Temperatures Outside the Optimal Range for Helicobacter pylori Increase Its Harboring within Candida Yeast Cells
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Strains
2.2. Strains Culture
2.3. Growth Curves of H. pylori and Candida Strains at 4 °C, 25 °C, 37 °C or 40 °C
2.4. Co-Cultures of H. pylori Strains with C. albicans Strains
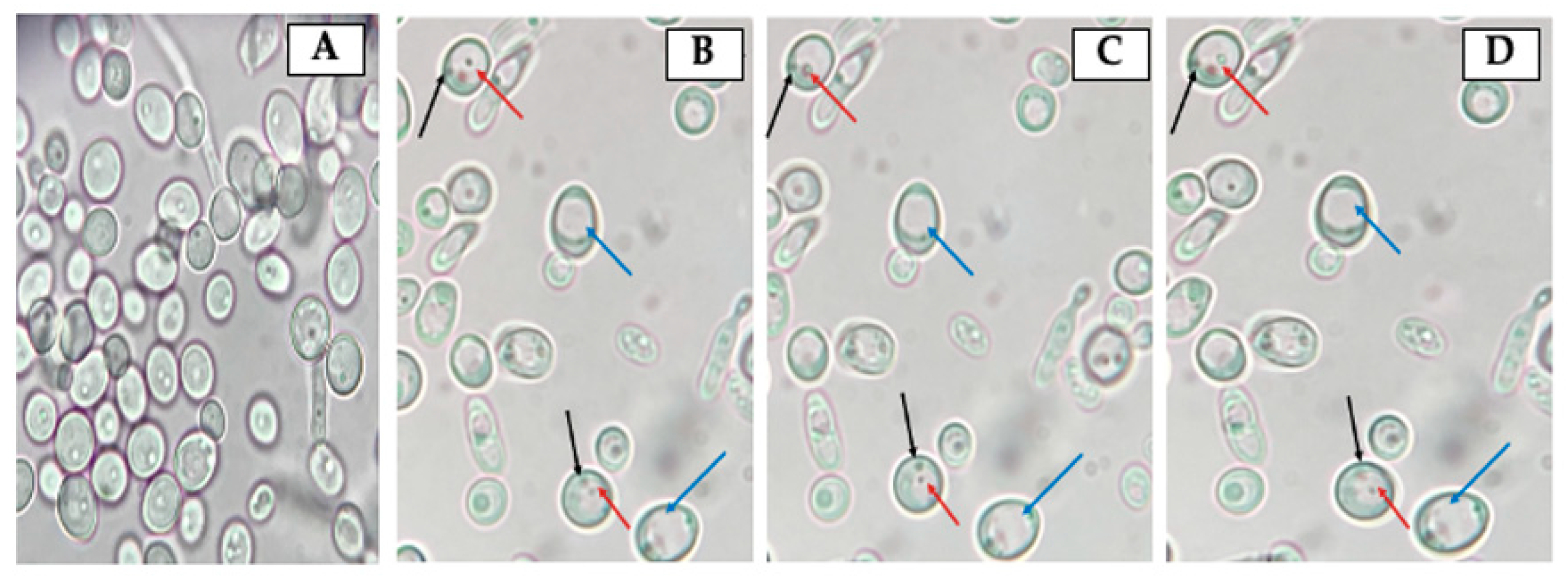
2.5. Search for Yeast Cells Containing Bacteria-Like Bodies (Y-BLBs)
2.6. Identification of Intra-Yeast BLBs as H. pylori Using the FISH Technique
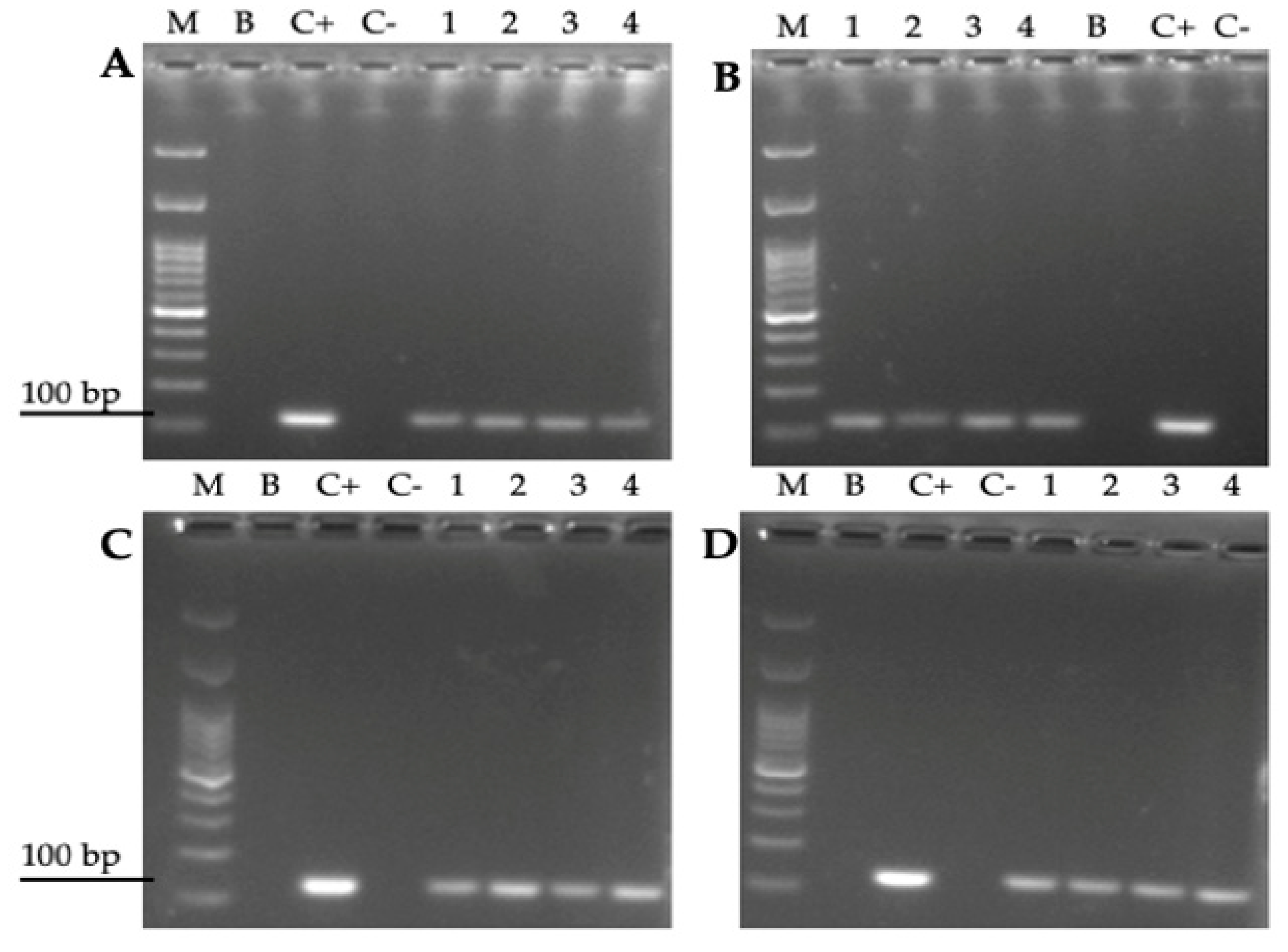
2.7. Detection of the H. pylori 16S rRNA Gene in Total DNA Extracted from Y-BLBs
2.8. Cell Viability Assay
2.9. Statistical Analysis
3. Results
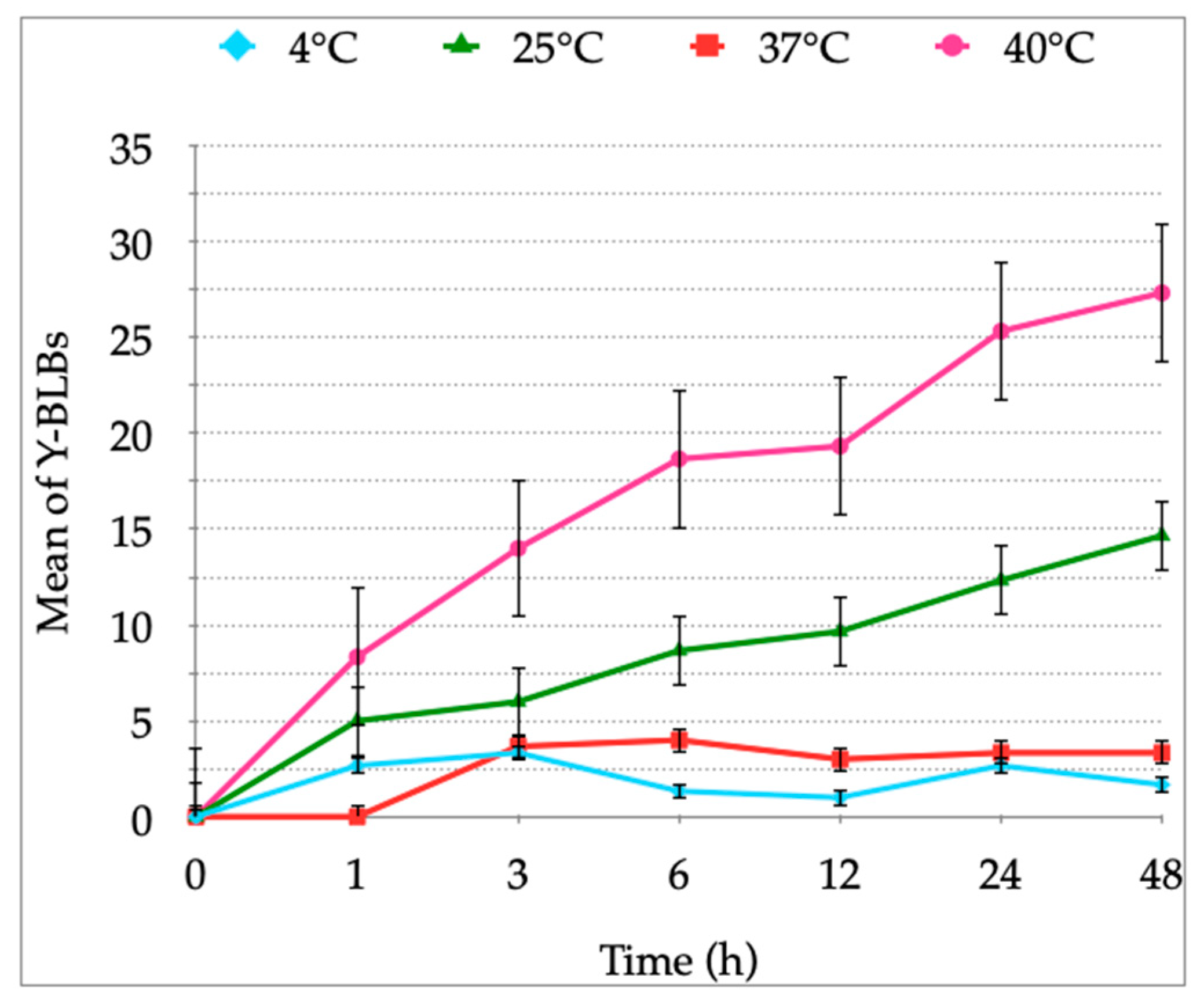
3.1. Growth Curves of H. pylori and Candida Strains at Different Temperatures
3.2. Identification of Bacteria-Like Bodies (BLBs) within Yeast Cells after Co-Culturing Both Microorganisms at Different Temperatures
3.3. Confirmation, by FISH, That BLBs Correspond to Intra-Yeast H. pylori
3.4. Amplification of the H. pylori 16S rRNA Gene from Total DNA Extracted from Yeast Cells Previously Co-Cultured with the Bacterium
3.5. Cell Viability Assays
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hathroubi, S.; Servetas, S.L.; Windham, I.; Merrell, D.S.; Ottemann, K.M. Helicobacter pylori Biofilm Formation and Its Potential Role in Pathogenesis. Microbiol. Mol. Biol. Rev. 2018, 82, 00001–00018. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Warren, J.R. Gastric Pathology Associated with Helicobacter pylori. Gastroenterol. Clin. N. Am. 2000, 29, 705–751. [Google Scholar] [CrossRef]
- Talaei, R.; Souod, N.; Momtaz, H.; Dabiri, H. Milk of livestock as a possible transmission route of Helicobacter pylori infection. Gastroenterol. Hepatol. Bed Bench 2015, 8, S30–S36. [Google Scholar]
- Quaglia, N.C.; Dambrosio, A.; Normanno, G.; Parisi, A.; Firinu, A.; Lorusso, V.; Celano, G.V. Survival of Helicobacter pylori in artificially contaminated ultrahigh temperature and pasteurized milk. Food Microbiol. 2007, 24, 296–300. [Google Scholar] [CrossRef] [PubMed]
- Quaglia, N.C.; Dambrosio, A. Helicobacter pylori: A foodborne pathogen? World J. Gastroenterol. 2018, 24, 3472–3487. [Google Scholar] [CrossRef]
- Siavoshi, F.; Sahraee, M.; Ebrahimi, H.; Sarrafnejad, A.; Saniee, P. Natural fruits, flowers, honey, and honeybees harbor Helicobacter pylori-positive yeasts. Helicobacter 2018, 23, e12471. [Google Scholar] [CrossRef]
- Velázquez, M.; Feirtag, J.M. Helicobacter pylori: Characteristics, pathogenicity, detection methods and mode of transmission implicating foods and water. Int. J. Food Microbiol. 1999, 53, 95–104. [Google Scholar] [CrossRef]
- Nilsson, H.-O.; Blom, J.; Abu Al-Soud, W.; Ljungh, A.A.; Andersen, L.P.; Wadström, T. Effect of Cold Starvation, Acid Stress, and Nutrients on Metabolic Activity of Helicobacter pylori. Appl. Environ. Microbiol. 2002, 68, 11–19. [Google Scholar] [CrossRef] [Green Version]
- Ratkowsky, D.A.; Olley, J.; McMeekin, T.A.; Ball, A. Relationship between temperature and growth rate of bacterial cultures. J. Bacteriol. 1982, 149, 1–5. [Google Scholar] [CrossRef] [Green Version]
- Huang, L.; Hwang, A.; Phillips, J. Effect of Temperature on Microbial Growth Rate-Mathematical Analysis: The Arrhenius and Eyring-Polanyi Connections. J. Food Sci. 2011, 76, E553–E560. [Google Scholar] [CrossRef]
- Charpe, A.; Sedani, S.; Murumkar, R.; Bhad, R. Effect of Temperature on Microbial Growth in Food during Storage. Multilogic Sci. 2019, 8, 56–58. [Google Scholar]
- Sjomina, O.; Pavlova, J.; Niv, Y.; Leja, M. Epidemiology of Helicobacter pylori infection. Helicobacter 2018, 23, e12514. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Porras, C.; Nodora, J.; Sexton, R.; Ferreccio, C.; Jimenez, S.; Dominguez, R.L.; Cook, P.; Anderson, G.; Morgan, D.R.; Baker, L.H.; et al. Epidemiology of Helicobacter pylori infection in six Latin American countries (SWOG Trial S0701). Cancer Causes Control. 2012, 24, 209–215. [Google Scholar] [CrossRef] [PubMed]
- O’Ryan, M.L.; Lucero, Y.; Rabello, M.; Mamani, N.; Salinas, A.M.; Peña, A.; Torres-Torreti, J.P.; Mejías, A.; Ramilo, O.; Suarez, N.; et al. Persistent and Transient Helicobacter pylori Infections in Early Childhood. Clin. Infect. Dis. 2015, 61, 211–218. [Google Scholar] [CrossRef]
- Khoder, G.; Muhammad, J.S.; Mahmoud, I.; Soliman, S.S.M.; Burucoa, C. Prevalence of Helicobacter pylori and Its Associated Factors among Healthy Asymptomatic Residents in the United Arab Emirates. Pathogens 2019, 8, 44. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Robinson, K.; Atherton, J.C. The Spectrum of Helicobacter-Mediated Diseases. Annu. Rev. Pathol. Mech. Dis. 2021, 16, 123–144. [Google Scholar] [CrossRef]
- Alba, C.; Blanco, A.; Alarcón, T. Antibiotic resistance in Helicobacter pylori. Curr. Opin. Infect. Dis. 2017, 30, 489–497. [Google Scholar] [CrossRef]
- Yang, T.; Li, H.; Chen, J.; Zeng, W.; Mao, J.; Zhang, Z.; Yang, J.; Yang, N.; Tu, M.; Zhang, J. Epidemiological study on antibiotic resistance among Helicobacter pylori in Taizhou district, Zhejiang, 2010–2013. Zhonghua Liu Xing Bing Xue Za Zhi 2014, 35, 704–707. [Google Scholar]
- Dang, B.N.; Graham, D.Y. Helicobacter pylori infection and antibiotic resistance: A WHO high priority? Nat. Rev. Gastroenterol. Hepatol. 2017, 14, 383–384. [Google Scholar] [CrossRef] [PubMed]
- Malfertheiner, P.; Megraud, F.; O’Morain, C.A.; Gisbert, J.P.; Kuipers, E.J.; Axon, A.T.; Bazzoli, F.; Gasbarrini, A.; Atherton, J.; Graham, D.Y.; et al. Management of Helicobacter pylori infection—the Maastricht V/Florence Consensus Report. Gut 2017, 66, 6–30. [Google Scholar] [CrossRef] [Green Version]
- Warren, J.R.; Marshall, B. Unidentified curved bacilli on gastric epithelium in active chronic gastritis. Lancet 1983, 1, 1273–1275. [Google Scholar]
- Mladenova, I.; Durazzo, M. Transmission of Helicobacter pylori. Minerva Gastroenterol. Dietol. 2018, 64, 251–254. [Google Scholar] [CrossRef]
- Cellini, L. Helicobacter pylori: A chameleon-like approach to life. World J. Gastroenterol. 2014, 20, 5575–5582. [Google Scholar] [CrossRef] [PubMed]
- De Francesco, V.; Zullo, A.; Hassan, C.; Giorgio, F.; Rosania, R.; Ierardi, E. Mechanisms of Helicobacter pylori antibiotic resistance: An updated appraisal. World J. Gastrointest. Pathophysiol. 2011, 2, 35–41. [Google Scholar] [CrossRef] [PubMed]
- Yonezawa, H.; Osaki, T.; Hanawa, T.; Kurata, S.; Ochiai, K.; Kamiya, S. Impact of Helicobacter pylori Biofilm Formation on Clarithromycin Susceptibility and Generation of Resistance Mutations. PLoS ONE 2013, 8, e73301. [Google Scholar] [CrossRef]
- Azevedo, N.F.; Almeida, C.; Cerqueira, L.; Dias, S.; Keevil, C.W.; Vieira, M.J. Coccoid Form of Helicobacter pylori as a Morphological Manifestation of Cell Adaptation to the Environment. Appl. Environ. Microbiol. 2007, 73, 3423–3427. [Google Scholar] [CrossRef] [Green Version]
- Chu, Y.-T.; Wang, Y.-H.; Wu, J.-J.; Lei, H.-Y. Invasion and Multiplication of Helicobacter pylori in Gastric Epithelial Cells and Implications for Antibiotic Resistance. Infect. Immun. 2010, 78, 4157–4165. [Google Scholar] [CrossRef] [Green Version]
- Dubois, A.; Borén, T. Helicobacter pylori is invasive and it may be a facultative intracellular organism. Cell. Microbiol. 2007, 9, 1108–1116. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, H.; Semino-Mora, C.; Dubois, A. Mechanism of H. pylori Intracellular Entry: An in vitro Study. Front. Cell. Infect. Microbiol. 2012, 2, 13. [Google Scholar] [CrossRef] [Green Version]
- Lai, C.-H.; Huang, J.-C.; Cheng, H.-H.; Wu, M.-C.; Huang, M.-Z.; Hsu, H.-Y.; Chen, Y.-A.; Hsu, C.-Y.; Pan, Y.-J.; Chu, Y.-T.; et al. Helicobacter pylori cholesterol glucosylation modulates autophagy for increasing intracellular survival in macrophages. Cell. Microbiol. 2018, 20, e12947. [Google Scholar] [CrossRef]
- Amieva, M.R.; Salama, N.R.; Tompkins, L.S.; Falkow, S. Helicobacter pylori enter and survive within multivesicular vacuoles of epithelial cells. Cell. Microbiol. 2002, 4, 677–690. [Google Scholar] [CrossRef]
- Moreno-Mesonero, L.; Moreno, Y.; Alonso, J.L.; Ferrus, M.A. DVC-FISH and PMA-qPCR techniques to assess the survival of Helicobacter pylori inside Acanthamoeba castellanii. Res. Microbiol. 2016, 167, 29–34. [Google Scholar] [CrossRef] [PubMed]
- Moreno-Mesonero, L.; Moreno, Y.; Alonso, J.L.; Ferrus, M.A. Detection of viable Helicobacter pylori inside free-living amoebae in wastewater and drinking water samples from Eastern Spain. Environ. Microbiol. 2017, 19, 4103–4112. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moreno-Mesonero, L.; Hortelano, I.; Moreno, Y.; Ferrús, M.A. Evidence of viable Helicobacter pylori and other bacteria of public health interest associated with free-living amoebae in lettuce samples by next generation sequencing and other molecular techniques. Int. J. Food Microbiol. 2020, 318, 108477. [Google Scholar] [CrossRef]
- Siavoshi, F.; Taghikhani, A.; Malekzadeh, R.; Sarrafnejad, A.; Kashanian, M.; Jamal, A.S.; Saniee, P.; Sadeghi, S.; Sharifi, A.H. The role of mother’s oral and vaginal yeasts in transmission of Helicobacter pylori to neonates. Arch. Iran. Med. 2013, 16, 288–294. [Google Scholar]
- Salmanian, A.-H.; Siavoshi, F.; Akbari, F.; Afshari, A.; Malekzadeh, R. Yeast of the oral cavity is the reservoir of Heliobacter pylori. J. Oral Pathol. Med. 2008, 37, 324–328. [Google Scholar] [CrossRef]
- Salmanian, A.-H.; Siavoshi, F.; Beyrami, Z.; Latifi-Navid, S.; Tavakolian, A.; Sadjadi, A. Foodborne Yeasts Serve as Reservoirs of Helicobacter pylori. J. Food Saf. 2011, 32, 152–160. [Google Scholar] [CrossRef]
- Sánchez-Alonzo, K.; Parra-Sepúlveda, C.; Vergara, L.; Bernasconi, H.; García-Cancino, A. Detection of Helicobacter pylori in oral yeasts from students of a Chilean university. Rev. Assoc. Médica Bras. 2020, 66, 1509–1514. [Google Scholar] [CrossRef]
- García-Ríos, E.; Morard, M.; Parts, L.; Liti, G.; Guillamón, J.M. The genetic architecture of low-temperature adaptation in the wine yeast Saccharomyces cerevisiae. BMC Genom. 2017, 18, 1–13. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Takagi, Y.; Fukano, H.; Shimozato, K.; Tanaka, R.; Horii, T.; Kawamoto, F.; Kanbe, T. Genotypes of Candida albicans isolated from healthy individuals and their distribution in patients with oral candidiasis. J. Infect. Chemother. 2013, 19, 1072–1079. [Google Scholar] [CrossRef]
- Angebault, C.; Djossou, F.; Abélanet, S.; Permal, E.; Ben Soltana, M.; Diancourt, L.; Bouchier, C.; Woerther, P.-L.; Catzeflis, F.; Andremont, A.; et al. Candida albicans Is Not Always the Preferential Yeast Colonizing Humans: A Study in Wayampi Amerindians. J. Infect. Dis. 2013, 208, 1705–1716. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bandara, H.M.H.N.; Panduwawala, C.P.; Samaranayake, L.P. Biodiversity of the human oral mycobiome in health and disease. Oral Dis. 2019, 25, 363–371. [Google Scholar] [CrossRef] [PubMed]
- Lemos-Carolino, M.; Madeira-Lopes, A.; van Uden, N. The temperature profile of the pathogenic yeast Candida albicans. J. Basic Microbiol. 1982, 22, 705–709. [Google Scholar] [CrossRef]
- Ansorg, R.; Schmid, E.N. Adhesion of Helicobacter pylori to yeast cells. Zent. Bakteriol. 1998, 288, 501–508. [Google Scholar] [CrossRef]
- Rai, P.; Chakraborty, S.B. Giant fungal gastric ulcer in an immunocompetent individual. Saudi J. Gastroenterol. Off. J. Saudi Gastroenterol. Assoc. 2012, 18, 282–284. [Google Scholar] [CrossRef]
- Ince, A.T.; Kocaman, O.; Ismailova, M.; Tozlu, M.; Gücin, Z.; Iraz, M. A rare co-existence of Helicobacter pylori, Candida albicans and Candida kefyr in a giant gastric ulcer. Turk. J. Gastroenterol. 2014, 25, 435–436. [Google Scholar] [CrossRef]
- Sánchez-Alonzo, K.; Belmar, L.; Parra-Sepúlveda, C.; Bernasconi, H.; Campos, V.L.; Smith, C.T.; Sáez, K.; García-Cancino, A. Antibiotics as a Stressing Factor Triggering the Harboring of Helicobacter pylori J99 within Candida albicans ATCC10231. Pathogens 2021, 10, 382. [Google Scholar] [CrossRef]
- Sánchez-Alonzo, K.; Silva-Mieres, F.; Arellano-Arriagada, L.; Parra-Sepúlveda, C.; Bernasconi, H.; Smith, C.T.; Campos, V.L.; García-Cancino, A. Nutrient Deficiency Promotes the Entry of Helicobacter pylori Cells into Candida Yeast Cells. Biology 2021, 10, 426. [Google Scholar] [CrossRef]
- Sánchez-Alonzo, K.; Parra-Sepúlveda, C.; Vega, S.; Bernasconi, H.; Campos, V.L.; Smith, C.T.; Sáez, K.; García-Cancino, A. In Vitro Incorporation of Helicobacter pylori into Candida albicans Caused by Acidic pH Stress. Pathogens 2020, 9, 489. [Google Scholar] [CrossRef]
- Rüssmann, H.; Kempf, V.A.J.; Koletzko, S.; Heesemann, J.; Autenrieth, I.B. Comparison of Fluorescent in Situ Hybridization and Conventional Culturing for Detection of Helicobacter pylori in Gastric Biopsy Specimens. J. Clin. Microbiol. 2001, 39, 304–308. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, H.; Huaiyang, Z.; Li, J.; Gao, H.; Li, P.; Zhou, H. The impact of temperature on microbial diversity and AOA activity in the Tengchong Geothermal Field, China. Sci. Rep. 2015, 5, 17056. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aragno, M. Responses of Microorganisms to Temperature. In Physiological Plant Ecology I: Responses to the Physical Environment; Lange, O.L., Nobel, P.S., Osmond, C.B., Ziegler, H., Eds.; Springer: Berlin/Heidelberg, Germany, 1981; pp. 339–369. [Google Scholar]
- Jiang, X.; Doyle, M.P. Effect of Environmental and Substrate Factors on Survival and Growth of Helicobacter pylori. J. Food Prot. 1998, 61, 929–933. [Google Scholar] [CrossRef] [PubMed]
- Krzyżek, P.; Gościniak, G. Morphology of Helicobacter pylori as a result of peptidoglycan and cytoskeleton rearrangements. Gastroenterol. Rev. 2018, 13, 182–195. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kusters, J.G.; Gerrits, M.M.; van Strijp, J.A.G.; Vandenbroucke-Grauls, C.M. Coccoid forms of Helicobacter pylori are the morphologic manifestation of cell death. Infect. Immun. 1997, 65, 3672–3679. [Google Scholar] [CrossRef] [Green Version]
- Nadeem, S.G.; Shafiq, A.; Hakim, S.T.; Anjum, Y.; Kazm, S.U. Effect of Growth Media, pH and Temperature on Yeast to Hyphal Transition in Candida albicans. Open J. Med Microbiol. 2013, 3, 185–192. [Google Scholar] [CrossRef] [Green Version]
- Kandror, O.; Bretschneider, N.; Kreydin, E.; Cavalieri, D.; Goldberg, A.L. Yeast Adapt to Near-Freezing Temperatures by STRE/Msn2,4-Dependent Induction of Trehalose Synthesis and Certain Molecular Chaperones. Mol. Cell 2004, 13, 771–781. [Google Scholar] [CrossRef]
- Pérez, M.C.P.; González, A.; Moreno, Y.; Ferrus, M.A. Helicobacter pylori growth pattern in reference media and extracts from selected minimally processed vegetables. Food Control. 2018, 86, 389–396. [Google Scholar] [CrossRef]
- Sudbery, P.; Gow, N.; Berman, J. The distinct morphogenic states of Candida albicans. Trends Microbiol. 2004, 12, 317–324. [Google Scholar] [CrossRef]
- Sun, L.; Liao, K.; Wang, D. Effects of Magnolol and Honokiol on Adhesion, Yeast-Hyphal Transition, and Formation of Biofilm by Candida albicans. PLoS ONE 2015, 10, e0117695. [Google Scholar] [CrossRef] [Green Version]
- Kumar, K.; Askari, F.; Sahu, M.S.; Kaur, R. Candida glabrata: A Lot More Than Meets the Eye. Microorganisms 2019, 7, 39. [Google Scholar] [CrossRef] [Green Version]
- Rodrigues, M.E.; Gomes, F.; Rodrigues, C.F. Candida spp./Bacteria Mixed Biofilms. J. Fungi 2019, 6, 5. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- O’Donnell, L.E.; Millhouse, E.; Sherry, L.; Kean, R.; Malcolm, J.; Nile, C.J.; Ramage, G. Polymicrobial Candida biofilms: Friends and foe in the oral cavity. FEMS Yeast Res. 2015, 15, 15. [Google Scholar] [CrossRef] [Green Version]






Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sánchez-Alonzo, K.; Arellano-Arriagada, L.; Castro-Seriche, S.; Parra-Sepúlveda, C.; Bernasconi, H.; Benavidez-Hernández, H.; Campos, V.L.; Sáez, K.; Smith, C.T.; García-Cancino, A. Temperatures Outside the Optimal Range for Helicobacter pylori Increase Its Harboring within Candida Yeast Cells. Biology 2021, 10, 915. https://doi.org/10.3390/biology10090915
Sánchez-Alonzo K, Arellano-Arriagada L, Castro-Seriche S, Parra-Sepúlveda C, Bernasconi H, Benavidez-Hernández H, Campos VL, Sáez K, Smith CT, García-Cancino A. Temperatures Outside the Optimal Range for Helicobacter pylori Increase Its Harboring within Candida Yeast Cells. Biology. 2021; 10(9):915. https://doi.org/10.3390/biology10090915
Chicago/Turabian StyleSánchez-Alonzo, Kimberly, Luciano Arellano-Arriagada, Susana Castro-Seriche, Cristian Parra-Sepúlveda, Humberto Bernasconi, Héctor Benavidez-Hernández, Víctor L. Campos, Katia Sáez, Carlos T. Smith, and Apolinaria García-Cancino. 2021. "Temperatures Outside the Optimal Range for Helicobacter pylori Increase Its Harboring within Candida Yeast Cells" Biology 10, no. 9: 915. https://doi.org/10.3390/biology10090915
APA StyleSánchez-Alonzo, K., Arellano-Arriagada, L., Castro-Seriche, S., Parra-Sepúlveda, C., Bernasconi, H., Benavidez-Hernández, H., Campos, V. L., Sáez, K., Smith, C. T., & García-Cancino, A. (2021). Temperatures Outside the Optimal Range for Helicobacter pylori Increase Its Harboring within Candida Yeast Cells. Biology, 10(9), 915. https://doi.org/10.3390/biology10090915







