Impact of Lifestyle Behaviors on Postprandial Hyperglycemia during Continuous Glucose Monitoring in Adult Males with Overweight/Obesity but without Diabetes
Abstract
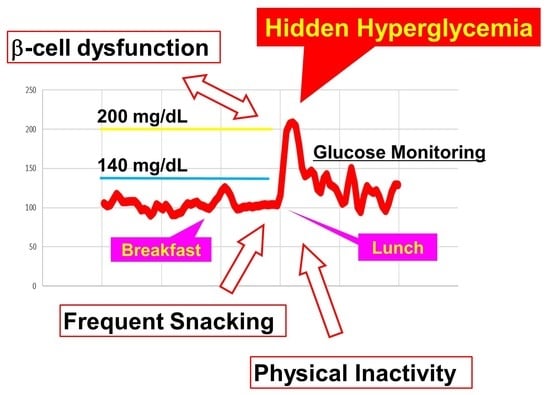
:1. Introduction
2. Methods
2.1. Study Participants
2.2. Assessing Glycemia
2.3. Insulin Secretion/Resistance Indices
2.4. Study Protocol
2.5. Determination of Snacking and Drinking Frequencies
2.6. Assessment of Physical Activity
2.7. Data Analysis for CGM
2.8. Statistical Analysis
2.9. Ethics Statement
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
Abbreviations
References
- Kahn, S.E.; Hull, R.L.; Utzschneider, K.M. Mechanisms linking obesity to insulin resistance and type 2 diabetes. Nature 2006, 444, 840–846. [Google Scholar] [CrossRef]
- Fonseca, V.A. Defining and Characterizing the Progression of Type 2 Diabetes. Diabetes Care 2009, 32 (Suppl. 2), S151–S156. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, C.; Cohrs, C.M.; Stertmann, J.; Bozsak, R.; Speier, S. Human beta cell mass and function in diabetes: Recent advances in knowledge and technologies to understand disease pathogenesis. Mol. Metab. 2017, 6, 943–957. [Google Scholar] [CrossRef]
- Defronzo, R.A. Banting lecture. from the triumvirate to the ominous octet: A new paradigm for the treatment of type 2 diabetes mellitus. Diabetes 2009, 58, 773–795. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Butler, A.E.; Janson, J.; Bonner-Weir, S.; Ritzel, R.; Rizza, R.A.; Butler, P.C. β-Cell Deficit and Increased β-Cell Apoptosis in Humans with Type 2 Diabetes. Diabetes 2003, 52, 102–110. [Google Scholar] [CrossRef] [Green Version]
- Holman, R.R. Assessing the Potential for Alpha-Glucosidase Inhibitors in Prediabetic States. Diabetes Res. Clin. Pract. 1998, 40 (Suppl. 1), S21–S25. [Google Scholar] [CrossRef]
- Kahn, S.E. The Importance of β-Cell Failure in the Development and Progression of Type 2 Diabetes. J. Clin. Endocrinol. Metab. 2001, 86, 4047–4058. [Google Scholar]
- Eizirik, D.L.; Korbutt, G.S.; Hellerström, C. Prolonged exposure of human pancreatic islets to high glucose concentrations in vitro impairs the beta-cell function. J. Clin. Investig. 1992, 90, 1263–1268. [Google Scholar] [CrossRef] [Green Version]
- Borg, R.; Kuenen, J.C.; Carstensen, B.; Zheng, H.; Nathan, D.M.; Heine, R.J.; Nerup, J.; Borch-Johnsen, K.; Witte, D.R.; on behalf of the ADAG Study Group. Real-life glycaemic profiles in non-diabetic individuals with low fasting glucose and normal HbA1c: The A1C-Derived Average Glucose (ADAG) study. Diabetologia 2010, 53, 1608–1611. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kishimoto, I.; Ohashi, A. Hyperglycemia During Continuous Glucose Monitoring in Obese/Overweight Male Individuals Without Diabetes. J. Diabetes Sci. Technol. 2021. [Google Scholar] [CrossRef]
- Singh, B.; Saxena, A. Surrogate markers of insulin resistance: A review. World J. Diabetes 2010, 1, 36–47. [Google Scholar] [CrossRef] [PubMed]
- Matsuda, M.; DeFronzo, R.A. Insulin sensitivity indices obtained from oral glucose tolerance testing: Comparison with the euglycemic insulin clamp. Diabetes Care 1999, 22, 1462–1470. [Google Scholar] [CrossRef]
- American Diabetes Association. Classification and Diagnosis of Diabetes: Standards of Medical Care in Diabetes—2020. Diabetes Care 2020, 43, S14–S31. [Google Scholar] [CrossRef] [Green Version]
- Vittinghoff, E.; McCulloch, C.E. Relaxing the Rule of Ten Events per Variable in Logistic and Cox Regression. Am. J. Epidemiol. 2017, 165, 710–718. [Google Scholar] [CrossRef] [Green Version]
- Ceriello, A.; Colagiuri, S. International Diabetes Federation guideline for management of postmeal glucose: A review of recommendations. Diabet. Med. 2008, 25, 1151–1156. [Google Scholar] [CrossRef] [Green Version]
- American Diabetes Association. Postprandial Blood Glucose. Diabetes Care 2001, 24, 775–778. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Verhoeven, A.A.C.; Adriaanse, M.A.; Evers, C.; de Ridder, D.T.D. The power of habits: Unhealthy snacking behaviour is primarily predicted by habit strength. Br. J. Health Psychol. 2012, 17, 758–770. [Google Scholar] [CrossRef]
- Mahmood, L.; Flores-Barrantes, P.; Moreno, L.A.; Manios, Y.; Gonzalez-Gil, E.M. The Influence of Parental Dietary Behaviors and Practices on Children’s Eating Habits. Nutrients 2021, 13, 1138. [Google Scholar] [CrossRef] [PubMed]
- Rezende, L.F.M.; Sá, T.H.; Mielke, G.I.; Viscondi, J.Y.K.; Rey-López, J.P.; Garcia, L.M.T. All-Cause Mortality Attributable to Sitting Time: Analysis of 54 Countries Worldwide. Am. J. Prev. Med. 2016, 51, 253–263. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wen, C.P.; Wai, J.P.M.; Tsai, M.K.; Yang, Y.C.; Cheng, T.Y.D.; Lee, M.C.; Chan, H.T.; Tsao, C.K.; Tsai, S.P.; Wu, X. Minimum amount of physical activity for reduced mortality and extended life expectancy: A prospective cohort study. Lancet 2011, 378, 1244–1253. [Google Scholar] [CrossRef]
- Patel, A.V.; Bernstein, L.; Deka, A.; Feigelson, H.S.; Campbell, P.T.; Gapstur, S.M.; Colditz, G.A.; Thun, M.J. Leisure Time Spent Sitting in Relation to Total Mortality in a Prospective Cohort of US Adults. Am. J. Epidemiol. 2010, 172, 419–429. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Duvivier, B.M.F.M.; Schaper, N.C.; Hesselink, M.K.C.; van Kan, L.; Stienen, N.; Winkens, B.; Koster, A.; Savelberg, H.H.C.M. Breaking Sitting with Light Activities vs. Structured Exercise: A Randomised Crossover Study Demonstrating Benefits for Glycaemic Control and Insulin Sensitivity in Type 2 Diabetes. Diabetologia 2017, 60, 490–498. [Google Scholar] [CrossRef] [PubMed] [Green Version]
| Parameters | CGM Max | TAR > 140 | TAR > 200 | % of ≥140 Peak per Meal | % of ≥200 Peak per Meal |
|---|---|---|---|---|---|
| HbA1c, % | 0.52 | 0.72 | 0.39 | 0.65 | 0.37 |
| 1,5-AG, μg/mL | −0.33 | ||||
| OGTT PG 0, mg/dL | 0.34 | ||||
| OGTT PG 30, mg/dL | 0.40 | 0.35 | |||
| OGTT PG 60, mg/dL | 0.33 | 0.43 | 0.40 | ||
| OGTT PG 120, mg/dL | |||||
| OGTT IRI 0, μU/mL | 0.38 | ||||
| OGTT IRI 30, μU/mL | |||||
| OGTT IRI 60, μU/mL | 0.35 | ||||
| OGTT IRI 120, μU/mL | 0.42 | 0.49 | 0.35 | 0.40 | |
| Insulinogenic index | |||||
| HOMA-β | |||||
| HOMA-IR | 0.41 | ||||
| Matsuda index | −0.33 | −0.52 | −0.39 | ||
| Disposition index | −0.48 | −0.52 | −0.40 | −0.44 | −0.38 |
| QUICKI | −0.45 | −0.34 |
| Parameters | CGM Max | TAR > 140 | TAR > 200 | % of ≥140 Peak per Meal | % of ≥200 Peak per Meal |
|---|---|---|---|---|---|
| Skip breakfast (4–10 a.m.), % of days | 0.33 | ||||
| Late dinner (10 p.m.), % of days | |||||
| Drinking habits, days per week | −0.35 | ||||
| Snacking habits, days per week | 0.33 | 0.41 | 0.42 | 0.39 | |
| Drinking frequency, times per day | −0.34 | −0.38 | |||
| Snacking frequency, times per day | 0.39 | ||||
| Average walking step counts, steps per day | |||||
| Maximal walking step counts, steps per day | |||||
| Minimal walking step counts, steps per day |
| Parameters | Category | n | CGM Max | TAR > 140 | TAR > 200 | % of ≥140 Peak per Meal | % of ≥200 Peak per Meal |
|---|---|---|---|---|---|---|---|
| Skip breakfast (4–10 a.m.) | ≥once during the study | 17 | 207 (175–228) | 11.0 (5.3–19.9) | 0.4 (0–0.92) | 71.4 (34.3–83.3) | 4.76 (0–10.3) |
| none | 19 | 184 (172–213) | 9.4 (2.7–15.5) | 0 (0–0.46) | 42.9 (23.8–66.7) | 0 (0–4.76) | |
| p value | 0.241 | 0.384 | 0.14 | 0.188 | 0.093 | ||
| Late dinner (10 p.m.) | ≥once during the study | 12 | 206 (190–234) | 11.5 (8.5–17.8) | 0.41 (0–0.73) | 64.3 (52.2–75.5) | 4.76 (0–8.81) |
| none | 24 | 184 (168–215 | 8.5 (2.4–15.8) | 0 (0–0.81) | 43.9 (23.6–76.8) | 0 (0–5.42) | |
| p value | 0.07 | 0.159 | 0.21 | 0.383 | 0.275 | ||
| Drinking habits | yes | 11 | 180 (172–187) | 5.2 (2.3–11.4) | 0 (0–0) | 40.9 (23.8–63.2) | 0 (0–0) |
| no | 25 | 205 (176–220) | 11.7 (5.3–17.7) | 0.25 (0–0.92) | 65 (33.2–88.2) | 4.76 (0–9.76) | |
| p value | 0.144 | 0.175 | 0.053 | 0.311 | 0.028 * | ||
| Snacking habits | yes | 12 | 214 (181–233) | 12.9 (8.8–18.5) | 0.59 (0–1.31) | 72.4 (56.3–88.6) | 4.88 (0–16.3) |
| no | 24 | 187 (168–213) | 8.5 (2.6–14.0) | 0 (0–0.45) | 43.9 (22.9–70.2) | 0 (0–4.76) | |
| p value | 0.093 | 0.07 | 0.038 * | 0.029 * | 0.056 | ||
| Drinking frequency | ≥once during the study | 11 | 180 (172–187) | 5.24 (2.3–11.4) | 0 (0–0) | 40.9 (23.8–63.2) | 0 (0–0) |
| None during the study | 25 | 205 (176–220) | 11.7 (5.3–17.7) | 0.25 (0–0.92) | 65 (33.2–77.2) | 4.76 (0–9.76) | |
| p value | 0.144 | 0.175 | 0.053 | 0.311 | 0.028 * | ||
| Snacking frequency | ≥once a day | 8 | 226 (207–247) | 13.4 (8.9–21.2) | 0.7 (0.3–2.82) | 77.2 (57.6–94.2) | 8.1 (4.45–33) |
| <once a day | 28 | 184 (168–211) | 9.5 (2.6–15.0) | 0 (0–0.52) | 48.6 (22.9–70.2) | 0 (0–4.76) | |
| p value | 0.003 * | 0.048 * | 0.005 * | 0.007 * | 0.005 * | ||
| Average daily step counts | ≥the median (6968 steps per day) | 18 | 187 (159–216) | 8 (1.6–14.2) | 0 (0–0.74) | 52.2 (18.9–69) | 0 (0–5.83) |
| <the median | 17 | 206 (175–224) | 11.3 (5.1–16.4) | 0.3 (0–0.81) | 65.7 (35.6–90.5) | 4.55 (0–9.64) | |
| p value | 0.241 | 0.156 | 0.331 | 0.156 | 0.349 | ||
| Maximal daily step counts | ≥the median (11,937 steps per day) | 18 | 196 (177–225) | 9.7 (3.6–15.9) | 0.13 (0–1) | 59.1 (22–74) | 2.17 (0–10.1) |
| <the median | 17 | 187 (171–214) | 10.9 (3.7–15.1) | 0 (0–0.51) | 52.4 (33.8–84.4) | 0 (0–5.16) | |
| p value | 0.447 | 0.921 | 0.46 | 0.78 | 0.517 | ||
| Minimal daily step counts | ≥the median (2681 steps per day) | 18 | 181 (164–195) | 6.4 (1.9–12.4) | 0 (0–0.14) | 41.9 (20.8–65.4) | 0 (0–1.09) |
| <the median | 17 | 212 (191–228) | 12.2 (6.7–19.9) | 0.46 (0–0.92) | 71.4 (43.9–84.4) | 4.76 (0–10.3) | |
| p value | 0.026 * | 0.027 * | 0.02 * | 0.056 | 0.01 * |
| Univariate Analysis | Model 1 | Model 2 | Model 3 | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Odds | 95% CI | p value | Odds | 95% CI | p value | Odds | 95% CI | p value | |
| Disposition index ≤1.57 | 12.6 | 1.4–117.6 | 0.007 * | - | - | - | - | - | - |
| Snacking frequency ≥once per day | - | - | - | 12.6 | 1.4–117.6 | 0.007 * | - | - | - |
| Minimal step category ≤2499 | - | - | - | - | - | - | 8.4 | 1.8–38.6 | 0.003 * |
| Bivariate Analysis | Model 4 | Model 5 | Model 6 | ||||||
| Odds | 95% CI | p value | Odds | 95% CI | p value | Odds | 95% CI | p value | |
| Disposition index ≤1.57 | 12.3 | 1.6–262.9 | 0.014 * | - | - | - | 11.1 | 1.3–247.0 | 0.024 * |
| Snacking frequency ≥once per day | 12.3 | 1.6–262.9 | 0.014 * | 11.1 | 1.3–247.0 | 0.024 * | - | - | - |
| Minimal step category ≤2499 | - | - | - | 7 | 1.4–42.4 | 0.016 * | 7 | 1.4–42.4 | 0.016 * |
| Trivariate Analysis | Model 7 | ||||||||
| Odds | 95% CI | p value | |||||||
| Disposition index ≤1.57 | 14.5 | 1.4–376.4 | 0.022 * | ||||||
| Snacking frequency ≥once per day | 14.5 | 1.4–376.4 | 0.022 * | ||||||
| Minimal step category ≤2499 | 6.6 | 1.1–54.7 | 0.036 * | ||||||
| Parameters | Snacking Habits Category | p Value | |||||
|---|---|---|---|---|---|---|---|
| Snacking Habits (+) (n = 12) | Snacking Habits (−) (n = 24) | ||||||
| Median | IQR, Lower | IQR, Upper | Median | IQR, Lower | IQR, Upper | ||
| Age, years | 54.0 | 50.5 | 56.3 | 56.0 | 52.3 | 58.0 | 0.187 |
| BMI, kg/m2 | 27.7 | 26.3 | 31.8 | 27.9 | 26.5 | 29.2 | 0.737 |
| HbA1c, % | 5.5 | 5.3 | 5.9 | 5.3 | 5.1 | 5.5 | 0.039 * |
| 1,5-AG, μg/mL | 19.6 | 11.8 | 26.5 | 20.2 | 15.4 | 24.1 | 0.801 |
| HOMA-β | 142.0 | 106.6 | 277.5 | 87.9 | 61.5 | 128.8 | 0.002 * |
| HOMA-IR | 2.6 | 2.1 | 4.2 | 1.6 | 1 | 2.3 | 0.001 * |
| Insulinogenic index | 0.9 | 0.4 | 1.6 | 0.6 | 0.3 | 1.0 | 0.46 |
| Matsuda index | 2.7 | 1.3 | 3.8 | 4.9 | 3.0 | 7.3 | 0.006 * |
| Disposition index | 1.5 | 1.4 | 4.4 | 2.9 | 2.0 | 4.7 | 0.159 |
| QUICKI | 0.33 | 0.31 | 0.34 | 0.36 | 0.34 | 0.38 | 0.001 * |
| OGTT PG 0, mg/dL | 90.5 | 84.8 | 98.3 | 92.5 | 86.8 | 96.8 | 0.724 |
| OGTT PG 30, mg/dL | 167.5 | 134 | 192.3 | 153.0 | 137.3 | 175.3 | 0.46 |
| OGTT PG 60, mg/dL | 170.5 | 162.0 | 218.3 | 179.0 | 146.0 | 193.0 | 0.557 |
| OGTT PG 120, mg/dL | 125.0 | 95.3 | 159.3 | 110.5 | 95.5 | 127.8 | 0.261 |
| OGTT IRI 0, μU/mL | 11.5 | 9.4 | 22.8 | 6.8 | 5.0 | 9.7 | 0.001 * |
| OGTT IRI 30, μU/mL | 61.5 | 39.9 | 110.6 | 49.3 | 29.6 | 73.6 | 0.202 |
| OGTT IRI 60, μU/mL | 97.8 | 56.4 | 188.2 | 55.5 | 43.0 | 132 | 0.093 |
| OGTT IRI 120, μU/mL | 116 | 39.1 | 180.6 | 41.0 | 24.7 | 66.9 | 0.017 * |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kishimoto, I.; Ohashi, A. Impact of Lifestyle Behaviors on Postprandial Hyperglycemia during Continuous Glucose Monitoring in Adult Males with Overweight/Obesity but without Diabetes. Nutrients 2021, 13, 3092. https://doi.org/10.3390/nu13093092
Kishimoto I, Ohashi A. Impact of Lifestyle Behaviors on Postprandial Hyperglycemia during Continuous Glucose Monitoring in Adult Males with Overweight/Obesity but without Diabetes. Nutrients. 2021; 13(9):3092. https://doi.org/10.3390/nu13093092
Chicago/Turabian StyleKishimoto, Ichiro, and Akio Ohashi. 2021. "Impact of Lifestyle Behaviors on Postprandial Hyperglycemia during Continuous Glucose Monitoring in Adult Males with Overweight/Obesity but without Diabetes" Nutrients 13, no. 9: 3092. https://doi.org/10.3390/nu13093092
APA StyleKishimoto, I., & Ohashi, A. (2021). Impact of Lifestyle Behaviors on Postprandial Hyperglycemia during Continuous Glucose Monitoring in Adult Males with Overweight/Obesity but without Diabetes. Nutrients, 13(9), 3092. https://doi.org/10.3390/nu13093092