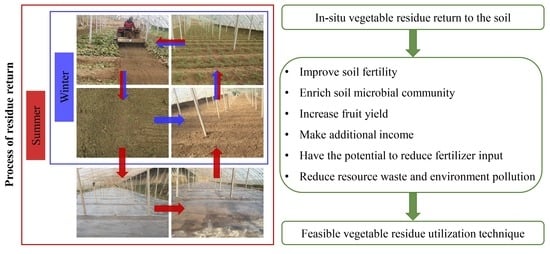
Techno-Economic Feasibility of In Situ Vegetable Residue Return in the Chinese Solar Greenhouse
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Site Description
2.2. Experimental Design
2.3. Soil Physicochemical Characteristics
2.4. Soil Microbial Communities
2.5. Fruit Yield and Physicochemical Characteristics of Vegetable Residues
2.6. Statistical Analysis
3. Results and Discussion
3.1. Vegetable Residue Return Improved Soil Physicochemical Characteristics
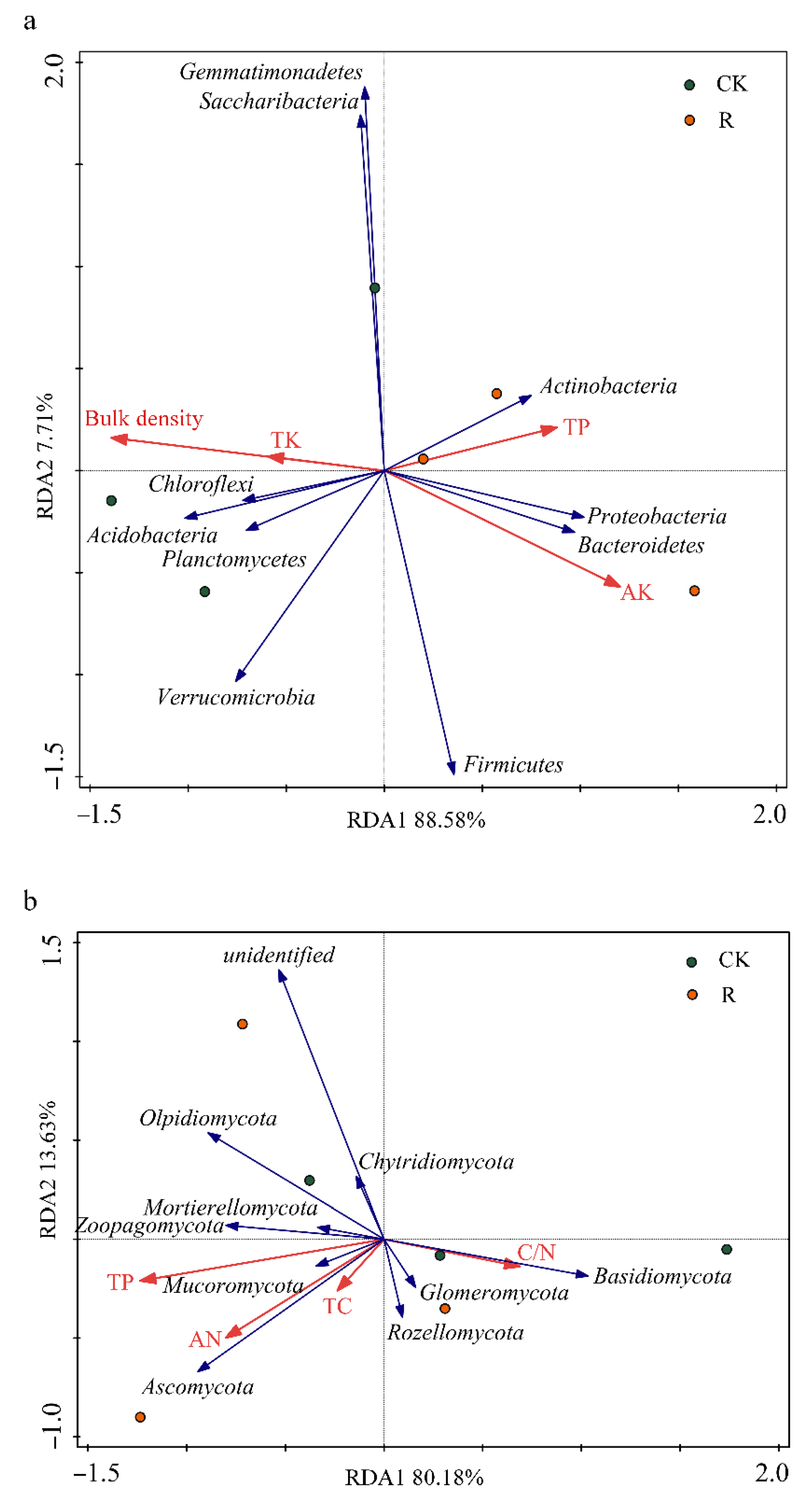
3.2. Changes in Microbial Communities
3.2.1. The Alpha Diversity of the Microbial Communities Affected by Vegetable Residue Return
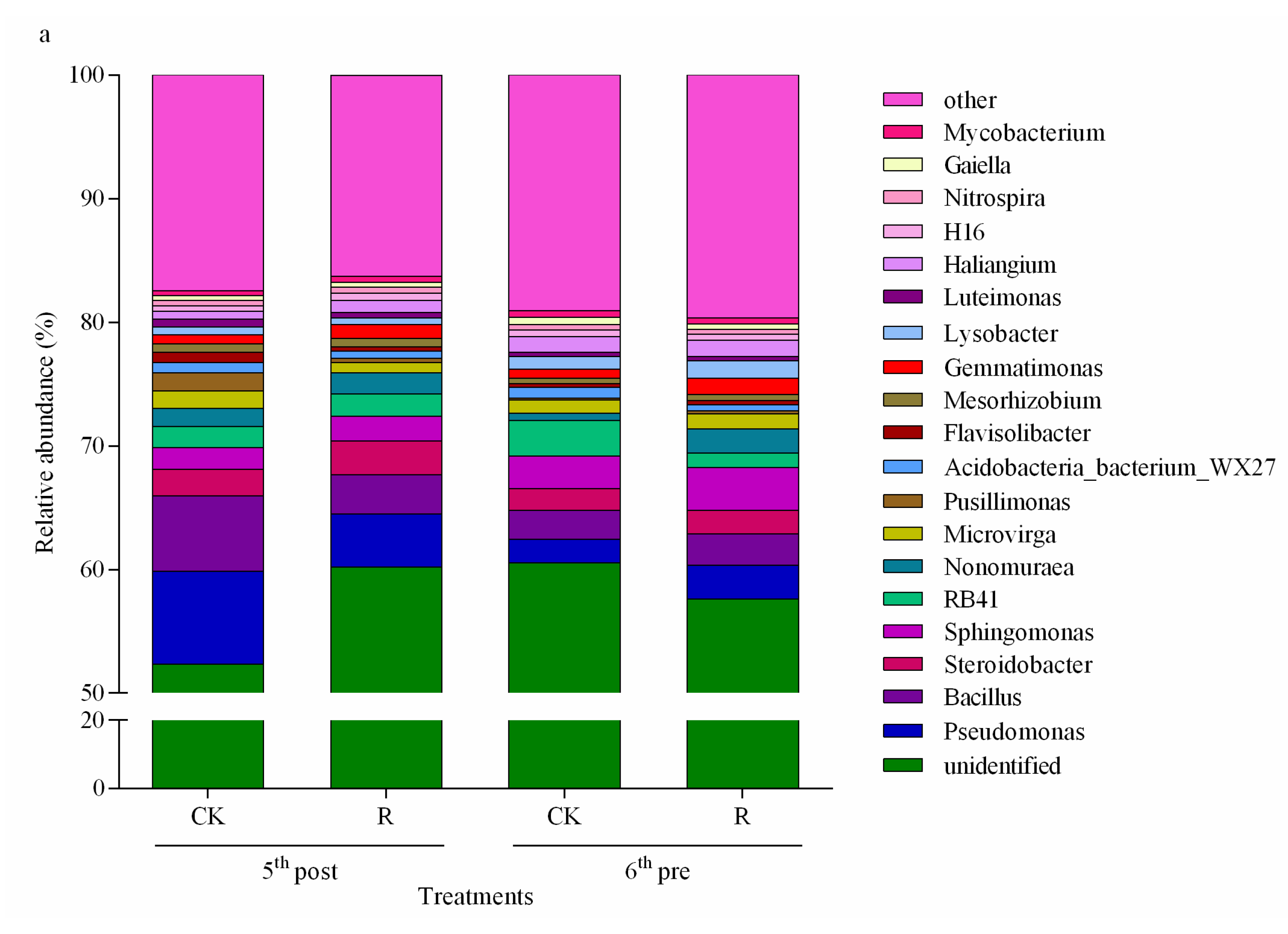
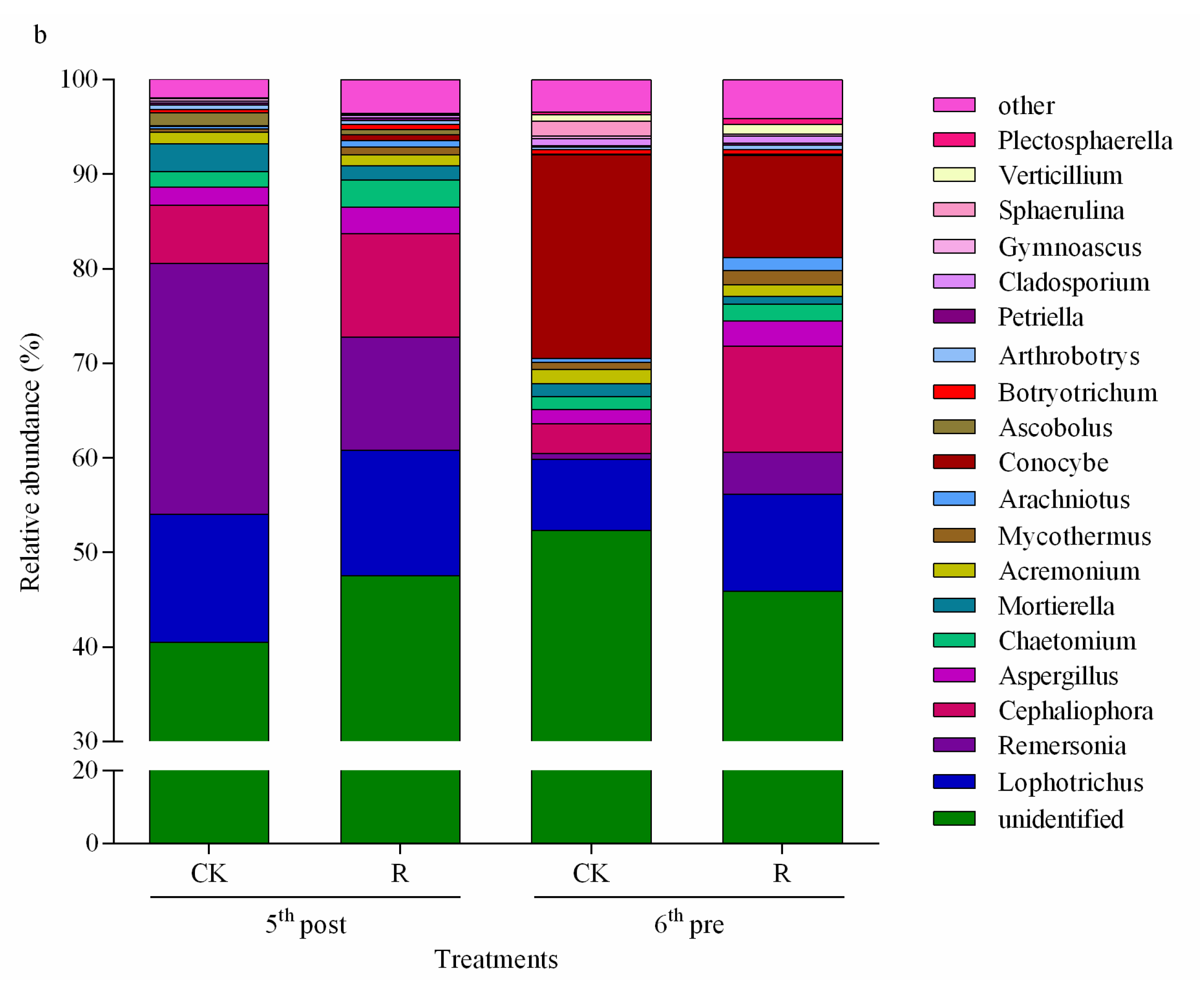
3.2.2. Dominant Taxa at the Phylum Level Affected by Vegetable Residue Return
3.2.3. Dominant Taxa at the Genus Level Affected by Vegetable Residue Return
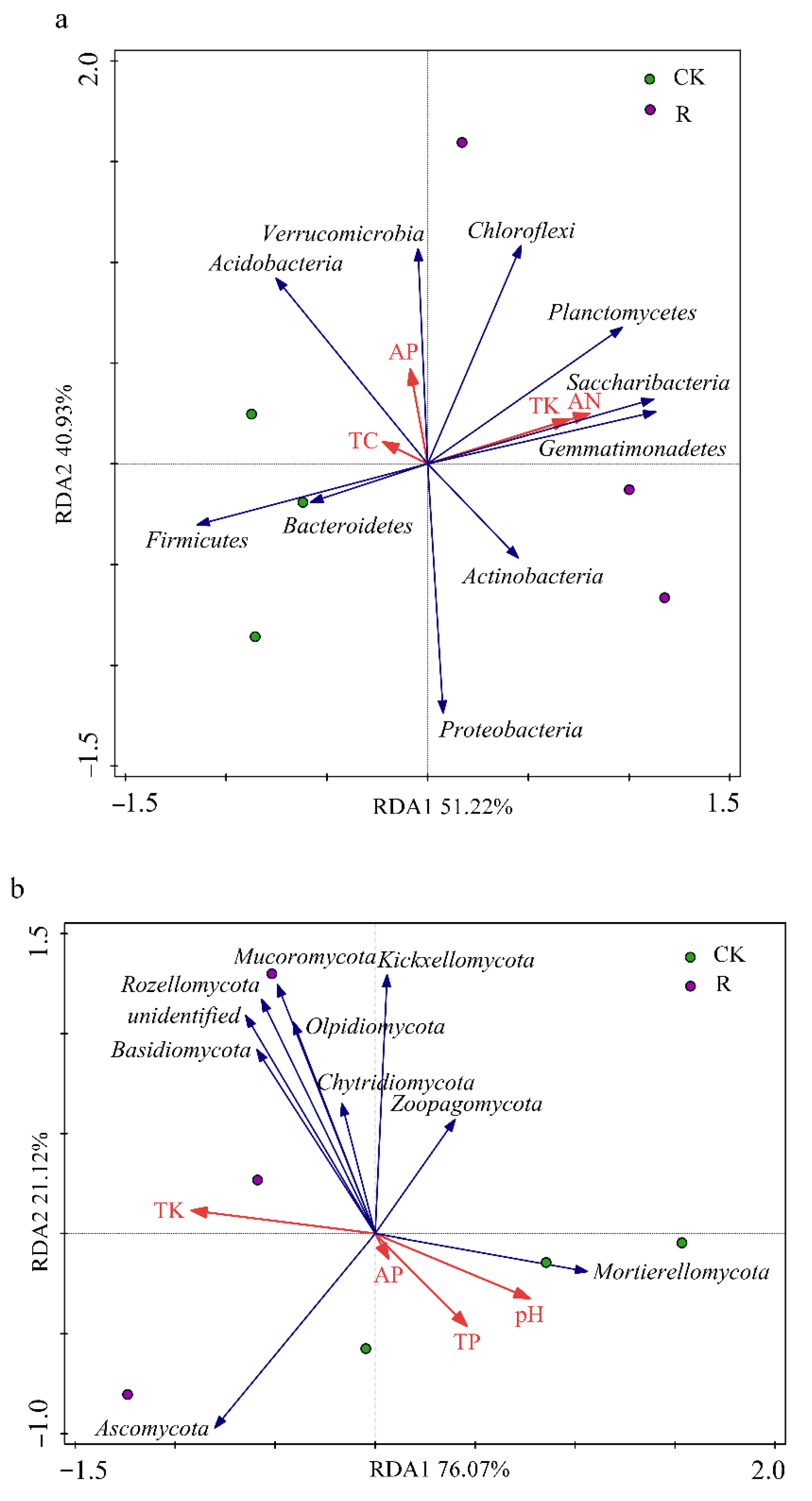
3.3. Correlations of Environmental Factors with the Abundances of the 10 Most Abundant Microbial Taxa
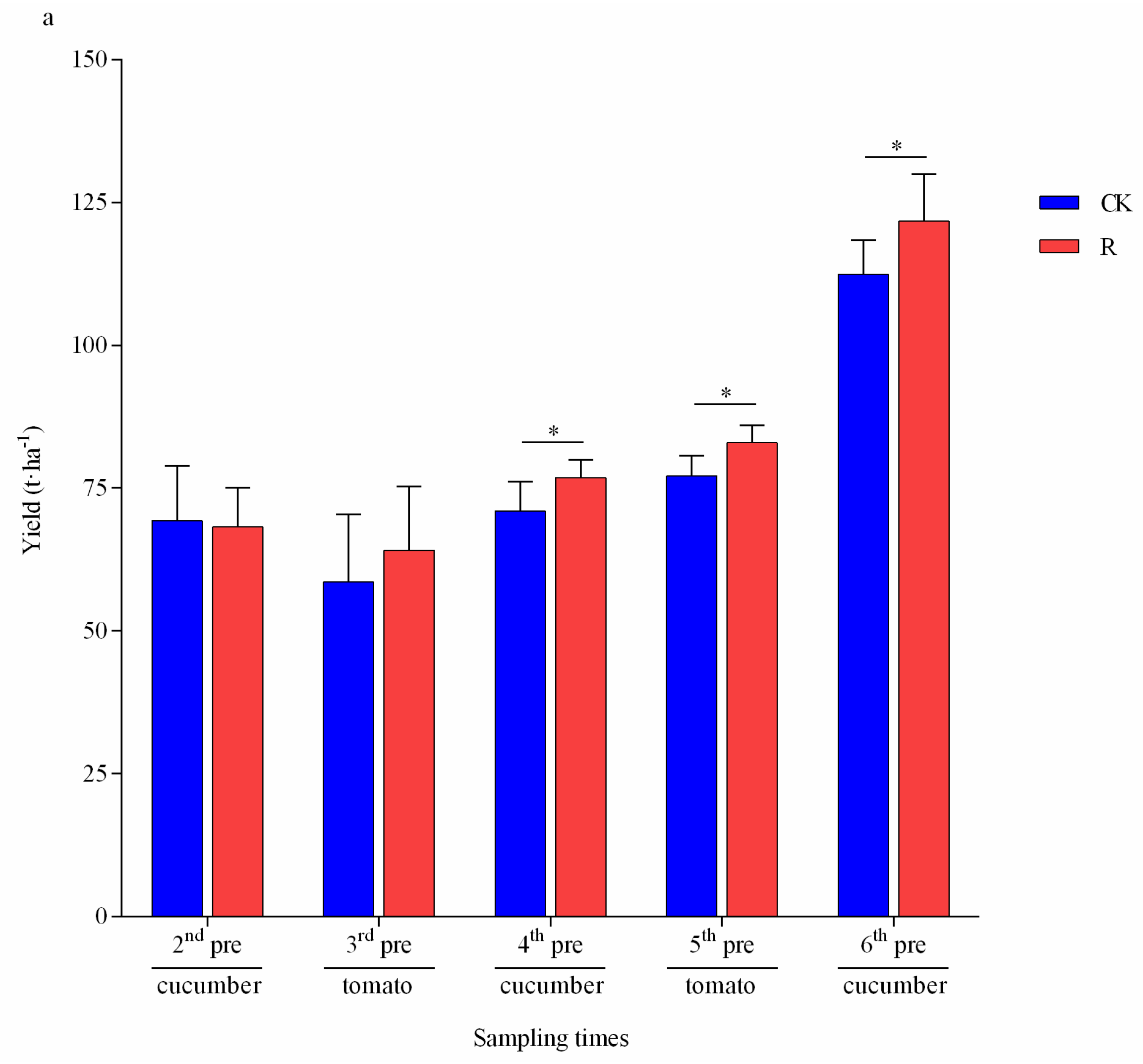
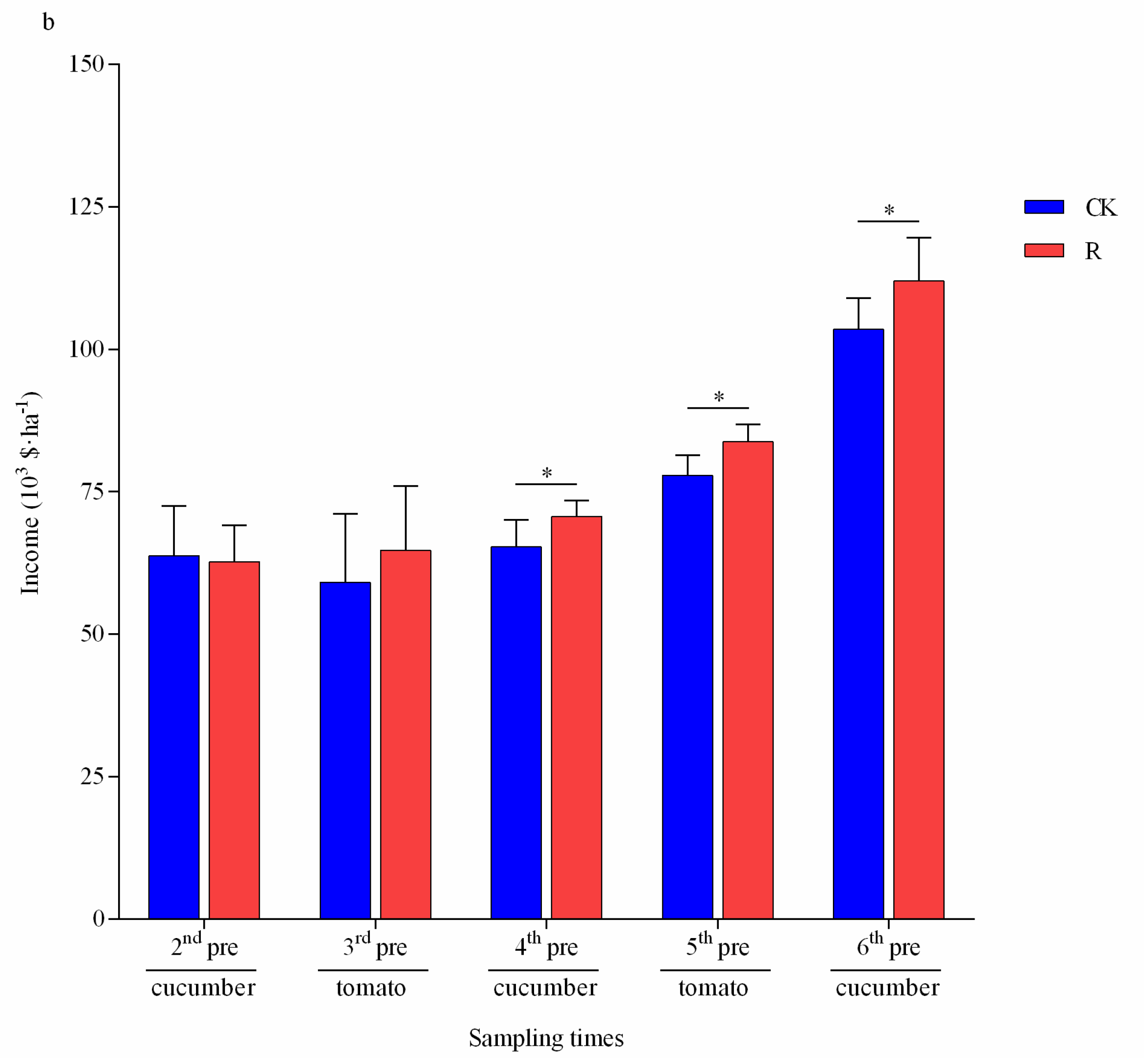
3.4. Physicochemical Characteristics of Vegetable Residue and Vegetable Growth
3.5. Feasibility Assessment
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Du, P.X.; Han, X.; Gao, J.Y.; Chen, Q.; Li, Y.M. Potential analysis on high efficient utilization of waste vegetable resources in China. China Veg. 2015, 1, 15–20. [Google Scholar] [CrossRef]
- Zhang, Z.H.; Ma, Z.H. Overview of Chinese protected vegetable industry and key points of development during the 13th five-year plan: Interview with Zhang Zhenhe, vice president of China vegetable association. China Veg. 2017, 5, 1–5. [Google Scholar] [CrossRef]
- Ros, M.; de Souza Oliveira Filho, J.; Murcia, M.D.P.; Bustamante, M.A.; Moral, R.; Coll, M.D.; Santisima-Trinidad, A.B.L.; Pascual, J.A. Mesophilic anaerobic digestion of pig slurry and fruit and vegetable waste: Dissection of the microbial community structure. J. Clean. Prod. 2017, 156, 757–765. [Google Scholar] [CrossRef]
- Cui, H.-Y.; Zhang, S.-B.; Zhao, M.-Y.; Zhao, Y.; Wei, Z.-M. Parallel faction analysis combined with two-dimensional correlation spectroscopy reveal the characteristics of mercury-composting-derived dissolved organic matter interactions. J. Hazard. Mater. 2020, 384, 121395. [Google Scholar] [CrossRef]
- Mahmoodi, P.; Karimi, K.; Taherzadeh, M.J. Hydrothermal processing as pretreatment for efficient production of ethanol and biogas from municipal solid waste. Bioresour. Technol. 2018, 261, 166–175. [Google Scholar] [CrossRef] [PubMed]
- Bouallagui, H.; Lahdheb, H.; Ben Romdan, E.; Rachdi, B.; Hamdi, M. Improvement of fruit and vegetable waste anaerobic digestion performance and stability with co-substrates addition. J. Environ. Manag. 2009, 90, 1844–1849. [Google Scholar] [CrossRef] [PubMed]
- Awasthi, M.K.; Sarsaiya, S.; Wainaina, S.; Rajendran, K.; Kumar, S.; Quan, W.; Duan, Y.; Awasthi, S.K.; Chen, H.; Pandey, A.; et al. A critical review of organic manure biorefinery models toward sustainable circular bioeconomy: Technological challenges, advancements, innovations, and future perspectives. Renew. Sustain. Energy Rev. 2019, 111, 115–131. [Google Scholar] [CrossRef]
- Sánchez, J.Ó.; Ospina, D.A.; Montoya, S. Compost supplementation with nutrients and microorganisms in composting process. Waste Manag. 2017, 69, 136–153. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhao, Y.; Chen, Y.; Lu, Q.; Li, M.; Wang, X.; Wei, Y.; Xie, X.; Wei, Z. A regulating method for reducing nitrogen loss based on enriched ammonia-oxidizing bacteria during composting. Bioresour. Technol. 2016, 221, 276–283. [Google Scholar] [CrossRef]
- Meena, M.D.; Yadav, R.K.; Narjary, B.; Yadav, G.; Jat, H.S.; Sheoran, P.; Meena, M.K.; Antil, R.S.; Meena, B.L.; Singh, H.V.; et al. Municipal solid waste (MSW): Strategies to improve salt affected soil sustainability: A review. Waste Manag. 2019, 84, 38–53. [Google Scholar] [CrossRef]
- Wang, R.; Zhao, Y.; Xie, X.; Mohamed, T.A.; Zhu, L.; Tang, Y.; Chen, Y.; Wei, Z. Role of NH3 recycling on nitrogen fractions during sludge composting. Bioresour. Technol. 2020, 295, 122175. [Google Scholar] [CrossRef]
- Wang, X.-D.; He, C.; Cheng, H.-Y.; Liu, B.-Y.; Li, S.-S.; Wang, Q.; Liu, Y.; Zhao, X.; Zhang, H.-L. Responses of greenhouse gas emissions to residue returning in China’s croplands and influential factors: A meta-analysis. J. Environ. Manag. 2021, 289, 112486. [Google Scholar] [CrossRef] [PubMed]
- Jiang, W.; Yan, T.; Chen, B. Impact of media channels and social interactions on the adoption of straw return by Chinese farmers. Sci. Total. Environ. 2021, 756, 144078. [Google Scholar] [CrossRef]
- Fernández-Gómez, M.J.; Romero, E.; Nogales, R. Feasibility of vermicomposting for vegetable greenhouse waste recycling. Bioresour. Technol. 2010, 101, 9654–9660. [Google Scholar] [CrossRef] [PubMed]
- He, S.; Liang, B.; Wu, D.; Wang, Y.; Li, J. Nutrient demand of tomato in the facility vegetable field. Acta Agric. Boreali-Sin. 2020, 35, 61–67. [Google Scholar] [CrossRef]
- Rayment, G.E.; Lyons, D.J. Soil Chemical Methods—Australasia; CSIRO Publishing: Collingwood, Australia, 2011. [Google Scholar]
- Qi, Y.; Chen, T.; Pu, J.; Yang, F.; Shukla, M.K.; Chang, Q. Response of soil physical, chemical and microbial biomass properties to land use changes in fixed desertified land. Catena 2018, 160, 339–344. [Google Scholar] [CrossRef]
- Tian, X. Study on ICP-AES Analysis Method of Simultaneously High-Speed Determination for Quick-Acting P and K in the Soil. Chin. J. Spectrosc. Lab. 1997, 14, 40–43. Available online: https://en.cnki.com.cn/Article_en/CJFDTotal-GPSS199704010.htm (accessed on 7 July 2021).
- Hao, H.-P.; Li, H.; Jiang, C.-D.; Tang, Y.-D.; Shi, L. Ion micro-distribution in varying aged leaves in salt-treated cucumber seedlings. Plant Physiol. Biochem. 2018, 129, 71–76. [Google Scholar] [CrossRef]
- Zhang, X.; Tian, X.; Ma, L.; Feng, B.; Liu, Q.; Yuan, L.; Fan, C.; Huang, H.; Yang, Q. Biodiversity of the Symbiotic Bacteria Associated with Toxic Marine Dinoflagellate Alexandrium tamarense. J. Biosci. Med. 2015, 03, 23–28. [Google Scholar] [CrossRef] [Green Version]
- Bengtsson-Palme, J.; Ryberg, M.; Hartmann, M.; Branco, S.M.; Wang, Z.; Godhe, A.; De Wit, P.; Sánchez-García, M.; Ebersberger, I.; Sousa, F.; et al. Improved software detection and extraction of ITS1 and ITS2 from ribosomal ITS sequences of fungi and other eukaryotes for analysis of environmental sequencing data. Methods Ecol. Evol. 2013, 4, 914–919. [Google Scholar] [CrossRef]
- Edgar, R.C. UPARSE: Highly accurate OTU sequences from microbial amplicon reads. Nat. Methods 2013, 10, 996–998. [Google Scholar] [CrossRef]
- Gao, Y.; Yu, H.; Liu, P.; Ma, C.; Li, Q.; Jiang, W. Ending composting during the thermophilic phase improves cultivation substrate properties and increasing winter cucumber yield. Waste Manag. 2018, 79, 260–272. [Google Scholar] [CrossRef] [PubMed]
- Tong, C.; Xiao, H.; Tang, G.; Wang, H.; Huang, T.; Xia, H.; Keith, S.J.; Li, Y.; Liu, S.; Wu, J. Long-term fertilizer effects on organic carbon and total nitrogen and coupling relationships of C and N in paddy soils in subtropical China. Soil Till. Res. 2009, 106, 8–14. [Google Scholar] [CrossRef]
- Yanni, S.F.; Whalen, J.K.; Simpson, M.J.; Janzen, H.H. Plant lignin and nitrogen contents control carbon dioxide production and nitrogen mineralization in soils incubated with Bt and non-Bt corn residues. Soil Biol. Biochem. 2011, 43, 63–69. [Google Scholar] [CrossRef]
- Saha, P.; Miah, M.; Hossain, A.; Rahman, F.; Saleque, M. Contribution of rice straw to potassium supply in rice-fallow-rice cropping pattern. Bangladesh J. Agric. Res. 2010, 34, 633–643. [Google Scholar] [CrossRef] [Green Version]
- Sheng, X.F.; He, L.Y. Solubilization of potassium-bearing minerals by a wild-type strain of Bacillus edaphicus and its mutants and increased potassium uptake by wheat. Can. J. Microbiol. 2006, 52, 66–72. [Google Scholar] [CrossRef]
- Gao, M.; Liang, F.; Yu, A.; Li, B.; Yang, L. Evaluation of stability and maturity during forced-aeration composting of chicken manure and sawdust at different C/N ratios. Chemosphere 2010, 78, 614–619. [Google Scholar] [CrossRef] [PubMed]
- Wong, J.; Mak, K.; Chan, N.; Lam, A.; Fang, M.; Zhou, L.; Wu, Q.; Liao, X. Co-composting of soybean residues and leaves in Hong Kong. Bioresour. Technol. 2001, 76, 99–106. [Google Scholar] [CrossRef]
- Chen, D.; Xing, W.; Lan, Z.; Saleem, M.; Wu, Y.; Hu, S.; Bai, Y. Direct and indirect effects of nitrogen enrichment on soil organisms and carbon and nitrogen mineralization in a semi-arid grassland. Funct. Ecol. 2019, 33, 175–187. [Google Scholar] [CrossRef] [Green Version]
- Chen, S.H.; Zhu, Z.L.; Wu, J.; Liu, D.H.; Wang, C.Q. Decomposition characteristics of straw return to soil and its effect on soil fertility in Purple Hilly Region. J. Soil Water Conserv. 2006, 20, 141–144. [Google Scholar] [CrossRef]
- Hou, L.; Chen, X.; Kuhn, L.; Huang, J. The effectiveness of regulations and technologies on sustainable use of crop residue in Northeast China. Energy Econ. 2019, 81, 519–527. [Google Scholar] [CrossRef]
- Li, H.; Dai, M.; Dai, S.; Dong, X. Current status and environment impact of direct straw return in China’s cropland—A review. Ecotoxicol. Environ. Saf. 2018, 159, 293–300. [Google Scholar] [CrossRef] [PubMed]
- DeBruyn, J.M.; Nixon, L.T.; Fawaz, M.N.; Johnson, A.M.; Radosevich, M. Global Biogeography and Quantitative Seasonal Dynamics of Gemmatimonadetes in Soil. Appl. Environ. Microbiol. 2011, 77, 6295–6300. [Google Scholar] [CrossRef] [Green Version]
- Liu, L.; Wang, S.; Guo, X.; Zhao, T.; Zhang, B. Succession and diversity of microorganisms and their association with physicochemical properties during green waste thermophilic composting. Waste Manag. 2018, 73, 101–112. [Google Scholar] [CrossRef]
- Fierer, N.; Bradford, M.A.; Jackson, R.B. Toward an ecological classification of soil bacteria. Ecology 2007, 88, 1354–1364. [Google Scholar] [CrossRef]
- Chen, S.; Waghmode, T.R.; Sun, R.; Kuramae, E.; Hu, C.; Liu, B. Root-associated microbiomes of wheat under the combined effect of plant development and nitrogen fertilization. Microbiome 2019, 7, 1–13. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- He, X.; Han, L.; Fu, B.; Du, S.; Liu, Y.; Huang, G. Effect and microbial reaction mechanism of rice straw biochar on pore methane production during mainstream large-scale aerobic composting in China. J. Clean. Prod. 2019, 215, 1223–1232. [Google Scholar] [CrossRef]
- Schmidt-Dannert, C. Biocatalytic portfolio of Basidiomycota. Curr. Opin. Chem. Biol. 2016, 31, 40–49. [Google Scholar] [CrossRef] [Green Version]
- Shiwen, W.; Jing, R.; Ting, H.; Guanru, Q.; Haihui, H.; Wenhui, L.; Wu, F.; Kai, P. Evaluation of soil enzyme activities and microbial communities in tomato continuous cropping soil treated with jerusalem artichoke residues. Commun. Soil Sci. Plant Anal. 2018, 49, 2727–2740. [Google Scholar] [CrossRef]
- Fudou, R.; Jojima, Y.; Iizuka, T.; Yamanaka, S. Haliangium ochraceum gen. nov., sp. nov. and Haliangium tepidum sp. nov.: Novel moderately halophilic myxobacteria isolated from coastal saline environments. J. Gen. Appl. Microbiol. 2002, 48, 109–115. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barron, G.L.; Morikawa, C.; Saikawa, M. New Cephaliophora species capturing rotifers and tardigrades. Can. J. Bot. 1990, 68, 685–690. [Google Scholar] [CrossRef]
- Pavlou, G.; Vakalounakis, D. Biological control of root and stem rot of greenhouse cucumber, caused by Fusarium oxysporum f. sp. radicis-cucumerinum, by lettuce soil amendment. Crop. Prot. 2005, 24, 135–140. [Google Scholar] [CrossRef]
- Ogundeji, A.O.; Li, Y.; Liu, X.; Meng, L.; Sang, P.; Mu, Y.; Wu, H.; Ma, Z.; Hou, J.; Li, S. Eggplant by grafting enhanced with biochar recruits specific microbes for disease suppression of Verticillium wilt. Appl. Soil Ecol. 2021, 163, 103912. [Google Scholar] [CrossRef]
- Ilić, Z.S.; Milenković, L.; Stanojević, L.; Cvetković, D.; Fallik, E. Effects of the modification of light intensity by color shade nets on yield and quality of tomato fruits. Sci. Hortic. 2012, 139, 90–95. [Google Scholar] [CrossRef]
- Sun, W.; Qian, X.; Gu, J.; Wang, X.-J.; Duan, M.-L. Mechanism and Effect of Temperature on Variations in Antibiotic Resistance Genes during Anaerobic Digestion of Dairy Manure. Sci. Rep. 2016, 6, 30237. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, S.; Song, L. Succession of bacterial community structure and metabolic function during solid waste decomposition. Bioresour. Technol. 2019, 291, 121865. [Google Scholar] [CrossRef]
- Bastian, F.; Bouziri, L.; Nicolardot, B.; Ranjard, L. Impact of wheat straw decomposition on successional patterns of soil microbial community structure. Soil Biol. Biochem. 2009, 41, 262–275. [Google Scholar] [CrossRef]
- Wang, X.; Wu, H.; Dai, K.; Zhang, D.; Feng, Z.; Zhao, Q.; Wu, X.; Jin, K.; Cai, D.; Oenema, O.; et al. Tillage and crop residue effects on rainfed wheat and maize production in northern China. Field Crop. Res. 2012, 132, 106–116. [Google Scholar] [CrossRef]
- Xia, L.; Lam, S.K.; Wolf, B.; Kiese, R.; Chen, D.; Butterbach-Bahl, K. Trade-offs between soil carbon sequestration and reactive nitrogen losses under straw return in global agroecosystems. Glob. Chang. Biol. 2018, 24, 5919–5932. [Google Scholar] [CrossRef]
- Cao, Y.; Gao, Y.; Li, J.; Tian, Y. Straw composts, gypsum and their mixtures enhance tomato yields under continuous saline water irrigation. Agric. Water Manag. 2019, 223, 105721. [Google Scholar] [CrossRef]
- Sui, N.; Zhou, Z.; Yu, C.; Liu, R.; Yang, C.; Zhang, F.; Song, G.; Meng, Y. Yield and potassium use efficiency of cotton with wheat straw incorporation and potassium fertilization on soils with various conditions in the wheat–cotton rotation system. Field Crop. Res. 2015, 172, 132–144. [Google Scholar] [CrossRef]
- Zhao, S.; He, P.; Qiu, S.; Jia, L.; Liu, M.; Jin, J.; Johnston, A.M. Long-term effects of potassium fertilization and straw return on soil potassium levels and crop yields in north-central China. Field Crop. Res. 2014, 169, 116–122. [Google Scholar] [CrossRef]

| Samplings | pH | TC (g·kg−1) | TN (g·kg−1) | C/N | TP (g·kg−1) | TK (g·kg−1) | AN (mg·kg−1) | AP (mg·kg−1) | AK (mg·kg−1) | Dry Weight (kg·ha−1) |
|---|---|---|---|---|---|---|---|---|---|---|
| Soil | 7.01 | 20.28 | 2.25 | 9.05 | 1.53 | 18.74 | 208.06 | 36.35 | 428.46 | - |
| Residues | - | 286.41 | 24.12 | 11.99 | 3.52 | 16.29 | - | - | - | 1955.84 |
| Sampling Times | Treatments | pH | Bulk Density (g·cm−3) | TC (g·kg−1) | TN (g·kg−1) | C/N | TP (g·kg−1) | TK (g·kg−1) | AN (mg·kg−1) | AP (mg·kg−1) | AK (mg·kg−1) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1st post (2017.09.01) | CK | 7.12 | 0.96 | 23.16 | 3.50 | 6.65 | 1.86 | 20.84 | 218.75 | 17.01 | 514.76 |
| R | 6.86 * | 0.92 | 29.52 * | 5.34 * | 5.55 * | 2.05 * | 20.93 | 271.25 * | 16.48 | 606.73 * | |
| 2nd pre (2017.12.21) | CK | 7.23 | 1.25 | 26.43 | 2.99 | 8.87 | 2.52 | 20.62 | 175.00 | 39.36 | 457.98 |
| R | 6.84 * | 1.13 * | 25.06 | 3.15 | 7.99 * | 2.42 | 20.66 | 201.25 * | 45.00 * | 536.73 * | |
| 2nd post (2018.02.02) | CK | 7.11 | 1.11 | 24.00 | 3.28 | 7.32 | 2.52 | 22.57 | 180.83 | 43.71 | 637.14 |
| R | 6.91 * | 1.16 | 23.66 | 3.64 * | 6.50 * | 2.48 | 22.40 | 221.67 * | 40.12 | 737.17 * | |
| 3rd pre (2018.07.26) | CK | 7.44 | 1.31 | 15.18 | 1.97 | 7.70 | 2.46 | 21.45 | 151.67 | 44.66 | 411.43 |
| R | 7.17 * | 1.18 * | 15.65 | 2.16 | 7.26 * | 2.47 | 20.08 | 186.67 * | 45.08 | 487.19 * | |
| 3rd post (2018.11.02) | CK | 7.32 | 1.06 | 16.41 | 2.46 | 6.68 | 2.78 | 22.58 | 134.33 | 23.52 | 693.75 |
| R | 6.97 * | 0.94 * | 16.10 | 2.58 | 6.24 * | 2.63 | 22.02 | 143.87 * | 29.87 * | 767.65 * | |
| 4th pre (2019.07.15) | CK | 7.72 | 1.18 | 16.88 | 2.01 | 8.42 | 2.78 | 21.19 | 129.89 | 31.37 | 582.58 |
| R | 7.64 * | 1.08 * | 16.89 | 2.11 * | 8.01 | 2.46 * | 20.23 * | 136.34 * | 30.25 | 638.08 * | |
| 4th post (2019.09.01) | CK | 7.63 | 1.21 | 17.28 | 2.12 | 8.17 | 2.66 | 20.74 | 118.99 | 36.90 | 724.62 |
| R | 7.5 * | 1.11 * | 20.80 * | 2.41 | 8.71 | 2.79 | 20.11 * | 148.22 * | 38.67 * | 883.43 * | |
| 5th pre (2020.07.15) | CK | 8.04 | 1.27 | 17.00 | 2.04 | 8.38 | 2.65 | 16.22 | 134.73 | 34.99 | 389.93 |
| R | 7.96 * | 1.12 * | 19.21 * | 2.17 | 8.84 | 2.75 | 16.80 * | 170.06 * | 35.74 | 435.55 * | |
| 5th post (2020.09.01) | CK | 7.88 | 1.18 | 18.92 | 2.48 | 7.63 | 2.89 | 17.32 | 103.35 | 43.55 | 769.95 |
| R | 7.72 * | 1.06 | 18.73 | 2.64 * | 7.08 | 2.64 | 17.75 * | 115.75 * | 44.29 | 847.90 * | |
| 6th pre (2020.12.21) | CK | 8.08 | 1.19 | 19.23 | 2.01 | 9.57 | 2.54 | 18.04 | 90.09 | 43.91 | 605.95 |
| R | 8.01 * | 1.11 * | 18.62 | 2.18 * | 8.53 * | 2.71 * | 18.19 | 101.63 * | 43.00 | 662.35 * |
| Sampling Time | Treatments | Bacteria | Fungi | ||
|---|---|---|---|---|---|
| Chao1 | Shannon | Chao1 | Shannon | ||
| 5th post (2020.09.01) | CK | 3016.73 | 9.16 | 438.58 | 4.17 |
| R | 3320.29 * | 9.54 * | 497.31 | 4.71 | |
| 6th pre (2020.12.21) | CK | 3447.14 | 9.83 | 464.47 | 4.43 |
| R | 3574.52 | 9.79 | 532.10 | 5.07 * | |
| Sampling Times | Residues Species | Treatments | Dry Weight (kg·ha−1) | TC (g·kg−1) | TN (g·kg−1) | C/N | TP (g·kg−1) | TK (g·kg−1) |
|---|---|---|---|---|---|---|---|---|
| 2nd pre (2017.12.21) | Cucumber | CK | 2014.43 | 376.35 | 31.11 | 12.16 | 3.65 | 25.34 |
| R | 1640.51 | 384.31 * | 31.61 | 12.18 | 3.48 | 25.73 | ||
| 3rd pre (2018.07.26) | Tomato | CK | 2355.90 | 319.72 | 31.86 | 9.96 | 3.64 | 41.90 |
| R | 2380.74 | 325.74 | 35.76 * | 9.05 * | 5.24 * | 51.40 * | ||
| 4th pre (2019.07.15) | Cucumber | CK | 2173.99 | 310.05 | 21.55 | 14.41 | 3.24 | 24.44 |
| R | 2594.54 * | 325.63 * | 22.09 | 14.81 | 3.39 | 30.43 * | ||
| 5th pre (2020.07.15) | Tomato | CK | 2129.83 | 313.58 | 28.49 | 10.93 | 3.26 | 50.02 |
| R | 2643.95 * | 313.22 | 30.44 | 10.34 | 3.57 | 51.32 | ||
| 6th pre (2020.12.21) | Cucumber | CK | 1432.81 | 352.55 | 39.61 | 8.94 | 5.77 | 37.06 |
| R | 1567.76 * | 362.26 | 39.71 | 9.16 | 7.05 * | 41.29 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wei, X.; Li, Y.; Fan, X.; He, C.; Yan, Y.; Sun, M.; Ding, C.; Wang, J.; Yu, X. Techno-Economic Feasibility of In Situ Vegetable Residue Return in the Chinese Solar Greenhouse. Agronomy 2021, 11, 1828. https://doi.org/10.3390/agronomy11091828
Wei X, Li Y, Fan X, He C, Yan Y, Sun M, Ding C, Wang J, Yu X. Techno-Economic Feasibility of In Situ Vegetable Residue Return in the Chinese Solar Greenhouse. Agronomy. 2021; 11(9):1828. https://doi.org/10.3390/agronomy11091828
Chicago/Turabian StyleWei, Xiaoxuan, Yansu Li, Xiaoguang Fan, Chaoxing He, Yan Yan, Mintao Sun, Chaowu Ding, Jun Wang, and Xianchang Yu. 2021. "Techno-Economic Feasibility of In Situ Vegetable Residue Return in the Chinese Solar Greenhouse" Agronomy 11, no. 9: 1828. https://doi.org/10.3390/agronomy11091828