Integration of the Process for Production of Ethyl Acetate by an Enhanced Extraction Process
Abstract
:1. Introduction
1.1. Synthesis Methods
1.2. Purification Method
- (1)
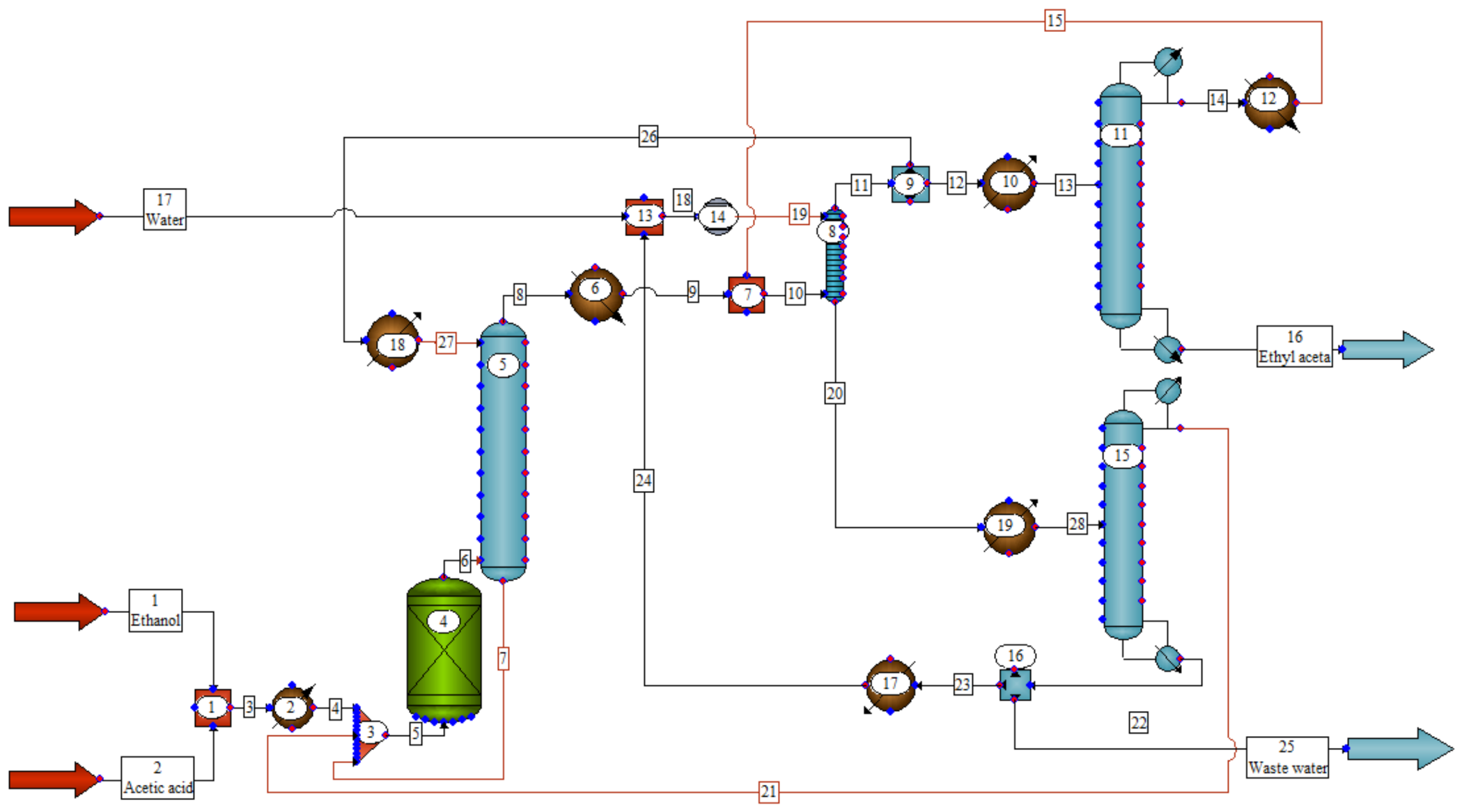
- First is the process of rectifying the reaction mixture, i.e., stripping acetic acid by a rectification column and distilling the ethyl acetate–ethanol–water (EA–ET–W) ternary azeotrope. In order to generate an azeotrope by the distillation of reaction products, and to avoid accumulating water in the reactor, an azeotropic agent should be fed to the column. By esterification of the acetic acid with ethanol, some excess water is released (approximately 17%) [29] which is more than contained in the azeotrope mixture (approximately 7.8%). Therefore, the azeotropic agent is the organic phase in the extraction process, recycled to the column. This fraction is richer in ethyl acetate than the azeotrope (>93%) [8], so it binds the excess water.
- (2)
- After azeotropic distillation, there is an extraction process. To obtain enriched phase of ethyl acetate from the distillation product, it is necessary to wash it with water and extract ethanol. The ethanol content of the organic mixture affects the solubility of water in this phase. The higher is the content of alcohol, the greater the water content in the organic phase. By extracting ethanol, water is also removed from the organic phase [8].
- (3)
- The final stage is the process of product rectification. Enriched phase of ethyl acetate is fed to the rectification column, where, by distilling the triple azeotrope, a pure ethyl acetate is obtained as the bottom product [8].
2. Methodology
- -
- a closed circuit of the extractant, demineralized water, reducing the consumption of the fresh extractant, but also a deep recovery of raw materials, i.e., ethyl acetate and ethanol from wastewater, significantly reducing the TOL;
- -
- cooling the azeotrope before extraction (Streams 8 and 14, Figure 1) from 70 °C down to 30 °C (HTXR 6, 12, Figure 1), shifting a phase equilibrium towards higher concentrations of EA in the organic phase, resulting in a reduced reflux on the azeotropic column as well as the reduced flowrate of the water phase directed to the wastewater treatment plant.
2.1. Thermodynamic Model
2.2. Kinetics of Esterification Reaction
3. Results
3.1. Optimization of the Reaction System
3.2. Optimization of Azeotropic Distillation
3.3. Optimization of the Extraction System
3.4. Optimization of Product Distillation
3.5. Optimization of Wastewater Distillation
3.6. Mass and Heat Balance of the Different Process Aproaches
4. Discussion
5. Conclusions
- ethanol consumption was reduced from 0.531 to 0.524 t/tproduct (−1.2%);
- the amount of process water was reduced from 2.18 to 1.42 t/tproduct (−34.9%);
- the product quality was improved from 98.0% to 99.9%;
- wastewater production was reduced from 2.36 to 1.61 t/tproduct (−31.8%) and the TOC/COD was reduced almost nine times.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A
| Stream No. | Molar Flow kmol/h | Mass Flow kg/h | Temp. °C | Pres. Bar | Component Mass % | |||
|---|---|---|---|---|---|---|---|---|
| Ethanol | Water | Ethyl Acetate | Acetic Acid | |||||
| 1 | 141.3 | 6511 | 15 | 1 | 100.00 | 0.00 | 0.00 | 0.00 |
| 2 | 141.1 | 8472 | 15 | 1 | 0.00 | 0.00 | 0.00 | 100.00 |
| 3 | 282.4 | 14,983 | 15 | 1 | 43.46 | 0.00 | 0.00 | 56.54 |
| 4 | 282.4 | 14,983 | 70 | 1 | 43.46 | 0.00 | 0.00 | 56.54 |
| 5 | 1223.3 | 47,670 | 77 | 1 | 28.65 | 22.96 | 25.67 | 22.72 |
| 6 | 1223.3 | 47,670 | 90 | 1 | 15.02 | 28.29 | 51.74 | 4.96 |
| 7 | 611.2 | 13,504 | 88 | 1 | 5.60 | 73.38 | 3.57 | 17.45 |
| 8 | 1354.8 | 90,166 | 71 | 1 | 8.00 | 6.45 | 85.54 | 0.01 |
| 9 | 1354.8 | 90,166 | 30 | 1 | 8.00 | 6.45 | 85.54 | 0.01 |
| 10 | 1520.4 | 100,880 | 30 | 1 | 7.48 | 6.67 | 85.84 | 0.01 |
| 11 | 1049.7 | 79,150 | 21 | 1 | 1.45 | 3.99 | 94.55 | 0.01 |
| 12 | 307.0 | 23,151 | 21 | 1 | 1.45 | 3.99 | 94.55 | 0.01 |
| 13 | 307.0 | 23,151 | 71 | 1 | 1.45 | 3.99 | 94.55 | 0.01 |
| 14 | 165.6 | 10,715 | 70 | 1 | 3.09 | 8.57 | 88.34 | 0.00 |
| 15 | 165.6 | 10,715 | 30 | 1 | 3.09 | 8.57 | 88.34 | 0.00 |
| 16 | 141.4 | 12,436 | 77 | 1 | 0.04 | 0.04 | 99.90 | 0.02 |
| 17 | 969.4 | 17,464 | 20 | 1 | 0.00 | 100.00 | 0.00 | 0.00 |
| 18 | 5600.9 | 100,920 | 20 | 1 | 0.02 | 99.97 | 0.00 | 0.01 |
| 19 | 5600.9 | 100,920 | 20 | 1 | 0.02 | 99.97 | 0.00 | 0.01 |
| 20 | 6071.6 | 122,650 | 24 | 1 | 5.23 | 85.17 | 9.59 | 0.01 |
| 21 | 329.8 | 19,187 | 71 | 1 | 33.32 | 5.41 | 61.28 | 0.00 |
| 22 | 5741.4 | 103,456 | 100 | 1 | 0.02 | 99.96 | 0.00 | 0.02 |
| 23 | 4631.5 | 83,456 | 100 | 1 | 0.02 | 99.96 | 0.00 | 0.02 |
| 24 | 4631.5 | 83,456 | 20 | 1 | 0.02 | 99.96 | 0.00 | 0.02 |
| 25 | 1109.9 | 20,000 | 100 | 1 | 0.02 | 99.96 | 0.00 | 0.02 |
| 26 | 742.7 | 56,000 | 21 | 1 | 1.45 | 3.99 | 94.55 | 0.01 |
| 27 | 742.7 | 56,000 | 70 | 1 | 1.45 | 3.99 | 94.55 | 0.01 |
| 28 | 6071.2 | 122,643 | 70 | 1 | 5.23 | 85.17 | 9.59 | 0.01 |
| Stream No. | Molar Flow kmol/h | Mass Flow kg/h | Temp. °C | Pres. Bar | Component Mass % | |||
|---|---|---|---|---|---|---|---|---|
| Ethanol | Water | Ethyl Acetate | Acetic Acid | |||||
| 1 | 141.3 | 6511 | 15 | 1 | 100.00 | 0.00 | 0.00 | 0.00 |
| 2 | 141.1 | 8472 | 15 | 1 | 0.00 | 0.00 | 0.00 | 100.00 |
| 3 | 282.4 | 14,983 | 15 | 1 | 43.46 | 0.00 | 0.00 | 56.54 |
| 4 | 282.4 | 14,983 | 70 | 1 | 43.46 | 0.00 | 0.00 | 56.54 |
| 5 | 1352.7 | 53,292 | 77 | 1 | 27.49 | 22.86 | 29.02 | 20.64 |
| 6 | 1352.7 | 53,292 | 90 | 1 | 15.29 | 27.62 | 52.34 | 4.74 |
| 7 | 676.0 | 14,912 | 88 | 1 | 5.86 | 73.55 | 3.67 | 16.93 |
| 8 | 1493.9 | 98,380 | 70 | 1 | 8.53 | 6.68 | 84.78 | 0.01 |
| 9 | 1493.9 | 98,380 | 70 | 1 | 8.53 | 6.68 | 84.78 | 0.01 |
| 10 | 1724.2 | 113,256 | 70 | 1 | 7.86 | 6.93 | 85.21 | 0.01 |
| 11 | 1189.1 | 87,314 | 27 | 1 | 1.86 | 4.70 | 93.43 | 0.01 |
| 12 | 372.0 | 27,314 | 27 | 1 | 1.86 | 4.70 | 93.43 | 0.01 |
| 13 | 372.0 | 27,314 | 71 | 1 | 1.86 | 4.70 | 93.43 | 0.01 |
| 14 | 230.4 | 14,880 | 70 | 1 | 3.40 | 8.57 | 88.03 | 0.00 |
| 15 | 230.4 | 14,880 | 70 | 1 | 3.40 | 8.57 | 88.03 | 0.00 |
| 16 | 141.5 | 12,434 | 77 | 1 | 0.02 | 0.07 | 99.90 | 0.01 |
| 17 | 970.2 | 17,478 | 20 | 1 | 0.00 | 100.00 | 0.00 | 0.00 |
| 18 | 6286.9 | 113,276 | 20 | 1 | 0.01 | 99.98 | 0.00 | 0.01 |
| 19 | 6286.9 | 113,276 | 20 | 1 | 0.01 | 99.98 | 0.00 | 0.01 |
| 20 | 6821.3 | 139,203 | 41 | 1 | 5.24 | 84.04 | 10.72 | 0.01 |
| 21 | 394.6 | 23,405 | 71 | 1 | 31.06 | 5.19 | 63.75 | 0.00 |
| 22 | 6426.7 | 115,797 | 100 | 1 | 0.02 | 99.97 | 0.00 | 0.01 |
| 23 | 5316.7 | 95,797 | 100 | 1 | 0.02 | 99.97 | 0.00 | 0.01 |
| 24 | 5316.7 | 95,797 | 20 | 1 | 0.02 | 99.97 | 0.00 | 0.01 |
| 25 | 1110.0 | 20,000 | 100 | 1 | 0.02 | 99.97 | 0.00 | 0.01 |
| 26 | 817.1 | 60,000 | 27 | 1 | 1.86 | 4.70 | 93.43 | 0.01 |
| 27 | 817.1 | 60,000 | 70 | 1 | 1.86 | 4.70 | 93.43 | 0.01 |
| 28 | 6821.3 | 139,203 | 70 | 1 | 5.24 | 84.04 | 10.72 | 0.01 |
| Stream No. | Molar Flow kmol/h | Mass Flow kg/h | Temp. °C | Pres. Bar | Component Mass % | |||
|---|---|---|---|---|---|---|---|---|
| Ethanol | Water | Ethyl Acetate | Acetic Acid | |||||
| 1 | 143.2 | 6595 | 15 | 1 | 100.00 | 0.00 | 0.00 | 0.00 |
| 2 | 141.1 | 8472 | 15 | 1 | 0.00 | 0.00 | 0.00 | 100.00 |
| 3 | 284.2 | 15,068 | 15 | 1 | 43.77 | 0.00 | 0.00 | 56.23 |
| 4 | 284.2 | 15,068 | 70 | 1 | 43.77 | 0.00 | 0.00 | 56.23 |
| 5 | 1296.7 | 51,047 | 77 | 1 | 25.04 | 23.01 | 27.46 | 24.49 |
| 6 | 1296.7 | 51,047 | 90 | 1 | 12.31 | 27.99 | 51.82 | 7.89 |
| 7 | 694.0 | 16,516 | 88 | 1 | 5.72 | 65.19 | 4.71 | 24.38 |
| 8 | 1424.1 | 94,531 | 71 | 1 | 6.84 | 6.81 | 86.35 | 0.00 |
| 9 | 1424.1 | 94,531 | 70 | 1 | 6.84 | 6.81 | 86.35 | 0.00 |
| 10 | 1590.0 | 105,218 | 70 | 1 | 6.53 | 6.99 | 86.48 | 0.00 |
| 11 | 1141.4 | 83,373 | 27 | 1 | 1.88 | 4.86 | 93.26 | 0.00 |
| 12 | 320.0 | 23,373 | 27 | 1 | 1.88 | 4.86 | 93.26 | 0.00 |
| 13 | 320.0 | 23,373 | 71 | 1 | 1.88 | 4.86 | 93.26 | 0.00 |
| 14 | 165.9 | 10,687 | 70 | 1 | 3.80 | 8.57 | 87.64 | 0.00 |
| 15 | 165.9 | 10,687 | 70 | 1 | 3.80 | 8.57 | 87.64 | 0.00 |
| 16 | 154.1 | 12,686 | 73 | 1 | 0.26 | 1.74 | 98.00 | 0.00 |
| 17 | 1533.0 | 27,618 | 20 | 1 | 0.00 | 100.00 | 0.00 | 0.00 |
| 18 | 5834.7 | 105,212 | 20 | 1 | 0.15 | 99.84 | 0.00 | 0.00 |
| 19 | 5834.7 | 105,212 | 20 | 1 | 0.15 | 99.84 | 0.00 | 0.00 |
| 20 | 6283.1 | 127,055 | 41 | 1 | 4.30 | 85.27 | 10.42 | 0.00 |
| 21 | 318.4 | 19,460 | 71 | 1 | 26.94 | 5.02 | 68.03 | 0.00 |
| 22 | 5964.8 | 107,594 | 99 | 1 | 0.21 | 99.79 | 0.00 | 0.00 |
| 23 | 4301.6 | 77,594 | 99 | 1 | 0.21 | 99.79 | 0.00 | 0.00 |
| 24 | 4301.6 | 77,594 | 20 | 1 | 0.21 | 99.79 | 0.00 | 0.00 |
| 25 | 1663.1 | 30,000 | 99 | 1 | 0.21 | 99.79 | 0.00 | 0.00 |
| 26 | 821.4 | 60,000 | 27 | 1 | 1.88 | 4.86 | 93.26 | 0.00 |
| 27 | 821.4 | 60,000 | 70 | 1 | 1.88 | 4.86 | 93.26 | 0.00 |
| 28 | 6283.1 | 127,055 | 70 | 1 | 4.30 | 85.27 | 10.42 | 0.00 |
References
- Kerton, F.M.; Marriott, R. Alternative Solvents for Green Chemistry, 2nd ed.; RSC Publishing: Cambridge, UK, 2013. [Google Scholar] [CrossRef]
- López-Porfiri, P.; Gorgojo, P.; Gonzalez-Miquel, M. Green Solvent Selection Guide for Biobased Organic Acid Recovery. ACS Sustain. Chem. Eng. 2020, 8, 8958–8969. [Google Scholar] [CrossRef]
- Technavio. Global Green and Bio Solvents Market 2016–2020. Ind. Biotechnol. 2016, 12, 4. [Google Scholar]
- Directive 2010/75/EU of the European Parliament and of the Council. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=celex%3A32010L0075 (accessed on 20 July 2021).
- Inoue, K.; Iwasaki, M.; Matsui, K. Process for Producing Ethyl Acetate. U.S. Patent US5241106A, 19 October 1992. [Google Scholar]
- Dalena, F.; Basile, A.; Rossi, C. Bioenergy Systems for the Future: Prospects for Biofuels and Biohydrogen; Woodhead Publishing: Sawston, UK, 2017. [Google Scholar]
- Lilja, J.; Murzin, D.Y.; Salmi, T.; Aumo, J.; Mäki-Arvela, P.; Sundell, M. Esterification of different acids over heterogeneous and homogeneous catalysts and correlation with the Taft equation. J. Mol. Catal. A Chem. 2002, 182–183, 555–563. [Google Scholar] [CrossRef]
- Yadav, G.D.; Mehta, P.H. Heterogeneous Catalysis in Esterification Reactions: Preparation of Phenethyl Acetate and Cyclohexyl Acetate by Using a Variety of Solid Acidic Catalysts. Ind. Eng. Chem. Res. 1994, 33, 2198–2208. [Google Scholar] [CrossRef]
- Bai, Y.; Zeng, S.; Bai, L.; Gao, H.; Zhou, Z.; Zhang, X. Highly Efficient Dehydration of Ethyl Acetate using Strong Hy-drophilic Ionic Liquids. Ind. Eng. Chem. Res. 2020, 59, 16751–16761. [Google Scholar] [CrossRef]
- Oleksik, J.; Krasodomski, W. Zastosowanie cieczy jonowych i katalizatorów stałych w syntezie biokomponentów pali-wowych. Naft. Gaz 2015, 4, 256–265. [Google Scholar]
- He, R.; Zou, Y.; Dong, Y.; Muhammad, Y.; Subhan, S.; Tong, Z. Kinetic study and process simulation of esterification of acetic acid and ethanol catalyzed by [HSO3-bmim][HSO4]. Chem. Eng. Res. Des. 2018, 137, 235–245. [Google Scholar] [CrossRef]
- Wu, K.; Chen, Y.W. An efficient two-phase reaction of ethyl acetate production in modified ZSM-5 zeolites. Appl. Catal. 2003, 257, 33–42. [Google Scholar] [CrossRef]
- Park, J.R.; Kwak, B.K.; Park, D.S. Effect of acid type in WO X clusters on the esterification of ethanol with acetic acid. Korean J. Chem. Eng. 2012, 29, 1695–1699. [Google Scholar] [CrossRef]
- Wang, D.; Han, Z. Production Method of Ethyl Acetate by Means of Condensation of Acetaldehyde. CN. Patent CN1245794A, 21 August 1998. [Google Scholar]
- Seki, T.; Nakajo, T.; Onaka, M. The Tishchenko Reaction: A Classic and Practical Tool for Ester Synthesis. Chem. Lett. 2006, 35, 8. [Google Scholar] [CrossRef]
- A Feasibility Study Analysing Various Process Routes of the Production of Ethyl Acetate. Available online: https://ukdiss.com/examples/production-ethyl-acetate-routes.php?vref=1 (accessed on 20 July 2021).
- Crane Robert, A.; Brown Stephen, H. Ethyl Acetate Synthesis from Ethylene and Acetic Acid Using Solid Acid Catalysts. U.S. Patent US5973193, 26 October 1999. [Google Scholar]
- Gregory, R.; Smith, D.J.H.; Westlake, D.J. The production of ethyl acetate from ethylene and acetic acid using clay catalysts. Clay Miner. 1983, 18, 431–435. [Google Scholar] [CrossRef]
- Inui, K.; Kurabayashi, T.; Sato, S. Direct synthesis of ethyl acetate from ethanol over Cu-Zn-Zr-Al-O catalyst. Appl. Catal. A Gen. 2002, 237, 53–61. [Google Scholar] [CrossRef]
- Colley, S.W.; Fawcett, C.R.; Rathmell, C.; Tuck, M.W.M. Process for the Preparation of Ethyl. Acetate. Patent EP1117631B1, 25 July 2001. [Google Scholar]
- Kirk-Othmer. Esterification. In Kirk-Othmer Encyclopedia of Chemical Technology; John Wiley & Sons: Lansing, MI, USA, 2007; Volume 9, pp. 1–37. [Google Scholar]
- Toth, A.J. Comprehensive evaluation and comparison of advanced separation methods on the separation of ethyl ace-tate-ethanol-water highly non-ideal mixture. Sep. Purif. Technol. 2019, 224, 490–508. [Google Scholar] [CrossRef]
- Gil, I.D.; Gómez, J.M.; Rodríguez, G. Control of an extractive distillation process to dehydrate ethanol using glycerol as entrainer. Comput. Chem. Eng. 2012, 39, 129–142. [Google Scholar] [CrossRef]
- Zhigang, L.; Chengyue, L.; Biaohua, C. Extractive Distillation: A Review. Sep. Purif. Rev. 2003, 32, 121–213. [Google Scholar]
- Berg, L.; Ratanapupech, P. Separation of Ethyl Acetate from Ethanol and Water by Extractive Distillation. U.S. Patent US4379028, 30 March 1982. [Google Scholar]
- Zhang, X.H.; Liu, Q.L.; Xiong, Y.; Zhu, A.M.; Chen, Y.; Zhang, Q.G. Pervaporation dehydration of ethyl ace-tate/ethanol/water azeotrope using chitosan/poly (vinyl pyrrolidone) blend membranes. J. Membr. Sci. 2009, 327, 274–280. [Google Scholar] [CrossRef]
- Liu, Y.; Hu, T.; Zhao, J.; Lu, L.; Muhammad, Y.; Lan, P.; He, R.; Zou, Y.; Tong, Z. Synthesis and application of PDMS/OP-POSS membrane for the pervaporative recovery of n-butyl acetate and ethyl acetate from aqueous media. J. Membr. Sci. 2019, 591, 117324. [Google Scholar] [CrossRef]
- Penkova, A.; Polotskaya, G.; Toikka, A. Pervaporation composite membranes for ethyl acetate production. Chem. Eng. Process. Process. Intensif. 2015, 87, 81–87. [Google Scholar] [CrossRef]
- Horan, K.A.; Murphy, C.D.; Stephens, R.M.; Warner, R.J.; Windhorst, K.A. Process Improvement for Continuous Ethyl Acetate Production. U.S. Patent US6768021B2, 22 December 1999. [Google Scholar]
- Calvar, N.; Gonz’alez, B.; Dominguez, A. Esterification of acetic acid with ethanol: Reaction kinetics and operation in a packed bed reactive distillation column. Chem. Eng. Process. 2007, 46, 1317–1323. [Google Scholar] [CrossRef]
- Arce, A.; Alonso, L.; Vidal, I. Liquid-Liquid Equilibria of the Systems Ethyl Acetate + Ethanol + Water, Butyl Acetate + Ethanol + Water, and Ethyl Acetate + Butyl Acetate + Water. J. Chem. Eng. Jpn. 1999, 32, 440–444. [Google Scholar] [CrossRef]
- Griswold, J.; Chu, P.L.; Winsauer, W.O. Phase Equilibria in Ethyl Alcohol–Ethyl Acetate–Water System. Ind. Eng. Chem. 1949, 41, 2352–2358. [Google Scholar] [CrossRef]
- Trofimova, M.; Sadaev, A.; Samarov, A.; Golikova, A.; Tsvetov, N.; Toikka, M.; Toikka, A. Liquid-liquid equilibrium of acetic acid—Ethanol—Ethyl Acetate—Water quaternary system: Data review and new results at 323.15 K and 333.15 K. Fluid Phase Equilibria 2020, 503, 112321. [Google Scholar] [CrossRef]
- Kang, Y.W.; Lee, Y.Y.; Lee, W.K. Vapor-Liquid Equilibria with Chemical Reaction Equilibrium—Systems Containing Acetic Acid, Ethyl Alcohol, Water, and Ethyl Acetate. J. Chem. Eng. Jpn. 1992, 25, 649–655. [Google Scholar] [CrossRef] [Green Version]
- Trofimova, M.; Toikka, M.; Toikka, A. Solubility, liquid–liquid equilibrium and critical states for the quaternary system acetic acid–ethanol–ethyl acetate–water at 293.15 K. Fluid Phase Equilibria 2012, 313, 46–51. [Google Scholar] [CrossRef]
- Atalay, F.S. Kinetics of the Esterification Reaction Between Ethanol and Acetic Acid. Dev. Chem. Eng. Miner. Process. 2008, 2, 181–184. [Google Scholar] [CrossRef]
- Gursahani, K.I.; Alcalá, R.; Cortright, R.D.; Dumesic, J.A. Reaction kinetics measurements and analysis of reaction pathways for conversions of acetic acid, ethanol, and ethyl acetate over silica-supported Pt. Appl. Catal. 2001, 222, 369–392. [Google Scholar] [CrossRef]
- Bell, R.P.; Dowding, A.L.; Noble, J.A. The kinetics of ester hydrolysis in concentrated aqueous acids. J. Chem. Soc. 1955, 3106–3110. [Google Scholar] [CrossRef]
- Ahmed Zeki, N.S.; Al-Hassani Haider, M.H.; Al-Jendeel, A. Kinetic Study of Esterification Reaction. Al-Khwarizmi Eng. J. 2010, 6, 33–42. [Google Scholar]







| Rectification Columns | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Heat duty [kW] | R/D | No. of Stages | Feed Stage No. | |||||||||
| S1 | S2 | S3 | S1 | S2 | S3 | S1 | S2 | S3 | S1 | S2 | S3 | |
| Rectification column SCDS 5 | - | - | - | 0.62 * | 0.61 * | 0.64 * | 8 | 8 | 8 | 8 | 8 | 8 |
| Rectification column SCDS 11 | −6593 | −9180.3 | −6611.6 | 3 | 3 | 3 | 35 | 35 | 35 | 15 | 15 | 15 |
| 6629.8 | 9219.2 | 6629.8 | ||||||||||
| Rectification column SCDS 15 | −10,186.5 | −12,127.2 | 10,186.5 | 2 | 2 | 2 | 18 | 18 | 18 | 9 | 9 | 9 |
| 13,768.6 | 16,136.6 | 13,768.6 | ||||||||||
| Reactors | ||||||||||||
| Heat Duty [kW] | Process Temperature [°C] | Volume of Apparatus [m3] | ||||||||||
| S1 | S2 | S3 | S1 | S2 | S3 | S1 | S2 | S3 | ||||
| Reactor evaporator KREA 4 | 12,171.2 | 13,586.2 | 12,747.5 | 85–90 | 85–90 | 85–90 | 300 | 300 | 300 | |||
| Extractors | ||||||||||||
| Process Temperature [°C] | W/A | |||||||||||
| S1 | S2 | S3 | S1 | S2 | S3 | |||||||
| Extractor EXTR 8 | 30 | 70 | 70 | 1 | 1 | 1 | ||||||
| Heat Exchangers | ||||||||||||
| Heat Duty [kW] | ||||||||||||
| S1 | S2 | S3 | ||||||||||
| Heat exchanger HTXR 2 | 535.6 | 535.6 | 535.6 | |||||||||
| Heat exchanger HTXR 6 | −15,658.6 | −14,855.8 | −14,014.1 | |||||||||
| Heat exchanger HTXR 10 | 673.4 | 713.5 | 609 | |||||||||
| Heat exchanger HTXR 12 | −267.4 | 0 | 0 | |||||||||
| Heat exchanger HTXR 17 | −7726.1 | −8869.3 | −8108.6 | |||||||||
| Heat exchanger HTXR 18 | 6088 | 4332.9 | 3947.5 | |||||||||
| Heat exchanger HTXR 19 | 1595 | 1541.1 | 1562 | |||||||||
| Improved Strategy S1 | Classic Strategy S2 (Heat Duty > S1) | Classic Strategy S3 (Heat Duty = S1) | |
|---|---|---|---|
| Raw Materials | |||
| Ethanol t/tproduct | 0.524 | 0.524 | 0.531 |
| Acetic acid t/tproduct | 0.681 | 0.681 | 0.681 |
| Process water t/tproduct | 1.42 | 1.42 | 2.18 |
| Product | |||
| Product quality % | 99.9 | 99.9 | 98.0 |
| Energy | |||
| Total heating kW/tproduct | 3333.9 | 3704.7 | 3704.7 |
| Total cooling kW/tproduct | 3251.1 | 3621.9 | 3621.9 |
| Wastewater | |||
| Wastewater t/tproduct | 1.61 | 1.61 | 2.36 |
| TOC mg/L | 131.7 | 131.7 | 1099.7 |
| COD mgO2/L | 470.9 | 470.9 | 4388.4 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Piotrowski, W.; Kubica, R. Integration of the Process for Production of Ethyl Acetate by an Enhanced Extraction Process. Processes 2021, 9, 1425. https://doi.org/10.3390/pr9081425
Piotrowski W, Kubica R. Integration of the Process for Production of Ethyl Acetate by an Enhanced Extraction Process. Processes. 2021; 9(8):1425. https://doi.org/10.3390/pr9081425
Chicago/Turabian StylePiotrowski, Wojciech, and Robert Kubica. 2021. "Integration of the Process for Production of Ethyl Acetate by an Enhanced Extraction Process" Processes 9, no. 8: 1425. https://doi.org/10.3390/pr9081425
APA StylePiotrowski, W., & Kubica, R. (2021). Integration of the Process for Production of Ethyl Acetate by an Enhanced Extraction Process. Processes, 9(8), 1425. https://doi.org/10.3390/pr9081425






