Anchoring the CerEla1.0 Genome Assembly to Red Deer (Cervus elaphus) and Cattle (Bos taurus) Chromosomes and Specification of Evolutionary Chromosome Rearrangements in Cervidae
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Samples and Karyotype Analysis
2.2. Chromosome Orthology and Breakpoint Site Prediction
2.3. FISH Probes
2.4. FISH
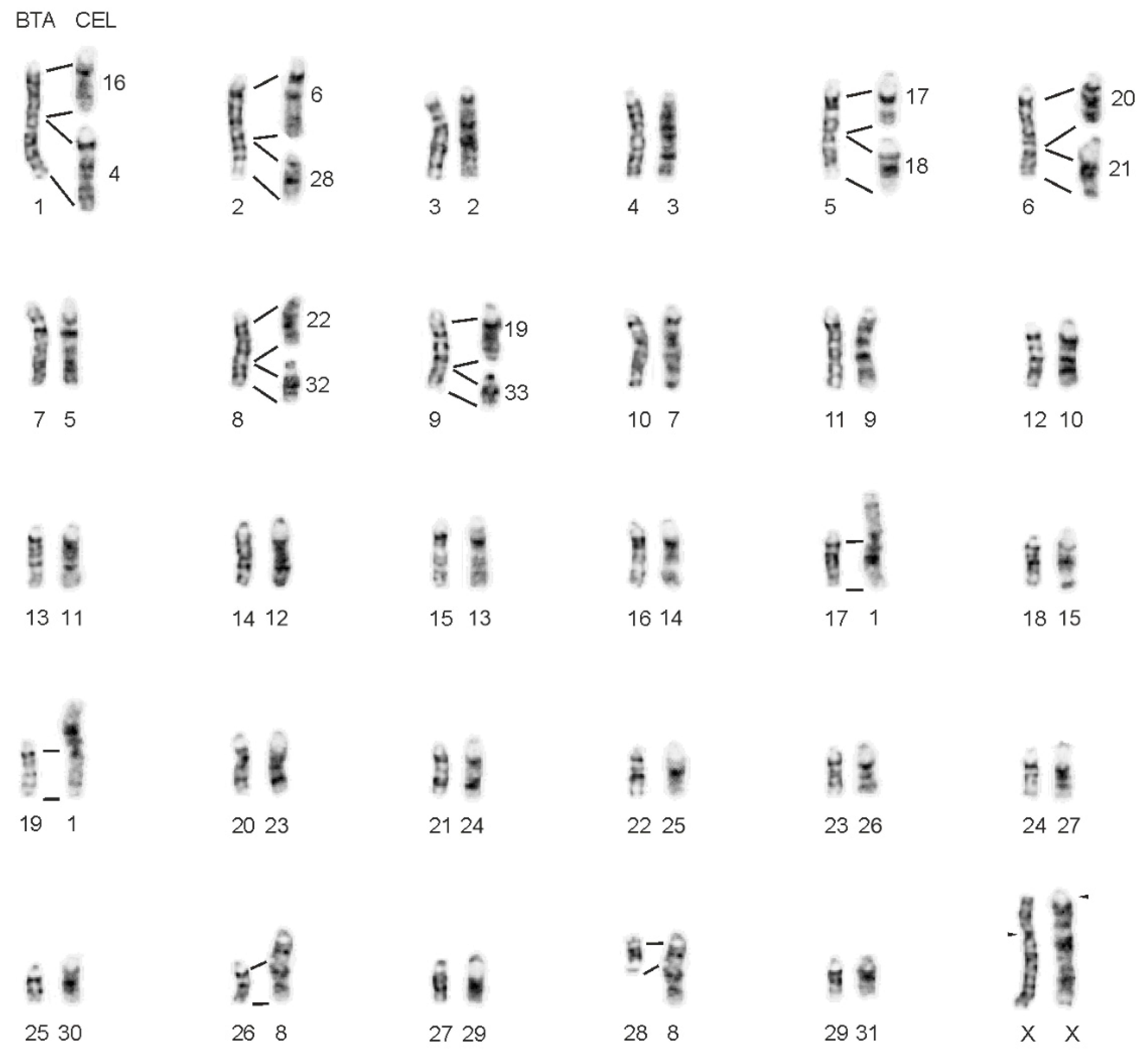
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wilson, D.E.; Reeder, D.M. Mammal Species of the World: A Taxonomic and Geographic Reference; Johns Hopkins University Press: Baltimore, MD, USA, 2005; ISBN 978-0-8018-8221-0. [Google Scholar]
- Wurster, D.H.; Benirschke, K. Indian Muntjac, Muntiacus Muntjak: A Deer with a Low Diploid Chromosome Number. Science 1970, 168, 1364–1366. [Google Scholar] [CrossRef]
- Nietzel, H. Chromosome Evolution of Cervidae: Karyotypic and Molecular Aspects. In Cytogenetics: Basic and Applied Aspects; Obe, G., Basler, A., Eds.; Springer: Berlin/Heidelberg, Germany, 1987; ISBN 978-3-642-72804-4. [Google Scholar]
- Fontana, F.; Rubini, M. Chromosomal Evolution in Cervidae. BioSystems 1990, 24, 157–174. [Google Scholar] [CrossRef]
- Huang, L.; Chi, J.; Nie, W.; Wang, J.; Yang, F. Phylogenomics of Several Deer Species Revealed by Comparative Chromosome Painting with Chinese Muntjac Paints. Genetica 2006, 127, 25–33. [Google Scholar] [CrossRef] [PubMed]
- Rubini, M.; Negri, E.; Fontana, F. Standard Karyotype and Chromosomal Evolution of the Fallow Deer (Dama dama L.). Cytobios 1990, 64, 155–161. [Google Scholar] [PubMed]
- Bonnet-Garnier, A.; Claro, F.; Thévenon, S.; Gautier, M.; Hayes, H. Identification by R-Banding and FISH of Chromosome Arms Involved in Robertsonian Translocations in Several Deer Species. Chromosome Res. 2003, 11, 649–663. [Google Scholar] [CrossRef] [PubMed]
- Duarte, J.M.B.; Jorge, W. Morphologic and Cytogenetic Description of the Small Red Brocket (Mazama Bororo Duarte, 1996) in Brazil. Mammalia 2009, 67, 403–410. [Google Scholar] [CrossRef]
- Yang, F.; O’Brien, P.C.; Wienberg, J.; Ferguson-Smith, M.A. A Reappraisal of the Tandem Fusion Theory of Karyotype Evolution in Indian Muntjac Using Chromosome Painting. Chromosome Res. 1997, 5, 109–117. [Google Scholar] [CrossRef]
- Chi, J.; Fu, B.; Nie, W.; Wang, J.; Graphodatsky, A.S.; Yang, F. New Insights into the Karyotypic Relationships of Chinese Muntjac (Muntiacus Reevesi), Forest Musk Deer (Moschus berezovskii) and Gayal (Bos frontalis). Cytogenet. Genome Res. 2005, 108, 310–316. [Google Scholar] [CrossRef] [PubMed]
- Chi, J.X.; Huang, L.; Nie, W.; Wang, J.; Su, B.; Yang, F. Defining the Orientation of the Tandem Fusions That Occurred during the Evolution of Indian Muntjac Chromosomes by BAC Mapping. Chromosoma 2005, 114, 167–172. [Google Scholar] [CrossRef] [PubMed]
- Dementyeva, P.V.; Trifonov, V.A.; Kulemzina, A.I.; Graphodatsky, A.S. Reconstruction of the Putative Cervidae Ancestral Karyotype by Chromosome Painting of Siberian Roe Deer (Capreolus pygargus) with Dromedary Probes. Cytogenet. Genome Res. 2010, 128, 228–235. [Google Scholar] [CrossRef] [PubMed]
- Frohlich, J.; Kubickova, S.; Musilova, P.; Cernohorska, H.; Muskova, H.; Vodicka, R.; Rubes, J. Karyotype Relationships among Selected Deer Species and Cattle Revealed by Bovine FISH Probes. PLoS ONE 2017, 12, e0187559. [Google Scholar] [CrossRef] [Green Version]
- Bonnet, A.; Thévenon, S.; Claro, F.; Gautier, M.; Hayes, H. Cytogenetic Comparison between Vietnamese Sika Deer and Cattle: R-Banded Karyotypes and FISH Mapping. Chromosome Res. 2001, 9, 673–687. [Google Scholar] [CrossRef]
- De Lorenzi, L.; Planas, J.; Rossi, E.; Malagutti, L.; Parma, P. New Cryptic Karyotypic Differences between Cattle (Bos Taurus) and Goat (Capra hircus). Chromosome Res. 2015, 23, 225–235. [Google Scholar] [CrossRef]
- Bana, N.Á.; Nyiri, A.; Nagy, J.; Frank, K.; Nagy, T.; Stéger, V.; Schiller, M.; Lakatos, P.; Sugár, L.; Horn, P.; et al. The Red Deer Cervus Elaphus Genome CerEla1.0: Sequencing, Annotating, Genes, and Chromosomes. Mol. Genet. Genom. 2018, 293, 665–684. [Google Scholar] [CrossRef]
- Slate, J.; Van Stijn, T.C.; Anderson, R.M.; McEwan, K.M.; Maqbool, N.J.; Mathias, H.C.; Bixley, M.J.; Stevens, D.R.; Molenaar, A.J.; Beever, J.E.; et al. A Deer (Subfamily Cervinae) Genetic Linkage Map and the Evolution of Ruminant Genomes. Genetics 2002, 160, 1587–1597. [Google Scholar] [CrossRef]
- Alkan, C.; Sajjadian, S.; Eichler, E.E. Limitations of Next-Generation Genome Sequence Assembly. Nat. Methods 2011, 8, 61–65. [Google Scholar] [CrossRef] [Green Version]
- Ariyadasa, R.; Stein, N. Advances in BAC-Based Physical Mapping and Map Integration Strategies in Plants. J. Biomed. Biotechnol. 2012, 2012, 184854. [Google Scholar] [CrossRef] [Green Version]
- Damas, J.; O’Connor, R.; Farré, M.; Lenis, V.P.E.; Martell, H.J.; Mandawala, A.; Fowler, K.; Joseph, S.; Swain, M.T.; Griffin, D.K.; et al. Upgrading Short-Read Animal Genome Assemblies to Chromosome Level Using Comparative Genomics and a Universal Probe Set. Genome Res. 2017, 27, 875–884. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lewin, H.A.; Graves, J.A.M.; Ryder, O.A.; Graphodatsky, A.S.; O’Brien, S.J. Precision Nomenclature for the New Genomics. Gigascience 2019, 8, giz086. [Google Scholar] [CrossRef]
- Groves, C.; Grubb, P. Ungulate Taxonomy, 1st ed.; Johns Hopkins University Press: Baltimore, MD, USA, 2011; ISBN 978-1-4214-0093-8. [Google Scholar]
- Cernohorska, H.; Kubickova, S.; Vahala, J.; Robinson, T.J.; Rubes, J. Cytotypes of Kirk’s Dik-Dik (Madoqua Kirkii, Bovidae) Show Multiple Tandem Fusions. Cytogenet. Genome Res. 2011, 132, 255–263. [Google Scholar] [CrossRef] [PubMed]
- Cernohorska, H.; Kubickova, S.; Vahala, J.; Rubes, J. Molecular Insights into X;BTA5 Chromosome Rearrangements in the Tribe Antilopini (Bovidae). Cytogenet. Genome Res. 2012, 136, 188–198. [Google Scholar] [CrossRef]
- Seabright, M. A Rapid Banding Technique for Human Chromosomes. Lancet 1971, 2, 971–972. [Google Scholar] [CrossRef]
- O’Brien, S.J.; Graphodatsky, A.S.; Perelman, P.L. (Eds.) Atlas of Mammalian Chromosomes, 2nd ed.; Wiley-Blackwell: Hoboken, NJ, USA, 2020; ISBN 978-1-119-41803-0. [Google Scholar]
- Vozdova, M.; Kubickova, S.; Cernohorska, H.; Fröhlich, J.; Vodicka, R.; Rubes, J. Comparative Study of the Bush Dog (Speothos Venaticus) Karyotype and Analysis of Satellite DNA Sequences and Their Chromosome Distribution in Six Species of Canidae. Cytogenet. Genome Res. 2019, 159, 88–96. [Google Scholar] [CrossRef] [PubMed]
- Proskuryakova, A.A.; Kulemzina, A.I.; Perelman, P.L.; Makunin, A.I.; Larkin, D.M.; Farré, M.; Kukekova, A.V.; Lynn Johnson, J.; Lemskaya, N.A.; Beklemisheva, V.R.; et al. X Chromosome Evolution in Cetartiodactyla. Genes 2017, 8, 216. [Google Scholar] [CrossRef] [Green Version]
- Partipilo, G.; D’Addabbo, P.; Lacalandra, G.M.; Liu, G.E.; Rocchi, M. Refinement of Bos Taurus Sequence Assembly Based on BAC-FISH Experiments. BMC Genom. 2011, 12, 639. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lorenzi, L.D.; Parma, P. Identification of Some Errors in the Genome Assembly of Bovidae by FISH. CGR 2020, 160, 85–93. [Google Scholar] [CrossRef] [PubMed]
- BAC Resource Consortium, T.; Cheung, V.G.; Nowak, N.; Jang, W.; Kirsch, I.R.; Zhao, S.; Chen, X.-N.; Furey, T.S.; Kim, U.-J.; Kuo, W.-L.; et al. Integration of Cytogenetic Landmarks into the Draft Sequence of the Human Genome. Nature 2001, 409, 953–958. [Google Scholar] [CrossRef] [Green Version]
- Herzog, S. The Karyotype of the Red Deer (Cervus elaphus L.). Caryologia 1987, 40, 299–305. [Google Scholar] [CrossRef] [Green Version]
- Huang, L.; Chi, J.; Wang, J.; Nie, W.; Su, W.; Yang, F. High-Density Comparative BAC Mapping in the Black Muntjac (Muntiacus Crinifrons): Molecular Cytogenetic Dissection of the Origin of MCR 1p+4 in the X1X2Y1Y2Y3 Sex Chromosome System. Genomics 2006, 87, 608–615. [Google Scholar] [CrossRef] [Green Version]
- Cernohorska, H.; Kubickova, S.; Kopecna, O.; Kulemzina, A.I.; Perelman, P.L.; Elder, F.F.B.; Robinson, T.J.; Graphodatsky, A.S.; Rubes, J. Molecular Cytogenetic Insights to the Phylogenetic Affinities of the Giraffe (Giraffa Camelopardalis) and Pronghorn (Antilocapra Americana). Chromosome Res. 2013, 21, 447–460. [Google Scholar] [CrossRef]
- Cernohorska, H.; Kubickova, S.; Kopecna, O.; Vozdova, M.; Matthee, C.A.; Robinson, T.J.; Rubes, J. Nanger, Eudorcas, Gazella, and Antilope Form a Well-Supported Chromosomal Clade within Antilopini (Bovidae, Cetartiodactyla). Chromosoma 2014, 124, 235–247. [Google Scholar] [CrossRef] [PubMed]
- Kiazim, L.G.; O’Connor, R.E.; Larkin, D.M.; Romanov, M.N.; Narushin, V.G.; Brazhnik, E.A.; Griffin, D.K. Comparative Mapping of the Macrochromosomes of Eight Avian Species Provides Further Insight into Their Phylogenetic Relationships and Avian Karyotype Evolution. Cells 2021, 10, 362. [Google Scholar] [CrossRef] [PubMed]
- Gallagher, D.S.; Davis, S.K.; De Donato, M.; Burzlaff, J.D.; Womack, J.E.; Taylor, J.F.; Kumamoto, A.T. A Molecular Cytogenetic Analysis of the Tribe Bovini (Artiodactyla: Bovidae: Bovinae) with an Emphasis on Sex Shromosome Morphology and NOR Distribution. Chromosome Res. 1999, 7, 481–492. [Google Scholar] [CrossRef]
- Rubes, J.; Musilova, P.; Kopecna, O.; Kubickova, S.; Cernohorska, H.; Kulemsina, A.I. Comparative Molecular Cytogenetics in Cetartiodactyla. Cytogenet. Genome Res. 2012, 137, 194–207. [Google Scholar] [CrossRef] [PubMed]
- Gallagher, D.S., Jr.; Womack, J.E. Chromosome Conservation in the Bovidae. J. Hered. 1992, 83, 287–298. [Google Scholar] [CrossRef] [PubMed]
- Abril, V.V.; Duarte, J.M.B. Chromosome Polymorphism in the Brazilian Dwarf Brocket Deer, Mazama Nana (Mammalia, Cervidae). Genet. Mol. Biol. 2008, 31, 53–57. [Google Scholar] [CrossRef] [Green Version]
- Abril, V.V.; Carnelossi, E.A.G.; González, S.; Duarte, J.M.B. Elucidating the Evolution of the Red Brocket Deer Mazama Americana Complex (Artiodactyla; Cervidae). Cytogenet. Genome Res. 2010, 128, 177–187. [Google Scholar] [CrossRef]
- Duarte, J.M.B.; González, S. Neotropical Cervidology: Biology and Medicine of Latin American Deer; Funep: Jaboticabal, Brazil; IUCN: Gland, Switzerland, 2010; ISBN 978-85-7805-046-7. [Google Scholar]





| Species | Latin Name | Abbrev. | 2n | FNa | Bia | X | Fused BTA Orthologues |
|---|---|---|---|---|---|---|---|
| Red deer | Cervus elaphus | CEL | 68 | 68 | 2 | A | 17/19 |
| White-lipped deer | Cervus albirostris | CAL | 66 | 68 | 4 | A | 17/19, 25/6prox |
| Rusa deer | Rusa timorensis | RTI | 60 | 68 | 10 | A | 17/19, 5prox/22, 2dist/7, 5dist/8prox, 5prox/22, 18/3 |
| Eld’s deer | Rucervus eldii | REL | 58 | 68 | 12 | A | 17/19, 2dist/7, 5dist/8prox, 5prox/10, 18/1prox, 22/1dist |
| Roe deer | Capreolus capreolus | CCA | 70 | 68 | 0 | B | |
| Reindeer | Rangifer tarandus | RTA | 70 | 70 | 2 | B | |
| Moose | Alces alces | AAL | 68 | 70 | 4 | B | 29/17 |
| White-tailed deer | Odocoileus virginianus | OVI | 70 | 70 | 2 | B |
| Red Deer (CerEla1.0) | CEL Chr | Cattle (ARS-UCD1.2) | Comments * | ||||
|---|---|---|---|---|---|---|---|
| Pseudochr | INSDC | Size (Mb) | BTA Chr | RefSeq | Size (Mb) | ||
| 1 | CM0080008.1 | 104.5 | 13 | 15 | NC_037342.1 | 85.01 | |
| 2 | CM0080009.1 | 63.26 | 31 | 29 | NC_037356.1 | 51.1 | Reverse |
| 3 | CM0080010.1 | 88.46 | 17 | 5prox (1–70 Mb) | NC_037332.1 | 120.09 | 1–55 Mb of BTA5 |
| 4 | CM0080011.1 | 81.2 | 15 | 18 | NC_037345.1 | 65.82 | |
| 5 | CM0080012.1 | 178.03 | 1 | 17/19 | NC_037344.1 | 73.17 | |
| NC_037346.1 | 63.45 | ||||||
| 6 | CM0080013.1 | 73.11 | 21 | 6dist (64–118 Mb) | NC_037333.1 | 117.81 | Reverse, 70–118 Mb of BTA6 |
| 7 | CM0080014.1 | 66.84 | 26 | 23 | NC_037350.1 | 52.5 | |
| 8 | CM0080015.1 | 55.92 | 28 | 2dist (94–136 Mb) | NC_037329.1 | 136.23 | Reverse, 80–136 Mb of BTA2 |
| 9 | CM0080016.1 | 141.95 | 5 | 7 | NC_037334.1 | 110.68 | |
| 10 | CM0080017.1 | 55.94 | 30 | 25 | NC_037352.1 | 42.35 | |
| 11 | CM0080018.1 | 140.39 | 9 | 11 | NC_037338.1 | 106.98 | Reverse |
| 12 | CM0080019.1 | 127.78 | 7 | 10 | NC_037337.1 | 103.31 | Reverse |
| 13 | CM0080020.1 | 89.79 | 24 | 21 | NC_037348.1 | 69.86 | |
| 14 | CM0080021.1 | 103.59 | 14 | 16 | NC_037343.1 | 81.01 | |
| 15 | CM0080022.1 | 125.28 | 8 | 28/26 | NC_037355.1 | 45.94 | |
| NC_037353.1 | 51.99 | ||||||
| 16 | CM0080023.1 | 62.95 | 32 | 8dist (64–112 Mb) | NC_037335.1 | 113.32 | Reverse, 69–112 Mb of BTA8 |
| 17 | CM0080024.1 | 79.72 | 20 | 6prox (1–63 Mb) | NC_037333.1 | 117.81 | 1–66 Mb of BTA6 |
| 18 | CM0080025.1 | 152.66 | 3 | 4 | NC_037331.1 | 120 | |
| 19 | CM0080026.1 | 127.24 | 4 | 1dist (59–158 Mb) | NC_037328.1 | 158.53 | Rearranged, 57–158 Mb of BTA1 |
| 20 | CM0080027.1 | 149.34 | 2 | 3 | NC_037330.1 | 121.01 | |
| 21 | CM0080028.1 | 107.36 | 12 | 14 | NC_037341.1 | 82.4 | |
| 22 | CM0080029.1 | 63.92 | 18 | 5dist (71–121 Mb) | NC_037332.1 | 120.09 | Reverse, 60–121 Mb of BTA5 |
| 23 | CM0080030.1 | 109.47 | 11 | 13 | NC_037340.1 | 83.47 | |
| 24 | CM0080031.1 | 78.16 | 25 | 22 | NC_037349.1 | 60.77 | |
| 25 | CM0080032.1 | 96.54 | 23 | 20 | NC_037347.1 | 71.97 | |
| 26 | CM0080033.1 | 55.1 | 33 | 9dist (64–106 Mb) | NC_037336.1 | 105.45 | 65–106 Mb of BTA9 |
| 27 | CM0080034.1 | 84.64 | 27 | 24 | NC_037351.1 | 62.32 | |
| 28 | CM0080035.1 | 82.07 | 19 | 9prox (1–62 Mb) | NC_037336.1 | 105.45 | 1–64 Mb of BTA9 |
| 29 | CM0080036.1 | 80.17 | 22 | 8prox (1–63 Mb) | NC_037335.1 | 113.32 | 1–64 Mb of BTA8 |
| 30 | CM0080037.1 | 117.8 | 10 | 12 | NC_037339.1 | 87.22 | |
| 31 | CM0080038.1 | 75.46 | 16 | 1prox (1–58 Mb) | NC_037328.1 | 158.53 | 1–51 Mb of BTA1 |
| 32 | CM0080039.1 | 60.01 | 29 | 27 | NC_037354.1 | 45.61 | |
| 33 | CM0080040.1 | 121.43 | 6 | 2prox (1–92 Mb) | NC_037329.1 | 136.23 | 1–71 Mb of BTA2 |
| X | CM008041.1 | 181.54 | X | NC_037357.1 | 139.01 | ||
| Y | CM008042.1 | 4.03 | - | - | - | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vozdova, M.; Kubickova, S.; Cernohorska, H.; Fröhlich, J.; Rubes, J. Anchoring the CerEla1.0 Genome Assembly to Red Deer (Cervus elaphus) and Cattle (Bos taurus) Chromosomes and Specification of Evolutionary Chromosome Rearrangements in Cervidae. Animals 2021, 11, 2614. https://doi.org/10.3390/ani11092614
Vozdova M, Kubickova S, Cernohorska H, Fröhlich J, Rubes J. Anchoring the CerEla1.0 Genome Assembly to Red Deer (Cervus elaphus) and Cattle (Bos taurus) Chromosomes and Specification of Evolutionary Chromosome Rearrangements in Cervidae. Animals. 2021; 11(9):2614. https://doi.org/10.3390/ani11092614
Chicago/Turabian StyleVozdova, Miluse, Svatava Kubickova, Halina Cernohorska, Jan Fröhlich, and Jiri Rubes. 2021. "Anchoring the CerEla1.0 Genome Assembly to Red Deer (Cervus elaphus) and Cattle (Bos taurus) Chromosomes and Specification of Evolutionary Chromosome Rearrangements in Cervidae" Animals 11, no. 9: 2614. https://doi.org/10.3390/ani11092614






