hospital bleeding and long-term mortality in patients with ST-elevation myocardial infarction treated with primary percutaneous coronary intervention. J. Am. Coll. Cardiol. 2012, 60, B161–B162. [Google Scholar] [CrossRef] [Green Version]
- Mrdovic, I.; Savic, L.; Krljanac, G. Simple risk algorithm to predict serious bleeding in patients with ST-segment elevation myocardial infarction undergoing primary percutaneous coronary intervention: RISK-PCI bleeding score. Circ. J. 2013, 77, 1719–1727. [Google Scholar] [CrossRef] [Green Version]
- Al-Daydamony, M.M.; Farag, E.M. CRUSADE bleeding score as a predictor of bleeding events in patients with acute coronary syndrome in Zagazig University Hospital. Indian Heart J. 2016, 68, 632–638. [Google Scholar] [CrossRef] [Green Version]
- Jinatongthai, P.; Khaisombut, N.; Likittanasombat, K.; Chaiyakunapruk, N.; Watcharathanakij, S.; Nathisuwan, S. Use of the CRUSADE bleeding risk score in the prediction of major bleeding for patients with acute coronary syndrome receiving enoxaparin in Thailand. Heart Lung Circ. 2014, 23, 1051–1058. [Google Scholar] [CrossRef]
- Amador, P.; Santos, J.F.; Gonçalves, S.; Seixo, F.; Soares, L. Comparison of ischemic and bleeding risk scores in non-ST elevation acute coronary syndromes. Acute Card. Care 2011, 13, 68–75. [Google Scholar] [CrossRef]
- Speer, M.Y.; Yang, H.Y.; Brabb, T.; Leaf, E.; Look, A.; Lin, W.L.; Frutkin, A.; Dichek, D.; Giachelli, C.M. Smooth muscle cells give rise to osteochondrogenic precursors and chondrocytes in calcifying arteries. Circ. Res. 2009, 104, 733–741. [Google Scholar] [CrossRef]
- Brnic, D.; Martinovic, D.; Zivkovic, P.M.; Tokic, D.; Vilovic, M.; Rusic, D.; Tadin Hadjina, I.; Libers, C.; Glumac, S.; Supe-Domic, D.; et al. Inactive matrix Gla protein is elevated in patients with inflammatory bowel disease. World J. Gastroenterol. 2020, 26, 4866–4877. [Google Scholar] [CrossRef]
- Schurgers, L.J.; Spronk, H.M.; Skepper, J.N.; Hackeng, T.M.; Shanahan, C.M.; Vermeer, C.; Weissberg, P.L.; Proudfoot, D.J. Post-translational modifications regulate matrix Gla protein function: Importance for inhibition of vascular smooth muscle cell calcification. Thromb. Haemost. 2007, 5, 2503–2511. [Google Scholar] [CrossRef] [PubMed]
- Dalmeijer, G.W.; van der Schouw, Y.T.; Magdeleyns, E.; Ahmed, N.; Vermeer, C.; Beulens, J.W. The effect of menaquinone-7 supplementation on circulating species of matrix Gla protein. Atherosclerosis 2012, 225, 397–402. [Google Scholar] [CrossRef] [PubMed]
- Price, P.A.; Rice, J.S.; Williamson, M.K. Conserved phosphorylation of serines in the Ser-X-Glu/Ser(P) sequences of the vitamin K-dependent matrix Gla protein from shark, lamb, rat, cow, and human. Protein Sci. 1994, 3, 822–830. [Google Scholar] [CrossRef] [Green Version]
- Price, P.A.; Urist, M.R.; Otawara, Y. Matrix Gla protein, a new gamma-carboxyglutamic acid-containing protein which is associated with the organic matrix of bone. Biochem. Biophys. Res. Commun. 1983, 117, 765–771. [Google Scholar] [CrossRef]
- Price, P.A.; Williamson, M.K. Primary structure of bovine matrix Gla protein, a new vitamin K-dependent bone protein. J. Biol. Chem. 1985, 260, 14971–14975. [Google Scholar] [CrossRef]
- Cranenburg, E.C.; Koos, R.; Schurgers, L.J.; Magdeleyns, E.J.; Schoonbrood, T.H.; Landewé, R.B.; Brandenburg, V.M.; Bekers, O.; Vermeer, C. Characterisation and potential diagnostic value of circulating matrix Gla protein (MGP) species. Thromb. Haemost. 2010, 104, 811–822. [Google Scholar] [CrossRef]
- Ibanez, B.; James, S.; Agewall, S.; Antunes, M.J.; Bucciarelli-Ducci, C.; Bueno, H.; Caforio, A.L.P.; Crea, F.; Goudevenos, J.A.; Halvorsen, S.; et al. ESC Scientific Document Group. 2017 ESC Guidelines for the management of acute myocardial infarction in patients presenting with ST-segment elevation: The Task Force for the management of acute myocardial infarction in patients presenting with ST-segment elevation of the European Society of Cardiology (ESC). Eur. Heart J. 2018, 39, 119–177. [Google Scholar]
- Collet, J.P.; Thiele, H.; Barbato, E.; Barthélémy, O.; Bauersachs, J.; Bhatt, D.L.; Dendale, P.; Dorobantu, M.; Edvardsen, T.; Folliguet, T.; et al. ESC Scientific Document Group. 2020 ESC Guidelines for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation. Eur. Heart J. 2020, 37, ehaa575. [Google Scholar]
- Lee, K.L.; Woodlief, L.H.; Topol, E.J.; Weaver, W.D.; Betriu, A.; Col, J.; Simoons, M.; Aylward, P.; Van de Werf, F.; Califf, R.M. Predictors of 30-day mortality in the era of reperfusion for acute myocardial infarction. Results from an international trial of 41,021 patients. GUSTO-I Investigators. Circulation 1995, 91, 1659–1668. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schurgers, L.J.; Joosen, I.A.; Laufer, E.M.; Chatrou, M.L.; Herfs, M.; Winkens, M.H.; Westenfeld, R.; Veulemans, V.; Krueger, T.; Shanahan, C.M. Vitamin K-antagonists accelerate atherosclerotic calcification and induce a vulnerable plaque phenotype. PLoS ONE 2012, 7, e43229. [Google Scholar] [CrossRef] [Green Version]
- Dalmeijer, G.W.; van der Schouw, Y.T.; Vermeer, C.; Magdeleyns, E.J.; Schurgers, L.J.; Beulens, J.W. Circulating matrix Gla protein is associated with coronary artery calcification and vitamin K status in healthy women. J. Nutr. Biochem. 2013, 24, 624–628. [Google Scholar] [CrossRef]
- Mayer, O., Jr.; Seidlerová, J.; Bruthans, J.; Filipovský, J.; Timoracká, K.; Vaněk, J.; Cerná, L.; Wohlfahrt, P.; Cífková, R.; Theuwissen, E.; et al. Desphospho-uncarboxylated matrix Gla-protein is associated with mortality risk in patients with chronic stable vascular disease. Atherosclerosis 2014, 235, 162–168. [Google Scholar] [CrossRef] [PubMed]
- Wei, F.F.; Trenson, S.; Verhamme, P.; Vermeer, C.; Staessen, J.A. Vitamin K-Dependent Matrix Gla Protein as Multifaceted Protector of Vascular and Tissue Integrity. Hypertension 2019, 73, 1160–1169. [Google Scholar] [CrossRef]
- van Rein, N.; le Cessie, S.; van Vliet, I.P.; Reitsma, P.H.; van der Meer, F.J.; Lijfering, W.M.; Cannegieter, S.C. Increased risk of major bleeding after a minor bleed during treatment with vitamin K antagonists is determined by fixed common risk factors. J. Thromb. Haemost. 2016, 14, 948–952. [Google Scholar] [CrossRef] [Green Version]
- Kamal, A.H.; Tefferi, A.; Pruthi, R.K. How to interpret and pursue an abnormal prothrombin time, activated partial thromboplastin time, and bleeding time in adults. Mayo Clin. Proc. 2007, 82, 864–873. [Google Scholar] [CrossRef] [Green Version]
- Bilalic, A.; Ticinovic Kurir, T.; Kumric, M.; Borovac, J.A.; Matetic, A.; Supe-Domic, D.; Bozic, J. Circulating Levels of Dephosphorylated-Uncarboxylated Matrix Gla Protein in Patients with Acute Coronary Syndrome. Molecules 2021, 26, 1108. [Google Scholar] [CrossRef] [PubMed]
- Hong, Y.J.; Jeong, M.H.; Choi, Y.H.; Ma, E.H.; Ko, J.S.; Lee, M.G.; Park, K.H.; Sim, D.S.; Yoon, N.S.; Youn, H.J.; et al. Differences in intravascular ultrasound findings in culprit lesions in infarct-related arteries between ST segment elevation myocardial infarction and non-ST segment elevation myocardial infarction. J. Cardiol. 2010, 56, 15–22. [Google Scholar] [CrossRef] [Green Version]
- Bjørklund, G.; Svanberg, E.; Dadar, M.; Card, D.J.; Chirumbolo, S.; Harrington, D.J.; Aaseth, J. The Role of Matrix Gla Protein (MGP) in Vascular Calcification. Curr. Med. Chem. 2020, 27, 1647–1660. [Google Scholar] [CrossRef] [PubMed]
- Ueland, T.; Dahl, C.P.; Gullestad, L.; Aakhus, S.; Broch, K.; Skårdal, R.; Vermeer, C.; Aukrust, P.; Schurgers, L.J. Circulating levels of non-phosphorylated undercarboxylated matrix Gla protein are associated with disease severity in patients with chronic heart failure. Clin. Sci. 2011, 121, 119–127. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mohamed, M.O.; Kinnaird, T.; Anderson, R.; Rashid, M.; Martin, G.P.; Freeman, P.; Kwok, C.S.; Myint, P.K.; Zaman, A.G.; Mamas, M.A. Combinations of bleeding and ischemic risk and their association with clinical outcomes in acute coronary syndrome. Int. J. Cardiol. 2019, 290, 7–14. [Google Scholar] [CrossRef] [PubMed]
- Cordero, A.; Rodriguez-Manero, M.; García-Acuña, J.M.; López-Palop, R.; Cid, B.; Carrillo, P.; Agra-Bermejo, R.; González-Salvado, V.; Iglesias-Alvarez, D.; Bertomeu-Martínez, V.; et al. Additive value of the CRUSADE score to the GRACE score for mortality risk prediction in patients with acute coronary syndromes. Int. J. Cardiol. 2017, 245, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.K.M.; Mehta, O.H.; Liao, Y.B.; Wang, M.T.M.; Stewart, R.; White, H. Meta-Analysis of Bleeding Scores Performance for Acute Coronary Syndrome. Heart Lung Circ. 2020, 29, 1749–1757. [Google Scholar] [CrossRef]
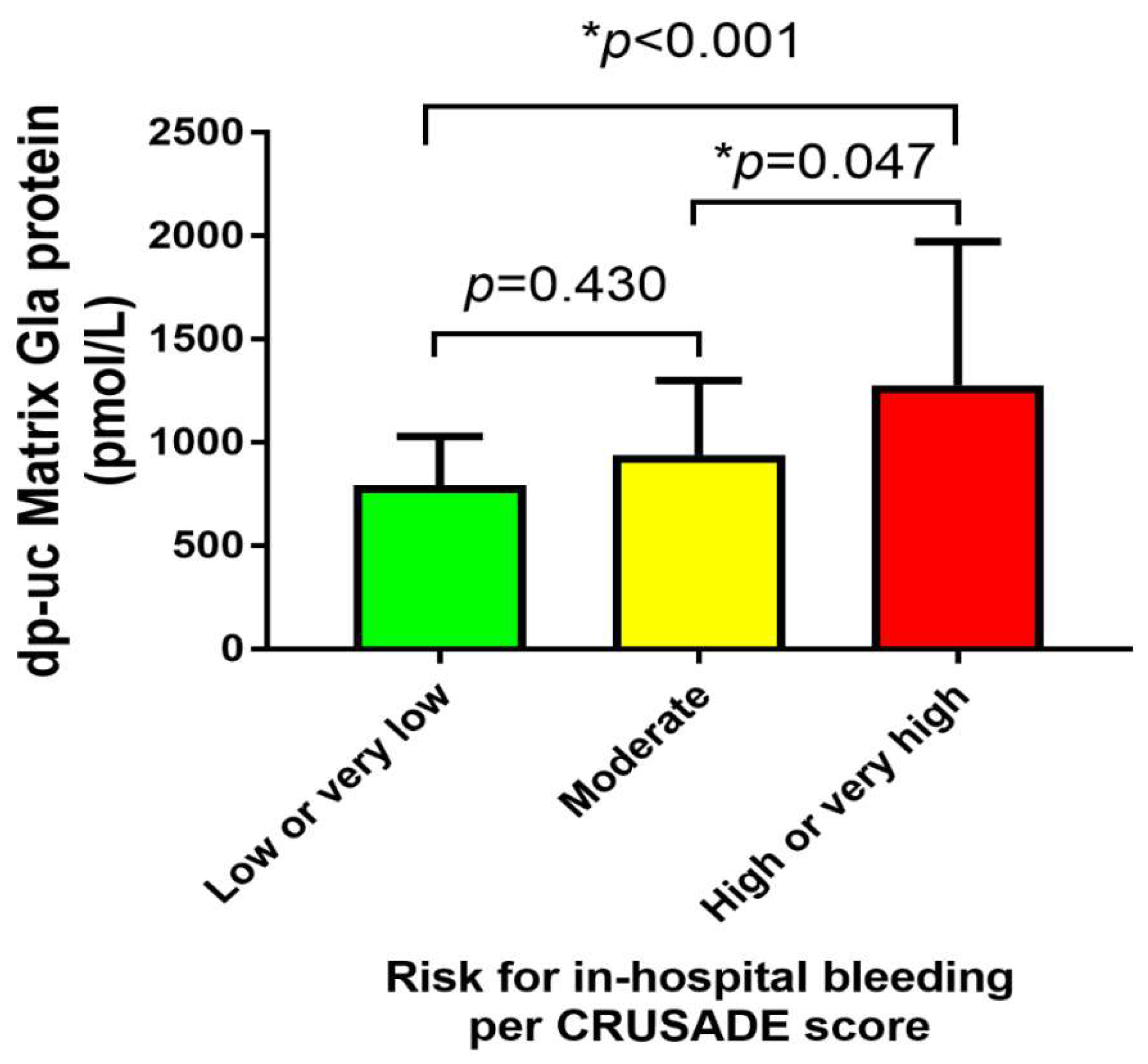


| Variable | Overall (n = 80) | STEMI (n = 40) | NSTEMI (n = 40) | p-Value |
|---|---|---|---|---|
| Age, years | 69.2 ± 7.5 | 68.4 ± 7.2 | 70.0 ± 7.7 | 0.441 1 |
| Male sex | 61 (76%) | 29 (72%) | 32 (80%) | 0.621 2 |
| Body mass index, kg/m2 | 27.0 ± 2.5 | 27.4 ± 2.7 | 26.7 ± 2.2 | 0.594 1 |
| Admission heart rate, bpm | 75.1 ± 16.8 | 76.7 ± 18.7 | 73.5 ± 14.7 | 0.205 1 |
| Systolic blood pressure, mmHg | 133.9 ± 21.6 | 132.7 ± 21.7 | 135.1 ± 21.7 | 0.699 1 |
| Diastolic blood pressure, mmHg | 78.4 ± 12.5 | 79.2 ± 11.9 | 77.6 ± 13.3 | 0.087 1 |
| CRUSADE score, points | 29.9 ± 12.3 | 27.4 ± 9.9 | 32.4 ± 14.0 | 0.0111 |
| GRACE score, points | 125.9 ± 12.8 | 126.1 ± 20.5 | 125.8 ± 16.6 | 0.168 1 |
| Left ventricular ejection fraction, % | 50.2 ± 10.3 | 50.2 ± 9.6 | 50.4 ± 11.3 | 0.192 1 |
| Estimated glomerular filtration rate, mL/min | 69.2 ± 19.0 | 72.5 ± 16.1 | 65.9 ± 21.2 | 0.0291 |
| Urea, mmol/L | 7.9 ± 3.3 | 7.3 ± 2.2 | 8.5 ± 4.1 | 0.0391 |
| Creatinine, μmol/L | 97.6 ± 36.5 | 89.8 ± 22.0 | 105.4 ± 45.7 | 0.0341 |
| Erythrocytes ×1012/L | 4.63 ± 0.52 | 4.63 ± 0.56 | 4.62 ± 0.48 | 0.690 1 |
| Hematocrit, L/L | 0.463 ± 0.05 | 0.513 ± 0.06 | 0.413 ± 0.05 | 0.114 1 |
| Leukocytes, ×109/L | 10.2 ± 2.9 | 10.9 ± 2.7 | 9.6 ± 3.0 | 0.580 1 |
| Thrombocytes, ×109/L | 233.9 ± 63.1 | 233.7 ± 59.1 | 234.1 ± 67.5 | 0.619 1 |
| Glucose, mmol/L | 8.3 ± 3.8 | 8.2 ± 3.4 | 8.4 ± 4.3 | 0.239 1 |
| C-reactive protein, mg/L * | 16.5 ± 33.2 | 18.6 ± 40.8 | 14.3 ± 23.5 | 0.317 3 |
| hs-cTnI at admission, ng/L * | 404.7 ± 560.7 | 446.3 ± 645.7 | 363.1 ± 464.9 | 0.073 3 |
| Prothrombin time-INR | 1.10 ± 0.41 | 1.08 ± 0.27 | 1.13 ± 0.53 | 0.585 1 |
| Activated partial thromboplastin time, s | 24.3 ± 4.2 | 24.1 ± 4.5 | 24.4 ± 4.0 | 0.730 1 |
| dp-ucMGP, pmol/L | 931.8 ± 446.7 | 765.1 ± 166.2 | 1098.6 ± 565.2 | 0.0011 |
| Multivessel disease | 12 (16.4%) | 2 (5.1%) | 10 (29.4%) | 0.0054 |
| Arterial hypertension | 49 (61.3%) | 25 (62.5%) | 24 (60%) | 0.818 2 |
| Diabetes mellitus type II | 13 (16.3%) | 4 (10%) | 9 (22.5%) | 0.130 4 |
| Smoking | 35 (43.8%) | 20 (50%) | 15 (37.5%) | 0.260 2 |
| Beta-blocker use | 28 (35%) | 12 (30%) | 16 (40%) | 0.348 2 |
| ACE inhibitor or ARB use | 40 (50%) | 20 (50%) | 20 (50%) | 1.000 2 |
| Calcium channel blocker use | 14 (17.5%) | 8 (20%) | 6 (15%) | 0.556 2 |
| Statin use | 16 (20%) | 5 (12.5) | 11 (27.5%) | 0.094 2 |
| Variable | Odds Ratio | 95% CI | p-Value |
|---|---|---|---|
| Age, years | 1.043 | 0.946–1.150 | 0.393 |
| dp-ucMGP, pmol/L | 1.004 | 1.002–1.006 | 0.023 |
| Thrombocyte count, ×109/L | 1.010 | 0.998–1.022 | 0.062 |
| Prothrombin time-INR | 0.734 | 0.186–2.896 | 0.659 |
| aPTT, s | 1.067 | 0.922–1.234 | 0.384 |
| Multivessel disease | 2.770 | 0.485–15.824 | 0.252 |
| ACS type | 0.832 | 0.156–4.429 | 0.829 |
| Variable | Lowest Dp-ucMGP Tertile | Highest Dp-ucMGP Tertile | p-Value * |
|---|---|---|---|
| Age, years | 67.6 ± 6.8 | 71.5 ± 7.8 | 0.065 |
| CRUSADE bleeding score, points | 25.7 ± 10.7 | 37.4 ± 13.6 | <0.001 |
| GRACE score, points | 124 ± 22 | 131 ± 16 | 0.188 |
| eGFR, mL/min | 77.5 ± 16.5 | 56.9 ± 19.4 | <0.001 |
| Hematocrit, L/L | 0.44 ± 0.04 | 0.39 ± 0.05 | 0.004 |
| Thrombocytes, ×109/L | 234 ± 64 | 239 ± 64 | 0.829 |
| Prothrombin time-INR | 0.99 ± 0.05 | 1.30 ± 0.07 | 0.038 |
| aPTT, s | 23.0 ± 1.7 | 26.4 ± 6.3 | 0.012 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bilalic, A.; Kurir, T.T.; Borovac, J.A.; Kumric, M.; Supe-Domic, D.; Vilovic, M.; Martinovic, D.; Bozic, J. Association of Dephosphorylated-Uncarboxylated Matrix Gla Protein and Risk of Major Bleeding in Patients Presenting with Acute Myocardial Infarction. Life 2021, 11, 733. https://doi.org/10.3390/life11080733
Bilalic A, Kurir TT, Borovac JA, Kumric M, Supe-Domic D, Vilovic M, Martinovic D, Bozic J. Association of Dephosphorylated-Uncarboxylated Matrix Gla Protein and Risk of Major Bleeding in Patients Presenting with Acute Myocardial Infarction. Life. 2021; 11(8):733. https://doi.org/10.3390/life11080733
Chicago/Turabian StyleBilalic, Admira, Tina Ticinovic Kurir, Josip A. Borovac, Marko Kumric, Daniela Supe-Domic, Marino Vilovic, Dinko Martinovic, and Josko Bozic. 2021. "Association of Dephosphorylated-Uncarboxylated Matrix Gla Protein and Risk of Major Bleeding in Patients Presenting with Acute Myocardial Infarction" Life 11, no. 8: 733. https://doi.org/10.3390/life11080733
APA StyleBilalic, A., Kurir, T. T., Borovac, J. A., Kumric, M., Supe-Domic, D., Vilovic, M., Martinovic, D., & Bozic, J. (2021). Association of Dephosphorylated-Uncarboxylated Matrix Gla Protein and Risk of Major Bleeding in Patients Presenting with Acute Myocardial Infarction. Life, 11(8), 733. https://doi.org/10.3390/life11080733









