Acute Reperfusion Therapies for Acute Ischemic Stroke
Abstract
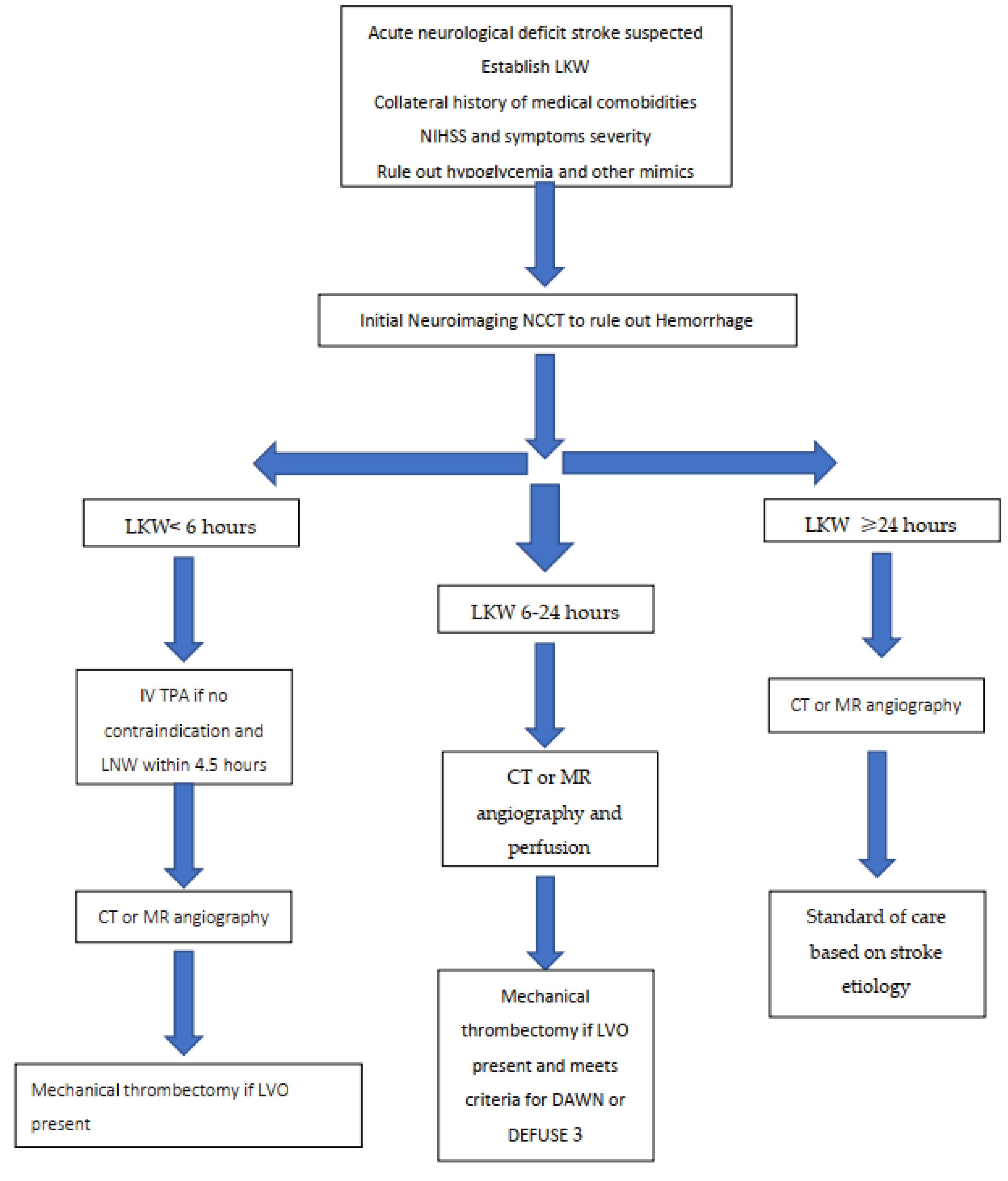
:1. Introduction
2. Basic Principles of Acute Stroke Reperfusion Therapy
3. Acute Reperfusion Treatment Options
3.1. Intravenous Thrombolysis
3.1.1. Alteplase
3.1.2. Tenecteplase
3.2. Endovascular Mechanical Thrombectomy
Special Considerations in Thrombectomy Treatment and Combination Therapy
4. Future Directions
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Virani, S.S.; Alonso, A.; Aparicio, H.J.; Benjamin, E.J.; Bittencourt, M.S.; Callaway, C.W.; Carson, A.P.; Chamberlain, A.M.; Cheng, S.; Delling, F.N.; et al. Heart Disease and Stroke Statistics—2021 Update. Circulation 2021, 143, e254–e743. [Google Scholar] [CrossRef]
- Saver, J.L. Time Is Brain—Quantified. Stroke 2006, 37, 263–266. [Google Scholar] [CrossRef] [Green Version]
- Gerber, J.C.; Petrova, M.; Krukowski, P.; Kuhn, M.; Abramyuk, A.; Bodechtel, U.; Dzialowski, I.; Engellandt, K.; Kitzler, H.; Pallesen, L.-P.; et al. Collateral state and the effect of endovascular reperfusion therapy on clinical outcome in ischemic stroke patients. Brain Behav. 2016, 6, e00513. [Google Scholar] [CrossRef]
- Lima, F.O.; Furie, K.L.; Silva, G.S.; Lev, M.H.; Camargo, É.C.; Singhal, A.B.; Harris, G.J.; Halpern, E.F.; Koroshetz, W.J.; Smith, W.S.; et al. The Pattern of Leptomeningeal Collaterals on CT Angiography Is a Strong Predictor of Long-Term Functional Outcome in Stroke Patients With Large Vessel Intracranial Occlusion. Stroke 2010, 41, 2316–2322. [Google Scholar] [CrossRef] [Green Version]
- Marks, M.P.; Lansberg, M.G.; Michael, M.; Jean-Marc, O.; Matus, S.; Stephanie, K.; Ryan, M.; Manabu, I.; Greg, Z.; Roland, B.; et al. Effect of Collateral Blood Flow on Patients Undergoing Endovascular Therapy for Acute Ischemic Stroke. Stroke 2014, 45, 1035–1039. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Leng, X.; Fang, H.; Leung, T.W.H.; Mao, C.; Miao, Z.; Liu, L.; Wong, K.S.; Liebeskind, D.S. Impact of collaterals on the efficacy and safety of endovascular treatment in acute ischaemic stroke: A systematic review and meta-analysis. J. Neurol. Neurosurg. Psychiatry 2016, 87, 537. [Google Scholar] [CrossRef] [PubMed]
- Cao, W.; Yassi, N.; Sharma, G.; Yan, B.; Desmond, P.M.; Davis, S.M.; Campbell, B.C. V Diagnosing acute lacunar infarction using CT perfusion. J. Clin. Neurosci. 2016, 29, 70–72. [Google Scholar] [CrossRef]
- Rudilosso, S.; Urra, X.; San Román, L.; Laredo, C.; López-Rueda, A.; Amaro, S.; Oleaga, L.; Chamorro, Á. Perfusion Deficits and Mismatch in Patients with Acute Lacunar Infarcts Studied with Whole-Brain CT Perfusion. Am. J. Neuroradiol. 2015, 36, 1407. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hjort, N.; Christensen, S.; Sølling, C.; Ashkanian, M.; Wu, O.; Røhl, L.; Gyldensted, C.; Andersen, G.; Østergaard, L. Ischemic injury detected by diffusion imaging 11 minutes after stroke. Ann. Neurol. 2005, 58, 462–465. [Google Scholar] [CrossRef] [PubMed]
- Benson, J.C.; Payabvash, S.; Mortazavi, S.; Zhang, L.; Salazar, P.; Hoffman, B.; Oswood, M.; McKinney, A.M. CT Perfusion in Acute Lacunar Stroke: Detection Capabilities Based on Infarct Location. Am. J. Neuroradiol. 2016, 37, 2239. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Albers, G.W.; Thijs, V.N.; Wechsler, L.; Kemp, S.; Schlaug, G.; Skalabrin, E.; Bammer, R.; Kakuda, W.; Lansberg, M.G.; Shuaib, A.; et al. Magnetic resonance imaging profiles predict clinical response to early reperfusion: The diffusion and perfusion imaging evaluation for understanding stroke evolution (DEFUSE) study. Ann. Neurol. 2006, 60, 508–517. [Google Scholar] [CrossRef]
- Kakuda, W.; Lansberg, M.G.; Thijs, V.N.; Kemp, S.M.; Bammer, R.; Wechsler, L.R.; Moseley, M.E.; Parks, M.P.; Albers, G.W. Optimal Definition for PWI/DWI Mismatch in Acute Ischemic Stroke Patients. J. Cereb. Blood Flow Metab. 2008, 28, 887–891. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- The National Institute of Neurological Disorders and Stroke rt-PA Stroke Study Group. Tissue Plasminogen Activator for Acute Ischemic Stroke. N. Engl. J. Med. 1995, 333, 1581–1588. [Google Scholar] [CrossRef] [PubMed]
- Khatri, P.; Kleindorfer, D.O.; Devlin, T.; Sawyer Jr, R.N.; Starr, M.; Mejilla, J.; Broderick, J.; Chatterjee, A.; Jauch, E.C.; Levine, S.R.; et al. Effect of Alteplase vs Aspirin on Functional Outcome for Patients With Acute Ischemic Stroke and Minor Nondisabling Neurologic Deficits: The PRISMS Randomized Clinical Trial. JAMA 2018, 320, 156–166. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hacke, W.; Donnan, G.; Fieschi, C.; Kaste, M.; von Kummer, R.; Broderick, J.P.; Brott, T.; Frankel, M.; Grotta, J.C.; Haley, E.C., Jr.; et al. Association of outcome with early stroke treatment: Pooled analysis of ATLANTIS, ECASS, and NINDS rt-PA stroke trials. Lancet 2004, 363, 768–774. [Google Scholar] [CrossRef] [PubMed]
- Hacke, W.; Kaste, M.; Bluhmki, E.; Brozman, M.; Dávalos, A.; Guidetti, D.; Larrue, V.; Lees, K.R.; Medeghri, Z.; Machnig, T.; et al. Thrombolysis with Alteplase 3 to 4.5 Hours after Acute Ischemic Stroke. N. Engl. J. Med. 2008, 359, 1317–1329. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Emberson, J.; Lees, K.R.; Lyden, P.; Blackwell, L.; Albers, G.; Bluhmki, E.; Brott, T.; Cohen, G.; Davis, S.; Donnan, G.; et al. Effect of treatment delay, age, and stroke severity on the effects of intravenous thrombolysis with alteplase for acute ischaemic stroke: A meta-analysis of individual patient data from randomised trials. Lancet 2014, 384, 1929–1935. [Google Scholar] [CrossRef] [Green Version]
- Lansberg, M.G.; Schrooten, M.; Bluhmki, E.; Thijs, V.N.; Saver, J.L. Treatment Time-Specific Number Needed to Treat Estimates for Tissue Plasminogen Activator Therapy in Acute Stroke Based on Shifts Over the Entire Range of the Modified Rankin Scale. Stroke 2009, 40, 2079–2084. [Google Scholar] [CrossRef] [Green Version]
- Dong, Y.; Cao, W.; Cheng, X.; Fang, K.; Wu, F.; Yang, L.; Xie, Y.; Dong, Q. Low-dose intravenous tissue plasminogen activator for acute ischaemic stroke: An alternative or a new standard? Stroke Vasc. Neurol. 2016, 1, 115–121. [Google Scholar] [CrossRef] [Green Version]
- Kazunori, T.; Masatoshi, K.; Masaki, N.; Yoshiaki, S.; Jyoji, N.; Eisuke, F.; Kazumi, K.; Hiroshi, Y.; Yasushi, O.; Yasuhiro, H.; et al. Routine Use of Intravenous Low-Dose Recombinant Tissue Plasminogen Activator in Japanese Patients. Stroke 2009, 40, 3591–3595. [Google Scholar] [CrossRef]
- Anderson, C.S.; Robinson, T.; Lindley, R.I.; Arima, H.; Lavados, P.M.; Lee, T.-H.; Broderick, J.P.; Chen, X.; Chen, G.; Sharma, V.K.; et al. Low-Dose versus Standard-Dose Intravenous Alteplase in Acute Ischemic Stroke. N. Engl. J. Med. 2016, 374, 2313–2323. [Google Scholar] [CrossRef] [PubMed]
- Mackey, J.; Kleindorfer, D.; Sucharew, H.; Moomaw, C.J.; Kissela, B.M.; Alwell, K.; Flaherty, M.L.; Woo, D.; Khatri, P.; Adeoye, O.; et al. Population-based study of wake-up strokes. Neurology 2011, 76, 1662–1667. [Google Scholar] [CrossRef] [Green Version]
- Fink, J.N.; Kumar, S.; Horkan, C.; Linfante, I.; Selim, M.H.; Caplan, L.R.; Schlaug, G. The Stroke Patient Who Woke Up. Stroke 2002, 33, 988–993. [Google Scholar] [CrossRef] [Green Version]
- Rimmele, D.; Thomalla, G. Wake-Up Stroke: Clinical Characteristics, Imaging Findings, and Treatment Option—An Update. Front. Neurol. 2014, 5, 35. [Google Scholar] [CrossRef] [Green Version]
- Petkova, M.; Rodrigo, S.; Lamy, C.; Oppenheim, G.; Touzé, E.; Mas, J.-L.; Méder, J.-F.; Oppenheim, C. MR Imaging Helps Predict Time from Symptom Onset in Patients with Acute Stroke: Implications for Patients with Unknown Onset Time. Radiology 2010, 257, 782–792. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aoki, J.; Kimura, K.; Iguchi, Y.; Shibazaki, K.; Sakai, K.; Iwanaga, T. FLAIR can estimate the onset time in acute ischemic stroke patients. J. Neurol. Sci. 2010, 293, 39–44. [Google Scholar] [CrossRef] [PubMed]
- Thomalla, G.; Rossbach, P.; Rosenkranz, M.; Siemonsen, S.; Krützelmann, A.; Fiehler, J.; Gerloff, C. Negative fluid-attenuated inversion recovery imaging identifies acute ischemic stroke at 3 h or less. Ann. Neurol. 2009, 65, 724–732. [Google Scholar] [CrossRef]
- Thomalla, G.; Simonsen, C.Z.; Boutitie, F.; Andersen, G.; Berthezene, Y.; Cheng, B.; Cheripelli, B.; Cho, T.-H.; Fazekas, F.; Fiehler, J.; et al. MRI-Guided Thrombolysis for Stroke with Unknown Time of Onset. N. Engl. J. Med. 2018, 379, 611–622. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tsivgoulis, G.; Katsanos, A.H.; Malhotra, K.; Sarraj, A.; Barreto, A.D.; Köhrmann, M.; Krogias, C.; Ahmed, N.; Caso, V.; Schellinger, P.D.; et al. Thrombolysis for acute ischemic stroke in the unwitnessed or extended therapeutic time window. Neurology 2020, 94, e1241. [Google Scholar] [CrossRef]
- Ma, H.; Campbell, B.C.V.; Parsons, M.W.; Churilov, L.; Levi, C.R.; Hsu, C.; Kleinig, T.J.; Wijeratne, T.; Curtze, S.; Dewey, H.M.; et al. Thrombolysis Guided by Perfusion Imaging up to 9 Hours after Onset of Stroke. N. Engl. J. Med. 2019, 380, 1795–1803. [Google Scholar] [CrossRef] [PubMed]
- Campbell, B.C.V.; Ma, H.; Ringleb, P.A.; Parsons, M.W.; Churilov, L.; Bendszus, M.; Levi, C.R.; Hsu, C.; Kleinig, T.J.; Fatar, M.; et al. Extending thrombolysis to 4·5–9 h and wake-up stroke using perfusion imaging: A systematic review and meta-analysis of individual patient data. Lancet 2019, 394, 139–147. [Google Scholar] [CrossRef] [Green Version]
- Powers, W.J.; Rabinstein, A.A.; Ackerson, T.; Adeoye, O.M.; Bambakidis, N.C.; Becker, K.; Biller, J.; Brown, M.; Demaerschalk, B.M.; Hoh, B.; et al. Guidelines for the Early Management of Patients With Acute Ischemic Stroke: 2019 Update to the 2018 Guidelines for the Early Management of Acute Ischemic Stroke: A Guideline for Healthcare Professionals From the American Heart Association/American Stroke. Stroke 2019, 50, e344–e418. [Google Scholar] [CrossRef] [PubMed]
- Guillermin, A.; Yan, D.J.; Perrier, A.; Marti, C. Safety and efficacy of tenecteplase versus alteplase in acute coronary syndrome: A systematic review and meta-analysis of randomized trials. Arch. Med. Sci. 2016, 12, 1181–1187. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Logallo, N.; Novotny, V.; Assmus, J.; Kvistad, C.E.; Alteheld, L.; Rønning, O.M.; Thommessen, B.; Amthor, K.-F.; Ihle-Hansen, H.; Kurz, M.; et al. Tenecteplase versus alteplase for management of acute ischaemic stroke (NOR-TEST): A phase 3, randomised, open-label, blinded endpoint trial. Lancet Neurol. 2017, 16, 781–788. [Google Scholar] [CrossRef]
- Campbell, B.C.V.; Mitchell, P.J.; Churilov, L.; Yassi, N.; Kleinig, T.J.; Dowling, R.J.; Yan, B.; Bush, S.J.; Dewey, H.M.; Thijs, V.; et al. Tenecteplase versus Alteplase before Thrombectomy for Ischemic Stroke. N. Engl. J. Med. 2018, 378, 1573–1582. [Google Scholar] [CrossRef] [PubMed]
- Burgos, A.M.; Saver, J.L. Evidence that Tenecteplase Is Noninferior to Alteplase for Acute Ischemic Stroke. Stroke 2019, 50, 2156–2162. [Google Scholar] [CrossRef]
- Khismatullin, R.R.; Nagaswami, C.; Shakirova, A.Z.; Vrtková, A.; Procházka, V.; Gumulec, J.; Mačák, J.; Litvinov, R.I.; Weisel, J.W. Quantitative Morphology of Cerebral Thrombi Related to Intravital Contraction and Clinical Features of Ischemic Stroke. Stroke 2020, 51, 3640–3650. [Google Scholar] [CrossRef]
- Bhatia, R.; Hill, M.D.; Shobha, N.; Menon, B.; Bal, S.; Kochar, P.; Watson, T.; Goyal, M.; Demchuk, A.M. Low Rates of Acute Recanalization With Intravenous Recombinant Tissue Plasminogen Activator in Ischemic Stroke. Stroke 2010, 41, 2254–2258. [Google Scholar] [CrossRef] [Green Version]
- Joonsang, Y.; Jang-Hyun, B.; Hyungjong, P.; Dongbeom, S.; Kyoungsub, K.; Gun, H.I.; Dae, K.Y.; Hyun, K.S.; Sun, L.H.; Hwan, A.S.; et al. Thrombus Volume as a Predictor of Nonrecanalization After Intravenous Thrombolysis in Acute Stroke. Stroke 2018, 49, 2108–2115. [Google Scholar] [CrossRef]
- Riedel, C.H.; Zimmerman, P.; Jensen-Kondering, U.; Stingele, R.; Deuschl, G.; Jansen, O. The Importance of Size. Stroke 2011, 42, 1775–1777. [Google Scholar] [CrossRef]
- Moftakhar, P.; English, J.D.; Cooke, D.L.; Kim, W.T.; Stout, C.; Smith, W.S.; Dowd, C.F.; Higashida, R.T.; Halbach, V.V.; Hetts, S.W. Density of Thrombus on Admission CT Predicts Revascularization Efficacy in Large Vessel Occlusion Acute Ischemic Stroke. Stroke 2013, 44, 243–245. [Google Scholar] [CrossRef] [Green Version]
- Rai, A.T.; Seldon, A.E.; Boo, S.; Link, P.S.; Domico, J.R.; Tarabishy, A.R.; Lucke-Wold, N.; Carpenter, J.S. A population-based incidence of acute large vessel occlusions and thrombectomy eligible patients indicates significant potential for growth of endovascular stroke therapy in the USA. J. Neurointerv. Surg. 2017, 9, 722–726. [Google Scholar] [CrossRef] [Green Version]
- Kidwell, C.S.; Jahan, R.; Gornbein, J.; Alger, J.R.; Nenov, V.; Ajani, Z.; Feng, L.; Meyer, B.C.; Olson, S.; Schwamm, L.H.; et al. A Trial of Imaging Selection and Endovascular Treatment for Ischemic Stroke. N. Engl. J. Med. 2013, 368, 914–923. [Google Scholar] [CrossRef] [Green Version]
- Ciccone, A.; Valvassori, L.; Nichelatti, M.; Sgoifo, A.; Ponzio, M.; Sterzi, R.; Boccardi, E. Endovascular Treatment for Acute Ischemic Stroke. N. Engl. J. Med. 2013, 368, 904–913. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Broderick, J.P.; Palesch, Y.Y.; Demchuk, A.M.; Yeatts, S.D.; Khatri, P.; Hill, M.D.; Jauch, E.C.; Jovin, T.G.; Yan, B.; Silver, F.L.; et al. Endovascular Therapy after Intravenous t-PA versus t-PA Alone for Stroke. N. Engl. J. Med. 2013, 368, 893–903. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bracard, S.; Ducrocq, X.; Mas, J.L.; Soudant, M.; Oppenheim, C.; Moulin, T.; Guillemin, F. Mechanical thrombectomy after intravenous alteplase versus alteplase alone after stroke (THRACE): A randomised controlled trial. Lancet Neurol. 2016, 15, 1138–1147. [Google Scholar] [CrossRef]
- Jovin, T.G.; Chamorro, A.; Cobo, E.; de Miquel, M.A.; Molina, C.A.; Rovira, A.; San Román, L.; Serena, J.; Abilleira, S.; Ribó, M.; et al. Thrombectomy within 8 Hours after Symptom Onset in Ischemic Stroke. N. Engl. J. Med. 2015, 372, 2296–2306. [Google Scholar] [CrossRef] [Green Version]
- Saver, J.L.; Goyal, M.; Bonafe, A.; Diener, H.-C.; Levy, E.I.; Pereira, V.M.; Albers, G.W.; Cognard, C.; Cohen, D.J.; Hacke, W.; et al. Stent-Retriever Thrombectomy after Intravenous t-PA vs. t-PA Alone in Stroke. N. Engl. J. Med. 2015, 372, 2285–2295. [Google Scholar] [CrossRef] [Green Version]
- Berkhemer, O.A.; Fransen, P.S.S.; Beumer, D.; van den Berg, L.A.; Lingsma, H.F.; Yoo, A.J.; Schonewille, W.J.; Vos, J.A.; Nederkoorn, P.J.; Wermer, M.J.H.; et al. A randomized trial of intraarterial treatment for acute ischemic stroke. N. Engl. J. Med. 2015, 372, 11–20. [Google Scholar] [CrossRef] [Green Version]
- Campbell, B.C.V.; Mitchell, P.J.; Kleinig, T.J.; Dewey, H.M.; Churilov, L.; Yassi, N.; Yan, B.; Dowling, R.J.; Parsons, M.W.; Oxley, T.J.; et al. Endovascular Therapy for Ischemic Stroke with Perfusion-Imaging Selection. N. Engl. J. Med. 2015, 372, 1009–1018. [Google Scholar] [CrossRef] [Green Version]
- Goyal, M.; Demchuk, A.M.; Menon, B.K.; Eesa, M.; Rempel, J.L.; Thornton, J.; Roy, D.; Jovin, T.G.; Willinsky, R.A.; Sapkota, B.L.; et al. Randomized assessment of rapid endovascular treatment of ischemic stroke. N. Engl. J. Med. 2015, 372, 1019–1030. [Google Scholar] [CrossRef] [PubMed]
- Goyal, M.; Menon, B.K.; van Zwam, W.H.; Dippel, D.W.J.; Mitchell, P.J.; Demchuk, A.M.; Dávalos, A.; Majoie, C.B.L.M.; van der Lugt, A.; de Miquel, M.A.; et al. Endovascular thrombectomy after large-vessel ischaemic stroke: A meta-analysis of individual patient data from five randomised trials. Lancet 2016, 387, 1723–1731. [Google Scholar] [CrossRef]
- Nogueira, R.G.; Jadhav, A.P.; Haussen, D.C.; Bonafe, A.; Budzik, R.F.; Bhuva, P.; Yavagal, D.R.; Ribo, M.; Cognard, C.; Hanel, R.A.; et al. Thrombectomy 6 to 24 Hours after Stroke with a Mismatch between Deficit and Infarct. N. Engl. J. Med. 2017, 378, 11–21. [Google Scholar] [CrossRef] [PubMed]
- Albers, G.W.; Marks, M.P.; Kemp, S.; Christensen, S.; Tsai, J.P.; Ortega-Gutierrez, S.; Kim-Tenser, M.; Leslie-Mazwi, T.; Sarraj, A.; Kasner, S.E.; et al. Thrombectomy for Stroke at 6 to 16 Hours with Selection by Perfusion Imaging. N. Engl. J. Med. 2017, 378, 708–718. [Google Scholar] [CrossRef]
- Ohara, T.; Menon, B.K.; Al-Ajlan, F.S.; Horn, M.; Najm, M.; Al-Sultan, A.; Puig, J.; Dowlatshahi, D.; Calleja Sanz, A.I.; Sohn, S.-I.; et al. Thrombus Migration and Fragmentation After Intravenous Alteplase Treatment. Stroke 2021, 52, 203–212. [Google Scholar] [CrossRef]
- Yang, P.; Zhang, Y.; Zhang, L.; Zhang, Y.; Treurniet, K.M.; Chen, W.; Peng, Y.; Han, H.; Wang, J.; Wang, S.; et al. Endovascular Thrombectomy with or without Intravenous Alteplase in Acute Stroke. N. Engl. J. Med. 2020, 382, 1981–1993. [Google Scholar] [CrossRef]
- Campbell, B.C.V.; Mitchell, P.J.; Churilov, L.; Yassi, N.; Kleinig, T.J.; Yan, B.; Dowling, R.J.; Bush, S.J.; Dewey, H.M.; Thijs, V.; et al. Tenecteplase versus alteplase before endovascular thrombectomy (EXTEND-IA TNK): A multicenter, randomized, controlled study. Int. J. Stroke 2017, 13, 328–334. [Google Scholar] [CrossRef]
| Inclusion Criteria |
| Age ≥ 18 years |
| Persistent disabling neurological deficits suggestive of an acute ischemic stroke |
| Onset of symptoms between 3 to 4.5 h from LKW (Level I) or Wake-up and unknown time of onset of symptoms, based on DWI-FLAIR imaging mismatch (level II) |
| Exclusion Criteria |
| Significant head trauma or prior stroke in the previous 3 months |
| Symptoms suggestive of SAH |
| History of previous ICH |
| Intraaxial-Intracranial neoplasm |
| Recent intracranial or intraspinal surgery within prior 3 months |
| Gastrointestinal malignancy or hemorrhage within 21 days |
| Known aortic arch dissection or infective endocarditis |
| Elevated blood pressure (systolic > 185 mm Hg or diastolic > 110 mm Hg) |
| Active internal bleeding in the past 21 days |
| Acute bleeding diathesis, including but not limited to: |
| Platelet count < 100,000/mm3 |
| Therapeutic doses of low molecular weight heparin received within 24 h (e.g., to treat VTE and ACS); this exclusion does not apply to prophylactic doses (e.g., to prevent VTE) |
| Current use of anticoagulant with INR > 1.7 or PT > 15 s |
| Current use (i.e., last dose within 48 h in a patient with normal renal function) of a direct thrombin inhibitor or direct factor Xa inhibitor with evidence of anticoagulant effect by laboratory tests such as aPTT, INR, ECT, TT, or appropriate factor Xa activity assays |
| Blood glucose concentration < 50 mg/dL (2.7 mmol/L) unless corrected prior to administration |
| CT Head demonstrating multilobar infarction or hypodensity involving >1/3 cerebral hemisphere |
| Extensive regions of obvious hypodensity consistent with irreversible injury |
| Careful considerations in which IV alteplase can be considered (weighing risks and benefits) |
| Arterial puncture at noncompressible site in previous 7 days |
| Symptoms most consistent with a stroke mimic |
| Seizure at onset with postictal residual neurological impairments |
| Only minor or rapidly improving stroke symptoms |
| Pregnancy |
| Known systemic malignancy with reasonable life expectancy and no known coagulopathy |
| Pre-existing disability with mRS of ≥2 |
| Major surgery or serious trauma (excluding head trauma) within previous 14 days |
| Previous gastrointestinal or urinary tract hemorrhage (beyond 21 days) |
| Recent acute myocardial infarction (within previous 3 months) |
| Untreated intracranial vascular malformation |
| Large (≥10 mm), untreated, unruptured intracranial aneurysm |
| MR CLEAN | ESCAPE | EXTEND IA | SWIFT PRIME | REVASCAT | THRACE | |
|---|---|---|---|---|---|---|
| Patients number (treatment vs. control) | 500 (233 vs. 267) | 315 (165 vs. 150) | 70 (35 vs. 35) | 195 (98 vs. 98) | 206 (103 vs. 103) | 414 (204 vs. 208) |
| Median age in years (treatment vs. control) | 65.8 vs. 65.7 | 71 vs. 70 | 68.6 vs. 70.2 | 65 vs. 66 | 65.7 vs. 67.2 | 66 vs. 68 |
| LKW to randomization | 6 | 12 h, image to puncture <60 min | 6 | 6 h, image to puncture <90 min | 3.7 h | 4.5 h, onset to groin puncture 5 h |
| Inclusion criteria | Age ≥ 18, NIHSS ≥ 2 | Age ≥ 18, any NIHSS (disabling) Barthel index > 90 | Age ≥ 18, any NIHSS, premorbid mRS < 2 | Age 18–80 NIHSS ≥ 8 Premorbid mRS < 2 | Age 18–80 NIHSS ≥ 6 Premorbid mRS < 2 | Age 18–80 NIHSS 10–25 |
| Imaging | CTA +/− CTP Any ASPECT | CT ASPECTS 6–10 with good collaterals >50% of MCA | CTA/CTP (core < 70 mL) | CTA +/−CTP ASPECT 6–10 No cervical ICA occlusion | CTA ASPECT 7–10 | CTA or MRA Any ASPECT |
| NIHSS (treatment vs. control) | 17 vs. 18 | 16 vs. 17 | 17 vs. 13 | 17 vs. 17 | 17 vs. 17 | 18 vs. 17 |
| ASPECT (treatment vs. control) | 9 vs. 9 | 9 vs. 9 | Not reported | 9 vs. 9 | 7 vs. 8 | Median not reported |
| TPA (treatment vs. control) | 87.1 vs. 90.6 | 72.7 vs. 78.7 | 100 vs. 100 | 100 vs. 100 | 68.0 vs. 77.7 | 100 vs. 100 |
| Median onset to groin puncture in min | 260 | 185 | 210 | 224 | 269 | 250 |
| Onset to reperfusion in min | Not reported | 241 | 248 | 250 | 355 | 303 |
| M1 occlusion | 66.1 vs. 62 | 68.1 vs. 71.4 | 57 vs. 51 | 67 vs. 77 | 64.7 vs. 64.4 | 86 vs. 79 |
| TICI score 2b-3% | 58.7 | 72.4 | 86 | 88 | 65.7 | 69 |
| MRS 0–2 at 90 days % | 32.6 vs. 19.1 OR 1.7 (95% CI 1.2–2.3) | 53 vs. 29.3 OR 1.8 (95% CI 1.4 2.4) | 71 vs. 40 OR 4.2 (95% CI 1.4–12) | 60.2 vs. 35.5 OR 1.7 (95% CI 1.2–2.3) | 43.7 vs. 28.2 OR 2.1 (95% CI 1.1–4.0) | 53 vs. 42 OR 1.55 (95% CI 1.05–2.30) |
| MRS 0–2 at 90 days NNT | 7.1 | 4.2 | 3.2 | 4 | 6.3 | 9.1 |
| sICH% | 6 vs. 5.2 | 3.6 vs. 2.7 | 1 vs. 3 | 1 vs. 3 | 1.9 vs. 1.9 | 2 vs. 2 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Imran, R.; Mohamed, G.A.; Nahab, F. Acute Reperfusion Therapies for Acute Ischemic Stroke. J. Clin. Med. 2021, 10, 3677. https://doi.org/10.3390/jcm10163677
Imran R, Mohamed GA, Nahab F. Acute Reperfusion Therapies for Acute Ischemic Stroke. Journal of Clinical Medicine. 2021; 10(16):3677. https://doi.org/10.3390/jcm10163677
Chicago/Turabian StyleImran, Rajeel, Ghada A Mohamed, and Fadi Nahab. 2021. "Acute Reperfusion Therapies for Acute Ischemic Stroke" Journal of Clinical Medicine 10, no. 16: 3677. https://doi.org/10.3390/jcm10163677
APA StyleImran, R., Mohamed, G. A., & Nahab, F. (2021). Acute Reperfusion Therapies for Acute Ischemic Stroke. Journal of Clinical Medicine, 10(16), 3677. https://doi.org/10.3390/jcm10163677





