Isolation, Characterization and Identification of a New Lysinibacillus fusiformis Strain ZC from Metlaoui Phosphate Laundries Wastewater: Bio-Treatment Assays
Abstract
:1. Introduction
2. Material and Methods
2.1. Study Site and Sampling
2.2. Physicochemical Characterization of Phosphate Laundries Wastewaters
2.3. Isolation of Bacteria from Phosphate Laundries Wastewaters
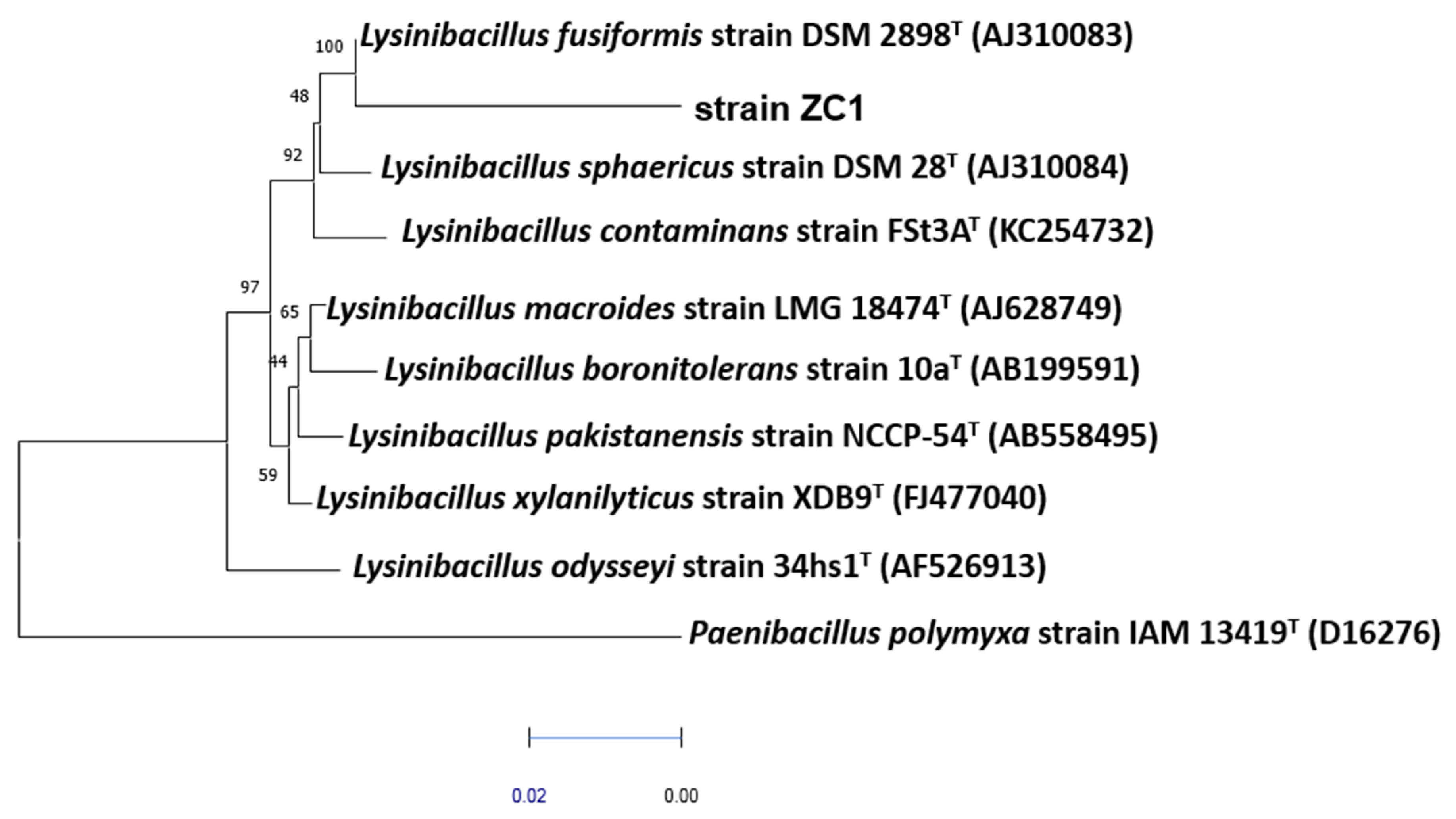
2.4. DNA Extraction Protocol, Bacterial Identification Using 16S rDNA Sequencing and Phylogenetic Analysis
2.5. Nucleotide Sequence Accession Number
2.6. Characterization of the Bacterial ZC Strain
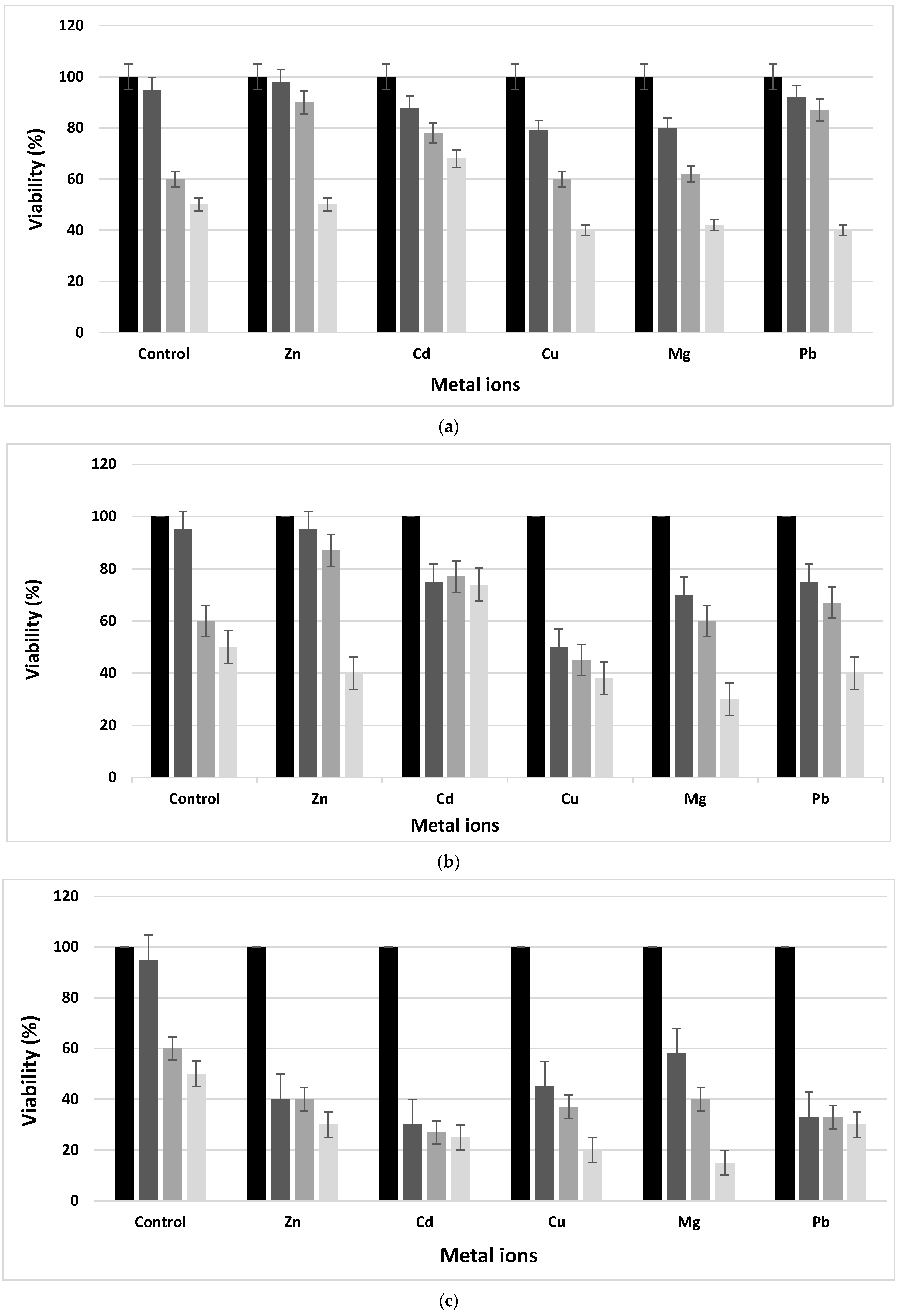
2.6.1. MTT Colorimetric Assay
2.6.2. Enzymatic Profile of Strain ZC
2.6.3. Determination of Heavy Metal Content Using Atomic Absorption Spectrometry (AAS) Technique in the Absence and in the Presence of ZC Strain
2.6.4. Biofilm Formation Assays by L. fusiformis
Phenotypic (Qualitative) Characterization of Slime-Producing Bacteria
Characterization of Semi-Quantitative Slime-Producing Bacteria
2.6.5. Quantitative Adherence Assays
2.6.6. Cell Surface Hydrophobicity
2.6.7. Antibiotic Susceptibility Testing
2.7. Bio-Treatment Assays of Phosphate Laundries Wastewaters
2.8. Statistical Analysis
3. Result and Discussion
3.1. Physicochemical Characteristics of Phosphates Laundries Wastewater
3.2. Phylogenetic Identification of Strain ZC
3.3. MTT Assay
3.4. Determination of Heavy Metal Content Using Atomic Absorption Spectrometry (AAS) Technique
3.5. Enzymatic Changes
3.6. Qualitative Slime Production
3.7. Quantitative Adhesion and Culture Cells Adherence Assays
3.8. Cell Surface Hydrophobicity
3.9. Antibiotic Sensitivity of Bacteria
3.10. Bio-Treatment Assays of Phosphate Laundries Wastewater Using Lysinibacillus fusiformis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mekki, A.; Awali, A.; Aloui, F.; Loukil, S.; Sayadi, S. Characterization and toxicity assessment of wastewater from rock phosphate processing in Tunisia. Mine Water Environ. 2017, 36, 502–507. [Google Scholar] [CrossRef]
- Tijani, A.; Fakhfakh, H. Les projets sociaux sont-ils des leviers de création de la valeur? J. Bus. Econ. 2011, 1, 94–105. [Google Scholar]
- Galfati, I.; Bilal, E.; Abdallah, H.; Beji Sassi, A. Geochemistry of solid effluents and phosphate ore washed from Métlaoui-Gafsa basin, Tunisia. Rom. J. Miner. Deposits. 2014, 87, 83–86. [Google Scholar]
- Mekki, A.; Sayadi, S. Study of heavy metal accumulation and residual toxicity in soil saturated with phosphate processing wastewater. Water Air Soil Pollut. 2017, 228, 215–224. [Google Scholar] [CrossRef] [Green Version]
- Srarfi, F.; Rachdi, R.; Bol, R.; Gocke, M.I.; Brahim, N.; SlimShimi, N. Stream sediments geochemistry and the influence of flood phosphate mud in mining area, Metlaoui, Western south of Tunisia. Environ. Earth. Sci. 2019, 78, 211–223. [Google Scholar] [CrossRef]
- Moula, A.; Borgi, M.A.; Loukil, S.; Chaieb, M.; Mekki, A. Assessment of phosphate laundries waste water phytotoxicity and biotreatment assays. Clean Soil Air Water 2020, 48, 1–8. [Google Scholar] [CrossRef]
- Chen, H.; Teng, Y.; Lu, S.; Wang, Y.; Wang, J. Contamination features and health risk of soil heavy metals in China. Sci. Total Environ. 2015, 512, 143–153. [Google Scholar] [CrossRef]
- François, F.; Lombard, C.; Guigner, J.-M.; Soreau, P.; Brian-Jaisson, F.; Martino, G.; Vandervennet, M.; Garcia, D.; Molinier, A.-L.; Pignol, D.; et al. Isolation and characterization of environmental bacteria capable of extracellular biosorption of mercury. Appl. Environ. Microbiol. 2012, 78, 1097–1106. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Anyanwu, C.U.; Nwankwo, S.C.; Moneke, A.N. Soil bacterial response to introduced metal stress. Int. J. Basic Appl. Sci. 2011, 11, 109–115. [Google Scholar]
- Gall, J.E.; Boyd, R.S.; Rajakaruna, N. Transfer of heavy metals through terrestrial food webs: A review. Environ. Monit. Assess. 2015, 187, 201–221. [Google Scholar] [CrossRef] [Green Version]
- Choi, A.L.; Sun, G.; Zhang, Y.; Grandjean, P. Developmental fluoride neurotoxicity: A systematic review and meta-analysis. Environ. Health Perspect. 2012, 120, 1362–1368. [Google Scholar] [CrossRef] [Green Version]
- Siddiquee, S.; Rovina, K.; Al Azad, S.; Naher, L.; Suryani, S.; Chaikaew, P. Heavy metal contaminants removal from wastewater using the potential filamentous fungi biomass: A review. J. Microb. Biochem. Technol. 2015, 7, 384–393. [Google Scholar] [CrossRef]
- Srivastava, N.K.; Majumder, C.B. Novel biofiltration methods for the treatment of heavy metals from industrial wastewater. J. Hazard. Mater. 2008, 151, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Alloway, B.J. Heavy Metals and Metalloids as Micronutrient for Plants and Animals; Springer: Dordrecht, The Netherlands, 2013; pp. 195–209. [Google Scholar]
- Lakherwal, D. Adsorption of heavy metals: A review. Int. J. Environ. Res. Dev. 2014, 4, 41–48. [Google Scholar]
- Igiri, B.E.; Okoduwa, S.I.R.; Idoko, G.O.; Akabuogu, E.P.; Adeyi, A.O.; Ejiogu, I.K. Toxicity and bioremediation of heavy metals contaminated ecosystem from tannery wastewater: A review. J. Toxicol. 2018, 2018, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Lemire, J.; Mailloux, R.; Auger, C.; Whalen, D.; Appanna, V.D. Pseudomonas fluorescens orchestrates a fine metabolic-balancing act to counter aluminium toxicity. Environ. Microbiol. 2010, 12, 1384–1390. [Google Scholar]
- Ben Younes, S.; Ellouze, M.; Sayadi, S. A comparative study of an industrial effluent treatment using enzymatic and alkaline adapted consortium assays. J. Chem. Technol. Biotechnol. 2013, 88, 563–571. [Google Scholar] [CrossRef]
- Jiang, H.; Dong, H.; Zhang, G.; Yu, B.; Chapman, L.R.; Fields, M.W. Microbial diversity in water and sediment of Lake Chaka, an athalasso haline lake in northwestern China. Appl. Environ. Microbiol. 2006, 72, 3832–3845. [Google Scholar] [CrossRef] [Green Version]
- Kouidhi, B.; Zmantar, T.; Bakhrouf, A. Anti-cariogenic and anti-biofilms activity of Tunisian propolis extract and its potential protective effect against cancer cells proliferation. Anaerobe 2010, 16, 566–571. [Google Scholar] [CrossRef]
- Ben Kahla-Nakbi, A.; Besbes, A.; Chaieb, K.; Rouabhia, M.; Bakhrouf, A. Investigation of several virulence properties among Vibrio alginolyticus strains isolated from diseased cultured fish in Tunisia. Dis. Aquat. Org. 2009, 86, 21–28. [Google Scholar] [CrossRef] [Green Version]
- Ben Younes, S.; Dallali, C.; Ellafi, A.; Bouslama, L.; Feriani, A.; Sayadi, S. Extracellular enzymatic activities of bacterial strains isolated from Tunisian biotopes: Decolorization and detoxification of indigo carmine. Cata. Lett. 2020, 151, 1248–1261. [Google Scholar] [CrossRef]
- Marzan, L.W.; Hossain, M.; Akter Mina, S.; Akter, Y.; Masudul Azad Chowdhury, A.M. Isolation and biochemical characterization of heavy-metal resistant bacteria from tannery effluent in Chittagong city, Bangladesh: Bioremediation viewpoint. Egy. J. Aquat. Res. 2017, 43, 65–74. [Google Scholar] [CrossRef]
- Freeman, D.J.; Falkiner, F.R.; Keane, C.T. New method for detecting slime production by coagulase negative staphylococci. J. Clin. Pathol. 1989, 42, 872–874. [Google Scholar] [CrossRef] [Green Version]
- Ellafi, A.; Denden, I.; Ben Abdallah, F.; Souissi, I.; Bakhrouf, A. Survival and adhesion ability of Shigella spp. strains after their incubation in seawater microcosms. World J. Microbiol. Biotechnol. 2009, 25, 1161–1168. [Google Scholar] [CrossRef]
- Ben Abdallah, F.; Chaieb, K.; Zmantar, T.; Kallel, H.; Bakhrouf, A. Adherence assays and slime production of vibrio alginolyticus and vibrio parahaemolyticus. Braz. J. Microbiol. 2009, 40, 394–398. [Google Scholar] [CrossRef] [PubMed]
- Chaieb, K.; Chehab, O.; Zmantar, T.; Rouabhia, M.; Mahdouani, K.; Bakhrouf, A. In vitro effect of pH and ethanol on biofilm formation by clinical ica-positive Staphylococcus epidermidis strains. Ann. Microbiol. 2007, 57, 431–437. [Google Scholar] [CrossRef]
- Chatti, A.; Daghfous, D.; Landoulsi, A. Effect of seqA mutation on Salmonella typhimurium virulence. J. Infect. 2007, 54, 241–245. [Google Scholar] [CrossRef] [PubMed]
- Van Loosdrecht, M.C.; Lyklema, J.; Norde, W.; Schraa, G.; Zehnder, A.J.B. The role of bacterial cell wall hydrophobicity in adhesion. Appl. Environ. Microbiol. 1987, 531, 1893–1897. [Google Scholar] [CrossRef] [Green Version]
- Jardine, J.; Mavumengwana, V.; Ubomba-Jaswa, E. Antibiotic resistance and heavy metal tolerance in cultured bacteria from hot springs as indicators of environmental intrinsic resistance and tolerance levels. Environ. Pollut. 2019, 249, 696–702. [Google Scholar] [CrossRef]
- El Rhaouat, O.; El Kherrati, I.; El khayyat, F.; Chiguer, H.; Ezziani, K.; Ibeda, A.; Fareh, M.; Saidi, Y.; El Kharim, K.; Belghyti, D. Physic-chemical evaluation of urban wastewater of the town of Sidi Kacem. Comput. Water Energy Environ. Eng. 2014, 3, 30–35. [Google Scholar] [CrossRef] [Green Version]
- Domingues, V.S.; de Souza Monteiro, A.; Júlio, A.D.L.; Queiroz, A.L.L.; dos Santos, V.L. Diversity of metal-resistant and tensoactive-producing culturable heterotrophic bacteria isolated from a copper mine in Brazilian Amazonia. Sci. Rep. 2020, 10, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Ünaldi Coral, M.N.; Korkmaz, H.; Arikan, B.; Coral, G. Plasmid mediated heavy metal resistances in Enterobacter spp. isolated from Sofulu landfill, in Adana, Turkey. Ann. Microbiol. 2005, 55, 175–179. [Google Scholar]
- Ministère des Affaires Locales et de l’Environnement, Official Printing Office of the Republic of Tunisia. Décret gouvernemental n° 2018-315 du 26 mars 2018, ANNEXE 1, Rejet dans le domaine public maritime, hydraulique et réseau public d’assainissement. J. Off. R. Tunis. 2018, 26, 824–827. (In French) [Google Scholar]
- APHA (American Public Health Association). Standard Methods for the Examination of Water and Wastewater, 21st ed.; American Public Health Association: Washington, DC, USA, 2005. [Google Scholar]
- Jarvis, I.; Burnett, W.C.; Nathan, Y.; Almbaydin, F.S.M.; Attia, A.K.M.; Castro, L.N.; Flicoteaux, R.; Hilmy, M.E.; Husain, V.; Qutawnah, A.A.; et al. Phosphorite geochemistry: State of-the-art and environmental concerns. Eclogae Geol. Helv. 1994, 87, 643–700. [Google Scholar]
- Jukes, T.H.; Cantor, C.R. Evolution of proteins molecules. In Mammalian Protein Metabolism; Munro, H.N., Ed.; Academic Press: New York, NY, USA, 1969; Volume 3, pp. 21–132. [Google Scholar]
- Kumar, S.; Stecher, G.; Li, M.; Knyaz, C.; Tamura, K. MEGA X: Molecular evolutionary genetics analysis across computing platforms. Mol. Biol. Evol. 2018, 35, 1547–1549. [Google Scholar] [CrossRef]
- Ashraf, M.A.; Maah, M.J.; Yusoff, I. Morphology, geology and water quality assessment of former tin mining catchment. Sci. World J. 2012, 1–15. [Google Scholar] [CrossRef] [Green Version]
- Gadd, G.M. Biosorption: Critical review of scientific rationale, environmental importance and significance for pollution treatment. J. Chem. Technol. Biotechnol. 2009, 84, 13–28. [Google Scholar] [CrossRef]
- Kalaimurugan, D.; Balamuralikrishnan, B.; Durairaj, K.; Vasudhevan, P.; Shivakumar, M.S.; Kaul, T.; Chang, S.W.; Ravindran, B.; Venkatesan, S. Isolation and characterization of heavy-metal-resistant bacteria and their applications in environmental bioremediation. Int. J. Environ. Sci. Technol. 2020, 17, 1455–1462. [Google Scholar] [CrossRef]
- Chellaiah, E.R. Cadmium (heavy metals) bioremediation by Pseudomonasaeruginosa: A minireview. Appl. Water Sci. 2018, 8, 154–163. [Google Scholar] [CrossRef] [Green Version]
- Xu, Y.; Xu, J.; Mao, D.; Luo, Y. Effect of the selective pressure of sub-lethal level of heavy metals on the fate and distribution of ARGs in the catchment scale. Environ. Pollut. 2016, 220, 900–908. [Google Scholar] [CrossRef]
- Mohamed, Z.A. Removal of cadmium and manganese by a non-toxic strain of the fresh water cyanobacterium Gloeothece magna. Water Res. 2001, 35, 4405–4409. [Google Scholar] [CrossRef]
- Outten, C.E.; Outten, F.W.; O’Halloran, T.V. DNA distortion mechanism for transcriptional activation by ZntR, a Zn(II)-responsive MerR Homologue in Escherichia coli. J. Biol. Chem. 1999, 274, 37517–37524. [Google Scholar] [CrossRef] [Green Version]
- Nies, D.H. Microbial heavy-metal resistance. Appl. Microbiol. Biotechnol. 1999, 51, 730–750. [Google Scholar] [CrossRef]
- Haferburg, G.; Kothe, E. Microbes and metals: Interactions in the environment. J. Basic Microbiol. 2007, 47, 453–467. [Google Scholar] [CrossRef]
- Nanda, M.; Kumar, V.; Sharma, D.K. Multimetal tolerance mechanisms in bacteria: The resistance strategies acquired by bacteria that can be exploited to ‘clean-up’ heavy metal contaminants from water. Aquat. Toxicol. 2019, 212, 1–10. [Google Scholar] [CrossRef]
- Chaturvedi, K.S.; Hung, C.S.; Crowley, J.R.; Stapleton, A.E.; Henderson, J.P. The siderophore yersiniabactin binds copper to protect pathogens during infection. Nat. Chem. Biol. 2012, 8, 731–736. [Google Scholar] [CrossRef] [Green Version]
- Harrison, J.J.; Ceri, H.; Turner, R.J. Multi metal resistance and tolerance in microbial biofilms. Nat. Rev. Microbiol. 2007, 5, 928–938. [Google Scholar] [CrossRef] [PubMed]
- Pulsawat, W.; Leksawasdi, N.; Rogers, P.L.; Foster, L.J.R. Anions effects on biosorption of Mn(II) by extracellular polymeric substance (EPS) from Rhizobium etli. Biotechnol. Lett. 2003, 25, 1267–1270. [Google Scholar] [CrossRef] [PubMed]
- Moreland, L.W. Rheumatology and Immunology Therapy: A to Z Essentials; Springer Science & Business Media: Berlin, Germany, 2004. [Google Scholar] [CrossRef]
- Sharaf, E.F.; El-Sayed, W.S.; Abosaif, R.M. Lecithinase-producing bacteria in commercial and home-made foods: Evaluation of toxic properties and identification of potent producers. J. Taibah Univ. Sci. 2014, 8, 207–215. [Google Scholar] [CrossRef]
- De Souza, A.P.; Gerlach, R.F.; Line, S.R.P. Inhibition of human gingival gelatinases (MMP-2 and MMP-9) by metal salts. Dent. Mater. 2000, 16, 103–108. [Google Scholar] [CrossRef]
- Touati, A.; Achour, W.; Abbassi, M.S.; Hassen, A.B. Détection des gènes ica et de la production de slime parmi des souches de Staphylococcus epidermidis isolées d’infections liées aux cathéters chez des patients neutropéniques. Pathol. Biol. 2007, 55, 277–282. [Google Scholar] [CrossRef]
- Prabhakaran, P.; Ashraf, M.A.; Aqma, W.S. Microbial stress response to heavy metals in the environment. RSC. Adv. 2016, 6, 109862–109877. [Google Scholar] [CrossRef]
- Hunt, S.M.; Werner, E.M.; Huang, B.; Hamilton, M.A.; Stewart, P.S. Hypothesis for the role of nutrient starvation in biofilm detachment. Appl. Environ. Microbiol. 2004, 70, 7418–7425. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mitzel, M.R.; Sand, S.; Whalen, J.K.; Tufenkji, N. Hydrophobicity of biofilm coatings influences the transport dynamics of polystyrene nanoparticles in biofilm-coated sand. Water Res. 2016, 92, 113–120. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Falcón García, C.; Kretschmer, M.; Lozano-Andrade, C.N.; Schönleitner, M.; Dragoŝ, A.; Kovács, Á.T.; Lieleg, O. Metal ions weaken the hydrophobicity and antibiotic resistance of Bacillus subtilis NCIB 3610 biofilms. NPJ Biofilms Microbiomes 2020, 6, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Martins, P.S.D.O.; Almeida, N.F.D.; Leite, S.G.F. Application of a bacterial extracellular polymeric substance in heavy metal adsorption in a co-contaminated aqueous system. Braz. J. Microbiol. 2008, 39, 780–786. [Google Scholar] [CrossRef] [Green Version]
- Zhang, M.; Chen, L.; Ye, C.; Yu, X. Co-selection of antibiotic resistance via copper shock loading on bacteria from a drinking water bio-filte. Environ. Pollut. 2018, 233, 132–141. [Google Scholar] [CrossRef]
- Livermore, D.M. Bacterial resistance: Origins, epidemiology, and impact. Clin. Infect. Dis. 2003, 36, 11–23. [Google Scholar] [CrossRef] [Green Version]
- Baker-Austin, C.; Wright, M.S.; Stepanauskas, R.; McArthur, J.V. Co-selection of antibiotic and metal resistance. Trends Microbiol. 2006, 14, 176–182. [Google Scholar] [CrossRef]
- Abdel-Aty, A.M.; Ammar, N.S.; Abdel Ghafar, H.H.; Ali, R.K. Biosorption of cadmium and lead from aqueous solution by fresh water alga Anabaena sphaerica biomass. J. Adv. Res. 2013, 4, 367–374. [Google Scholar] [CrossRef] [Green Version]
- Das, S.; Lyla, P.S.; Khan, S.A. Biogeochemical processes in the continental slope of Bay of Bengal: I. Bacterial solubilization of inorganic phosphate. Rev. Biol. Trop. 2007, 55, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Kulshreshtha, A.; Agrawal, R.; Barar, M.; Saxena, S. A review on bioremediation of heavy metals in contaminated water. IOSR J. Environ. Sci. Toxicol. Food Technol. 2014, 8, 44–50. [Google Scholar] [CrossRef]
- Vijayaraghavan, K.; Yun, Y.S. Bacterial biosorbents and biosorption. Biotechnol. Adv. 2008, 26, 266–291. [Google Scholar] [CrossRef] [PubMed]
- Akar, T.; Tunali, S.; Kiran, I. Botrytis cinerea as a new fungal biosorbent for removal of Pb(II) from aqueous solutions. Biochem. Eng. J. 2005, 25, 227–235. [Google Scholar] [CrossRef]

| Parameter, Unit | MPLW, SD | Tunisian National Standards | International Standars |
|---|---|---|---|
| pH, 25 °C | 7.5 ± 0.2 | 6.5–8.50 | 9.00 |
| EC, mS cm−1 | 3.2 ± 0.3 | 5.00 | 6.00 |
| Salinity, g L−1 | 2.1 ± 0.1 | NI | NI |
| Chlorosity, g L−1 | 1.1 ± 0.1 | NI | NI |
| TSS, g L−1 | 61.8 ± 1 | 0.03 | ≤0.03 |
| TS, g L−1 | 6.8 ± 0.9 | NI | NI |
| VSS, g L−1 | 70 ± 1 | NI | NI |
| Turbidity, NTU | 503 ± 3 | 70.00 | ≤30.00 |
| TOC, g L−1 | 3.7 ± 0.1 | NI | NI |
| COD, mg L−1 | 1155 ± 2 | 200.00 | ≤70.00 |
| BOD5, mg L−1 | 280 ± 2 | 50.00 | ≤50.00 |
| COD/BOD5 ratio | 4.1 ± 0 | 0.33 | ≤0.71 |
| CODsoluble, mg/L | 577 ± 2 | NI | NI |
| Mg, mg L−1 | 5655 ± 52 | NI | NI |
| Co/Mn, mg L−1 | 0 ± 0 | NI | NI |
| K, mg L−1 | 45.0 ± 0.5 | 50.00 | ≤30.00 |
| Fe, mg L−1 | 0.7 ± 0.0 | 5.00 | ≤5.00 |
| Cd, mg L−1 | 0.5 ± 0.0 | 0.10 | ≤0.10 |
| Cu, mg L−1 | 0.3 ± 0.0 | 0.50 | ≤0.25 |
| Zn, mg L−1 | 0.1 ± 0.0 | 0.50 | ≤1.00 |
| Pb, mg L−1 | 1.0 ± 0.3 | 0.10 | ≤0.10 |
| Total cultivable microflora (CFU mL−1) | 38.0 ± 3 × 104 | NI | NI |
| Initial Metal Ion Concentration (mM) | Metal Ion Concentration before Sonication (mM) | Metal Ion Concentration after Sonication (mM) | Accumulation/Adsorption (%) | |
|---|---|---|---|---|
| Cu | 1 | 0.8 ± 0 | 0.1 ± 0 | 10 ± 0 |
| 10 | 8.8 ± 0 | 1.1 ± 0 | 11 ± 0 | |
| 100 | 80 ± 3 | 19.5 ± 0.9 | 19.5 ± 0 | |
| Zn | 1 | 0.8 ± 0 | 0.1 ± 0 | 11 ± 0 |
| 10 | 8.5 ± 0.8 | 1.4 ± 0.1 | 14.8 ± 0 | |
| 100 | 80.8 ± 2 | 18.4 ± 0.8 | 18.4 ± 0 | |
| Mg | 1 | 0.9 ± 0.1 | 0.1 ± 0 | 10 ± 0 |
| 10 | 8.4 ± 1 | 1.6 ± 0.1 | 16 ± 0 | |
| 100 | 70 ± 4 | 28.9 ± 1 | 28.9 ± 1 | |
| Cd | 1 | 0.9 ± 0 | 0 ± 0 | 4 ± 0 |
| 10 | 8.3 ± 1 | 1.5 ± 0 | 15.4 ± 0 | |
| 100 | 61.6 ± 2 | 38.4 ± 2 | 38.4 ± 2 | |
| Pb | 1 | 0.8 ± 0 | 0.2 ± 0 | 20.0 ± 0 |
| 10 | 6.4 ± 0.1 | 3.5 ± 0.5 | 35.0 ± 0.5 | |
| 100 | 54.5 ± 1 | 45.3 ± 1 | 45.3 ± 1 |
| ZC Strain | Cu (mM) | Zn (mM) | Mg (mM) | Cd (mM) | Pb (mM) | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 10 | 100 | 1 | 10 | 100 | 1 | 10 | 100 | 1 | 10 | 100 | 1 | 10 | 100 | ||
| Lipase | − | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + |
| Gelatinase | + | + | + | + | + | + | − | + | + | + | + | + | + | + | + | + |
| Caseinase | + | − | − | − | + | + | + | + | + | − | + | + | + | + | + | + |
| Amylase | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + |
| Lecithinase | + | + | + | + | + | − | − | + | + | + | + | + | + | + | + | + |
| Hemolysin | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − |
| ZC Strain | Cu (mM) | Zn (mM) | Mg (mM) | Cd (mM) | Pb (mM) | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 10 | 100 | 1 | 10 | 100 | 1 | 10 | 100 | 1 | 10 | 100 | 1 | 10 | 100 | ||
| OD570 | 0.1 ± 0 | 0.1 ± 0 | 0.1 ± 0 | 0.3 ± 0 | 0.2 ± 0 | 0.2 ± 0 | 0.2 ± 0 | 0.2 ± 0 | 0.3 ± 0 | 0.3 ± 0 | 0.2 ± 0 | 0.2 ± 0 | 0.2 ± 0 | 0.2 ± 0 | 0.2 ± 0 | 0.2 ± 0 |
| Adhesion (%) | 1.2 ± 0.2 | 1.2 ± 0.1 | 2.6 ± 0.2 | 1.8 ± 0.1 | 1.3 ± 0.0 | 2.9 ± 0.2 | 2.8 ± 0.3 | 1.4 ± 0.4 | 3.2 ± 0.3 | 3.0 ± 0.0 | 1.3 ± 0.2 | 1.6 ± 0.4 | 1.5 ± 0.6 | 1.3 ± 0.1 | 2.3 ± 0.1 | 2.2 ± 0.3 |
| Control Strain | [Metal Ion] (mM) | Cu | Zn | Mg | Cd | Pb | |
|---|---|---|---|---|---|---|---|
| Hexane | 36.3 ± 2 | 1 | 14 ± 0.3 | 59 ± 1 | 53 ± 2 | 33 ± 3 | 43 ± 3 |
| 10 | 13 ± 1 | 27 ± 1 | 56 ± 1 | 47 ± 2 | 46 ± 1 | ||
| 100 | 27 ± 2 | 37 ± 1 | 65 ± 2 | 55 ± 2 | 56 ± 1 | ||
| Decane | 69.7 ± 1 | 1 | 18 ± 1 | 16 ± 1 | 18 ± 1 | 17 ± 1 | 14 ± 1 |
| 10 | 29 ± 2 | 42 ± 2 | 61 ± 1 | 48 ± 1 | 31 ± 1 | ||
| 100 | 58 ± 3 | 22 ± 2 | 42 ± 1 | 41 ± 1 | 40 ± 1 | ||
| Chloroform | 31.3 ± 2 | 1 | 32 ± 2 | 24 ± 1 | 10 ± 1 | 17 ± 2 | 17 ± 1 |
| 10 | 22 ± 1 | 11 ± 1 | 16 ± 1 | 20 ± 1 | 13 ± 1 | ||
| 100 | 31 ± 2 | 8 ± 1 | 9 ± 0.8 | 11 ± 0.6 | 7 ± 1 |
| Antibiotics | ZC Strain | Cu (mM) | Zn (mM) | Mg (mM) | Cd (mM) | Pb (mM) | ||||||||||
| 1 | 10 | 100 | 1 | 10 | 100 | 1 | 10 | 100 | 1 | 10 | 100 | 1 | 10 | 100 | ||
| Penicillin | I | I | S | S | I | I | S | R | I | I | S | S | S | S | S | S |
| Tobramycin | S | R | R | R | R | R | R | R | R | R | R | R | R | R | R | R |
| Neomycin | S | R | R | R | R | R | R | R | R | R | R | R | R | R | R | R |
| Tetracyclin | S | R | R | R | R | R | R | R | R | R | R | R | R | R | R | R |
| Cefoxitin | S | R | R | R | R | R | R | R | R | R | R | R | R | R | R | R |
| Kanamycin | S | R | I | R | R | R | R | R | R | R | R | R | R | R | R | R |
| Netilmicin | S | R | R | R | R | S | I | R | R | I | R | R | R | R | R | R |
| MARindex | 0 | 0.8 | 0.7 | 0.8 | 0.8 | 0.7 | 0.7 | 1 | 0.8 | 0.7 | 0.8 | 0.8 | 0.8 | 0.8 | 0.8 | 0.8 |
| Cu (mg/L) | Zn (mg/L) | Mg (mg/L) | Cd (mg/mL) | Pb (mg/mL) | |
|---|---|---|---|---|---|
| Untreated MPLW | 0.3 ± 0 | 0.1 ± 0 | 5655 ± 52 | 0.05 ± 0 | 1 ± 0.3 |
| Treated MPLW | 0.06 ± 0 | 0.02 ± 0 | 84 ± 15 | 0.09 ± 0 | 0.01 ± 0 |
| Reff | 80.6 | 86.6 | 98.5 | 82.3 | 87.8 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Taieb, I.; Ben Younes, S.; Messai, B.; Mnif, S.; Mzoughi, R.; Bakhrouf, A.; Jabeur, C.; Ayala Serrano, J.A.; Ellafi, A. Isolation, Characterization and Identification of a New Lysinibacillus fusiformis Strain ZC from Metlaoui Phosphate Laundries Wastewater: Bio-Treatment Assays. Sustainability 2021, 13, 10072. https://doi.org/10.3390/su131810072
Taieb I, Ben Younes S, Messai B, Mnif S, Mzoughi R, Bakhrouf A, Jabeur C, Ayala Serrano JA, Ellafi A. Isolation, Characterization and Identification of a New Lysinibacillus fusiformis Strain ZC from Metlaoui Phosphate Laundries Wastewater: Bio-Treatment Assays. Sustainability. 2021; 13(18):10072. https://doi.org/10.3390/su131810072
Chicago/Turabian StyleTaieb, Ines, Sonia Ben Younes, Boutheina Messai, Sami Mnif, Ridha Mzoughi, Amina Bakhrouf, Chédia Jabeur, Juan Alfonso Ayala Serrano, and Ali Ellafi. 2021. "Isolation, Characterization and Identification of a New Lysinibacillus fusiformis Strain ZC from Metlaoui Phosphate Laundries Wastewater: Bio-Treatment Assays" Sustainability 13, no. 18: 10072. https://doi.org/10.3390/su131810072






