Environmental Exposures around Conception: Developmental Pathways Leading to Lifetime Disease Risk
Abstract
1. Introduction
2. Human Peri-Conception Undernutrition and Offspring Health
3. Mouse Peri-Conception Undernutrition and Offspring Health
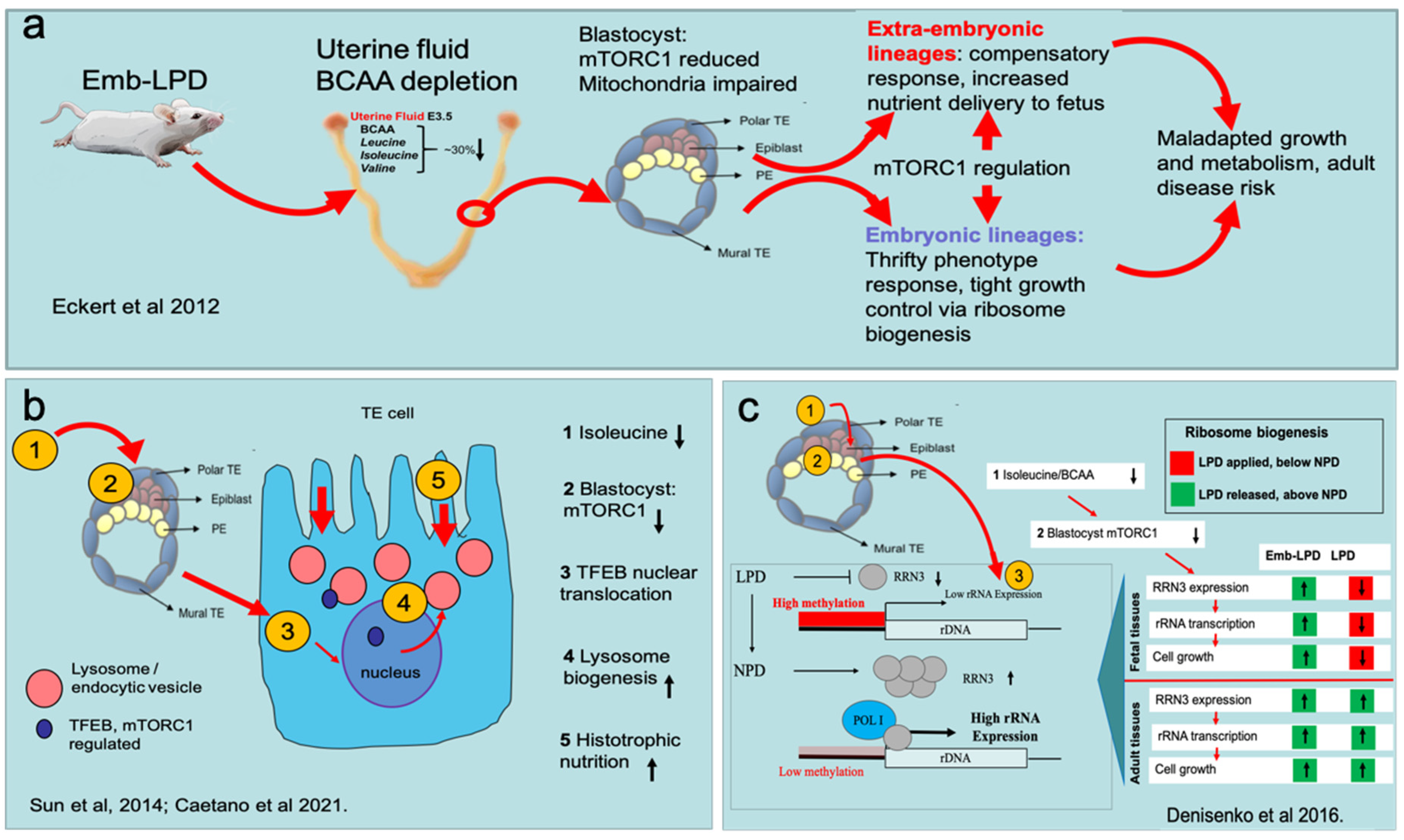
3.1. Embryo Induction of Altered Developmental Programming by Maternal Undernutrition
3.2. Blastocyst Nutrient Sensing Activates Compensatory Responses in Extra-Embryonic Lineages
3.3. Blastocyst Nutrient Sensing Coordinates the Foetal Growth Trajectory
3.4. From Peri-Conception Exposure to the Endgame of Disease Risk
4. From Diverse Peri-Conception Environment to a Convergent Adult Disease Risk
5. Conclusions and Thinking Ahead
Author Contributions
Funding
Institutional Review Board Statement
Conflicts of Interest
References
- Fleming, T.P.; Watkins, A.J.; Velazquez, M.A.; Mathers, J.C.; Prentice, A.M.; Stephenson, J.; Barker, M.; Saffery, R.; Yajnik, C.S.; Eckert, J.J.; et al. Origins of lifetime health around the time of conception: Causes and consequences. Lancet 2018, 391, 1842–1852. [Google Scholar] [CrossRef]
- Velazquez, M.A.; Fleming, T.P.; Watkins, A.J. Periconceptional environment and the developmental origins of disease. J. Endocrinol. 2019, 242, T33–T49. [Google Scholar] [CrossRef] [PubMed]
- Sinclair, K.D.; Karamitri, A.; Gardner, D.S. Dietary regulation of developmental programming in ruminants: Epigenetic modifications in the germline. Soc. Reprod. Fertil. Suppl. 2010, 67, 59–72. [Google Scholar] [CrossRef] [PubMed]
- Caton, J.S.; Crouse, M.S.; McLean, K.J.; Dahlen, C.R.; Ward, A.K.; Cushman, R.A.; Grazul-Bilska, A.T.; Neville, B.W.; Borowicz, P.P.; Reynolds, L.P. Maternal periconceptual nutrition, early pregnancy, and developmental outcomes in beef cattle. J. Anim. Sci. 2020, 98, skaa358. [Google Scholar] [CrossRef]
- Canovas, S.; Ross, P.J. Epigenetics in preimplantation mammalian development. Theriogenology 2016, 86, 69–79. [Google Scholar] [CrossRef] [PubMed]
- Hanna, C.W.; Demond, H.; Kelsey, G. Epigenetic regulation in development: Is the mouse a good model for the human? Hum. Reprod. Update 2018, 24, 556–576. [Google Scholar] [CrossRef]
- Watkins, A.J.; Dias, I.; Tsuro, H.; Allen, D.; Emes, R.D.; Moreton, J.; Wilson, R.; Ingram, R.J.M.; Sinclair, K.D. Paternal diet programs offspring health through sperm- and seminal plasma-specific pathways in mice. Proc. Natl. Acad. Sci. USA 2018, 115, 10064–10069. [Google Scholar] [CrossRef]
- Morgan, H.L.; Paganopoulou, P.; Akhtar, S.; Urquhart, N.; Philomin, R.; Dickinson, Y.; Watkins, A.J. Paternal diet impairs F1 and F2 offspring vascular function through sperm and seminal plasma specific mechanisms in mice. J. Physiol. 2020, 598, 699–715. [Google Scholar] [CrossRef] [PubMed]
- UNICEF(Ed.). The State of the World’s Children 2019. Children. In Food and Nutrition: Growing Well in a Changing World; United Nations Children’s Fund (UNICEF): New York, NY, USA, 2019. [Google Scholar]
- Roseboom, T.J. Epidemiological evidence for the developmental origins of health and disease: Effects of prenatal undernutrition in humans. J. Endocrinol. 2019, 242, T135–T144. [Google Scholar] [CrossRef] [PubMed]
- de Rooij, S.R.; Painter, R.C.; Phillips, D.I.; Osmond, C.; Michels, R.P.; Godsland, I.F.; Bossuyt, P.M.; Bleker, O.P.; Roseboom, T.J. Impaired insulin secretion after prenatal exposure to the Dutch famine. Diabetes Care 2006, 29, 1897–1901. [Google Scholar] [CrossRef]
- Ravelli, A.C.; van Der Meulen, J.H.; Osmond, C.; Barker, D.J.; Bleker, O.P. Obesity at the age of 50 y in men and women exposed to famine prenatally. Am. J. Clin. Nutr. 1999, 70, 811–816. [Google Scholar] [CrossRef] [PubMed]
- Roseboom, T.J.; van der Meulen, J.H.; Osmond, C.; Barker, D.J.; Ravelli, A.C.; Bleker, O.P. Plasma lipid profiles in adults after prenatal exposure to the Dutch famine. Am. J. Clin. Nutr. 2000, 72, 1101–1106. [Google Scholar] [CrossRef] [PubMed]
- Roseboom, T.J.; van der Meulen, J.H.; Osmond, C.; Barker, D.J.; Ravelli, A.C.; Schroeder-Tanka, J.M.; van Montfrans, G.A.; Michels, R.P.; Bleker, O.P. Coronary heart disease after prenatal exposure to the Dutch famine, 1944–1945. Heart 2000, 84, 595–598. [Google Scholar] [CrossRef] [PubMed]
- Painter, R.C.; de Rooij, S.R.; Bossuyt, P.M.; Simmers, T.A.; Osmond, C.; Barker, D.J.; Bleker, O.P.; Roseboom, T.J. Early onset of coronary artery disease after prenatal exposure to the Dutch famine. Am. J. Clin. Nutr. 2006, 84, 322–327. [Google Scholar] [CrossRef] [PubMed]
- van Abeelen, A.F.; Veenendaal, M.V.; Painter, R.C.; de Rooij, S.R.; Dijkgraaf, M.G.; Bossuyt, P.M.; Elias, S.G.; Grobbee, D.E.; Uiterwaal, C.S.; Roseboom, T.J. Survival effects of prenatal famine exposure. Am. J. Clin. Nutr. 2012, 95, 179–183. [Google Scholar] [CrossRef] [PubMed]
- Ekamper, P.; van Poppel, F.; Stein, A.D.; Lumey, L.H. Independent and additive association of prenatal famine exposure and intermediary life conditions with adult mortality between age 18–63 years. Soc. Sci. Med. 2014, 119, 232–239. [Google Scholar] [CrossRef] [PubMed]
- Hoek, H.W.; Susser, E.; Buck, K.A.; Lumey, L.H.; Lin, S.P.; Gorman, J.M. Schizoid personality disorder after prenatal exposure to famine. Am. J. Psychiatry 1996, 153, 1637–1639. [Google Scholar] [CrossRef]
- de Rooij, S.R.; Wouters, H.; Yonker, J.E.; Painter, R.C.; Roseboom, T.J. Prenatal undernutrition and cognitive function in late adulthood. Proc. Natl. Acad. Sci. USA 2010, 107, 16881–16886. [Google Scholar] [CrossRef]
- de Rooij, S.R.; Caan, M.W.; Swaab, D.F.; Nederveen, A.J.; Majoie, C.B.; Schwab, M.; Painter, R.C.; Roseboom, T.J. Prenatal famine exposure has sex-specific effects on brain size. Brain J. Neurol. 2016, 139, 2136–2142. [Google Scholar] [CrossRef]
- Franke, K.; Gaser, C.; Roseboom, T.J.; Schwab, M.; de Rooij, S.R. Premature brain aging in humans exposed to maternal nutrient restriction during early gestation. NeuroImage 2018, 173, 460–471. [Google Scholar] [CrossRef]
- Wang, P.X.; Wang, J.J.; Lei, Y.X.; Xiao, L.; Luo, Z.C. Impact of fetal and infant exposure to the Chinese Great Famine on the risk of hypertension in adulthood. PLoS ONE 2012, 7, e49720. [Google Scholar] [CrossRef]
- Xu, M.Q.; Sun, W.S.; Liu, B.X.; Feng, G.Y.; Yu, L.; Yang, L.; He, G.; Sham, P.; Susser, E.; St Clair, D.; et al. Prenatal malnutrition and adult schizophrenia: Further evidence from the 1959–1961 Chinese famine. Schizophr. Bull. 2009, 35, 568–576. [Google Scholar] [CrossRef] [PubMed]
- Heijmans, B.T.; Tobi, E.W.; Stein, A.D.; Putter, H.; Blauw, G.J.; Susser, E.S.; Slagboom, P.E.; Lumey, L.H. Persistent epigenetic differences associated with prenatal exposure to famine in humans. Proc. Natl Acad Sci. USA 2008, 105, 17046–17049. [Google Scholar] [CrossRef] [PubMed]
- Tobi, E.W.; Goeman, J.J.; Monajemi, R.; Gu, H.; Putter, H.; Zhang, Y.; Slieker, R.C.; Stok, A.P.; Thijssen, P.E.; Müller, F.; et al. DNA methylation signatures link prenatal famine exposure to growth and metabolism. Nat. Commun. 2014, 5, 5592. [Google Scholar] [CrossRef] [PubMed]
- Tobi, E.W.; Slieker, R.C.; Stein, A.D.; Suchiman, H.E.; Slagboom, P.E.; van Zwet, E.W.; Heijmans, B.T.; Lumey, L.H. Early gestation as the critical time-window for changes in the prenatal environment to affect the adult human blood methylome. Int. J. Epidemiol. 2015, 44, 1211–1223. [Google Scholar] [CrossRef] [PubMed]
- Tobi, E.W.; Slieker, R.C.; Luijk, R.; Dekkers, K.F.; Stein, A.D.; Xu, K.M.; Slagboom, P.E.; van Zwet, E.W.; Lumey, L.H.; Heijmans, B.T. DNA methylation as a mediator of the association between prenatal adversity and risk factors for metabolic disease in adulthood. Sci. Adv. 2018, 4, eaao4364. [Google Scholar] [CrossRef] [PubMed]
- Shen, L.; Li, C.; Wang, Z.; Zhang, R.; Shen, Y.; Miles, T.; Wei, J.; Zou, Z. Early-life exposure to severe famine is associated with higher methylation level in the IGF2 gene and higher total cholesterol in late adulthood: The Genomic Research of the Chinese Famine (GRECF) study. Clin. Epigenetics 2019, 11, 88. [Google Scholar] [CrossRef]
- Wang, Z.; Song, J.; Li, C.; Li, Y.; Shen, L.; Dong, B.; Zou, Z.; Ma, J. DNA methylation of the INSR gene as a mediator of the association between prenatal exposure to famine and adulthood waist circumference. Sci. Rep. 2020, 10, 12212. [Google Scholar] [CrossRef]
- Moore, S.E.; Cole, T.J.; Poskitt, E.M.; Sonko, B.J.; Whitehead, R.G.; McGregor, I.A.; Prentice, A.M. Season of birth predicts mortality in rural Gambia. Nature 1997, 388, 434. [Google Scholar] [CrossRef] [PubMed]
- Waterland, R.A.; Kellermayer, R.; Laritsky, E.; Rayco-Solon, P.; Harris, R.A.; Travisano, M.; Zhang, W.; Torskaya, M.S.; Zhang, J.; Shen, L.; et al. Season of conception in rural gambia affects DNA methylation at putative human metastable epialleles. PLoS Genet. 2010, 6, e1001252. [Google Scholar] [CrossRef] [PubMed]
- Dominguez-Salas, P.; Moore, S.E.; Baker, M.S.; Bergen, A.W.; Cox, S.E.; Dyer, R.A.; Fulford, A.J.; Guan, Y.; Laritsky, E.; Silver, M.J.; et al. Maternal nutrition at conception modulates DNA methylation of human metastable epialleles. Nat. Commun. 2014, 5, 3746. [Google Scholar] [CrossRef]
- Silver, M.J.; Kessler, N.J.; Hennig, B.J.; Dominguez-Salas, P.; Laritsky, E.; Baker, M.S.; Coarfa, C.; Hernandez-Vargas, H.; Castelino, J.M.; Routledge, M.N.; et al. Independent genomewide screens identify the tumor suppressor VTRNA2-1 as a human epiallele responsive to periconceptional environment. Genome Biol. 2015, 16, 118. [Google Scholar] [CrossRef]
- Kessler, N.J.; Waterland, R.A.; Prentice, A.M.; Silver, M.J. Establishment of environmentally sensitive DNA methylation states in the very early human embryo. Sci. Adv. 2018, 4, eaat2624. [Google Scholar] [CrossRef]
- Vaiserman, A. Season-of-birth phenomenon in health and longevity: Epidemiologic evidence and mechanistic considerations. J. Dev. Orig. Health Dis. 2020, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Maasen, K.; James, P.T.; Prentice, A.M.; Moore, S.E.; Fall, C.H.; Chandak, G.R.; Betts, M.; Silver, M.J.; Buxton, J.L. Periconceptional environment predicts leukocyte telomere length in a cross-sectional study of 7–9 year old rural Gambian children. Sci. Rep. 2020, 10, 9675. [Google Scholar] [CrossRef]
- D’Mello, M.J.; Ross, S.A.; Briel, M.; Anand, S.S.; Gerstein, H.; Paré, G. Association between shortened leukocyte telomere length and cardiometabolic outcomes: Systematic review and meta-analysis. Circ. Cardiovasc. Genet. 2015, 8, 82–90. [Google Scholar] [CrossRef] [PubMed]
- Kwong, W.Y.; Wild, A.E.; Roberts, P.; Willis, A.C.; Fleming, T.P. Maternal undernutrition during the preimplantation period of rat development causes blastocyst abnormalities and programming of postnatal hypertension. Development 2000, 127, 4195–4202. [Google Scholar] [CrossRef]
- Fleming, T.P.; Watkins, A.J.; Sun, C.; Velazquez, M.A.; Smyth, N.R.; Eckert, J.J. Do little embryos make big decisions? How maternal dietary protein restriction can permanently change an embryo. Reprod. Fertil. Dev. 2015, 27, 684–692. [Google Scholar] [CrossRef] [PubMed]
- Watkins, A.J.; Ursell, E.; Panton, R.; Papenbrock, T.; Hollis, L.; Cunningham, C.; Wilkins, A.; Perry, V.H.; Sheth, B.; Kwong, W.Y.; et al. Adaptive responses by mouse early embryos to maternal diet protect fetal growth but predispose to adult onset disease. Biol. Reprod. 2008, 78, 299–306. [Google Scholar] [CrossRef] [PubMed]
- Watkins, A.J.; Lucas, E.S.; Torrens, C.; Cleal, J.K.; Green, L.; Osmond, C.; Eckert, J.J.; Gray, W.P.; Hanson, M.A.; Fleming, T.P. Maternal low-protein diet during mouse pre-implantation development induces vascular dysfunction and altered renin-angiotensin-system homeostasis in the offspring. Br. J. Nutr. 2010, 103, 1762–1770. [Google Scholar] [CrossRef] [PubMed]
- Watkins, A.J.; Lucas, E.S.; Wilkins, A.; Cagampang, F.R.; Fleming, T.P. Maternal periconceptional and gestational low protein diet affects mouse offspring growth, cardiovascular and adipose phenotype at 1 year of age. PLoS ONE 2011, 6, e28745. [Google Scholar] [CrossRef]
- Gould, J.M.; Smith, P.J.; Airey, C.J.; Mort, E.J.; Airey, L.E.; Warricker, F.D.M.; Pearson-Farr, J.E.; Weston, E.C.; Gould, P.J.W.; Semmence, O.G.; et al. Mouse maternal protein restriction during preimplantation alone permanently alters brain neuron proportion and adult short-term memory. Proc. Natl. Acad. Sci. USA 2018, 115, E7398–E7407. [Google Scholar] [CrossRef]
- Lanham, S.A.; Smith, S.J.; Watkins, A.J.; Lucas, E.S.; MacCaoilte, N.; Oreffo, R.O.C.; Fleming, T.P.; Eckert, J.J. Periconception maternal low-protein diet adversely affects male mouse fetal bone growth and mineral density quality in late gestation. J. Dev. Orig. Health Dis. 2021, 12, 384–395. [Google Scholar] [CrossRef] [PubMed]
- Lanham, S.A.; Bertram, C.; Cooper, C.; Oreffo, R.O. Animal models of maternal nutrition and altered offspring bone structure—Bone development across the lifecourse. Eur. Cell Mater. 2011, 22, 321–332, discussion 332. [Google Scholar] [CrossRef]
- Eckert, J.J.; Porter, R.; Watkins, A.J.; Burt, E.; Brooks, S.; Leese, H.J.; Humpherson, P.G.; Cameron, I.T.; Fleming, T.P. Metabolic induction and early responses of mouse blastocyst developmental programming following maternal low protein diet affecting life-long health. PLoS ONE 2012, 7, e52791. [Google Scholar] [CrossRef] [PubMed]
- Schutt, A.K.; Blesson, C.S.; Hsu, J.W.; Valdes, C.T.; Gibbons, W.E.; Jahoor, F.; Yallampalli, C. Preovulatory exposure to a protein-restricted diet disrupts amino acid kinetics and alters mitochondrial structure and function in the rat oocyte and is partially rescued by folic acid. Reprod. Biol. Endocrinol. RBE 2019, 17, 12. [Google Scholar] [CrossRef] [PubMed]
- Baltz, J.M.; Zhou, C. Cell volume regulation in mammalian oocytes and preimplantation embryos. Mol. Reprod. Dev. 2012, 79, 821–831. [Google Scholar] [CrossRef] [PubMed]
- Houghton, F.D. Media composition: Amino acids and cellular homeostasis. Methods Mol. Biol. 2012, 912, 97–106. [Google Scholar] [CrossRef] [PubMed]
- Sturmey, R.G.; Brison, D.R.; Leese, H.J. Symposium: Innovative techniques in human embryo viability assessment. Assessing embryo viability by measurement of amino acid turnover. Reprod. Biomed. Online 2008, 17, 486–496. [Google Scholar] [CrossRef]
- Brison, D.R.; Houghton, F.D.; Falconer, D.; Roberts, S.A.; Hawkhead, J.; Humpherson, P.G.; Lieberman, B.A.; Leese, H.J. Identification of viable embryos in IVF by non-invasive measurement of amino acid turnover. Hum. Reprod. 2004, 19, 2319–2324. [Google Scholar] [CrossRef] [PubMed]
- Eckert, J.J.; Houghton, F.D.; Hawkhead, J.A.; Balen, A.H.; Leese, H.J.; Picton, H.M.; Cameron, I.T.; Fleming, T.P. Human embryos developing in vitro are susceptible to impaired epithelial junction biogenesis correlating with abnormal metabolic activity. Hum. Reprod. 2007, 22, 2214–2224. [Google Scholar] [CrossRef] [PubMed]
- Kelleher, A.M.; Burns, G.W.; Behura, S.; Wu, G.; Spencer, T.E. Uterine glands impact uterine receptivity, luminal fluid homeostasis and blastocyst implantation. Sci. Rep. 2016, 6, 38078. [Google Scholar] [CrossRef] [PubMed]
- Forde, N.; Simintiras, C.A.; Sturmey, R.; Mamo, S.; Kelly, A.K.; Spencer, T.E.; Bazer, F.W.; Lonergan, P. Amino acids in the uterine luminal fluid reflects the temporal changes in transporter expression in the endometrium and conceptus during early pregnancy in cattle. PLoS ONE 2014, 9, e100010. [Google Scholar] [CrossRef]
- Yang, Y.; Wang, L.; Chen, C.; Qi, H.; Baker, P.N.; Liu, X.; Zhang, H.; Han, T.L. Metabolic Changes of Maternal Uterine Fluid, Uterus, and Plasma during the Peri-implantation Period of Early Pregnancy in Mice. Reprod. Sci. 2020, 27, 488–502. [Google Scholar] [CrossRef] [PubMed]
- Petrie, L.; Duthie, S.J.; Rees, W.D.; McConnell, J.M. Serum concentrations of homocysteine are elevated during early pregnancy in rodent models of fetal programming. Br. J. Nutr. 2002, 88, 471–477. [Google Scholar] [CrossRef] [PubMed]
- Takahara, T.; Amemiya, Y.; Sugiyama, R.; Maki, M.; Shibata, H. Amino acid-dependent control of mTORC1 signaling: A variety of regulatory modes. J. Biomed. Sci. 2020, 27, 87. [Google Scholar] [CrossRef]
- Martin, P.M.; Sutherland, A.E.; Van Winkle, L.J. Amino acid transport regulates blastocyst implantation. Biol Reprod. 2003, 69, 1101–1108. [Google Scholar] [CrossRef]
- Van Winkle, L.J.; Tesch, J.K.; Shah, A.; Campione, A.L. System B0,+ amino acid transport regulates the penetration stage of blastocyst implantation with possible long-term developmental consequences through adulthood. Hum. Reprod. Update 2006, 12, 145–157. [Google Scholar] [CrossRef]
- Meyuhas, O. Ribosomal Protein S6 Phosphorylation: Four Decades of Research. Int. Rev. Cell Mol. Biol. 2015, 320, 41–73. [Google Scholar] [CrossRef] [PubMed]
- Chauvin, C.; Koka, V.; Nouschi, A.; Mieulet, V.; Hoareau-Aveilla, C.; Dreazen, A.; Cagnard, N.; Carpentier, W.; Kiss, T.; Meyuhas, O.; et al. Ribosomal protein S6 kinase activity controls the ribosome biogenesis transcriptional program. Oncogene 2014, 33, 474–483. [Google Scholar] [CrossRef]
- Velazquez, M.A.; Sheth, B.; Smith, S.J.; Eckert, J.J.; Osmond, C.; Fleming, T.P. Insulin and branched-chain amino acid depletion during mouse preimplantation embryo culture programmes body weight gain and raised blood pressure during early postnatal life. Biochim. Biophys. Acta Mol. Basis Dis. 2018, 1864, 590–600. [Google Scholar] [CrossRef]
- Sun, C.; Velazquez, M.A.; Marfy-Smith, S.; Sheth, B.; Cox, A.; Johnston, D.A.; Smyth, N.; Fleming, T.P. Mouse early extra-embryonic lineages activate compensatory endocytosis in response to poor maternal nutrition. Development 2014, 141, 1140–1150. [Google Scholar] [CrossRef]
- Bora, P.; Thamodaran, V.; Šušor, A.; Bruce, A.W. p38-Mitogen Activated Kinases Mediate a Developmental Regulatory Response to Amino Acid Depletion and Associated Oxidative Stress in Mouse Blastocyst Embryos. Front. Cell Dev. Biol. 2019, 7, 276. [Google Scholar] [CrossRef]
- Caetano, L.; Eckert, J.; Johnston, D.; Chatelet, D.; Tumbarello, D.; Smyth, N.; Ingamells, S.; Price, A.; Fleming, T. Blastocyst trophectoderm endocytic activation, a marker of adverse developmental programming. Reproduction 2021. [Google Scholar] [CrossRef]
- Rees, W.D. Manipulating the sulfur amino acid content of the early diet and its implications for long-term health. Proc. Nutr. Soc. 2002, 61, 71–77. [Google Scholar] [CrossRef] [PubMed]
- Steegers-Theunissen, R.P.; Twigt, J.; Pestinger, V.; Sinclair, K.D. The periconceptional period, reproduction and long-term health of offspring: The importance of one-carbon metabolism. Hum. Reprod. Update 2013, 19, 640–655. [Google Scholar] [CrossRef] [PubMed]
- Van Winkle, L.J.; Ryznar, R. One-Carbon Metabolism Regulates Embryonic Stem Cell Fate Through Epigenetic DNA and Histone Modifications: Implications for Transgenerational Metabolic Disorders in Adults. Front. Cell Dev. Biol. 2019, 7, 300. [Google Scholar] [CrossRef] [PubMed]
- Van Winkle, L.J.; Galat, V.; Iannaccone, P.M. Lysine Deprivation during Maternal Consumption of Low-Protein Diets Could Adversely Affect Early Embryo Development and Health in Adulthood. Int. J. Environ. Res. Public Health 2020, 17, 5462. [Google Scholar] [CrossRef]
- Mitchell, M.; Schulz, S.L.; Armstrong, D.T.; Lane, M. Metabolic and mitochondrial dysfunction in early mouse embryos following maternal dietary protein intervention. Biol. Reprod. 2009, 80, 622–630. [Google Scholar] [CrossRef]
- Chiaratti, M.R.; Malik, S.; Diot, A.; Rapa, E.; Macleod, L.; Morten, K.; Vatish, M.; Boyd, R.; Poulton, J. Is Placental Mitochondrial Function a Regulator that Matches Fetal and Placental Growth to Maternal Nutrient Intake in the Mouse? PLoS ONE 2015, 10, e0130631. [Google Scholar] [CrossRef]
- Casas-Terradellas, E.; Tato, I.; Bartrons, R.; Ventura, F.; Rosa, J.L. ERK and p38 pathways regulate amino acid signalling. Biochim. Biophys. Acta 2008, 1783, 2241–2254. [Google Scholar] [CrossRef]
- Rezatabar, S.; Karimian, A.; Rameshknia, V.; Parsian, H.; Majidinia, M.; Kopi, T.A.; Bishayee, A.; Sadeghinia, A.; Yousefi, M.; Monirialamdari, M.; et al. RAS/MAPK signaling functions in oxidative stress, DNA damage response and cancer progression. J. Cell. Physiol. 2019, 234, 14951–14965. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.J.; Pan, W.W.; Liu, S.B.; Shen, Z.F.; Xu, Y.; Hu, L.L. ERK/MAPK signalling pathway and tumorigenesis. Exp. Ther. Med. 2020, 19, 1997–2007. [Google Scholar] [CrossRef] [PubMed]
- Frum, T.; Halbisen, M.A.; Wang, C.; Amiri, H.; Robson, P.; Ralston, A. Oct4 cell-autonomously promotes primitive endoderm development in the mouse blastocyst. Dev. Cell 2013, 25, 610–622. [Google Scholar] [CrossRef] [PubMed]
- Meng, Y.; Moore, R.; Tao, W.; Smith, E.R.; Tse, J.D.; Caslini, C.; Xu, X.X. GATA6 phosphorylation by Erk1/2 propels exit from pluripotency and commitment to primitive endoderm. Dev. Biol. 2018, 436, 55–65. [Google Scholar] [CrossRef] [PubMed]
- Thamodaran, V.; Bruce, A.W. p38 (Mapk14/11) occupies a regulatory node governing entry into primitive endoderm differentiation during preimplantation mouse embryo development. Open Biol. 2016, 6, 160190. [Google Scholar] [CrossRef]
- Bedzhov, I.; Graham, S.J.; Leung, C.Y.; Zernicka-Goetz, M. Developmental plasticity, cell fate specification and morphogenesis in the early mouse embryo. Philos. Trans. R Soc. Lond. B Biol. Sci. 2014, 369, 20130538. [Google Scholar] [CrossRef]
- Gasperowicz, M.; Natale, D.R. Establishing three blastocyst lineages—Then what? Biol. Reprod. 2011, 84, 621–630. [Google Scholar] [CrossRef] [PubMed]
- Beckman, D.A.; Lloyd, J.B.; Brent, R.L. Quantitative studies on the mechanisms of amino acid supply to rat embryos during organogenesis. Reprod. Toxicol. 1998, 12, 197–200. [Google Scholar] [CrossRef]
- González, I.M.; Martin, P.M.; Burdsal, C.; Sloan, J.L.; Mager, S.; Harris, T.; Sutherland, A.E. Leucine and arginine regulate trophoblast motility through mTOR-dependent and independent pathways in the preimplantation mouse embryo. Dev. Biol. 2012, 361, 286–300. [Google Scholar] [CrossRef]
- Assemat, E.; Vinot, S.; Gofflot, F.; Linsel-Nitschke, P.; Illien, F.; Chatelet, F.; Verroust, P.; Louvet-Vallee, S.; Rinninger, F.; Kozyraki, R. Expression and role of cubilin in the internalization of nutrients during the peri-implantation development of the rodent embryo. Biol. Reprod. 2005, 72, 1079–1086. [Google Scholar] [CrossRef]
- Fleming, T.P.; Pickering, S.J. Maturation and polarization of the endocytotic system in outside blastomeres during mouse preimplantation development. J. Embryol. Exp. Morphol. 1985, 89, 175–208. [Google Scholar]
- Kelleher, A.M.; DeMayo, F.J.; Spencer, T.E. Uterine Glands: Developmental Biology and Functional Roles in Pregnancy. Endocr. Rev. 2019, 40, 1424–1445. [Google Scholar] [CrossRef] [PubMed]
- Puertollano, R.; Ferguson, S.M.; Brugarolas, J.; Ballabio, A. The complex relationship between TFEB transcription factor phosphorylation and subcellular localization. EMBO J. 2018, 37, e98804. [Google Scholar] [CrossRef]
- Drizyte-Miller, K.; Chen, J.; Cao, H.; Schott, M.B.; McNiven, M.A. The small GTPase Rab32 resides on lysosomes to regulate mTORC1 signaling. J. Cell Sci. 2020, 133, jcs236661. [Google Scholar] [CrossRef]
- Watkins, A.J.; Lucas, E.S.; Marfy-Smith, S.; Bates, N.; Kimber, S.J.; Fleming, T.P. Maternal nutrition modifies trophoblast giant cell phenotype and fetal growth in mice. Reproduction 2015, 149, 563–575. [Google Scholar] [CrossRef]
- Coan, P.M.; Vaughan, O.R.; Sekita, Y.; Finn, S.L.; Burton, G.J.; Constancia, M.; Fowden, A.L. Adaptations in placental phenotype support fetal growth during undernutrition of pregnant mice. J. Physiol. 2010, 588, 527–538. [Google Scholar] [CrossRef] [PubMed]
- Sun, C.; Denisenko, O.; Sheth, B.; Cox, A.; Lucas, E.S.; Smyth, N.R.; Fleming, T.P. Epigenetic regulation of histone modifications and Gata6 gene expression induced by maternal diet in mouse embryoid bodies in a model of developmental programming. BMC Dev. Biol. 2015, 15, 3. [Google Scholar] [CrossRef] [PubMed]
- Law, P.P.; Holland, M.L. Deciphering the Role of the Non-Coding Genome in Regulating Gene-Diet Interactions. Nutrients 2018, 10, 1831. [Google Scholar] [CrossRef] [PubMed]
- Gensous, N.; Ravaioli, F.; Pirazzini, C.; Gramignoli, R.; Ellis, E.; Storci, G.; Capri, M.; Strom, S.; Laconi, E.; Franceschi, C.; et al. Aging and Caloric Restriction Modulate the DNA Methylation Profile of the Ribosomal RNA Locus in Human and Rat Liver. Nutrients 2020, 12, 277. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, Y.; Tsuneoka, M. Control of Ribosomal RNA Transcription by Nutrients. In Gene Expression and Regulation in Mammalian Cells—Transcription Toward the Establishment of Novel Therapeutics; Uchiumi, F., Ed.; BoD: Norderstedt, Germany, 2018; pp. 25–51. [Google Scholar]
- McStay, B.; Grummt, I. The epigenetics of rRNA genes: From molecular to chromosome biology. Annu. Rev. Cell Dev. Biol. 2008, 24, 131–157. [Google Scholar] [CrossRef]
- Denisenko, O.; Lucas, E.S.; Sun, C.; Watkins, A.J.; Mar, D.; Bomsztyk, K.; Fleming, T.P. Regulation of ribosomal RNA expression across the lifespan is fine-tuned by maternal diet before implantation. Biochim. Biophys. Acta 2016, 1859, 906–913. [Google Scholar] [CrossRef] [PubMed]
- Torreira, E.; Louro, J.A.; Pazos, I.; González-Polo, N.; Gil-Carton, D.; Duran, A.G.; Tosi, S.; Gallego, O.; Calvo, O.; Fernández-Tornero, C. The dynamic assembly of distinct RNA polymerase I complexes modulates rDNA transcription. Elife 2017, 6, e20832. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Tornero, C. RNA polymerase I activation and hibernation: Unique mechanisms for unique genes. Transcription 2018, 9, 248–254. [Google Scholar] [CrossRef]
- Holland, M.L.; Lowe, R.; Caton, P.W.; Gemma, C.; Carbajosa, G.; Danson, A.F.; Carpenter, A.A.; Loche, E.; Ozanne, S.E.; Rakyan, V.K. Early-life nutrition modulates the epigenetic state of specific rDNA genetic variants in mice. Science 2016, 353, 495–498. [Google Scholar] [CrossRef]
- Hales, C.N.; Barker, D.J. The thrifty phenotype hypothesis. Br. Med. Bull. 2001, 60, 5–20. [Google Scholar] [CrossRef]
- Vaag, A.A.; Grunnet, L.G.; Arora, G.P.; Brøns, C. The thrifty phenotype hypothesis revisited. Diabetologia 2012, 55, 2085–2088. [Google Scholar] [CrossRef]
- Neel, J.V. Diabetes mellitus: A “thrifty” genotype rendered detrimental by “progress”? Am. J. Hum. Genet. 1962, 14, 353–362. [Google Scholar]
- Gosling, A.L.; Buckley, H.R.; Matisoo-Smith, E.; Merriman, T.R. Pacific Populations, Metabolic Disease and ‘Just-So Stories’: A Critique of the ‘Thrifty Genotype’ Hypothesis in Oceania. Ann. Hum. Genet. 2015, 79, 470–480. [Google Scholar] [CrossRef] [PubMed]
- Mayer, C.; Zhao, J.; Yuan, X.; Grummt, I. mTOR-dependent activation of the transcription factor TIF-IA links rRNA synthesis to nutrient availability. Genes Dev. 2004, 18, 423–434. [Google Scholar] [CrossRef] [PubMed]
- Venniyoor, A. PTEN: A Thrifty Gene That Causes Disease in Times of Plenty? Front. Nutr. 2020, 7, 81. [Google Scholar] [CrossRef] [PubMed]
- Dong, J.; Shin, N.; Chen, S.; Lei, J.; Burd, I.; Wang, X. Is there a definite relationship between placental mTOR signaling and fetal growth? Biol. Reprod. 2020, 103, 471–486. [Google Scholar] [CrossRef]
- Rosario, F.J.; Powell, T.L.; Gupta, M.B.; Cox, L.; Jansson, T. mTORC1 Transcriptional Regulation of Ribosome Subunits, Protein Synthesis, and Molecular Transport in Primary Human Trophoblast Cells. Front. Cell Dev. Biol. 2020, 8, 583801. [Google Scholar] [CrossRef]
- Laribee, R.N. Transcriptional and Epigenetic Regulation by the Mechanistic Target of Rapamycin Complex 1 Pathway. J. Mol. Biol. 2018, 430, 4874–4890. [Google Scholar] [CrossRef] [PubMed]
- Wadhwa, P.D.; Buss, C.; Entringer, S.; Swanson, J.M. Developmental origins of health and disease: Brief history of the approach and current focus on epigenetic mechanisms. Semin. Reprod. Med. 2009, 27, 358–368. [Google Scholar] [CrossRef] [PubMed]
- Gürke, J.; Hirche, F.; Thieme, R.; Haucke, E.; Schindler, M.; Stangl, G.I.; Fischer, B.; Navarrete Santos, A. Maternal Diabetes Leads to Adaptation in Embryonic Amino Acid Metabolism during Early Pregnancy. PLoS ONE 2015, 10, e0127465. [Google Scholar] [CrossRef]
- Kermack, A.J.; Finn-Sell, S.; Cheong, Y.C.; Brook, N.; Eckert, J.J.; Macklon, N.S.; Houghton, F.D. Amino acid composition of human uterine fluid: Association with age, lifestyle and gynaecological pathology. Hum. Reprod. 2015, 30, 917–924. [Google Scholar] [CrossRef] [PubMed]
- Kermack, A.J.; Lowen, P.; Wellstead, S.J.; Fisk, H.L.; Montag, M.; Cheong, Y.; Osmond, C.; Houghton, F.D.; Calder, P.C.; Macklon, N.S. Effect of a 6-week "Mediterranean" dietary intervention on in vitro human embryo development: The Preconception Dietary Supplements in Assisted Reproduction double-blinded randomized controlled trial. Fertil. Steril. 2020, 113, 260–269. [Google Scholar] [CrossRef]
- House, J.S.; Mendez, M.; Maguire, R.L.; Gonzalez-Nahm, S.; Huang, Z.; Daniels, J.; Murphy, S.K.; Fuemmeler, B.F.; Wright, F.A.; Hoyo, C. Periconceptional Maternal Mediterranean Diet Is Associated With Favorable Offspring Behaviors and Altered CpG Methylation of Imprinted Genes. Front. Cell Dev. Biol. 2018, 6, 107. [Google Scholar] [CrossRef] [PubMed]
- Broughton, D.E.; Moley, K.H. Obesity and female infertility: Potential mediators of obesity’s impact. Fertil. Steril. 2017, 107, 840–847. [Google Scholar] [CrossRef]
- Robker, R.L.; Akison, L.K.; Bennett, B.D.; Thrupp, P.N.; Chura, L.R.; Russell, D.L.; Lane, M.; Norman, R.J. Obese women exhibit differences in ovarian metabolites, hormones, and gene expression compared with moderate-weight women. J. Clin. Endocrinol. Metab. 2009, 94, 1533–1540. [Google Scholar] [CrossRef] [PubMed]
- Igosheva, N.; Abramov, A.Y.; Poston, L.; Eckert, J.J.; Fleming, T.P.; Duchen, M.R.; McConnell, J. Maternal Diet-Induced Obesity Alters Mitochondrial Activity and Redox Status in Mouse Oocytes and Zygotes. PLoS ONE 2010, 5, e10074. [Google Scholar] [CrossRef]
- Leary, C.; Leese, H.J.; Sturmey, R.G. Human embryos from overweight and obese women display phenotypic and metabolic abnormalities. Hum. Reprod. 2015, 30, 122–132. [Google Scholar] [CrossRef] [PubMed]
- Kelly, A.C.; Powell, T.L.; Jansson, T. Placental function in maternal obesity. Clin. Sci. 2020, 134, 961–984. [Google Scholar] [CrossRef] [PubMed]
- Baker, B.C.; Hayes, D.J.; Jones, R.L. Effects of micronutrients on placental function: Evidence from clinical studies to animal models. Reproduction 2018, 156, R69–R82. [Google Scholar] [CrossRef]
- Schröder-Heurich, B.; Springer, C.J.P.; von Versen-Höynck, F. Vitamin D Effects on the Immune System from Periconception through Pregnancy. Nutrients 2020, 12, 1432. [Google Scholar] [CrossRef]
- Sinclair, K.D.; Allegrucci, C.; Singh, R.; Gardner, D.S.; Sebastian, S.; Bispham, J.; Thurston, A.; Huntley, J.F.; Rees, W.D.; Maloney, C.A.; et al. DNA methylation, insulin resistance, and blood pressure in offspring determined by maternal periconceptional B vitamin and methionine status. Proc. Natl. Acad. Sci. USA 2007, 104, 19351–19356. [Google Scholar] [CrossRef]
- Maloney, C.A.; Hay, S.M.; Young, L.E.; Sinclair, K.D.; Rees, W.D. A methyl-deficient diet fed to rat dams during the peri-conception period programs glucose homeostasis in adult male but not female offspring. J. Nutr. 2011, 141, 95–100. [Google Scholar] [CrossRef]
- Clare, C.E.; Brassington, A.H.; Kwong, W.Y.; Sinclair, K.D. One-Carbon Metabolism: Linking Nutritional Biochemistry to Epigenetic Programming of Long-Term Development. Annu. Rev. Anim. Biosci. 2019, 7, 263–287. [Google Scholar] [CrossRef]
- Kawakubo-Yasukochi, T.; Morioka, M.; Ohe, K.; Yasukochi, A.; Ozaki, Y.; Hazekawa, M.; Nishinakagawa, T.; Ono, K.; Nakamura, S.; Nakashima, M. Maternal folic acid depletion during early pregnancy increases sensitivity to squamous tumor formation in the offspring in mice. J. Dev. Orig. Health Dis. 2019, 10, 683–691. [Google Scholar] [CrossRef]
- Penailillo, R.S.; Eckert, J.J.; Burton, M.A.; Burdge, G.C.; Fleming, T.P.; Lillycrop, K.A. High maternal folic acid intake around conception alters mouse blastocyst lineage allocation and expression of key developmental regulatory genes. Mol. Reprod. Dev. 2021, 88, 261–273. [Google Scholar] [CrossRef] [PubMed]
- Vecoli, C.; Pulignani, S.; Andreassi, M.G. Genetic and Epigenetic Mechanisms Linking Air Pollution and Congenital Heart Disease. J. Cardiovasc. Dev. Dis. 2016, 3, 32. [Google Scholar] [CrossRef] [PubMed]
- Green, M.P.; Harvey, A.J.; Finger, B.J.; Tarulli, G.A. Endocrine disrupting chemicals: Impacts on human fertility and fecundity during the peri-conception period. Environ. Res. 2021, 194, 110694. [Google Scholar] [CrossRef] [PubMed]
- Heindel, J.J.; Blumberg, B. Environmental Obesogens: Mechanisms and Controversies. Annu. Rev. Pharmacol. Toxicol. 2019, 59, 89–106. [Google Scholar] [CrossRef]
- Howard, S.G. Developmental Exposure to Endocrine Disrupting Chemicals and Type 1 Diabetes Mellitus. Front. Endocrinol. 2018, 9, 513. [Google Scholar] [CrossRef]
- Kleijkers, S.H.M.; Mantikou, E.; Slappendel, E.; Consten, D.; van Echten-Arends, J.; Wetzels, A.M.; van Wely, M.; Smits, L.J.M.; van Montfoort, A.P.A.; Repping, S.; et al. Influence of embryo culture medium (G5 and HTF) on pregnancy and perinatal outcome after IVF: A multicenter RCT. Hum. Reprod. 2016, 31, 2219–2230. [Google Scholar] [CrossRef]
- Ceelen, M.; van Weissenbruch, M.M.; Vermeiden, J.P.; van Leeuwen, F.E.; Delemarre-van de Waal, H.A. Cardiometabolic differences in children born after in vitro fertilization: Follow-up study. J. Clin. Endocrinol. Metab. 2008, 93, 1682–1688. [Google Scholar] [CrossRef]
- Gkourogianni, A.; Kosteria, I.; Telonis, A.G.; Margeli, A.; Mantzou, E.; Konsta, M.; Loutradis, D.; Mastorakos, G.; Papassotiriou, I.; Klapa, M.I.; et al. Plasma metabolomic profiling suggests early indications for predisposition to latent insulin resistance in children conceived by ICSI. PLoS ONE 2014, 9, e94001. [Google Scholar] [CrossRef]
- Valenzuela-Alcaraz, B.; Serafini, A.; Sepulveda-Martinez, A.; Casals, G.; Rodriguez-Lopez, M.; Garcia-Otero, L.; Cruz-Lemini, M.; Bijnens, B.; Sitges, M.; Balasch, J.; et al. Postnatal persistence of fetal cardiovascular remodelling associated with assisted reproductive technologies: A cohort study. BJOG 2018, 126, 291–298. [Google Scholar] [CrossRef]
- Chen, W.; Peng, Y.; Ma, X.; Kong, S.; Tan, S.; Wei, Y.; Zhao, Y.; Zhang, W.; Wang, Y.; Yan, L.; et al. Integrated multi-omics reveal epigenomic disturbance of assisted reproductive technologies in human offspring. EBioMedicine 2020, 61, 103076. [Google Scholar] [CrossRef]
- Novakovic, B.; Lewis, S.; Halliday, J.; Kennedy, J.; Burgner, D.P.; Czajko, A.; Kim, B.; Sexton-Oates, A.; Juonala, M.; Hammarberg, K.; et al. Assisted reproductive technologies are associated with limited epigenetic variation at birth that largely resolves by adulthood. Nat. Commun. 2019, 10, 3922. [Google Scholar] [CrossRef] [PubMed]
- Watkins, A.J.; Platt, D.; Papenbrock, T.; Wilkins, A.; Eckert, J.J.; Kwong, W.Y.; Osmond, C.; Hanson, M.; Fleming, T.P. Mouse embryo culture induces changes in postnatal phenotype including raised systolic blood pressure. Proc. Natl. Acad. Sci. USA 2007, 104, 5449–5454. [Google Scholar] [CrossRef] [PubMed]
- Rexhaj, E.; Paoloni-Giacobino, A.; Rimoldi, S.F.; Fuster, D.G.; Anderegg, M.; Somm, E.; Bouillet, E.; Allemann, Y.; Sartori, C.; Scherrer, U. Mice generated by in vitro fertilization exhibit vascular dysfunction and shortened life span. J. Clin. Investig. 2013, 123, 5052–5060. [Google Scholar] [CrossRef] [PubMed]
- De Vos, A.; Santos-Ribeiro, S.; Van Landuyt, L.; Van de Velde, H.; Tournaye, H.; Verheyen, G. Birthweight of singletons born after cleavage-stage or blastocyst transfer in fresh and warming cycles. Hum. Reprod. 2018, 33, 196–201. [Google Scholar] [CrossRef]
- Shi, W.; Zhang, W.; Li, N.; Xue, X.; Liu, C.; Qu, P.; Shi, J.; Huang, C. Comparison of perinatal outcomes following blastocyst and cleavage-stage embryo transfer: Analysis of 10 years’ data from a single centre. Reprod. Biomed. Online 2019, 38, 967–978. [Google Scholar] [CrossRef]
- Aljahdali, A.; Airina, R.; Velazquez, M.A.; Sheth, B.; Wallen, K.; Osmond, C.; Watkins, A.J.; Eckert, J.J.; Smyth, N.R.; Fleming, T.P. The duration of embryo culture after mouse IVF differentially affects cardiovascular and metabolic health in male offspring. Hum. Reprod. 2020, 35, 2497–2514. [Google Scholar] [CrossRef]
- Hu, R.; Li, Y.; Yang, Y.; Liu, M. Mass spectrometry-based strategies for single-cell metabolomics. Mass Spectrom. Rev. 2021. [Google Scholar] [CrossRef]
- Yan, Z.; An, J.; Peng, Y.; Kong, S.; Liu, Q.; Yang, M.; He, Q.; Song, S.; Chen, Y.; Chen, W.; et al. DevOmics: An integrated multi-omics database of human and mouse early embryo. Brief. Bioinform. 2021, bbab208. [Google Scholar] [CrossRef]
- Rabaglino, M.B.; O’Doherty, A.; Bojsen-Møller Secher, J.; Lonergan, P.; Hyttel, P.; Fair, T.; Kadarmideen, H.N. Application of multi-omics data integration and machine learning approaches to identify epigenetic and transcriptomic differences between in vitro and in vivo produced bovine embryos. PLoS ONE 2021, 16, e0252096. [Google Scholar] [CrossRef]
- Kleijkers, S.H.; Eijssen, L.M.; Coonen, E.; Derhaag, J.G.; Mantikou, E.; Jonker, M.J.; Mastenbroek, S.; Repping, S.; Evers, J.L.; Dumoulin, J.C.; et al. Differences in gene expression profiles between human preimplantation embryos cultured in two different IVF culture media. Hum. Reprod. 2015, 30, 2303–2311. [Google Scholar] [CrossRef]
- Krisher, R.L.; Heuberger, A.L.; Paczkowski, M.; Stevens, J.; Pospisil, C.; Prather, R.S.; Sturmey, R.G.; Herrick, J.R.; Schoolcraft, W.B. Applying metabolomic analyses to the practice of embryology: Physiology, development and assisted reproductive technology. Reprod. Fertil. Dev. 2015, 27, 602–620. [Google Scholar] [CrossRef]
- Ntostis, P.; Kokkali, G.; Iles, D.; Huntriss, J.; Tzetis, M.; Picton, H.; Pantos, K.; Miller, D. Can trophectoderm RNA analysis predict human blastocyst competency? Syst. Biol. Reprod. Med. 2019, 65, 312–325. [Google Scholar] [CrossRef]
- Meistermann, D.; Bruneau, A.; Loubersac, S.; Reignier, A.; Firmin, J.; François-Campion, V.; Kilens, S.; Lelièvre, Y.; Lammers, J.; Feyeux, M.; et al. Integrated pseudotime analysis of human pre-implantation embryo single-cell transcriptomes reveals the dynamics of lineage specification. Cell Stem Cell 2021, 28, 1625–1640.e6. [Google Scholar] [CrossRef]
- Khurana, P.; Smyth, N.R.; Sheth, B.; Velazquez, M.A.; Eckert, J.J.; Fleming, T.P. Advanced maternal age perturbs mouse embryo development and alters the phenotype of derived embryonic stem cells. J. Dev. Orig. Health Dis. 2021, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Simbulan, R.K.; Di Santo, M.; Liu, X.; Lin, W.; Donjacour, A.; Maltepe, E.; Shenoy, A.; Borini, A.; Rinaudo, P. Embryonic stem cells derived from in vivo or in vitro-generated murine blastocysts display similar transcriptome and differentiation potential. PLoS ONE 2015, 10, e0117422. [Google Scholar] [CrossRef] [PubMed]
- Stephenson, J.; Heslehurst, N.; Hall, J.; Schoenaker, D.; Hutchinson, J.; Cade, J.E.; Poston, L.; Barrett, G.; Crozier, S.R.; Barker, M.; et al. Before the beginning: Nutrition and lifestyle in the preconception period and its importance for future health. Lancet 2018, 391, 1830–1841. [Google Scholar] [CrossRef]
- Fall, C.H.D.; Kumaran, K. Metabolic programming in early life in humans. Philos. Trans. R Soc. Lond. B Biol. Sci. 2019, 374, 20180123. [Google Scholar] [CrossRef] [PubMed]
- Thayer, Z.M.; Rutherford, J.; Kuzawa, C.W. The Maternal Nutritional Buffering Model: An evolutionary framework for pregnancy nutritional intervention. Evol. Med. Public Health 2020, 2020, 14–27. [Google Scholar] [CrossRef]
- Jacob, C.M.; Killeen, S.L.; McAuliffe, F.M.; Stephenson, J.; Hod, M.; Diaz Yamal, I.; Malhotra, J.; Mocanu, E.; McIntyre, H.D.; Kihara, A.B.; et al. Prevention of noncommunicable diseases by interventions in the preconception period: A FIGO position paper for action by healthcare practitioners. Int. J. Gynaecol. Obstet. 2020, 151 (Suppl. 1), 6–15. [Google Scholar] [CrossRef]
- Krebs, N.F.; Hambidge, K.M.; Westcott, J.L.; Garcés, A.L.; Figueroa, L.; Tsefu, A.K.; Lokangaka, A.L.; Goudar, S.S.; Dhaded, S.M.; Saleem, S.; et al. Growth from Birth Through Six Months for Infants of Mothers in the “Women First” Preconception Maternal Nutrition Trial. J. Pediatrics 2021, 229, 199–206.e194. [Google Scholar] [CrossRef]
- Salavati, N.; Bakker, M.K.; Lewis, F.; Vinke, P.C.; Mubarik, F.; Erwich, J.H.M.; van der Beek, E.M. Associations between preconception macronutrient intake and birth weight across strata of maternal BMI. PLoS ONE 2020, 15, e0243200. [Google Scholar] [CrossRef] [PubMed]
- Saffari, A.; Shrestha, S.; Issarapu, P.; Sajjadi, S.; Betts, M.; Sahariah, S.A.; Tomar, A.S.; James, P.; Dedaniya, A.; Yadav, D.K.; et al. Effect of maternal preconceptional and pregnancy micronutrient interventions on children’s DNA methylation: Findings from the EMPHASIS study. Am. J. Clin. Nutr. 2020, 112, 1099–1113. [Google Scholar] [CrossRef] [PubMed]
- Barker, M.; Dombrowski, S.U.; Colbourn, T.; Fall, C.H.D.; Kriznik, N.M.; Lawrence, W.T.; Norris, S.A.; Ngaiza, G.; Patel, D.; Skordis-Worrall, J.; et al. Intervention strategies to improve nutrition and health behaviours before conception. Lancet 2018, 391, 1853–1864. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fleming, T.P.; Sun, C.; Denisenko, O.; Caetano, L.; Aljahdali, A.; Gould, J.M.; Khurana, P. Environmental Exposures around Conception: Developmental Pathways Leading to Lifetime Disease Risk. Int. J. Environ. Res. Public Health 2021, 18, 9380. https://doi.org/10.3390/ijerph18179380
Fleming TP, Sun C, Denisenko O, Caetano L, Aljahdali A, Gould JM, Khurana P. Environmental Exposures around Conception: Developmental Pathways Leading to Lifetime Disease Risk. International Journal of Environmental Research and Public Health. 2021; 18(17):9380. https://doi.org/10.3390/ijerph18179380
Chicago/Turabian StyleFleming, Tom P., Congshan Sun, Oleg Denisenko, Laura Caetano, Anan Aljahdali, Joanna M. Gould, and Pooja Khurana. 2021. "Environmental Exposures around Conception: Developmental Pathways Leading to Lifetime Disease Risk" International Journal of Environmental Research and Public Health 18, no. 17: 9380. https://doi.org/10.3390/ijerph18179380
APA StyleFleming, T. P., Sun, C., Denisenko, O., Caetano, L., Aljahdali, A., Gould, J. M., & Khurana, P. (2021). Environmental Exposures around Conception: Developmental Pathways Leading to Lifetime Disease Risk. International Journal of Environmental Research and Public Health, 18(17), 9380. https://doi.org/10.3390/ijerph18179380




