Separation and Determination of Chemopreventive Phytochemicals of Flavonoids from Brassicaceae Plants
Abstract
:1. Introduction
2. Results
2.1. Extraction Yield
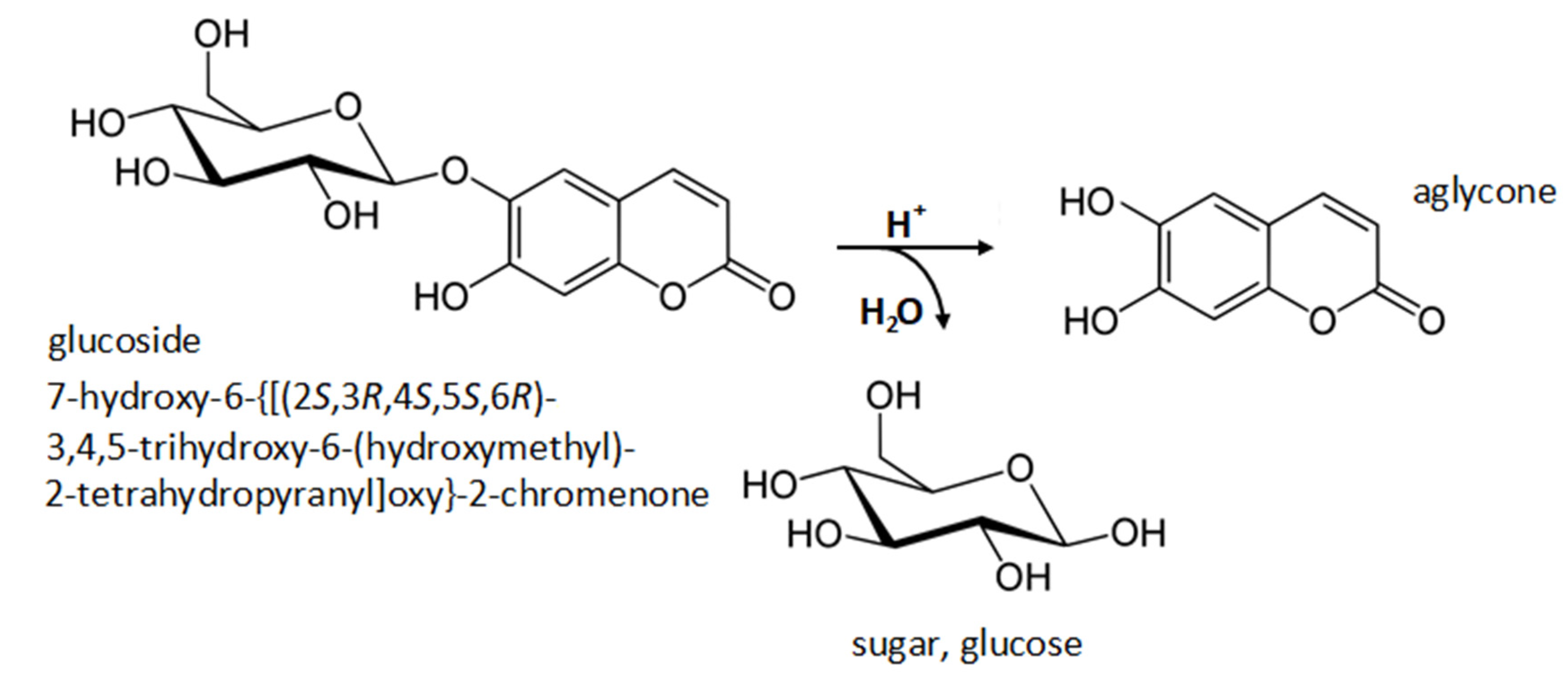
2.2. Hydrolysis of Extracts
2.3. Antioxidant Activity and Total Phenolics Contents in Plants Extracts
2.4. RP-ESI-UHPLC-MS/MS Method Validation
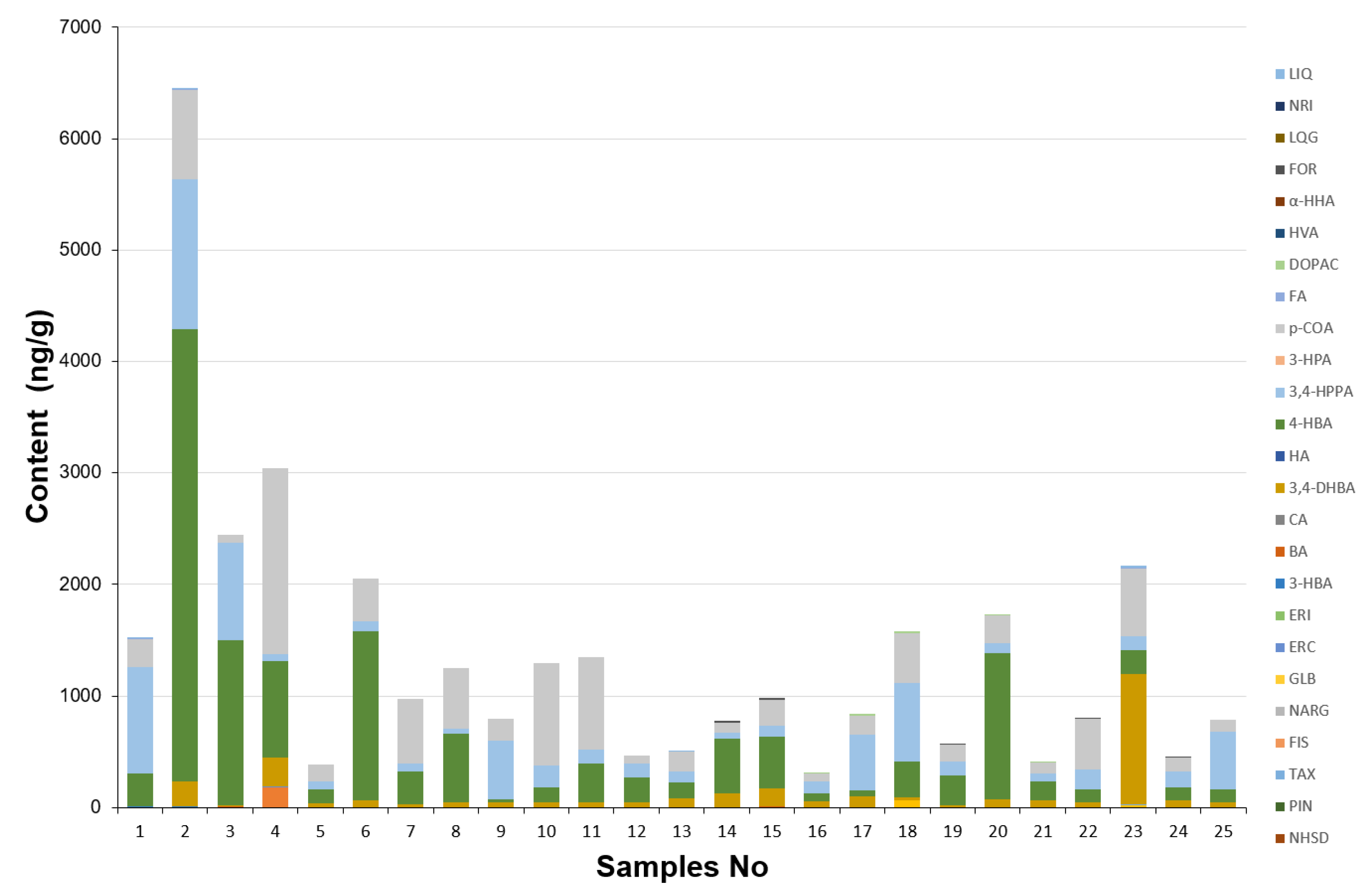
2.5. Determination of Phenolic Acids and Flavonoids in Brassicaceae Plants Extracts
3. Material and Methods
3.1. Analyzed Samples
3.2. Extract Preparation (Maceration)
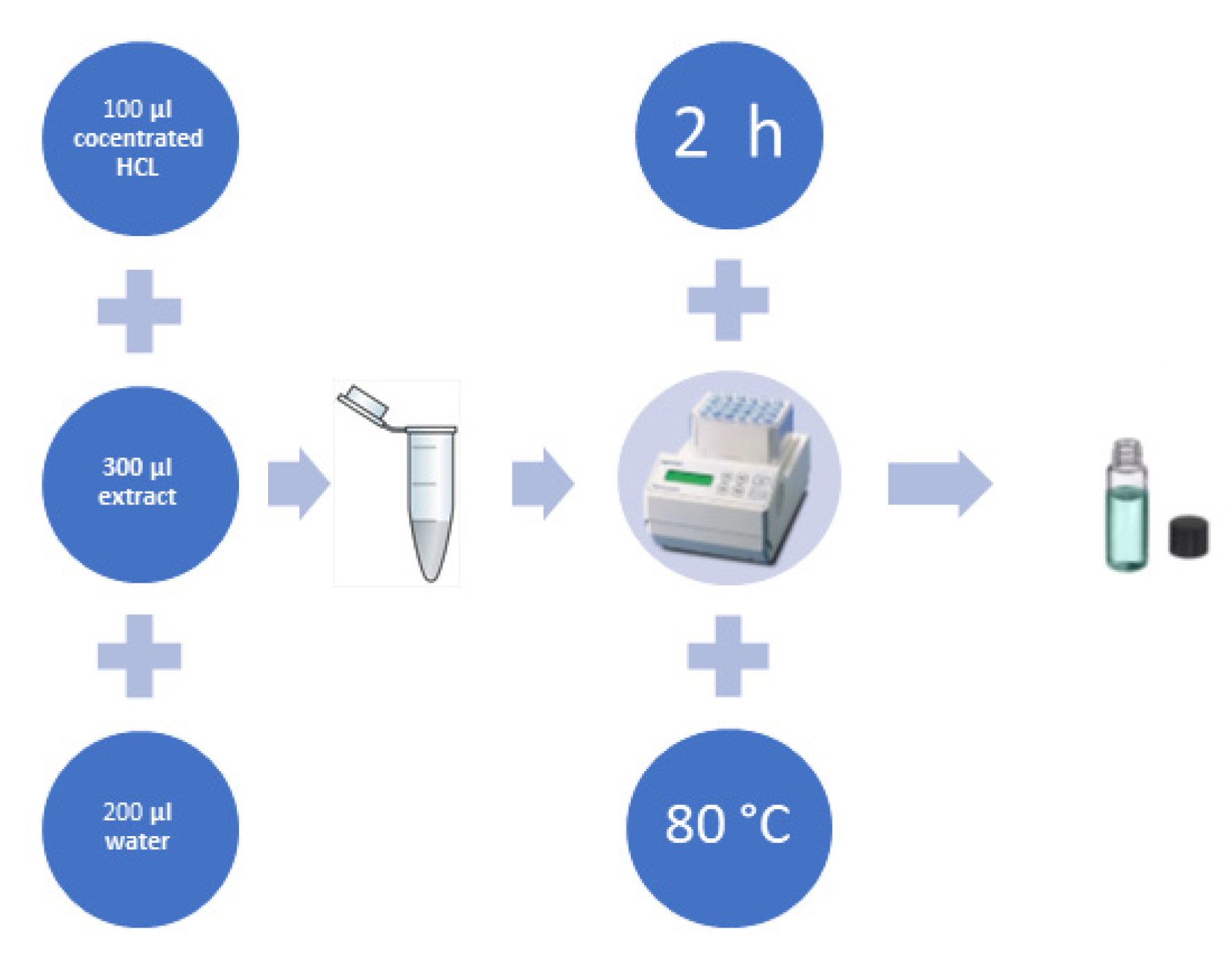
3.3. Hydrolysis of Plant Extracts
3.4. Chemicals and Reagents for Preliminary Experiments
3.5. Apparatus
3.6. Antioxidant Activity
3.7. Determination of Total Phenolics Contents in Plants Extracts
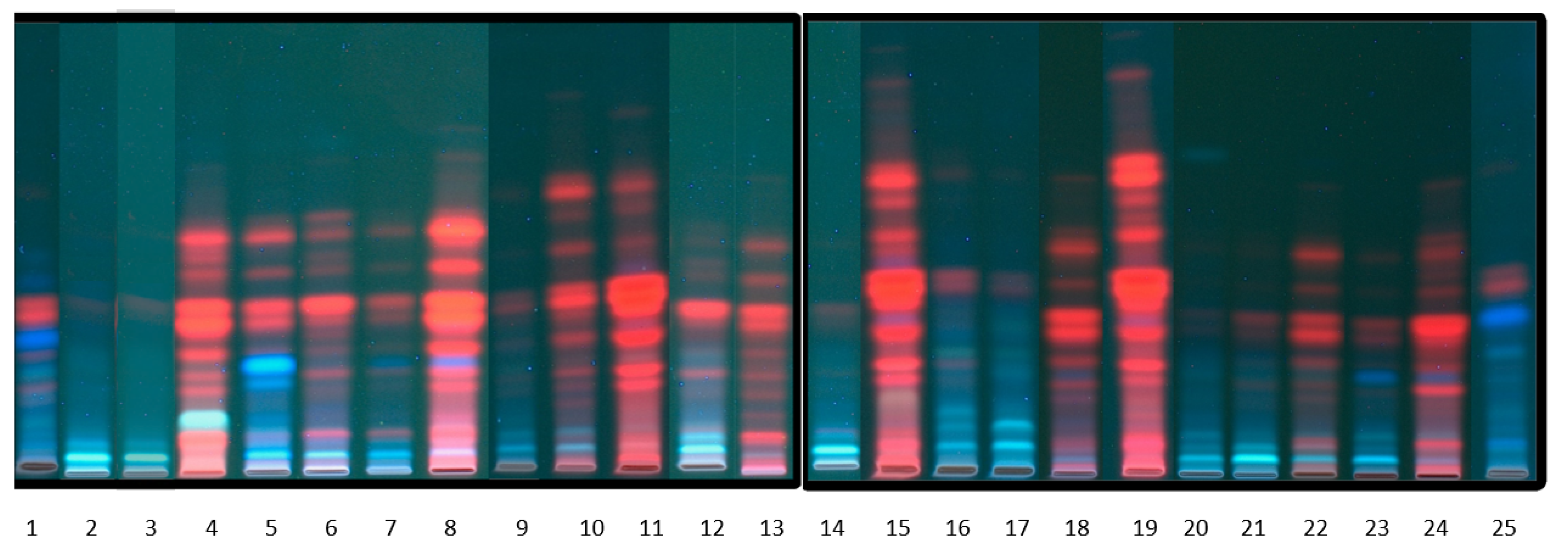
3.8. TLC Analysis
3.9. RP-UHPLC-ESI-MS/MS Method to Determine Selected Flavonoids
3.10. Methods Validation
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Warwick, S.I. Brassicaceae in Agriculture. In Genetics and Genomics of the Brassicaceae; Schmidt, R., Bancroft, I., Eds.; Springer: New York, NY, USA, 2011; Volume 9. [Google Scholar]
- Abu-Ghannam, N.; Jaiswal, A.K. Blanching as a treatment process: Effect on polyphenol and antioxidant capacity of cabbage. In Processing and Impact on Active Components in Food; Elsevier: Amsterdam, The Netherlands, 2015. [Google Scholar]
- Kusznierewicz, B.; Śmiechowska, A.; Bartoszek, A.; Namieśnik, J. The effect of heating and fermenting on antioxidant properties of white cabbage. Food Chem. 2008, 108, 853–861. [Google Scholar] [CrossRef]
- Kusznierewicza, B.; Bartoszek, A.; Wolska, L.; Drzewiecki, J.; Gorinstein, S.; Namieśnik, J. Partial characterization of white cabbages (Brassica oleracea var. capitata f. alba) from different regions by glucosinolates, bioactive compounds, total antioxidant activities and proteins. LWT-Food Sci. Technol. 2008, 41, 1–9. [Google Scholar]
- Koss-Mikołajczyk, I.; Kusznierewicz, B.; Wiczkowski, W.; Płatosz, N.; Bartoszek, A. Phytochemical composition and biological activities of differently pigmented cabbage (Brassica oleracea var. capitata) and cauliflower (Brassica oleracea var. botrytis) varieties. J. Sci. Food. Agric. 2019, 99, 5499–5507. [Google Scholar] [PubMed]
- Chantiratikul, A.; Pakmaruek, P.; Chinrasri, O.; Aengwanich, W.; Chookhampaeng, S.; Maneetong, S.; Chantiratikul, P. Efficacy of selenium from hydroponically produced selenium-enriched kale sprout (Brassica oleracea var. alboglabra L.) in broilers. Biol. Trace Elem. Res. 2015, 165, 96–102. [Google Scholar] [CrossRef]
- Kurilich, A.C.; Tsau, G.J.; Brown, A.; Howard, L.; Klein, B.P.; Jeffery, E.H.; Kushad, M.; Wallig, M.A.; Juvik, J.A. Carotene, tocopherol, and ascorbate contents in subspecies of brassica oleracea. J. Agric. Food Chem. 1999, 47, 1576–1581. [Google Scholar] [CrossRef] [PubMed]
- Naguib, A.E.M.M.; El-Baz, F.K.; Salama, Z.A.; Hanaa, H.A.E.B.; Ali, H.F.; Gaafar, A.A. Enhancement of phenolics, flavonoids and glucosinolates of Broccoli (Brassica olaracea var. Italica) as antioxidants in response to organic and bio-organic fertilizers. J. Saudi Soc. Agric. Sci. 2012, 11, 135–142. [Google Scholar]
- Kaulmann, A.; André, C.M.; Schneider, Y.J.; Hoffmann, L.; Bohn, T. Carotenoid and polyphenol bioaccessibility and cellular uptake from plum and cabbage varieties. Food. Chem. 2016, 197, 325–332. [Google Scholar]
- Cartea, M.E.; Francisco, M.; Soengas, P.; Velasco, P. Phenolic compounds in Brassica vegetables. Molecules 2011, 16, 251–280. [Google Scholar] [CrossRef] [PubMed]
- Possenti, M.; Baima, S.; Raffo, A.; Durazzo, A. Glucosinolates in food. In Glucosinolates, Reference Series in Phytochemistry; Giusti, A.M., Fausta, N.J.M., Mérillon, K., Ramawat, G., Eds.; Springer International Publishing: Cham, Switzerland, 2016. [Google Scholar]
- Moon, J.K.; Kim, J.R.; Ahn, Y.J.; Shibamoto, T. Analysis and Anti-Helicobacter Activity of Sulforaphane and Related Compounds Present in Broccoli (Brassica oleracea L.) Sprouts. J. Agric. Food Chem. 2010, 58, 6672–6677. [Google Scholar] [CrossRef]
- Rakhmetov, D.; Vergun, O.; Rakhmetova, S.; Fishchenko, V. Antioxidant potential of selected oil plants of Brassicaceaeburnett. Agrobiodivers. Impr. Nut. Health Life Qual. 2018, 2, 133–139. [Google Scholar]
- Kusznierewicz, B.; Lewandowska, J.; Kruszyna, A.; Piasek, A.; Śmiechowska, A.; Namiśnik, J.; Bartoszek, A. The antioxidative properties of white cabbage (Brassica oleracea var. capitata f. alba) fresh and submitted to culinary processing. J. Food Biochem. 2010, 34, 262–285. [Google Scholar]
- Seong, G.U.; Hwang, I.W.; Chung, S.K. Antioxidant capacities and polyphenolics of Chinese cabbage (Brassica rapa L. ssp. Pekinensis) leaves. Food Chem. 2016, 199, 612–618. [Google Scholar] [CrossRef] [PubMed]
- Taveira, M.; Pereira, D.M.; Sousa, C.; Ferreres, F.; Andrade, P.B.; Martins, A.; Pereira, J.A.; Valentão, P. In vitro cultures of Brassica oleracea L. var. Costata DC: Potential plant bioreactor for antioxidant phenolic compounds. J. Agric. Food Chem. 2009, 57, 1247–1252. [Google Scholar]
- Jeffery, E.H.; Brown, A.F.; Kurilich, A.C.; Keck, A.S.; Matusheski, N.; Klein, B.P.; Juvik, J.A. Variation in content of bioactive components in broccoli. J. Food Compos. Anal. 2003, 16, 323–330. [Google Scholar] [CrossRef]
- Lisiewska, Z.; Kmiecik, W. Effects of level of nitrogen fertilizer, processing conditions and period of storage of frozen broccoli and cauliflower on vitamin C retention. Food Chem. 1996, 57, 261–270. [Google Scholar] [CrossRef]
- Vallejo, F.; Tomás-Barberán, F.A.; García-Viguera, C. Potential bioactive compounds in health promotion from broccoli cultivars grown in Spain. J. Sci. Food Agric. 2002, 82, 1293–1297. [Google Scholar] [CrossRef]
- Berg, H.; Faulks, R.; Granado, H.F.; Hirschberg, J.; Olmedilla, B.; Sandmann, G.; Southon, S.; Stahl, W. The potential for the improvement of carotenoid levels in foods and the likely systemic effects. J. Sci. Food Agric. 2000, 80, 880–912. [Google Scholar] [CrossRef]
- FoodData Central. Available online: https://fdc.nal.usda.gov/index.html (accessed on 15 March 2021).
- Fimognari, C.; Hrelia, P. Sulforaphane as a promising molecule for fighting cancer. Mutat. Res. 2007, 635, 90–104. [Google Scholar] [CrossRef] [PubMed]
- Jaiswal, A.; Abu-Ghannam, N.; Gupta, S. A comparative study on the polyphenolic content, antibacterial activity and antioxidant capacity of different solvent extracts of brassica oleracea vegetables. Int. J. Food Sci. Technol. 2012, 47, 223–231. [Google Scholar] [CrossRef] [Green Version]
- Morales-López, J.; Centeno-Álvarez, M.; Nieto-Camacho, A.; López, M.G.; Pérez-Hernández, E.; Pérez-Hernández, N.; Fernández-Martínez, E. Evaluation of antioxidant and hepatoprotective effects of white cabbage essential oil. Pharm. Biol. 2017, 55, 233–241. [Google Scholar] [CrossRef]
- Aires, A.; Fernandes, C.; Carvalho, R.; Bennett, R.N.; Saavedra, M.J.; Rosa, E.A. Seasonal effects on bioactive compounds and antioxidant capacity of six economically important brassica vegetables. Molecules 2011, 16, 6816–6832. [Google Scholar] [CrossRef] [Green Version]
- Avato, P.; Argentieri, M.P. Brassicaceae: A rich source of health improving phytochemicals. Phytochem. Rev. 2015, 14, 1019–1033. [Google Scholar] [CrossRef]
- Avato, P.; D’Addabbo, T.; Leonetti, P.; Argentieri, M.P. Nematicidal potential of Brassicaceae. Phytochem. Rev. 2013, 12, 791–802. [Google Scholar] [CrossRef]
- Cléro, É.; Doyon, F.; Chungue, V.; Rachédi, F.; Boissin, J.L.; Sebbag, J.; Shan, L.; Rubino, C.; Vathaire, F. Dietary patterns, goitrogenic food, and thyroid cancer: A case-control study in French Polynesia. Nutr. Cancer 2012, 64, 929–936. [Google Scholar] [CrossRef]
- Wook, J.C.; Jeongseon, K. Factors and the risk of thyroid cancer: A review. Clin. Nutr. Res. 2014, 3, 75–88. [Google Scholar]
- Llorach, R.; Gil-Izquierdo, A.; Ferreres, F.; Tomás-Barberán, F.A. HPLC-DAD-MS/MS ESI characterization of unusual highly glycosylated acylated flavonoids from cauliflower (Brassica oleracea L. var. botrytis) agroindustrialbyproducts. J. Agric. Food Chem. 2003, 51, 3895–3899. [Google Scholar] [CrossRef]
- Jana, S.; Patel, D.; Patel, S.; Upadhyay, K.; Thadani, J.; Mandal, R.; Das, S.; Devkar, R. Anthocyanin rich extract of Brassica oleracea L. alleviates experimentally induced myocardial infarction. PLoS ONE 2017, 12, e0182137. [Google Scholar] [CrossRef]
- Giorgetti, L.; Giorgi, G.; Cherubini, E.; Gervasi, P.G.; Della Croce, C.M.; Longo, V.; Bellani, L. Screening and identification of major phytochemical compounds in seeds, sprouts and leaves of Tuscan black kale Brassica oleracea (L.) sspacephala (DC) var. sabellica L. Nat. Prod. Res. 2018, 32, 1617–1626. [Google Scholar] [CrossRef] [PubMed]
- Park, S.Y.; Lim, S.H.; Ha, S.H.; Yeo, Y.; Park, W.T.; Kwon, D.Y.; Park, S.U.; Kim, J.K. Metabolite profiling approach reveals the interface of primary and secondary metabolism in colored cauliflowers (Brassica oleracea L. ssp. botrytis). J. Agric. Food Chem. 2013, 61, 6999–7007. [Google Scholar] [CrossRef]
- Dong, M.; Tian, Z.; Ma, Y.; Yang, Z.; Ma, Z.; Wang, X.; Li, Y.; Jiang, H. Rapid screening and characterization of glucosinolates in 25 Brassicaceae tissues by UHPLC-Q-exactiveorbitrap-MS. Food Chem. 2021, 365, 130493. [Google Scholar] [CrossRef] [PubMed]
- Arena, K.; Cacciola, F.; Dugo, L.; Dugo, P.; Mondello, L. Determination of the metabolite content of Brassica juncea cultivars using comprehensive two-dimensional liquid chromatography coupled with a photodiode array and mass spectrometry detection. Molecules 2020, 25, 1235. [Google Scholar] [CrossRef] [Green Version]
- Šamec, D.; Pavlović, I.; Redovniković, I.R.; Salopek-Sondi, B. Comparative analysis of phytochemicals and activity of endogenous enzymes associated with their stability, bioavailability and food quality in five Brassicaceae sprouts. Food Chem. 2018, 269, 96–102. [Google Scholar] [CrossRef]
- Fratianni, F.; Cardinale, F.; Cozzolino, A.; Granese, T.; Pepe, S.; Riccardi, R.; Spigno, P.; Coppola, R.; Nazzaro, F. Polyphenol Composition and Antioxidant Activity of Two Autochthonous Brassicaceae of the Campania Region, Southern Italy. Food Nutr. Sci. 2014, 5, 66–70. [Google Scholar] [CrossRef] [Green Version]
- Heimler, D.; Vignolini, P.; Dini, M.G.; Vincieri, F.F.; Romani, A. Antiradical activity and polyphenol composition of local Brassicaceae edible varieties. Food Chem. 2006, 96, 464–469. [Google Scholar] [CrossRef]
- Mattila, P.; Hellström, J. Phenolic acids in potatoes, vegetables, and some of their products. J. Food Compos. Anal. 2007, 20, 152–160. [Google Scholar] [CrossRef]
- Kinoshita, T.; Kajiyama, K.; Hiraga, Y.; Takahashi, K.; Tamura, Y.; Mizutani, K. Isoflavan derivatives from Glycyrrhizaglabra (licorice). Heterocycles 1996, 43, 581–588. [Google Scholar] [CrossRef]
- Wang, J.; Wang, D.; Yu, J.; Liu, C.; Li, L.; Zhang, Y. Isolation of liquiritigenin-4′-apiosyl-glucoside and liquiritin from the root of Glycyrrhiza uralensis by high-performance centrifugal partition chromatography. J. Chromatogr. Sci. 2014, 52, 310–314. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, Y.W.; Kang, H.E.; Lee, M.G.; Hwang, S.J.; Kim, S.C.; Lee, C.H.; Kim, S.G. Liquiritigenin, a flavonoid aglycone from licorice, has a choleretic effect and the ability to induce hepatic transporters and phase-II enzymes. AJP-GI 2009, 296, G372–G381. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rodríguez-García, C.; Sánchez-Quesada, C.; Gaforio, J.J. Dietary Flavonoids as Cancer Chemopreventive Agents: An Updated Review of Human Studies. Antioxidants 2019, 8, 137. [Google Scholar] [CrossRef] [Green Version]
- Punvittayagul, C.; Wongpoomchai, R.; Taya, S.; Pompimon, W. Effect of pinocembrin isolated from Boesenbergiapandurata on xenobiotic-metabolizing enzymes in rat liver. Drug Metab. Lett. 2001, 5, 1–5. [Google Scholar] [CrossRef]
- Medjakovic, S.; Jungbauer, A. Red Clover Isoflavones Biochanin A and Formononetin are Potent Ligands of the Human Aryl Hydrocarbon Receptor. J. Steroid. Biochem. Mol. Biol. 2008, 108, 171–177. [Google Scholar] [CrossRef] [PubMed]
- Peterson, J.J.; Beecher, G.R.; Bhagwat, S.A.; Dwyer, J.T.; Gebhardt, S.E.; Haytowitz, D.B.; Holden, J.M. Flavanones in grapefruit, lemons, and limes: A compilation and review of the data from the analytical literature. J. Food Compos. Anal. 2006, 19, S74–S80. [Google Scholar] [CrossRef]
- Bajkacz, S.; Baranowska, I.; Buszewski, B.; Kowalski, B.; Ligor, M. Determination of Flavonoids and Phenolic Acids in Plant Materials Using SLE-SPE-UHPLC-MS/MS Method. Food Anal. Methods 2018, 11, 3563–3575. [Google Scholar] [CrossRef] [Green Version]
- United Nations Office on Drugs and Crime (UNODC). Guidance for the Validation of Analytical Methodology and Calibration of Equipment Used for Testing of Illicit Drugs in Seized Materials and Biological Specimens; United Nations Vienna: Wien, Austria, 2009. [Google Scholar]
- Wenzl, T.; Haedrich, J.; Schaechtele, A.; Robouch, P.; Stroka, J. Guidance Document on the Estimation of LOD and LOQ for Measurements in the Field of Contaminants in Feed and Food; Publications Office of the European Union: Luxemburg, 2016. [Google Scholar]

| No | Sample Type | The Extraction Yield (EY) [%] ± SD a |
|---|---|---|
| 1 | Radish–sprouts | 22.0 ± 2.11 |
| 2 | Radish–roots | 11.73 ± 1.18 |
| 3 | Radish–spots | 10.25 ± 1.04 |
| 4 | Radish–leaves | 6.22 ± 0.58 |
| 5 | Broccoli–sprouts | 19.96 ± 1.97 |
| 6 | Broccoli–flowers | 24.13 ± 2.42 |
| 7 | Brussels sprouts–leaves | 4.84 ± 0.35 |
| 8 | Kale–leaves | 16.86 ± 1.57 |
| 9 | Canola–roots | 8.00 ± 0.88 |
| 10 | Canola–spots | 11.88 ± 1.15 |
| 11 | Canola–leaves | 12.54 ± 1.19 |
| 12 | Conehead cabbage–leaves | 10.43 ± 1.02 |
| 13 | Kohlrabi–spots | 42.42 ± 4.15 |
| 14 | Kohlrabi–edible spot | 11.67 ± 1.09 |
| 15 | Kohlrabi–leaves | 14.23 ± 1.31 |
| 16 | Green cabbage–leaves | 16.06 ± 1.52 |
| 17 | Chinese cabbage–midvein | 23.22 ± 2.18 |
| 18 | Chinese cabbage–leaves | 13.59 ± 1.14 |
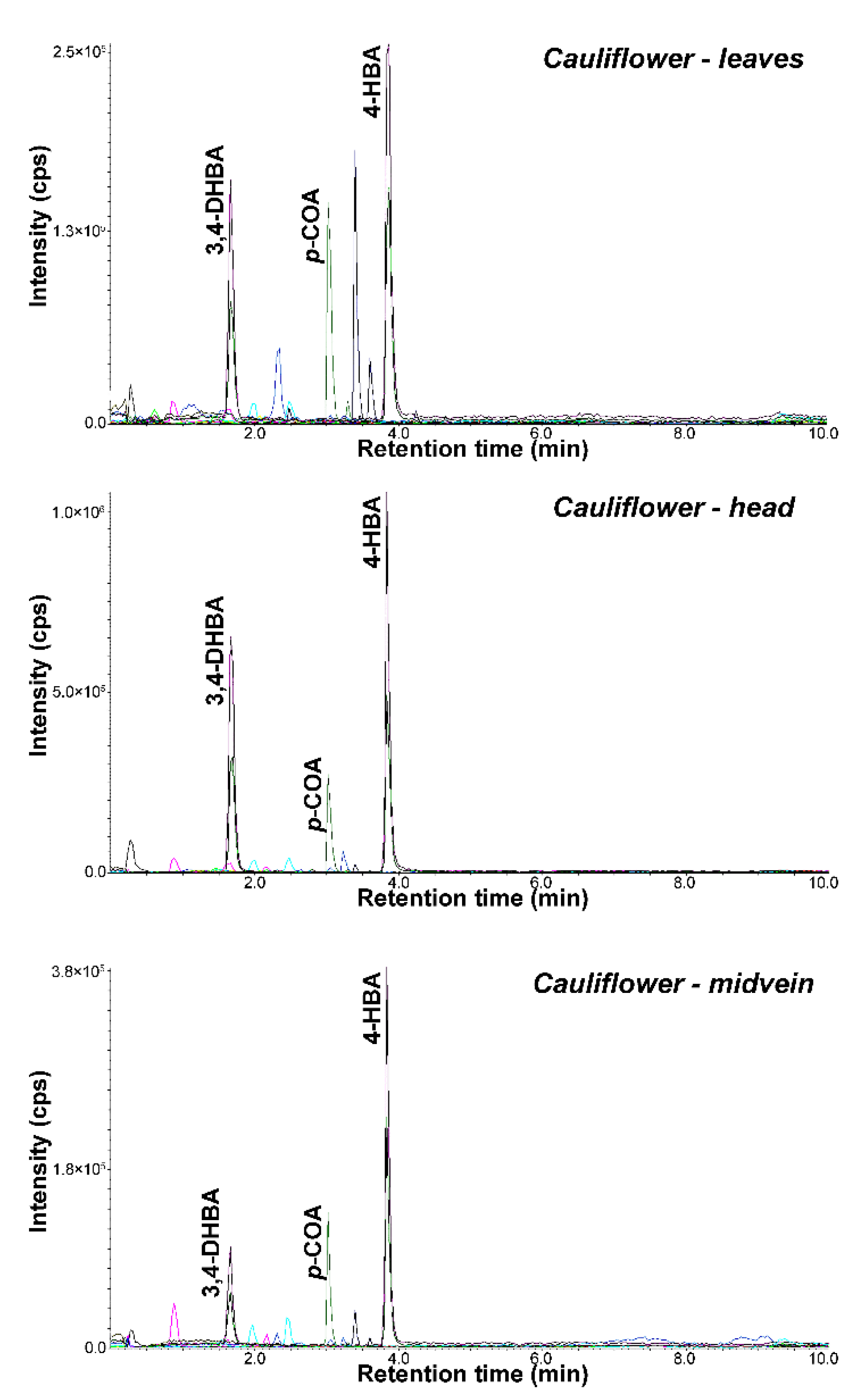
| 19 | Cauliflower–leaves | 7.27 ± 0.67 |
| 20 | Cauliflower–head | 18.73 ± 1.78 |
| 21 | Cauliflower–midvein | 28.75 ± 2.65 |
| 22 | Pak Choi cabbage–leaves | 17.49 ± 1.75 |
| 23 | Red cabbage–leaves | 15.10 ± 1.47 |
| 24 | Italian cabbage–leaves | 17.19 ± 1.57 |
| 25 | Kale–sprouts | 20.93 ± 2.13 |
| Sample No | %RSA | TEAC [mM] | GAE [mg/mL] | Total Phenolic Content [mg/g] |
|---|---|---|---|---|
| 1 | 86.73 ± 0.67 | 81.86 | 0.85 | 8.47 |
| 2 | 40.09 ± 0.18 | 24.44 | 0.47 | 4.67 |
| 3 | 41.12 ± 0.19 | 26.43 | 0.51 | 4.86 |
| 4 | 54.55 ± 0.64 | 14.36 | 0.59 | 5.87 |
| 5 | 84.35 ± 0.64 | 78.45 | 0.80 | 8.03 |
| 6 | 41.97 ± 0.09 | 18.22 | 0.44 | 4.37 |
| 7 | 40.29 ± 1.00 | 15.84 | 0.44 | 4.40 |
| 8 | 52.54 ± 0.18 | 23.14 | 0.66 | 6.62 |
| 9 | 30.89 ± 0.55 | 9.19 | 0.35 | 3.47 |
| 10 | 42.88 ± 0.28 | 13.39 | 0.41 | 4.10 |
| 11 | 51.24 ± 0.69 | 21.04 | 0.45 | 4.52 |
| 12 | 38.02 ± 2.20 | 47.77 | 0.48 | 4.77 |
| 13 | 45.97 ± 1.23 | 10.65 | 0.36 | 3.55 |
| 14 | 20.77 ± 1.83 | 23.53 | 0.40 | 3.99 |
| 15 | 63.89 ± 1.19 | 32.23 | 0.56 | 5.60 |
| 16 | 76.59 ± 0.09 | 87.16 | 0.63 | 6.27 |
| 17 | 80.23 ± 0.46 | 72.39 | 0.63 | 6.26 |
| 18 | 67.19 ± 0.18 | 45.87 | 0.47 | 4.67 |
| 19 | 45.86 ± 0.46 | 28.58 | 0.51 | 5.13 |
| 20 | 61.55 ± 0.28 | 43.98 | 0.43 | 4.33 |
| 21 | 42.43 ± 2.75 | 49.28 | 0.45 | 4.46 |
| 22 | 29.98 ± 0.94 | 27.35 | 0.43 | 4.33 |
| 23 | 58.12 ± 0.92 | 48.90 | 0.64 | 6.44 |
| 24 | 56.89 ± 0.64 | 71.25 | 0.59 | 5.89 |
| 25 | 60.65 ± 0.09 | 94.73 | 0.77 | 7.72 |
| Analyte | Linear Range (ng/g) | R2 | LOD/LOQ (ng/g) | Nominal Concentration (ng/g) | Intra-Day | Inter-Day | Matrix Effect ME (%) | ||
|---|---|---|---|---|---|---|---|---|---|
| CV a (%) | RE b (%) | CV a (%) | RE b (%) | ||||||
| 3,4-DHBA | 0.4–500 | 0.9951 | 0.13/0.4 | 300 | 2.14 | 1.36 | 3.63 | 2.51 | −4.58 |
| 10 | 3.84 | 2.41 | 5.47 | 3.96 | −6.98 | ||||
| α-HHA | 0.4–500 | 0.9924 | 0.13/0.4 | 300 | 4.12 | −2.15 | 6.18 | −3.74 | −8.47 |
| 10 | 5.47 | −3.47 | 7.56 | −5.25 | −7.36 | ||||
| DOPAC | 0.4–500 | 0.9974 | 0.13/0.4 | 300 | 1.73 | −4.28 | 3.58 | −5.87 | 2.57 |
| 10 | 3.69 | −5.39 | 5.17 | −6.39 | 4.65 | ||||
| 4-HBA | 0.4–500 | 0.9936 | 0.13/0.4 | 300 | 2.58 | −2.76 | 3.81 | −4.85 | 5.36 |
| 10 | 4.69 | −3.33 | 4.83 | −6.97 | 6.17 | ||||
| CA | 0.8–500 | 0.9947 | 0.26/0.8 | 300 | 1.93 | 2.15 | 4.51 | 4.28 | −5.12 |
| 10 | 2.17 | 4.87 | 6.37 | 5.78 | −6.47 | ||||
| HA | 0.4–500 | 0.9991 | 0.13/0.4 | 300 | 4.83 | −1.79 | 7.42 | −3.69 | −2.14 |
| 10 | 5.39 | −3.65 | 8.69 | −4.83 | −6.07 | ||||
| 3-HBA | 0.4–500 | 0.9989 | 0.13/0.4 | 300 | 2.11 | −0.94 | 4.41 | −2.81 | 3.19 |
| 10 | 4.78 | −2.75 | 5.93 | −5.12 | 5.47 | ||||
| 3-HPA | 0.4–500 | 0.9978 | 0.13/0.4 | 300 | 4.32 | 3.54 | 6.71 | 4.32 | 2.58 |
| 10 | 6.71 | 4.39 | 8.54 | 6.96 | 4.96 | ||||
| HVA | 0.8–500 | 0.9990 | 0.26/0.8 | 300 | 2.95 | −3.27 | 4.33 | −5.63 | −3.96 |
| 10 | 3.69 | −5.83 | 7.05 | −8.11 | −5.87 | ||||
| 3,4-HPPA | 20–500 | 0.9963 | 6.67/20 | 300 | 0.87 | −2.94 | 3.82 | −4.75 | −4.12 |
| 50 | 2.47 | −4.65 | 4.57 | −6.35 | −6.05 | ||||
| p-COA | 0.4–500 | 0.9952 | 0.13/0.4 | 300 | 3.98 | 1.23 | 5.71 | 2.85 | 4.78 |
| 10 | 4.72 | 3.67 | 6.87 | 5.78 | 5.39 | ||||
| FA | 0.4–500 | 0.9932 | 0.13/0.4 | 300 | 2.56 | 2.26 | 4.41 | 4.59 | −1.47 |
| 10 | 4.78 | 4.63 | 5.89 | 6.32 | −4.36 | ||||
| ERC | 0.4–500 | 0.9981 | 0.13/0.4 | 300 | 3.66 | −3.15 | 4.87 | −4.59 | 3.97 |
| 10 | 5.21 | −3.98 | 6.39 | −5.93 | 5.82 | ||||
| LIQ | 0.4–500 | 0.9994 | 0.13/0.4 | 300 | 4.17 | −4.71 | 5.71 | −6.47 | 2.74 |
| 10 | 6.41 | −5.02 | 8.53 | −7.89 | 4.69 | ||||
| RUT | 0.4–500 | 0.9993 | 0.13/0.4 | 300 | 3.25 | 2.47 | 4.52 | 4.52 | 3.55 |
| 10 | 4.17 | 3.69 | 5.36 | 6.36 | 5.41 | ||||
| TAX | 0.4–500 | 0.9947 | 0.13/0.4 | 300 | 2.87 | 4.52 | 4.23 | 5.29 | 2.69 |
| 10 | 4.63 | 5.87 | 6.87 | 7.85 | 3.74 | ||||
| BA | 0.8–500 | 0.9968 | 0.26/0.8 | 300 | 1.85 | −2.78 | 2.54 | −3.89 | 4.11 |
| 10 | 3.64 | −5.69 | 4.85 | −7.25 | 5.07 | ||||
| NRI | 0.8–500 | 0.9920 | 0.26/0.8 | 300 | 2.87 | −3.21 | 4.41 | −4.25 | −6.87 |
| 10 | 3.54 | −4.87 | 6.52 | −6.78 | −8.15 | ||||
| NARG | 0.8–500 | 0.9994 | 0.26/0.8 | 300 | 0.47 | −5.70 | 1.23 | −6.23 | 4.25 |
| 10 | 3.69 | −6.42 | 4.32 | −8.52 | 5.78 | ||||
| HSD | 0.4–500 | 0.9987 | 0.13/0.4 | 300 | 4.21 | 2.14 | 6.05 | 3.46 | 5.23 |
| 10 | 5.87 | 4.78 | 7.93 | 4.85 | 6.90 | ||||
| NHSD | 0.4–500 | 0.9965 | 0.13/0.4 | 300 | 3.78 | 3.58 | 5.25 | 5.39 | 2.14 |
| 10 | 4.93 | 4.96 | 6.38 | 6.87 | 4.79 | ||||
| FIS | 0.4–500 | 0.9971 | 0.13/0.4 | 300 | 2.41 | 1.64 | 4.21 | 2.58 | −3.67 |
| 10 | 5.87 | 2.83 | 7.28 | 4.65 | −4.52 | ||||
| LQG | 0.4–500 | 0.9939 | 0.13/0.4 | 300 | 4.28 | −5.22 | 6.48 | −6.87 | −6.21 |
| 10 | 6.14 | −5.89 | 8.52 | −7.41 | −7.56 | ||||
| ERI | 0.4–500 | 0.9914 | 0.13/0.4 | 300 | 5.02 | 1.74 | 6.35 | 2.69 | −5.47 |
| 10 | 5.89 | 3.69 | 6.98 | 4.78 | −8.73 | ||||
| QUE | 0.8–500 | 0.9975 | 0.26/0.8 | 300 | 2.73 | 4.14 | 4.11 | 6.15 | −5.21 |
| 10 | 3.85 | 5.63 | 5.28 | 7.25 | −6.78 | ||||
| NAR | 0.4–500 | 0.9993 | 0.13/0.4 | 300 | 1.73 | 2.54 | 2.55 | 4.32 | 1.63 |
| 10 | 4.62 | 4.36 | 5.87 | 5.69 | 2.87 | ||||
| HST | 0.8–500 | 0.9945 | 0.26/0.8 | 300 | 3.98 | −5.32 | 3.69 | −6.14 | 3.69 |
| 10 | 5.87 | −6.12 | 7.21 | −8.25 | 5.74 | ||||
| FOR | 0.4–500 | 0.9968 | 0.13/0.4 | 300 | 2.02 | −1.56 | 4.20 | −3.69 | 2.46 |
| 10 | 4.96 | −3.54 | 5.93 | −4.75 | 6.89 | ||||
| PIN | 0.4–500 | 0.9972 | 0.13/0.4 | 300 | 1.52 | 2.47 | 2.47 | 3.62 | 1.25 |
| 10 | 3.69 | 3.69 | 4.89 | 5.55 | 4.98 | ||||
| GLB | 0.4–500 | 0.9954 | 0.13/0.4 | 300 | 0.57 | 4.52 | 1.47 | 5.87 | −3.57 |
| 10 | 3.58 | 5.69 | 5.63 | 6.45 | −4.82 | ||||
| No | Sample Type | Latin Name |
|---|---|---|
| 1 | Radish–sprouts | Raphanus sativus var. Sativus |
| 2 | Radish–roots | Raphanus sativus var. Sativus |
| 3 | Radish–spots | Raphanus sativus var. Sativus |
| 4 | Radish–leaves | Raphanus sativus var. Sativus |
| 5 | Broccoli–sprouts | Brassica oleracea L. var. italica Plenck |
| 6 | Broccoli–flowers | Brassica oleracea L. var. italica Plenck |
| 7 | Brussels sprouts–leaves | Brassica oleracea L. var. gemmifera (DC.) Zenker |
| 8 | Kale–leaves | Brassica oleracea L. var. sabellica L. |
| 9 | Canola–roots | Brassica napus L. |
| 10 | Canola–spots | Brassica napus L. |
| 11 | Canola–leaves | Brassica napus L. |
| 12 | Conehead cabbage–leaves | Brassica oleracea ‘Cour di Bue Grosso’ |
| 13 | Kohlrabi–spots | Brassica oleracea var. gongylodes L. |
| 14 | Kohlrabi–edible spot | Brassica oleracea var. gongylodes L. |
| 15 | Kohlrabi–leaves | Brassica oleracea var. gongylodes L. |
| 16 | Green cabbage–leaves | Brassica oleracea L. var. capitata L. |
| 17 | Chinese cabbage–midvein | Brassica rapa L. subsp. pekinensis |
| 18 | Chinese cabbage–leaves | Brassica rapa L. subsp. pekinensis |
| 19 | Cauliflower–leaves | Brassica oleracea L. var. botrytis L. |
| 20 | Cauliflower–head | Brassica oleracea L. var. botrytis L. |
| 21 | Cauliflower–midvein | Brassica oleracea L. var.botrytis L. |
| 22 | Pak Choi cabbage–leaves | Brassica rapa L. subsp. chinensis Hanelt |
| 23 | Red cabbage–leaves | Brassica oleracea var. capitata f. rubra |
| 24 | Italian cabbage–leaves | Brassica oleracea L. var. sabauda L. |
| 25 | Kale–sprouts | Brassica oleracea L. var. sabellica L. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bajkacz, S.; Ligor, M.; Baranowska, I.; Buszewski, B. Separation and Determination of Chemopreventive Phytochemicals of Flavonoids from Brassicaceae Plants. Molecules 2021, 26, 4734. https://doi.org/10.3390/molecules26164734
Bajkacz S, Ligor M, Baranowska I, Buszewski B. Separation and Determination of Chemopreventive Phytochemicals of Flavonoids from Brassicaceae Plants. Molecules. 2021; 26(16):4734. https://doi.org/10.3390/molecules26164734
Chicago/Turabian StyleBajkacz, Sylwia, Magdalena Ligor, Irena Baranowska, and Bogusław Buszewski. 2021. "Separation and Determination of Chemopreventive Phytochemicals of Flavonoids from Brassicaceae Plants" Molecules 26, no. 16: 4734. https://doi.org/10.3390/molecules26164734
APA StyleBajkacz, S., Ligor, M., Baranowska, I., & Buszewski, B. (2021). Separation and Determination of Chemopreventive Phytochemicals of Flavonoids from Brassicaceae Plants. Molecules, 26(16), 4734. https://doi.org/10.3390/molecules26164734







