Application of Bioactive Quercetin in Oncotherapy: From Nutrition to Nanomedicine
Abstract
:1. Introduction

2. Chemistry of Quercetin and Its Derivatives

3. Sources, Absorption and Metabolism of Quercetin
4. Effect of Quercetin on Cancer Cell Biology
4.1. Inhibition of Cell Growth
4.2. Inhibition of Metastasis
4.3. Induction of Apoptosis
| Organ/Tissue | Carcinogen/Cancer Cell Lne | Mode of Study/Model System | Effect/Signaling Mechanism | Ref. |
|---|---|---|---|---|
| Breast | MCF-7 breast cancer cells | In vitro | Induce antiproliferative effect and apoptosis by increased Bcl-2 and decreased Bax expression | [95] |
| HCC1937 breast cancer cells | In vitro | Induce antiproliferative effect via PI3K-Akt/PKB pathway | [76] | |
| SK-Br3 breast cancer cells | In vitro | Growth inhibition by decreasing level of Her-2/neu protein and inhibition of PI3K-Akt signaling pathway | [77] | |
| SK-Br3 breast cancer cells | In vitro | Induce antiproliferative effect by suppressing hypoxia-inducible factor-1alpha (HIF-1alpha) accumulation and reduced vascular endothelial growth factor (VEGF) secretion | [96] | |
| 4T1 breast cancer cells | In vitro | Induce antiproliferative effect and apoptosis by regulating Wnt/β-catenin signaling pathway | [21] | |
| TPA-treated MCF-7 breast cancer cells | In vitro | Prevents metastasis by inhibiting TPA-induced PKC δ/ERK/AP-1-dependent matrix metalloproteinase-9 activation and migration | [86] | |
| MDA-MB-231 breast cancer cells | In vitro | Growth inhibition by arresting cell cycle and inducing and inducing apoptosis by regulating mitochondrial- and caspase-3-dependent pathways | [91] | |
| MCF-7 and MDA-MB-231 breast cancer cells | In vitro | induces apoptosis through suppression of Twist via p38MAPK pathway | [97] | |
| ERalpha-negative breast cancer cells | In vitro | Induce antiproliferative effect and apoptosis via p53-dependent pathway | [93] | |
| Pancreas | MIA PaCa-2 and BxPC-3 pancreatic cancer cells | In vitro and in vivo (nude mouse model) | Induce antiproliferative effect and apoptosis. Inhibits tumor growth | [74] |
| Colon | CX-1 colon cancer cells | In vitro | Induce antiproliferative effect by suppressing hypoxia-inducible factor-1α (HIF-1α) accumulation and reduced vascular endothelial growth factor (VEGF) secretion | [96] |
| SW480 colon cancer cells | In vitro | Growth inhibition via inhibiting cyclin D(1) and survivin expression as well regulating Wnt/β-catenin signaling pathway | [20] | |
| HT-29 and HCT116 colon cancer cells | In vitro | regulates the sestrin 2-AMPK-p38 MAPK signaling pathway and inducing apoptosis via increasing the generation of intracellular ROS in a p53-independent manner | [94] | |
| HT-29 colon cancer cells | In vitro and in vivo (male nude mice) | Induces apoptosis via AMPK signaling pathway and reduce tumor volume | [92] | |
| Prostate | LNCaP prostate cancer cells | In vitro | Induce antiproliferative effect by suppressing hypoxia-inducible factor-1α (HIF-1α) accumulation and reduced vascular endothelial growth factor (VEGF) secretion | [96] |
| Chemically induced prostate cancer | In vivo (Sprague-Dawley male rats) | Suppress tumor progression by inhibiting the EGFR signaling pathway, regulating cell adhesion molecules and decreased snail, slug, and twist mRNA levels | [79] | |
| PC-3 prostate cancer cells | In vitro | Prevent metastasis via regulating EGFR/PI3k/Akt/ERK1/2 pathway and by suppressing transcriptional repressors Snail, Slug and Twist | [98] | |
| Liver | HepG2 hepatic cancer cells | In vitro | Induce antiproliferative effect by downregulating phosphatidylinositol 3-kinase (PI3K) and protein kinase C (PKC) via induction of p53 | [71] |
| HepG2 hepatic cancer cells | In vitro | Induce growth inhibition by cell cycle arrest at G1 phase and increasing levels of Cdk inhibitors p21 and p27 and tumor suppressor p53 | [80] | |
| Lymphatic system | Dalton’s lymphoma ascite cell line | In vivo | Inhibiting cancer growth by down-regulation of PI3K-Akt1-p53 pathway and glycolytic metabolism | [78] |
| Dalton’s lymphoma ascite cell line | In vivo | Induction of apoptosis and modulation of PKC signaling with the reduction of oxidative stress | [99] | |
| Salivary glands | ACC salivary cancer cells | In vitro | Induce antiproliferative effect and apoptosis by down-regulating the PI3K/Akt/IKK-alpha/NF-kappaB signaling pathway. | [100] |
| Ovary | SKOV3 oarian cancer cells | In vitro | Inhibiting cell growth by decreasing cyclin D1 expression level linked to alterations in G1/S phase | [83] |
| SKOV3 oariancancer cells | In vitro and in vivo (SKOV-3 xenograft mice model) | Inducing apoptotic effect of tumor necrosis factor-related apoptosis-inducing ligand (TRAIL) via ROS mediated CCAAT enhancer-binding protein homologous protein (CHOP)-death receptor 5 pathway | [101] | |
| Bone | U2OS osteocarcoma cells | In vitro | Inhibiting cell growth by decreasing cyclin D1 expression level linked to alterations in G1/S phase | [83] |
| Cervix | HeLa cervical cancer cells | In vitro | Induce cell growth inhibition and mitochondria mediated apoptosis via p53 induction and NF-kappaB inhibition | [81] |
| HeLa cervical cancer cells | In vitro | Induce antiproliferative effect and apoptosis by promoting cytochrome release, ROS accumulation and inhibiting anti-apoptotic AKT and Bcl-2 expression. Also perform cell arrest at G2/M phase | [102] | |
| Lung | A549 lung cancer cells | In vitro | quercetin-3-glucuronide and quercetin-3'-sulfate enriched plasma induces cell growth inhibition by cell cycle arrest at the G (2)/M phase via downregulating cdk1 and cyclin B expression | [82] |
| Skin | JB6 P+ mouse epidermal cancer cells | In vitro | inhibit TNF-alpha-induced upregulation of MMP-9 and cell migration via p13K/Akt signaling pathway | [87] |
| Brain | U87 and U251 glioma cells | In vitro/in vivo (C6 glioma xenograft models) | Induce anti proliferative effect and autophagy | [103] |
| U373MG glioblastoma cells | In vitro | Induce cell growth inhibition through mitochondria mediated apoptosis via activating Caspase-, caspase-7, JNK and p53 level. showed cell cycle arrest at sub-G1 phase | [104] |
5. Enhancing Bioavailability and Bioactivity of Quercetin Using Nanoparticles and Its Application in Cancer Treatment and Diagnosis
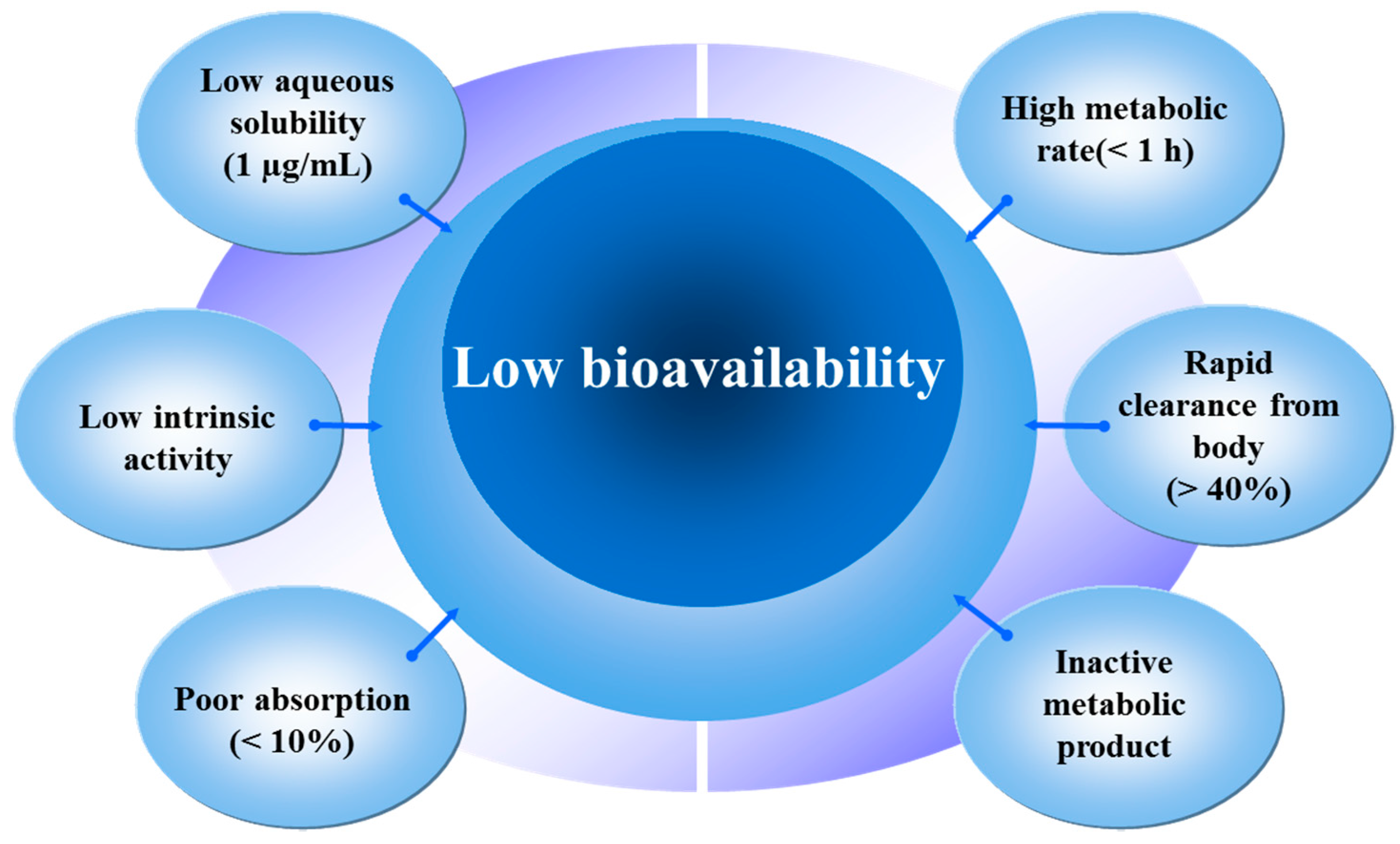
5.1. Silica Nanoparticles
5.2. PLGA and PLA Nanoparticles
5.3. Chitosan Nanoparticles
5.4. Liposomes
5.5. Other Nanoparticles
6. Future Perspectives and Limitations
7. Conclusions
| Delivery System | Cancer Type | Chemicals/Polymer Used | Size (d) nm | PDI | Entrapment Efficiency | In Vitro/In Vivo | Effective Dose | Effect | Ref. |
|---|---|---|---|---|---|---|---|---|---|
| Silica | JR8 human melanoma cell line | aminopropyl functionalized mesoporous silica nanoparticle | 250 ± 50 | NA | NA | In vitro | 60 µM | ~50% inhibition of cell proliferation at 72 h | [117] |
| Ex vivo (Porcine skin) | 0.27% w/w of quercetin in water/oil emulsion system | Higher amount of quercetin was retained in the skin as compared to control at 24 h | |||||||
| PLGA/PLA | A549 human lung adenocarcinoma epithelial cell line | PLGA (combination treatment of quercetin and etopside) | 153.4 ± 4.2 (etopside), 148.6 ± 1.6 (quercetin) | 0.058 ± 0.02 (etopside), 0.088 ± 0.03 (quercetin) | 63.88% ± 1.5% (etopside), 41.36% ± 3.4% (quercetin) | In vitro | 50 µM | Enhanced cytotoxic effect compared to free drugs combination at 72 h | [125] |
| MDA-MB231 human breast cancer cell line | PLA | 46 ± 6 | NA | 62% ± 3% | In vitro | 100 µg/mL | ~40% decrease in cell viability in 5 days | [126] | |
| DMBA induced Breast cancer | PLGA (coencapsulated quercetin and tamoxifen) | 185.3 ± 1.20 | 0.184 ± 0.004 | 67.16% ± 1.24% (tamoxifen), 68.60% ± 1.58% (quercetin) | In vitro | 10 µg/mL | increase in cell cytotoxicity | [129] | |
| In vivo (female SD rats) | 45 mg/kg (Oral, one time per week for 3 weeks | Tumor was reduced to ~32.36% after 30 days | |||||||
| HeLa cervical-tumor-derived cell line or IGROV-1 human ovarian carcinoma cell line | PEG-PLGA and Folic acid as targeting ligand | 155.0 ± 1.2 | <0.2 | 97.8 ± 0.14 | In vitro | 10 µM | ~56.63% reduction in cell viability of HeLa | [130] | |
| In vivo (female athymic nude and SHrN mice xenograft model) | 250 μL of 50 mg polymer/mL (single intravenous injection) | Folic acid enhances selective uptake of nanoparticles by folate receptor enriched cancer cells | |||||||
| MDA-MB-231 human breast cancer cell line and 4T1 murine mammary cancer cell line | MPEG-PLA | 155.3 ± 3.2 | 0.2 ± 0.05 | NA | In vitro | 13.5 µg/mL | ~38% lower cell viability compared to control | [131] | |
| In vivo (female BALB/c mouse xenograft model) | 0.5 mg/kg (peritumoral injection, every third day till day 19) | Reduced tumor size as compared to control | |||||||
| Chitosan | MiaPaCa2, Pancreatic cancer cell line | Chitosan | 300 | NA | 91% | In vitro | 10 µM to 100µM | Dose dependent cell inhibition | [144] |
| MiaPaCa2, Pancreatic cancer cell line | Chitosan (quercetin and 5-flourouracil dual drug loading) | 400 | NA | 95% (quercetin), 75% (flourouracil) | In vitro | 39.7 µM (quercetin) and 75 µM (flourouracil) | ~70% decrease in cell viability | [144] | |
| HepG2 human liver cancer cell line | Chirosan-quercetin conjugate loaded with paclitaxel | 185.8 ± 4.6 | 0.134 ± 0.056 | 85.63% ± 1.26% | In vitro | 0.01–100 µg/mL | Dose dependent cytotoxic effect with IC50 0.11 µg/mL | [146] | |
| In vivo (male ICR xenograft models) | 20 mg/kg (single oral dose) | ~71.22% reduction in tumor size | |||||||
| Liposomes | C6 glioma cell line | glyceryl behenate, soy lecithin, and cholesterol | 116.7 | NA | NA | In vitro | 0–400 μM | Induced necrotic cell death | [152] |
| MCF-7 human breast cancer cell line | Phosphotidyl choline | 100.974 ± 0.3 | NA | 40.7% ± 3.1% | In vitro | 50 mM/mL | ~83% inhibition in cell proliferation at 48 h | [153] | |
| MCF-7 and MDA-MB-231 human breast cancer cell line | Soy lecithin, glyceryl tridecanoate, glyceryl tripalmitate, vitamin E acetate, Kolliphor HS15 | 32 | 0.059 | 95% | In vitro | 50 µM | ~13.7% reduction in viability of MCF-7 cells and ~13.4% reduction in viability of MDA-MB-231 cells at 48 h | [162] | |
| Nanomicelles | A549 human lung cancer cell line | DSPE-PEG2000 Nanomicelles | 15.4–18.5 | <0.250 | ≥88.9% | In vitro | 100 µM | Decreased cell viability at 72 h | [155] |
| In vivo (female Rag-2M mice xenograft Model) | 30 mg/kg (three times per week for 3 weeks, perorally) | ~1.5 fold higher tumor growth inhibition than free quercetin control group | |||||||
| Nanoribbon | 4T1 murine mammary cancer cell line | Nanoribbon fabricated by atmospheric pressure PVD | 100–200 | NA | NA | In vitro | NA | ~57% reduction in cell viability | [156] |
Acknowledgments
Conflicts of Interest
References
- Xiao, Z.P.; Peng, Z.Y.; Peng, M.J.; Yan, W.B.; Ouyang, Y.Z.; Zhu, H.L. Flavonoids health benefits and their molecular mechanism. Mini-Rev. Med. Chem. 2011, 11, 169–177. [Google Scholar] [CrossRef] [PubMed]
- Haddad, A.Q.; Fleshner, N.; Nelson, C.; Saour, B.; Musquera, M.; Venkateswaran, V.; Klotz, L. Antiproliferative mechanisms of the flavonoids 2,2′-dihydroxychalcone and fisetin in human prostate cancer cells. Nutr. Cancer 2010, 62, 668–681. [Google Scholar] [CrossRef] [PubMed]
- Formica, J.V.; Regelson, W. Review of the biology of Quercetin and related bioflavonoids. Food Chem. Toxicol. 1995, 33, 1061–1080. [Google Scholar] [CrossRef]
- Kumar, S.; Pandey, A.K. Chemistry and biological activities of flavonoids: An overview. Sci. World J. 2013, 2013. [Google Scholar] [CrossRef] [PubMed]
- Moon, J.H.; Tsushida, T.; Nakahara, K.; Terao, J. Identification of quercetin 3-O-β-d-glucuronide as an antioxidative metabolite in rat plasma after oral administration of quercetin. Free Radic. Biol. Med. 2001, 30, 1274–1285. [Google Scholar] [CrossRef]
- Bors, W.; Michel, C.; Saran, M. Flavonoid antioxidants: Rate constants for reactions with oxygen radicals. Methods Enzymol. 1994, 234, 420–429. [Google Scholar] [PubMed]
- Prior, R.L. Fruits and vegetables in the prevention of cellular oxidative damage. Am. J. Clin. Nutr. 2003, 78, 570S–578S. [Google Scholar] [PubMed]
- Erkoç, Ş.; Erkoç, F.; Keskin, N. Theoretical investigation of quercetin and its radical isomers. J. Mol. Struct. THEOCHEM 2003, 631, 141–146. [Google Scholar] [CrossRef]
- Kampa, M.; Hatzoglou, A.; Notas, G.; Damianaki, A.; Bakogeorgou, E.; Gemetzi, C.; Kouroumalis, E.; Martin, P.-M.; Castanas, E. Wine antioxidant polyphenols inhibit the proliferation of human prostate cancer cell lines. Nutr. Cancer 2000, 37, 223–233. [Google Scholar] [CrossRef] [PubMed]
- American Cancer Society. Cancer Facts and Figures 2015; American Cancer Society: Atlanta, GA, USA, 2015. [Google Scholar]
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics, 2015. CA Cancer J. Clin. 2015, 65, 5–29. [Google Scholar] [CrossRef] [PubMed]
- Donaldson, M.S. Nutrition and cancer: A review of the evidence for an anti-cancer diet. Nutr. J. 2004, 3. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Riboli, E.; Norat, T. Epidemiologic evidence of the protective effect of fruit and vegetables on cancer risk. Am. J. Clin. Nutr. 2003, 78, 559S–569S. [Google Scholar] [PubMed]
- Nabavi, S.F.; Daglia, M.; Moghaddam, A.H.; Habtemariam, S.; Nabavi, S.M. Curcumin and liver disease: from chemistry to medicine. Compr. Rev. Food Sci. Food Saf. 2014, 13, 62–77. [Google Scholar] [CrossRef]
- Lee, K.W.; Bode, A.M.; Dong, Z. Molecular targets of phytochemicals for cancer prevention. Nat. Rev. Cancer 2011, 11, 211–218. [Google Scholar] [CrossRef] [PubMed]
- Goodman, M.; Bostick, R.M.; Kucuk, O.; Jones, D.P. Clinical trials of antioxidants as cancer prevention agents: Past, present, and future. Free Radic. Biol. Med. 2011, 51, 1068–1084. [Google Scholar] [CrossRef] [PubMed]
- Shu, L.; Cheung, K.L.; Khor, T.O.; Chen, C.; Kong, A.N. Phytochemicals: Cancer chemoprevention and suppression of tumor onset and metastasis. Cancer Metastasis Rev. 2010, 29, 483–502. [Google Scholar] [CrossRef] [PubMed]
- Bishayee, A. Cancer prevention and treatment with resveratrol: From rodent studies to clinical trials. Cancer Prev. Res. 2009, 2, 409–418. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Z.; Long, C.; Junming, T.; Qihuan, L.; Youshun, Z.; Chan, Z. Quercetin-induced apoptosis of HL-60 cells by reducing PI3K/Akt. Mol. Biol. Rep. 2012, 39, 7785–7793. [Google Scholar] [CrossRef] [PubMed]
- Shan, B.E.; Wang, M.X.; Li, R.Q. Quercetin inhibit human SW480 colon cancer growth in association with inhibition of cyclin D1 and survivin expression through Wnt/β-catenin signaling pathway. Cancer Investig. 2009, 27, 604–612. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Seo, E.M.; Sharma, A.R.; Ganbold, B.; Park, J.; Sharma, G.; Kang, Y.H.; Song, D.K.; Lee, S.S.; Nam, J.S. Regulation of Wnt signaling activity for growth suppression induced by quercetin in 4T1 murine mammary cancer cells. Int. J. Oncol. 2013, 43, 1319–1325. [Google Scholar] [PubMed]
- Huang, Y.T.; Hwang, J.J.; Lee, P.P.; Ke, F.C.; Huang, J.H.; Huang, C.J.; Kandaswami, C.; Middleton, E.; Lee, M.T. Effects of luteolin and quercetin, inhibitors of tyrosine kinase, on cell growth and metastasis-associated properties in A431 cells overexpressing epidermal growth factor receptor. Br. J. Pharmacol. 1999, 128, 999–1010. [Google Scholar] [CrossRef] [PubMed]
- Cai, X.; Fang, Z.; Dou, J.; Yu, A.; Zhai, G. Bioavailability of quercetin: Problems and promises. Curr. Med. Chem. 2013, 20, 2572–2582. [Google Scholar] [CrossRef] [PubMed]
- Khushnud, T.; Mousa, S.A. Potential role of naturally derived polyphenols and their nanotechnology delivery in cancer. Mol. Biotechnol. 2013, 55, 78–86. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Zhang, J.; Chen, M.; Wang, Y. Delivering flavonoids into solid tumors using nanotechnologies. Expert Opin. Drug Deliv. 2013, 10, 1411–1428. [Google Scholar] [CrossRef] [PubMed]
- Ferry, D.R.; Smith, A.; Malkhandi, J.; Fyfe, D.W.; deTakats, P.G.; Anderson, D.; Baker, J.; Kerr, D.J. Phase I clinical trial of the flavonoid quercetin: Pharmacokinetics and evidence for in vivo tyrosine kinase inhibition. Clin. Cancer. Res. 1996, 2, 659–668. [Google Scholar] [PubMed]
- Mukerjee, A.; Vishwanatha, J.K. Formulation, characterization and evaluation of curcumin-loaded PLGA nanospheres for cancer therapy. Anticancer Res. 2009, 29, 3867–3875. [Google Scholar] [PubMed]
- Jeetah, R.; Bhaw-Luximon, A.; Jhurry, D. Nanopharmaceutics: Phytochemical-based controlled or sustained drug-delivery systems for cancer treatment. J. Biomed. Nanotechnol. 2014, 10, 1810–1840. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A.R.; Kundu, S.K.; Nam, J.S.; Sharma, G.; Priya Doss, C.G.; Lee, S.S.; Chakraborty, C. Next generation delivery system for proteins and genes of therapeutic purpose: Why and how? Biomed. Res. Int. 2014, 2014, 327950. [Google Scholar] [CrossRef] [PubMed]
- Sharma, G.; Sharma, A.R.; Nam, J.S.; Doss, G.P.; Lee, S.S.; Chakraborty, C. Nanoparticle based insulin delivery system: The next generation efficient therapy for Type 1 diabetes. J. Nanobiotechnol. 2015, 13, 74. [Google Scholar] [CrossRef] [PubMed]
- Mulik, R.S.; Monkkonen, J.; Juvonen, R.O.; Mahadik, K.R.; Paradkar, A.R. Apoptosis-induced anticancer effect of transferrin-conjugated solid lipid nanoparticles of curcumin. Cancer Nanotechnol. 2012, 3, 65–81. [Google Scholar] [CrossRef] [PubMed]
- Morand, C.; Crespy, V.; Manach, C.; Besson, C.; Demigne, C.; Remesy, C. Plasma metabolites of quercetin and their antioxidant properties. Am. J. Physiol. 1998, 275, R212–R219. [Google Scholar] [PubMed]
- Cornard, J.P.; Dangleterre, L.; Lapouge, C. Computational and spectroscopic characterization of the molecular and electronic structure of the Pb(II)-quercetin complex. J. Phys. Chem. A 2005, 109, 10044–10051. [Google Scholar] [CrossRef] [PubMed]
- Mendoza-Wilson, A.M.A.; Glossman-Mitnik, D. CHIH-DFT determination of the molecular structure, infrared and ultraviolet spectra of the flavonoid quercetin. J. Mol. Str. THEOCHEM 2004, 681, 71–76. [Google Scholar] [CrossRef]
- Fischer, C.; Speth, V.; Fleig-Eberenz, S.; Neuhaus, G. Induction of zygotic polyembryos in wheat: Influence of auxin polar transport. Plant Cell 1997, 9, 1767–1780. [Google Scholar] [CrossRef] [PubMed]
- Williams, C.A.; Grayer, R.J. Anthocyanins and other flavonoids. Nat. Prod. Rep. 2004, 21, 539–573. [Google Scholar] [CrossRef] [PubMed]
- Harborne, J.B.; Williams, C.A. Advances in flavonoid research since 1992. Phytochemistry 2000, 55, 481–504. [Google Scholar] [CrossRef]
- Fang, N.; Yu, S.; Mabry, T.J. Flavonoids from Ageratina calophylla. Phytochemistry 1986, 25, 2684–2686. [Google Scholar] [CrossRef]
- Zeng, L.-M.; Wang, C.-J.; Su, J.-Y.; Li, D.; Owen, N.L.; Lu, Y.; Lu, N.; Zheng, Q.-T. Flavonoids from the red alga Acanthophora spicifera. Chin. J. Chem. 2001, 19, 1097–1100. [Google Scholar] [CrossRef]
- Dal Santo, S.; Tornielli, G.B.; Zenoni, S.; Fasoli, M.; Farina, L.; Anesi, A.; Guzzo, F.; Delledonne, M.; Pezzotti, M. The plasticity of the grapevine berry transcriptome. Genome Biol. 2013, 14. [Google Scholar] [CrossRef] [PubMed]
- Rice-Evans, C.; Miller, N.; Paganga, G. Antioxidant properties of phenolic compounds. Trends Plant Sci. 1997, 2, 152–159. [Google Scholar] [CrossRef]
- Heijnen, C.G.; Haenen, G.R.; Oostveen, R.M.; Stalpers, E.M.; Bast, A. Protection of flavonoids against lipid peroxidation: The structure activity relationship revisited. Free Radic. Res. 2002, 36, 575–581. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.P.; Liao, Z.P.; Yin, D.; Li, W.D.; Liu, D.; Li, Q.; Huang, Q.R.; Yang, Y.F.; He, M. The protective effects of Polygonum multiflorum stilbene glycoside preconditioning in an ischemia/reperfusion model of HUVECs. Acta Pharmacol. Sin. 2010, 31, 405–412. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Q.Y.; Huang, Y.; Chen, Z.Y. Interaction between flavonoids and alpha-tocopherol in human low density lipoprotein. J. Nutr. Biochem. 2000, 11, 14–21. [Google Scholar] [CrossRef]
- Borghetti, G.S.; Carini, J.P.; Honorato, S.B.; Ayala, A.P.; Moreira, J.C. F.; Bassani, V.L. Physicochemical properties and thermal stability of quercetin hydrates in the solid state. Thermochim. Acta 2012, 539, 109–114. [Google Scholar] [CrossRef]
- Ghosh, D.; Ghosh, S.; Sarkar, S.; Ghosh, A.; Das, N.; Das Saha, K.; Mandal, A.K. Quercetin in vesicular delivery systems: Evaluation in combating arsenic-induced acute liver toxicity associated gene expression in rat model. Chem. Biol. Interact. 2010, 186, 61–71. [Google Scholar] [CrossRef] [PubMed]
- Bischoff, S.C. Quercetin: Potentials in the prevention and therapy of disease. Curr. Opin. Clin. Nutr. Metab. Care 2008, 11, 733–740. [Google Scholar] [CrossRef] [PubMed]
- Harnly, J.M.; Doherty, R.F.; Beecher, G.R.; Holden, J.M.; Haytowitz, D.B.; Bhagwat, S.; Gebhardt, S. Flavonoid content of U.S. fruits, vegetables, and nuts. J. Agric. Food Chem. 2006, 54, 9966–9977. [Google Scholar] [CrossRef] [PubMed]
- Sampson, L.; Rimm, E.; Hollman, P.C.; de Vries, J.H.; Katan, M.B. Flavonol and flavone intakes in US health professionals. J. Am. Diet Assoc. 2002, 102, 1414–1420. [Google Scholar] [CrossRef]
- Brown, J.P. A review of the genetic effects of naturally occurring flavonoids, anthraquinones and related compounds. Mutat. Res. 1980, 75, 243–277. [Google Scholar] [CrossRef]
- Jones, E.; Hughes, R.E. Quercetin, flavonoids and the life-span of mice. Exp. Gerontol. 1982, 17, 213–217. [Google Scholar] [CrossRef]
- Johannot, L.; Somerset, S.M. Age-related variations in flavonoid intake and sources in the Australian population. Public Health Nutr. 2006, 9, 1045–1054. [Google Scholar] [CrossRef] [PubMed]
- Nishimuro, H.; Ohnishi, H.; Sato, M.; Ohnishi-Kameyama, M.; Matsunaga, I.; Naito, S.; Ippoushi, K.; Oike, H.; Nagata, T.; Akasaka, H.; et al. Estimated daily intake and seasonal food sources of quercetin in Japan. Nutrients 2015, 7, 2345–2358. [Google Scholar] [CrossRef] [PubMed]
- Jackson, M.J. The assessment of bioavailability of micronutrients: Introduction. Eur. J. Clin. Nutr. 1997, 51 (Suppl. 1), S1–S2. [Google Scholar] [PubMed]
- Tamura, G.; Gold, C.; Ferro-Luzzi, A.; Ames, B.N. Fecalase: A model for activation of dietary glycosides to mutagens by intestinal flora. Proc. Natl. Acad. Sci. USA 1980, 77, 4961–4965. [Google Scholar] [CrossRef] [PubMed]
- Crespy, V.; Morand, C.; Besson, C.; Manach, C.; Demigne, C.; Remesy, C. Quercetin, but not its glycosides, is absorbed from the rat stomach. J. Agric. Food Chem. 2002, 50, 618–621. [Google Scholar] [CrossRef] [PubMed]
- Crespy, V.; Morand, C.; Manach, C.; Besson, C.; Demigne, C.; Remesy, C. Part of quercetin absorbed in the small intestine is conjugated and further secreted in the intestinal lumen. Am. J. Physiol. 1999, 277, G120–G126. [Google Scholar] [PubMed]
- Nait Chabane, M.; al Ahmad, A.; Peluso, J.; Muller, C.D.; Ubeaud, G. Quercetin and naringenin transport across human intestinal Caco-2 cells. J. Pharm. Pharmacol. 2009, 61, 1473–1483. [Google Scholar] [CrossRef] [PubMed]
- Moon, J.H.; Nakata, R.; Oshima, S.; Inakuma, T.; Terao, J. Accumulation of quercetin conjugates in blood plasma after the short-term ingestion of onion by women. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2000, 279, R461–R467. [Google Scholar] [PubMed]
- Conquer, J.A.; Maiani, G.; Azzini, E.; Raguzzini, A.; Holub, B.J. Supplementation with quercetin markedly increases plasma quercetin concentration without effect on selected risk factors for heart disease in healthy subjects. J. Nutr. 1998, 128, 593–597. [Google Scholar] [PubMed]
- Walle, T.; Walle, U.K.; Halushka, P.V. Carbon Dioxide Is the Major Metabolite of Quercetin in Humans. J. Nutr. 2001, 131, 2648–2652. [Google Scholar] [PubMed]
- Justino, G.C.; Santos, M.R.; Canario, S.; Borges, C.; Florencio, M.H.; Mira, L. Plasma quercetin metabolites: Structure-antioxidant activity relationships. Arch. Biochem. Biophys. 2004, 432, 109–121. [Google Scholar] [CrossRef] [PubMed]
- Mullen, W.; Rouanet, J.M.; Auger, C.; Teissedre, P.L.; Caldwell, S.T.; Hartley, R.C.; Lean, M.E.; Edwards, C.A.; Crozier, A. Bioavailability of [2-(14)C]quercetin-4′-glucoside in rats. J. Agric. Food Chem. 2008, 56, 12127–12137. [Google Scholar] [CrossRef] [PubMed]
- Hong, Y.J.; Mitchell, A.E. Metabolic profiling of flavonol metabolites in human urine by liquid chromatography and tandem mass spectrometry. J. Agric. Food Chem. 2004, 52, 6794–6801. [Google Scholar] [CrossRef] [PubMed]
- Okamoto, T. Safety of quercetin for clinical application (Review). Int. J. Mol. Med. 2005, 16, 275–278. [Google Scholar] [CrossRef] [PubMed]
- Crebelli, R.; Aquilina, G.; Falcone, E.; Carere, A. Urinary and faecal mutagenicity in Sprague-Dawley rats dosed with the food mutagens quercetin and rutin. Food Chem. Toxicol. 1987, 25, 9–15. [Google Scholar] [CrossRef]
- Harwood, M.; Danielewska-Nikiel, B.; Borzelleca, J.F.; Flamm, G.W.; Williams, G.M.; Lines, T.C. A critical review of the data related to the safety of quercetin and lack of evidence of in vivo toxicity, including lack of genotoxic/carcinogenic properties. Food Chem. Toxicol. 2007, 45, 2179–2205. [Google Scholar] [CrossRef] [PubMed]
- Suolinna, E.M.; Buchsbaum, R.N.; Racker, E. The effect of flavonoids on aerobic glycolysis and growth of tumor cells. Cancer Res. 1975, 35, 1865–1872. [Google Scholar] [PubMed]
- Hosokawa, N.; Hirayoshi, K.; Nakai, A.; Hosokawa, Y.; Marui, N.; Yoshida, M.; Sakai, T.; Nishino, H.; Aoike, A.; Kawai, K.; et al. Flavonoids inhibit the expression of heat shock proteins. Cell Struct. Funct. 1990, 15, 393–401. [Google Scholar] [CrossRef] [PubMed]
- Scambia, G.; Ranelletti, F.O.; Panici, P.B.; Piantelli, M.; Bonanno, G.; de Vincenzo, R.; Ferrandina, G.; Rumi, C.; Larocca, L.M.; Mancuso, S. Inhibitory effect of quercetin on OVCA 433 cells and presence of type II oestrogen binding sites in primary ovarian tumours and cultured cells. Br. J. Cancer 1990, 62, 942–946. [Google Scholar] [CrossRef] [PubMed]
- Maurya, A.K.; Vinayak, M. Anticarcinogenic action of quercetin by downregulation of phosphatidylinositol 3-kinase (PI3K) and protein kinase C (PKC) via induction of p53 in hepatocellular carcinoma (HepG2) cell line. Mol. Biol. Rep. 2015, 42, 1419–1429. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, M.; Sakai, T.; Hosokawa, N.; Marui, N.; Matsumoto, K.; Fujioka, A.; Nishino, H.; Aoike, A. The effect of quercetin on cell cycle progression and growth of human gastric cancer cells. FEBS Lett. 1990, 260, 10–13. [Google Scholar] [CrossRef]
- Edwards, J.M.; Raffauf, R.F.; le Quesne, P.W. Antineoplastic activity and cytotoxicity of flavones, isoflavones, and flavanones. J. Nat. Prod. 1979, 42, 85–91. [Google Scholar] [CrossRef] [PubMed]
- Angst, E.; Park, J.L.; Moro, A.; Lu, Q.Y.; Lu, X.; Li, G.; King, J.; Chen, M.; Reber, H.A.; Go, V.L.; et al. The flavonoid quercetin inhibits pancreatic cancer growth in vitro and in vivo. Pancreas 2013, 42, 223–229. [Google Scholar] [CrossRef] [PubMed]
- Bruning, A. Inhibition of mTOR signaling by quercetin in cancer treatment and prevention. Anticancer Agents Med. Chem. 2013, 13, 1025–1031. [Google Scholar] [CrossRef] [PubMed]
- Gulati, N.; Laudet, B.; Zohrabian, V.M.; Murali, R.; Jhanwar-Uniyal, M. The antiproliferative effect of Quercetin in cancer cells is mediated via inhibition of the PI3K-Akt/PKB pathway. Anticancer Res. 2006, 26, 1177–1181. [Google Scholar] [PubMed]
- Jeong, J.H.; An, J.Y.; Kwon, Y.T.; Li, L.Y.; Lee, Y.J. Quercetin-induced ubiquitination and down-regulation of Her-2/neu. J. Cell. Biochem. 2008, 105, 585–595. [Google Scholar] [CrossRef] [PubMed]
- Maurya, A.K.; Vinayak, M. Quercetin regresses Dalton’s lymphoma growth via suppression of PI3K/AKT signaling leading to upregulation of p53 and decrease in energy metabolism. Nutr. Cancer 2015, 67, 354–363. [Google Scholar] [CrossRef] [PubMed]
- Firdous, A.B.; Sharmila, G.; Balakrishnan, S.; RajaSingh, P.; Suganya, S.; Srinivasan, N.; Arunakaran, J. Quercetin, a natural dietary flavonoid, acts as a chemopreventive agent against prostate cancer in an in vivo model by inhibiting the EGFR signaling pathway. Food Funct. 2014, 5, 2632–2645. [Google Scholar] [CrossRef] [PubMed]
- Mu, C.; Jia, P.; Yan, Z.; Liu, X.; Li, X.; Liu, H. Quercetin induces cell cycle G1 arrest through elevating Cdk inhibitors p21 and p27 in human hepatoma cell line (HepG2). Methods Find Exp. Clin. Pharmacol. 2007, 29, 179–183. [Google Scholar] [CrossRef] [PubMed]
- VidyaPriyadarsini, R.; SenthilMurugan, R.; Maitreyi, S.; Ramalingam, K.; Karunagaran, D.; Nagini, S. The flavonoid quercetin induces cell cycle arrest and mitochondria-mediated apoptosis in human cervical cancer (HeLa) cells through p53 induction and NF-kappaB inhibition. Eur. J. Pharmacol. 2010, 649, 84–91. [Google Scholar] [CrossRef] [PubMed]
- Yeh, S.L.; Yeh, C.L.; Chan, S.T.; Chuang, C.H. Plasma rich in quercetin metabolites induces G2/M arrest by upregulating PPAR-gamma expression in human A549 lung cancer cells. Planta Med. 2011, 77, 992–998. [Google Scholar] [CrossRef] [PubMed]
- Catanzaro, D.; Ragazzi, E.; Vianello, C.; Caparrotta, L.; Montopoli, M. Effect of Quercetin on Cell Cycle and Cyclin Expression in Ovarian Carcinoma and Osteosarcoma Cell Lines. Nat. Prod. Commun. 2015, 10, 1365–1368. [Google Scholar] [PubMed]
- Huang, C.Y.; Chan, C.Y.; Chou, I.T.; Lien, C.H.; Hung, H.C.; Lee, M.F. Quercetin induces growth arrest through activation of FOXO1 transcription factor in EGFR-overexpressing oral cancer cells. J. Nutr. Biochem. 2013, 24, 1596–1603. [Google Scholar] [CrossRef] [PubMed]
- Liabakk, N.B.; Talbot, I.; Smith, R.A.; Wilkinson, K.; Balkwill, F. Matrix metalloprotease 2 (MMP-2) and matrix metalloprotease 9 (MMP-9) type IV collagenases in colorectal cancer. Cancer Res. 1996, 56, 190–196. [Google Scholar] [PubMed]
- Lin, C.W.; Hou, W.C.; Shen, S.C.; Juan, S.H.; Ko, C.H.; Wang, L.M.; Chen, Y.C. Quercetin inhibition of tumor invasion via suppressing PKC δ/ERK/AP-1-dependent matrix metalloproteinase-9 activation in breast carcinoma cells. Carcinogenesis 2008, 29, 1807–1815. [Google Scholar] [CrossRef] [PubMed]
- Hwang, M.K.; Song, N.R.; Kang, N.J.; Lee, K.W.; Lee, H.J. Activation of phosphatidylinositol 3-kinase is required for tumor necrosis factor-alpha-induced upregulation of matrix metalloproteinase-9: Its direct inhibition by quercetin. Int. J. Biochem. Cell Biol. 2009, 41, 1592–1600. [Google Scholar] [CrossRef] [PubMed]
- Lee, L.T.; Huang, Y.T.; Hwang, J.J.; Lee, A.Y.; Ke, F.C.; Huang, C.J.; Kandaswami, C.; Lee, P.P.; Lee, M.T. Transinactivation of the epidermal growth factor receptor tyrosine kinase and focal adhesion kinase phosphorylation by dietary flavonoids: Effect on invasive potential of human carcinoma cells. Biochem. Pharmacol. 2004, 67, 2103–2114. [Google Scholar] [CrossRef] [PubMed]
- Jacobson, M.D.; Weil, M.; Raff, M.C. Programmed cell death in animal development. Cell 1997, 88, 347–354. [Google Scholar] [CrossRef]
- Lowe, S.W.; Lin, A.W. Apoptosis in cancer. Carcinogenesis 2000, 21, 485–495. [Google Scholar] [CrossRef] [PubMed]
- Chien, S.Y.; Wu, Y.C.; Chung, J.G.; Yang, J.S.; Lu, H.F.; Tsou, M.F.; Wood, W.G.; Kuo, S.J.; Chen, D.R. Quercetin-induced apoptosis acts through mitochondrial- and caspase-3-dependent pathways in human breast cancer MDA-MB-231 cells. Hum. Exp. Toxicol. 2009, 28, 493–503. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.J.; Kim, S.K.; Kim, B.S.; Lee, S.H.; Park, Y.S.; Park, B.K.; Kim, S.J.; Kim, J.; Choi, C.; Kim, J.S.; et al. Apoptotic effect of quercetin on HT-29 colon cancer cells via the AMPK signaling pathway. J. Agric. Food Chem. 2010, 58, 8643–8650. [Google Scholar] [CrossRef] [PubMed]
- Seo, H.S.; Ju, J.H.; Jang, K.; Shin, I. Induction of apoptotic cell death by phytoestrogens by up-regulating the levels of phospho-p53 and p21 in normal and malignant estrogen receptor alpha-negative breast cells. Nutr. Res. 2011, 31, 139–146. [Google Scholar] [CrossRef] [PubMed]
- Kim, G.T.; Lee, S.H.; Kim, J.I.; Kim, Y.M. Quercetin regulates the sestrin 2-AMPK-p38 MAPK signaling pathway and induces apoptosis by increasing the generation of intracellular ROS in a p53-independent manner. Int. J. Mol. Med. 2014, 33, 863–869. [Google Scholar] [CrossRef] [PubMed]
- Duo, J.; Ying, G.G.; Wang, G.W.; Zhang, L. Quercetin inhibits human breast cancer cell proliferation and induces apoptosis via Bcl-2 and Bax regulation. Mol. Med. Rep. 2012, 5, 1453–1456. [Google Scholar] [PubMed]
- Lee, D.H.; Lee, Y.J. Quercetin suppresses hypoxia-induced accumulation of hypoxia-inducible factor-1alpha (HIF-1alpha) through inhibiting protein synthesis. J. Cell. Biochem. 2008, 105, 546–553. [Google Scholar] [CrossRef] [PubMed]
- Ranganathan, S.; Halagowder, D.; Sivasithambaram, N.D. Quercetin Suppresses Twist to Induce Apoptosis in MCF-7 Breast Cancer Cells. PLoS ONE 2015, 10, e0141370. [Google Scholar] [CrossRef] [PubMed]
- Bhat, F.A.; Sharmila, G.; Balakrishnan, S.; Arunkumar, R.; Elumalai, P.; Suganya, S.; Raja Singh, P.; Srinivasan, N.; Arunakaran, J. Quercetin reverses EGF-induced epithelial to mesenchymal transition and invasiveness in prostate cancer (PC-3) cell line via EGFR/PI3K/Akt pathway. J. Nutr. Biochem. 2014, 25, 1132–1139. [Google Scholar] [CrossRef] [PubMed]
- Maurya, A.K.; Vinayak, M. Modulation of PKC signaling and induction of apoptosis through suppression of reactive oxygen species and tumor necrosis factor receptor 1 (TNFR1): Key role of quercetin in cancer prevention. Tumour Biol. 2015, 36, 8913–8924. [Google Scholar] [CrossRef] [PubMed]
- Sun, Z.J.; Chen, G.; Hu, X.; Zhang, W.; Liu, Y.; Zhu, L.X.; Zhou, Q.; Zhao, Y.F. Activation of PI3K/Akt/IKK-alpha/NF-kappaB signaling pathway is required for the apoptosis-evasion in human salivary adenoid cystic carcinoma: Its inhibition by quercetin. Apoptosis 2010, 15, 850–863. [Google Scholar] [CrossRef] [PubMed]
- Yi, L.; Zongyuan, Y.; Cheng, G.; Lingyun, Z.; Guilian, Y.; Wei, G. Quercetin enhances apoptotic effect of tumor necrosis factor-related apoptosis-inducing ligand (TRAIL) in ovarian cancer cells through reactive oxygen species (ROS) mediated CCAAT enhancer-binding protein homologous protein (CHOP)-death receptor 5 pathway. Cancer Sci. 2014, 105, 520–527. [Google Scholar] [CrossRef] [PubMed]
- Bishayee, K.; Ghosh, S.; Mukherjee, A.; Sadhukhan, R.; Mondal, J.; Khuda-Bukhsh, A.R. Quercetin induces cytochrome-c release and ROS accumulation to promote apoptosis and arrest the cell cycle in G2/M, in cervical carcinoma: Signal cascade and drug-DNA interaction. Cell Prolif. 2013, 46, 153–163. [Google Scholar] [CrossRef] [PubMed]
- Bi, Y.; Shen, C.; Li, C.; Liu, Y.; Gao, D.; Shi, C.; Peng, F.; Liu, Z.; Zhao, B.; Zheng, Z.; et al. Inhibition of autophagy induced by quercetin at a late stage enhances cytotoxic effects on glioma cells. Tumour Biol. 2015. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Moon, J.Y.; Ahn, K.S.; Cho, S.K. Quercetin induces mitochondrial mediated apoptosis and protective autophagy in human glioblastoma U373MG cells. Oxid. Med. Cell. Longev. 2013, 2013, 596496. [Google Scholar] [CrossRef] [PubMed]
- Hudson, S.P.; Padera, R.F.; Langer, R.; Kohane, D.S. The biocompatibility of mesoporous silicates. Biomaterials 2008, 29, 4045–4055. [Google Scholar] [CrossRef] [PubMed]
- Ambrogio, M.W.; Thomas, C.R.; Zhao, Y.L.; Zink, J.I.; Stoddart, J.F. Mechanized silica nanoparticles: A new frontier in theranostic nanomedicine. Acc. Chem. Res. 2011, 44, 903–913. [Google Scholar] [CrossRef] [PubMed]
- Fontecave, T.; Sanchez, C.; Azaïs, T.; Boissière, C. Chemical modification as a versatile tool for tuning stability of silica based mesoporous carriers in biologically relevant conditions. Chem. Mater. 2012, 24, 4326–4336. [Google Scholar] [CrossRef]
- Wang, Y.; Zhao, Q.; Han, N.; Bai, L.; Li, J.; Liu, J.; Che, E.; Hu, L.; Zhang, Q.; Jiang, T.; Wang, S. Mesoporous silica nanoparticles in drug delivery and biomedical applications. Nanomed. Nanotechnol. Biol. Med. 2015, 11, 313–327. [Google Scholar] [CrossRef] [PubMed]
- Andersson, J.; Rosenholm, J.; Areva, S.; Lindén, M. Influences of material characteristics on ibuprofen drug loading and release profiles from ordered micro- and mesoporous silica matrices. Chem. Mater. 2004, 16, 4160–4167. [Google Scholar] [CrossRef]
- Hu, Y.; Zhi, Z.; Zhao, Q.; Wu, C.; Zhao, P.; Jiang, H.; Jiang, T.; Wang, S. 3D cubic mesoporous silica microsphere as a carrier for poorly soluble drug carvedilol. Microporous Mesoporous Mater. 2012, 147, 94–101. [Google Scholar] [CrossRef]
- Souris, J.S.; Lee, C.H.; Cheng, S.H.; Chen, C.T.; Yang, C.S.; Ho, J.A.; Mou, C.Y.; Lo, L.W. Surface charge-mediated rapid hepatobiliary excretion of mesoporous silica nanoparticles. Biomaterials 2010, 31, 5564–5574. [Google Scholar] [CrossRef] [PubMed]
- Lu, J.; Liong, M.; Li, Z.; Zink, J.I.; Tamanoi, F. Biocompatibility, biodistribution, and drug-delivery efficiency of mesoporous silica nanoparticles for cancer therapy in animals. Small 2010, 6, 1794–1805. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Wang, J.; Bai, X.; Jiang, T.; Zhang, Q.; Wang, S. Mesoporous silica nanoparticles for increasing the oral bioavailability and permeation of poorly water soluble drugs. Mol. Pharm. 2012, 9, 505–513. [Google Scholar] [CrossRef] [PubMed]
- Ahern, R.J.; Hanrahan, J.P.; Tobin, J.M.; Ryan, K.B.; Crean, A.M. Comparison of fenofibrate-mesoporous silica drug-loading processes for enhanced drug delivery. Eur. J. Pharm. Sci. 2013, 50, 400–409. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Jiang, T.; Zhang, Q.; Wang, S. Inclusion of telmisartan in mesocellular foam nanoparticles: Drug loading and release property. Eur. J. Pharm. Biopharm. 2010, 76, 17–23. [Google Scholar] [CrossRef] [PubMed]
- Michelina, C.; Ferdinando, P.; Flavia, B.; Simona, P.; Sabina, M.; Paola, N.; Severina, P. Silica/quercetin sol-gel hybrids as antioxidant dental implant materials. Sci. Technol. Adv. Mater. 2015, 16, 035001. [Google Scholar]
- Sapino, S.; Ugazio, E.; Gastaldi, L.; Miletto, I.; Berlier, G.; Zonari, D.; Oliaro-Bosso, S. Mesoporous silica as topical nanocarriers for quercetin: Characterization and in vitro studies. Eur. J. Pharm. Biopharm. 2015, 89, 116–125. [Google Scholar] [CrossRef] [PubMed]
- Tang, L.; Cheng, J. Nonporous silica nanoparticles for nanomedicine application. Nano Today 2013, 8, 290–312. [Google Scholar] [CrossRef] [PubMed]
- Benezra, M.; Penate-Medina, O.; Zanzonico, P.B.; Schaer, D.; Ow, H.; Burns, A.; DeStanchina, E.; Longo, V.; Herz, E.; Iyer, S.; et al. Multimodal silica nanoparticles are effective cancer-targeted probes in a model of human melanoma. J. Clin. Investig. 2011, 121, 2768–2780. [Google Scholar] [CrossRef] [PubMed]
- Bradbury, M.S.; Phillips, E.; Montero, P.H.; Cheal, S.M.; Stambuk, H.; Durack, J.C.; Sofocleous, C.T.; Meester, R.J.; Wiesner, U.; Patel, S. Clinically-translated silica nanoparticles as dual-modality cancer-targeted probes for image-guided surgery and interventions. Integr. Biol. 2013, 5, 74–86. [Google Scholar] [CrossRef] [PubMed]
- Danhier, F.; Ansorena, E.; Silva, J.M.; Coco, R.; le Breton, A.; Preat, V. PLGA-based nanoparticles: An overview of biomedical applications. J. Control Release 2012, 161, 505–522. [Google Scholar] [CrossRef] [PubMed]
- Asghar, W.; Islam, M.; Wadajkar, A.S.; Yuan, W.; Ilyas, A.; Nguyen, K.T.; Iqbal, S.M. PLGA Micro- and Nanoparticles Loaded Into Gelatin Scaffold for Controlled Drug Release. IEEE Trans. Nanotechnol. 2012, 11, 546–553. [Google Scholar] [CrossRef]
- Hussein, A.S.; Abdullah, N.; Ahmadun, F.R. In vitro degradation of poly (d,l-lactide-co-glycolide) nanoparticles loaded with linamarin. IET Nanobiotechnol. 2013, 7, 33–41. [Google Scholar] [CrossRef] [PubMed]
- Gratton, S.E.; Ropp, P.A.; Pohlhaus, P.D.; Luft, J.C.; Madden, V.J.; Napier, M.E.; DeSimone, J.M. The effect of particle design on cellular internalization pathways. Proc. Natl. Acad. Sci. USA 2008, 105, 11613–11618. [Google Scholar] [CrossRef] [PubMed]
- Pimple, S.; Manjappa, A.S.; Ukawala, M.; Murthy, R.S. PLGA nanoparticles loaded with etoposide and quercetin dihydrate individually: In vitro cell line study to ensure advantage of combination therapy. Cancer Nanotechnol. 2012, 3, 25–36. [Google Scholar] [CrossRef] [PubMed]
- Pandey, S.K.; Patel, D.K.; Thakur, R.; Mishra, D.P.; Maiti, P.; Haldar, C. Anti-cancer evaluation of quercetin embedded PLA nanoparticles synthesized by emulsified nanoprecipitation. Int. J. Biol. Macromol. 2015, 75, 521–529. [Google Scholar] [CrossRef] [PubMed]
- Pool, H.; Quintanar, D.; de Figueroa, J.D.; Mano, C.M.; Bechara, J.E. H.; Godinez, L.A.; Mendoza, S. Antioxidant effects of quercetin and catechin encapsulated into PLGA nanoparticles. J. Nanomater. 2012, 2012. [Google Scholar] [CrossRef]
- Bishayee, K.; Khuda-Bukhsh, A.R.; Huh, S.O. PLGA-loaded gold-nanoparticles precipitated with quercetin downregulate HDAC-Akt activities controlling proliferation and activate p53-ROS crosstalk to induce apoptosis in hepatocarcinoma cells. Mol. Cells 2015, 38, 518–527. [Google Scholar] [CrossRef] [PubMed]
- Jain, A.K.; Thanki, K.; Jain, S. Co-encapsulation of tamoxifen and quercetin in polymeric nanoparticles: Implications on oral bioavailability, antitumor efficacy, and drug-induced toxicity. Mol. Pharm. 2013, 10, 3459–3474. [Google Scholar] [CrossRef] [PubMed]
- El-Gogary, R.I.; Rubio, N.; Wang, J.T.-W.; Al-Jamal, W.T.; Bourgognon, M.; Kafa, H.; Naeem, M.; Klippstein, R.; Abbate, V.; Leroux, F.; et al. Polyethylene glycol conjugated polymeric nanocapsules for targeted delivery of quercetin to folate-expressing cancer cells in vitro and in vivo. ACS Nano 2014, 8, 1384–1401. [Google Scholar] [CrossRef] [PubMed]
- Sharma, G.; Park, J.; Sharma, A.R.; Jung, J.S.; Kim, H.; Chakraborty, C.; Song, D.K.; Lee, S.S.; Nam, J.S. Methoxy poly(ethylene glycol)-poly(lactide) nanoparticles encapsulating quercetin act as an effective anticancer agent by inducing apoptosis in breast cancer. Pharm. Res. 2015, 32, 723–735. [Google Scholar] [CrossRef] [PubMed]
- Kumari, A.; Kumar, V.; Yadav, S.K. Plant extract synthesized PLA nanoparticles for controlled and sustained release of quercetin: A green approach. PLoS ONE 2012, 7, e41230. [Google Scholar] [CrossRef] [PubMed]
- Ravi Kumar, M.N.V. A review of chitin and chitosan applications. React. Funct. Polym. 2000, 46, 1–27. [Google Scholar] [CrossRef]
- Huang, M.; Khor, E.; Lim, L.Y. Uptake and cytotoxicity of chitosan molecules and nanoparticles: Effects of molecular weight and degree of deacetylation. Pharm. Res. 2004, 21, 344–353. [Google Scholar] [CrossRef] [PubMed]
- Ma, Z.; Lim, T.M.; Lim, L.Y. Pharmacological activity of peroral chitosan-insulin nanoparticles in diabetic rats. Int. J. Pharm. 2005, 293, 271–280. [Google Scholar] [CrossRef] [PubMed]
- Smith, J.M.; Dornish, M.; Wood, E.J. Involvement of protein kinase C in chitosan glutamate-mediated tight junction disruption. Biomaterials 2005, 26, 3269–3276. [Google Scholar] [CrossRef] [PubMed]
- Qiao, Y.; Bai, X.F.; Du, Y.G. Chitosan oligosaccharides protect mice from LPS challenge by attenuation of inflammation and oxidative stress. Int. Immunopharmacol. 2011, 11, 121–127. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.T.; Li, W.M.; Xu, G.; Li, X.Y.; Bai, X.F.; Wei, P.; Yu, C.; Du, Y.G. Chitosan oligosaccharides attenuate hydrogen peroxide-induced stress injury in human umbilical vein endothelial cells. Pharmacol. Res. 2009, 59, 167–175. [Google Scholar] [CrossRef] [PubMed]
- Singla, A.K.; Chawla, M. Chitosan: Some pharmaceutical and biological aspects—An update. J. Pharm. Pharmacol. 2001, 53, 1047–1067. [Google Scholar] [CrossRef] [PubMed]
- Sonvico, F.; Cagnani, A.; Rossi, A.; Motta, S.; di Bari, M.T.; Cavatorta, F.; Alonso, M.J.; Deriu, A.; Colombo, P. Formation of self-organized nanoparticles by lecithin/chitosan ionic interaction. Int. J. Pharm. 2006, 324, 67–73. [Google Scholar] [CrossRef] [PubMed]
- Raj, L.F.A.A.; Jonisha, R.; Revathi, B.; Jayalakshmy, E. Preparation and characterization of BSA and chitosan nanopartices for sustainable delivery system for quercetin. J. Appl. Pharm. Sci. 2015, 5, 1–5. [Google Scholar] [CrossRef]
- Sonaje, K.; Lin, K.J.; Tseng, M.T.; Wey, S.P.; Su, F.Y.; Chuang, E.Y.; Hsu, C.W.; Chen, C.T.; Sung, H.W. Effects of chitosan-nanoparticle-mediated tight junction opening on the oral absorption of endotoxins. Biomaterials 2011, 32, 8712–8721. [Google Scholar] [CrossRef] [PubMed]
- Feng, C.; Wang, Z.; Jiang, C.; Kong, M.; Zhou, X.; Li, Y.; Cheng, X.; Chen, X. Chitosan/O-carboxymethyl chitosan nanoparticles for efficient and safe oral anticancer drug delivery: In vitro and in vivo evaluation. Int. J. Pharm. 2013, 457, 158–167. [Google Scholar] [CrossRef] [PubMed]
- David, K.I.; Jaidev, L.R.; Sethuraman, S.; Krishnan, U.M. Dual drug loaded chitosan nanoparticles-sugar-coated arsenal against pancreatic cancer. Colloids Surf. B Biointerfaces 2015, 135, 689–698. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.S.; Jo, B.W.; Kim, Y.C. Enhanced paclitaxel bioavailability after oral administration of paclitaxel or prodrug to rats pretreated with quercetin. Eur. J. Pharm. Biopharm. 2004, 57, 313–318. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Chen, Y.; Dahmani, F.Z.; Yin, L.; Zhou, J.; Yao, J. Amphiphilic carboxymethyl chitosan-quercetin conjugate with P-gp inhibitory properties for oral delivery of paclitaxel. Biomaterials 2014, 35, 7654–7665. [Google Scholar] [CrossRef] [PubMed]
- Torchilin, V.P. Recent advances with liposomes as pharmaceutical carriers. Nat. Rev. Drug Discov. 2005, 4, 145–160. [Google Scholar] [CrossRef] [PubMed]
- Kundu, S.K.; Sharma, A.R.; Lee, S.S.; Sharma, G.; Doss, C.G.; Yagihara, S.; Kim, D.Y.; Nam, J.S.; Chakraborty, C. Recent trends of polymer mediated liposomal gene delivery system. Biomed. Res. Int. 2014, 2014, 934605. [Google Scholar] [CrossRef] [PubMed]
- Yuan, F.; Dellian, M.; Fukumura, D.; Leunig, M.; Berk, D.A.; Torchilin, V.P.; Jain, R.K. Vascular permeability in a human tumor xenograft: Molecular size dependence and cutoff size. Cancer Res. 1995, 55, 3752–3756. [Google Scholar] [PubMed]
- Stone, W.L.; Smith, M. Therapeutic uses of antioxidant liposomes. Mol. Biotechnol. 2004, 27, 217–230. [Google Scholar] [CrossRef]
- Zhang, L.; Gu, F.X.; Chan, J.M.; Wang, A.Z.; Langer, R.S.; Farokhzad, O.C. Nanoparticles in medicine: Therapeutic applications and developments. Clin. Pharmacol. Ther. 2008, 83, 761–769. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Wang, J.J.; Yang, G.Y.; Du, S.M.; Zeng, N.; Li, D.S.; Li, R.M.; Chen, J.Y.; Feng, J.B.; Yuan, S.H.; et al. Effects of quercetin nanoliposomes on C6 glioma cells through induction of type III programmed cell death. Int. J. Nanomed. 2012, 7, 271–280. [Google Scholar]
- Rezaei-Sadabady, R.; Eidi, A.; Zarghami, N.; Barzegar, A. Intracellular ROS protection efficiency and free radical-scavenging activity of quercetin and quercetin-encapsulated liposomes. Artif. Cells Nanomed. Biotechnol. 2014, 24, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Dian, L.; Yu, E.; Chen, X.; Wen, X.; Zhang, Z.; Qin, L.; Wang, Q.; Li, G.; Wu, C. Enhancing oral bioavailability of quercetin using novel soluplus polymeric micelles. Nanoscale Res. Lett. 2014, 9. [Google Scholar] [CrossRef] [PubMed]
- Tan, B.J.; Liu, Y.; Chang, K.L.; Lim, B.K.; Chiu, G.N. Perorally active nanomicellar formulation of quercetin in the treatment of lung cancer. Int. J. Nanomed. 2012, 7, 651–661. [Google Scholar]
- Han, Q.; Yang, R.; Li, J.; Liang, W.; Zhang, Y.; Dong, M.; Besenbacher, F.; Wang, C. Enhancement of biological activities of nanostructured hydrophobic drug species. Nanoscale 2012, 4, 2078–2082. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Zhang, J.; Zu, Y.J.; Nie, S.F.; Cao, J.; Wang, Q.; Nie, S.P.; Deng, Z.Y.; Xie, M.Y.; Wang, S. Biocompatible and biodegradable nanoparticles for enhancement of anti-cancer activities of phytochemicals. Chin. J. Nat. Med. 2015, 13, 641–652. [Google Scholar] [CrossRef]
- Nair, H.B.; Sung, B.; Yadav, V.R.; Kannappan, R.; Chaturvedi, M.M.; Aggarwal, B.B. Delivery of antiinflammatory nutraceuticals by nanoparticles for the prevention and treatment of cancer. Biochem. Pharmacol. 2010, 80, 1833–1843. [Google Scholar] [CrossRef] [PubMed]
- Santos, I.S.; Ponte, B.M.; Boonme, P.; Silva, A.M.; Souto, E.B. Nanoencapsulation of polyphenols for protective effect against colon-rectal cancer. Biotechnol. Adv. 2013, 31, 514–523. [Google Scholar] [CrossRef] [PubMed]
- Sanna, V.; Siddiqui, I.A.; Sechi, M.; Mukhtar, H. Nanoformulation of natural products for prevention and therapy of prostate cancer. Cancer Lett. 2013, 334, 142–151. [Google Scholar] [CrossRef] [PubMed]
- Parveen, S.; Misra, R.; Sahoo, S.K. Nanoparticles: A boon to drug delivery, therapeutics, diagnostics and imaging. Nanomedicine 2012, 8, 147–166. [Google Scholar] [CrossRef] [PubMed]
- Sun, M.; Nie, S.; Pan, X.; Zhang, R.; Fan, Z.; Wang, S. Quercetin-nanostructured lipid carriers: Characteristics and anti-breast cancer activities in vitro. Colloids Surf. B Biointerfaces 2014, 113, 15–24. [Google Scholar] [CrossRef] [PubMed]
© 2016 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nam, J.-S.; Sharma, A.R.; Nguyen, L.T.; Chakraborty, C.; Sharma, G.; Lee, S.-S. Application of Bioactive Quercetin in Oncotherapy: From Nutrition to Nanomedicine. Molecules 2016, 21, 108. https://doi.org/10.3390/molecules21010108
Nam J-S, Sharma AR, Nguyen LT, Chakraborty C, Sharma G, Lee S-S. Application of Bioactive Quercetin in Oncotherapy: From Nutrition to Nanomedicine. Molecules. 2016; 21(1):108. https://doi.org/10.3390/molecules21010108
Chicago/Turabian StyleNam, Ju-Suk, Ashish Ranjan Sharma, Lich Thi Nguyen, Chiranjib Chakraborty, Garima Sharma, and Sang-Soo Lee. 2016. "Application of Bioactive Quercetin in Oncotherapy: From Nutrition to Nanomedicine" Molecules 21, no. 1: 108. https://doi.org/10.3390/molecules21010108
APA StyleNam, J.-S., Sharma, A. R., Nguyen, L. T., Chakraborty, C., Sharma, G., & Lee, S.-S. (2016). Application of Bioactive Quercetin in Oncotherapy: From Nutrition to Nanomedicine. Molecules, 21(1), 108. https://doi.org/10.3390/molecules21010108








