Direct Selective Oxidation of Hydrogen Sulfide: Laboratory, Pilot and Industrial Tests
Abstract
:1. Introduction
- adsorption methods
- absorption methods
- catalytic methods
- multistage operation
- insufficient environmental safety, due to the presence of a high-temperature furnace in the technological chain, which is a source of toxic byproducts
- a limited range of applications (thus, it is impossible to treat gases with hydrogen sulfide contents below 20 vol.% or gas streams with flow rates below 1000 Nm3/h).
2. Direct Selective Oxidation in the Liquid Phase. RedOx Processes
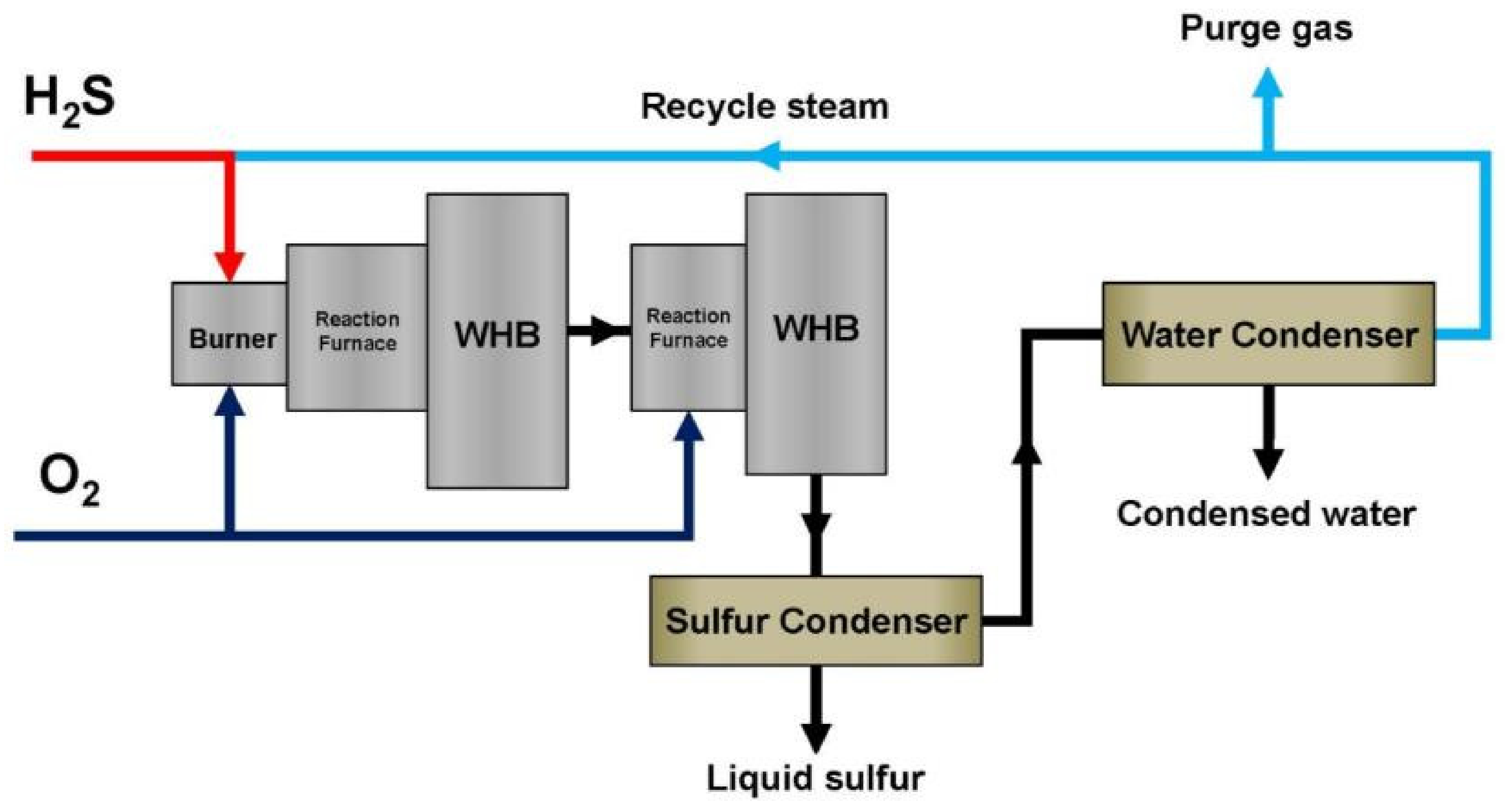
3. Claus Process
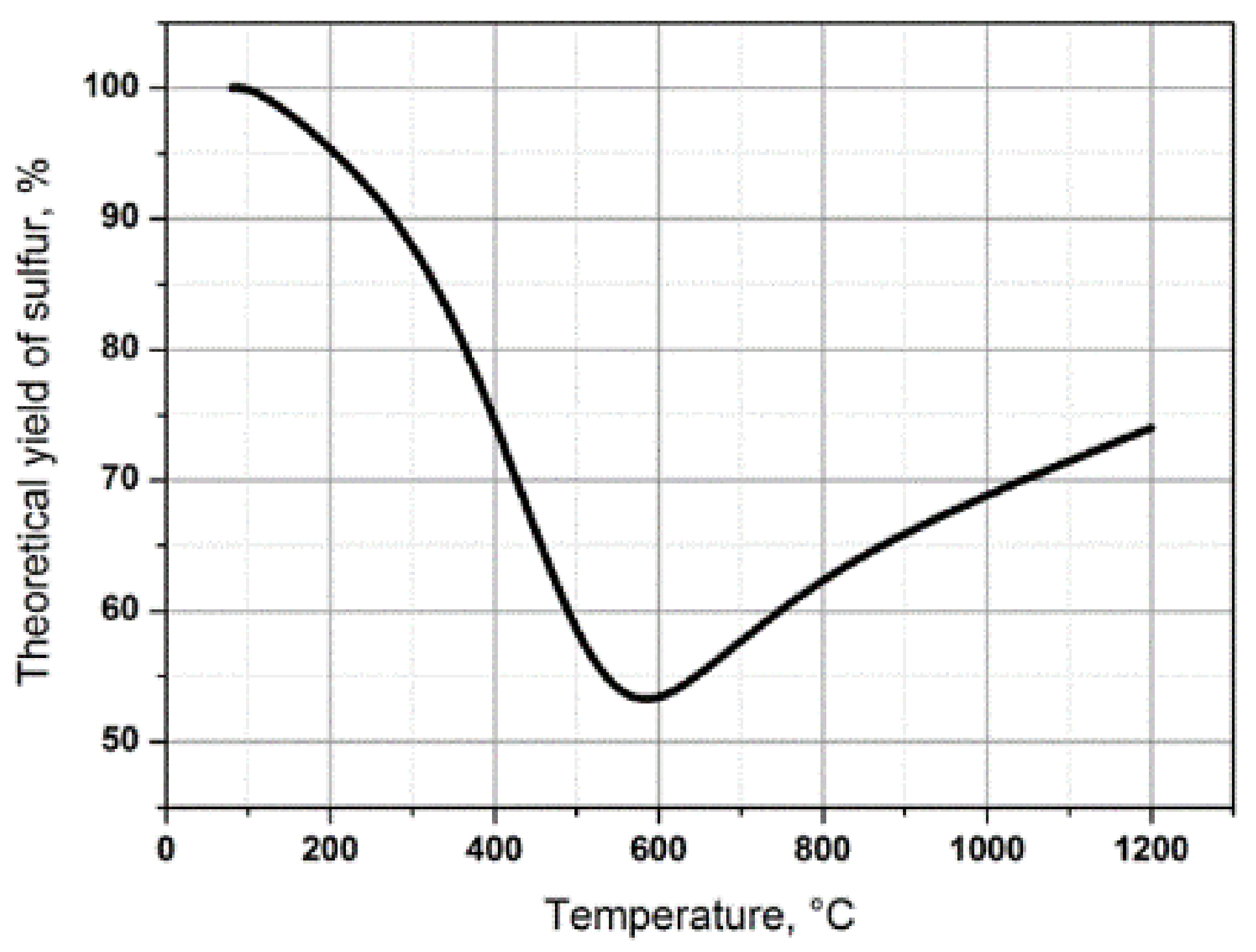
4. The Thermal Stage of the Claus Process. Process Conditions. Chemical Reactions Proceeding in the System
- The reheating of the initial gas streams, acid gas and air: Even at a concentration of hydrogen sulfide in the acid gas of 40 vol.%, it is necessary to heat initial gas streams to 300 °C to reach the lower threshold of the stable operation of the Claus furnace, i.e., 1050 °C. In practice, as the experience of operating Claus installations at the Orenburg GPP shows, considering the essential heat loss, the preheating temperature can be as high as 600 °C.
- The use of oxygen-enriched air as an oxidant: Even at hydrogen sulfide concentrations in the acid gas of 50%, the required oxygen concentration in the supplied air should be at least 50 vol.% in order to reach the lower threshold of the stable operation of the Claus furnace.
- Supply of hydrocarbon fuel gas to the flame furnace: A supply of fuel gas at 25–30% of the acid gas flow rate with a high H2S concentration will not provide the necessary temperature in the furnace to maintain stable operation. The heat of the combustion of hydrogen sulfide is utilized by heating chemically purified water, with water vapor production in a waste heat boiler. The hot gas passes through the boiler tubes and heats the water therein to boiling point. The gas cooled in the boiler is sent to the condenser, where it is cooled further to about 150 °C.
5. Catalytic Stage of the Claus Process. Catalysts Used
6. Claus Process. Enhancement. Oxygen Enrichment
7. PROClaus Process
8. SuperClaus Process
9. Modifications of the Claus Process
- The generation of additional synthesis gas
- Complete recovery of hydrogen sulfide in the form of elementary sulfur
- The utilization of carbon dioxide.
10. Modern Trends in the Field of Hydrogen Sulfide Treatment with the Formation of Elemental Sulfur. Direct Heterogeneous Catalytic Oxidation of Hydrogen Sulfide to Elemental Sulfur
- the single-step characteristic and continuity;
- “soft” conditions (T = 220–280 °C) due to the use of highly active catalysts, which allow for the oxidation of hydrogen sulfide directly in the composition of hydrocarbon.
11. Chemism of the Process of Direct Catalytic Oxidation of Hydrogen Sulfide
12. Main Types of Catalysts Used in the Process of Direct Heterogeneous Oxidation of Hydrogen Sulfide. Industrial Processes. Brief Description of the Most Common Catalysts for the Hydrogen Sulfide Oxidation Reaction with Oxygen to Elementary Sulfur
13. Activated Carbon. Catalysts Based on Activated Carbon
14. Catalysts Based on Carbon Nanotubes
15. Carbon Nanofiber-Based Catalysts
- burning out sulfur at elevated temperatures
- treatment of catalyst/sorbent with steam, with resulting hydrogen sulfide formation
- washing catalyst with an organic solvent, effectively dissolving sulfur.
16. Zeolite Catalysts for Direct Oxidation of Hydrogen Sulfide
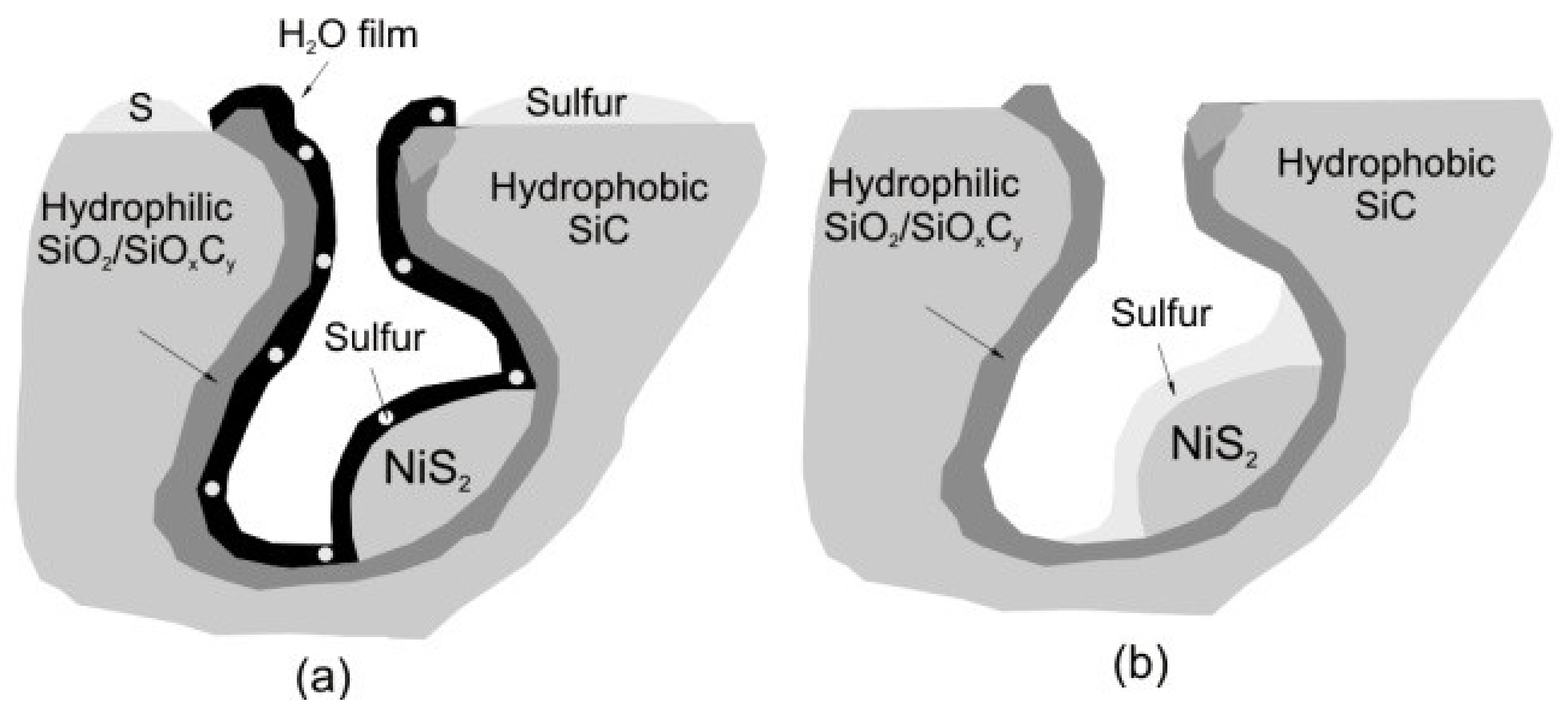
17. Catalysts Based on Sic
- The chemical inertness of the material allows the use of catalysts in aggressive media, providing high stability of catalysts;
- High SiC thermal conductivity (150 W/m·K) compared to alumina (15 W/m·K) ensures a uniform temperature distribution in the catalyst bed and prevents local overheating of the catalyst;
- SiC-based catalysts can be used to remove H2S from highly concentrated gases (>2 vol.%);
- The meso- and macroporous SiC structure allows the use of catalysts for the oxidation of hydrogen sulfide at temperatures below the dew point or in the presence of excess water.
18. Transition Metal Oxides
- The content of hydrogen sulfide in the feed, vol.% 20;
- Gas hourly space velocity, h−1 7200;
- Hydrogen sulfide/oxygen polar ratio 2/1;
- Temperature range of testing, °C 200–300;
- The geometric shape of catalysts spherical granules;
- Active component individual oxides of cobalt;
- manganese, chromium;
- Vanadium;
- The active component content, wt.% 0.1–0.6.
- Co > V > Fe = Cr > Mn > γ-Al2O3 (at T > 250 °C);
- V > Fe = Cr > Co > Mn > γ-Al2O3 (at T < 250 °C).
19. Description of Modern Industrial Methods Based on the Process of Direct H2S Oxidation
- Baseline magnesium-chromium oxide catalyst MgCr2O4/γ-Al2O3
- Iron oxide catalyst Fe2O3/γ-Al2O3
- γ-alumina γ-Al2O3.
20. Developments of the Boreskov Institute of Catalysis SB RAS Regarding the Creation of Processes of Heterogeneous Catalytic Oxidation of Hydrogen Sulfide for the Treatment of Various Gases
- The preservation of qualitative and quantitative composition of hydrocarbons, and
- The absence of hydrogen in the reaction products after the reactor.
- Hydrogen sulfide is almost completely removed on the first three dm of the catalyst bed; that is, its concentration drops significantly, i.e., to below the explosion limit.
- The reaction proceeds solely on the surface of the catalyst, so the transition of the process into the reactor volume proceeding according to the homogeneous chain (explosive) mechanism is excluded. Thus, the catalyst bed acts essentially as an effective flame arrester.
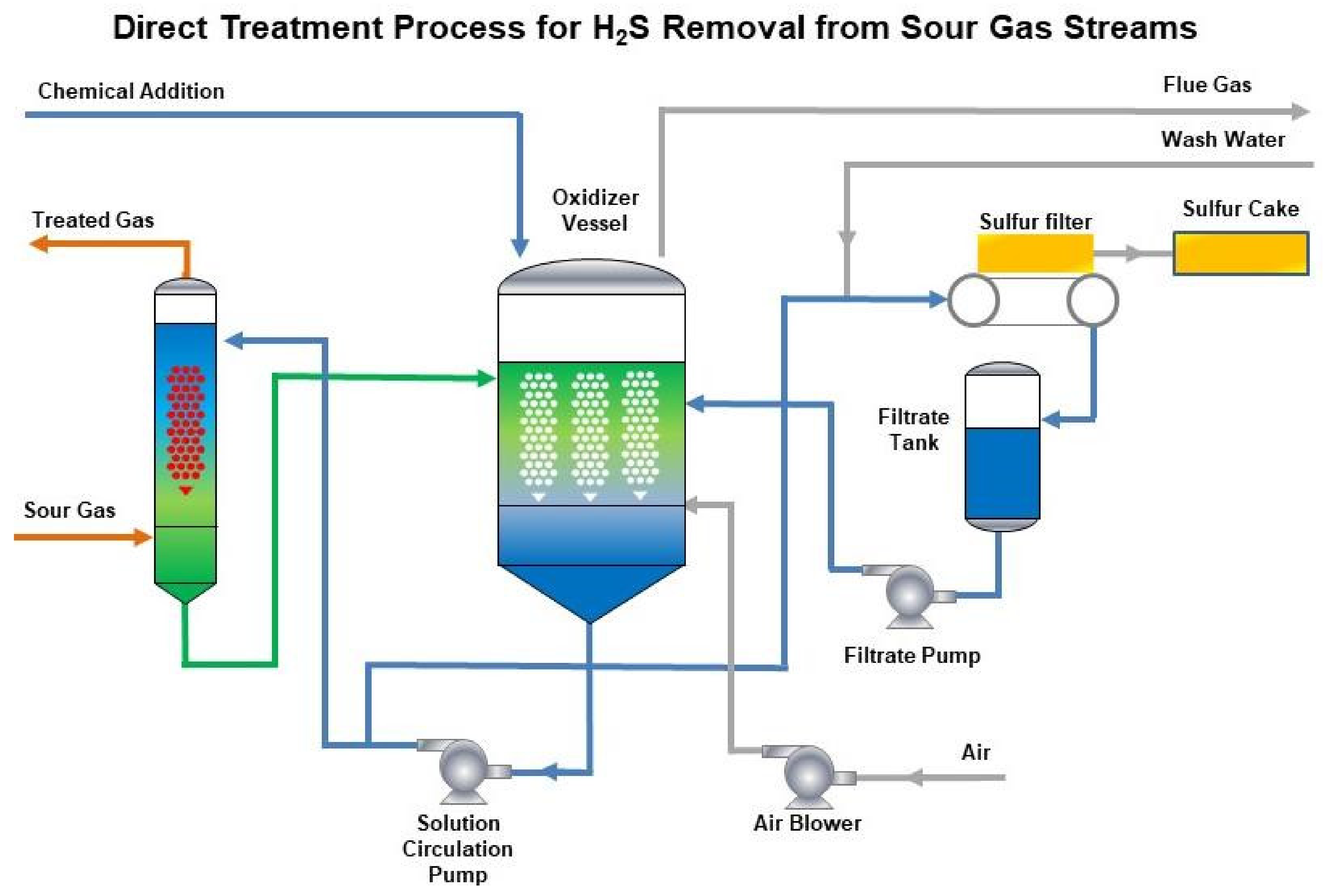
21. Installation for H2S Recovery from Acid Gas after the Amine Treatment of Oil-Associated Gases at Bavly Gas Shop of PJSC Tatneft
- Over 1 billion m3 of purified gas produced
- 6000 tons of hydrogen sulfide converted to elementary sulfur
- Emission of 12,000 tons of sulfur dioxide and sulfuric acid (340 railway tanks) into the atmosphere prevented;
- Environmental damage amounting to about 2.9 billion rubles avoided
- One-stage technology with computer control providing stable operation with variable parameters in terms of the acid gas (for example, hydrogen sulfide content).
22. Facility for the Purification of Gases Caused by Blowing-off Sour Crude Oil
- The basic design of the technology has been finalized.
- The design and working documentation have been presented.
- The various apparatus units have been fabricated (Figure 26);
- The block of the plant has been delivered to the customer (Figure 27);
- The technology achieves the direct catalytic oxidation of hydrogen sulfide via the use of acid gases. It is an alternative to the Claus process (MTU-0.5 Mini Plant, Republic of Kazakhstan).
23. Unit for the Direct Oxidation of Hydrogen Sulfide as a Component of the Associated Petroleum Gas
24. Conclusions
- continuity of the process that allows simultaneous gas purification and the production of a commodity, i.e., elemental sulfur;
- “soft” conditions for implementing the process (T = 220–280 °C) due to the use of a highly active catalyst.
- An installation for the purification of blow-off gases of high-sulfur crude oil
- An installation for the direct oxidation of hydrogen sulfide as an alternative to the conventional Claus Process
- An installation for the direct oxidation of hydrogen sulfide in the composition of oil-associated gases
- The production of commercial products, i.e., fuel gas and sulfur that correspond to technical standards (GOST 5542-87 and GOST 127.1-93, respectively)
- Extended operational range by H2S content in comparison with Claus units
- Substantial improvement of the environmental situation by avoiding hazardous emissions and the production of waste materials.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
Abbreviations
| AC | Activated carbon |
| APG | Associated petroleum gas |
| BAS | Broensted acid sites |
| CNF | Carbon nanofibers |
| CNT | Carbon nanotubes |
| DEA | Diethanolamine |
| EDTA | Ethylenediaminetetraacetic acid |
| DRS | Diffuse reflectance spectra |
| FRC | Federal Research Center |
| FTIR | Furier transform infrared |
| GHSV | Gas hourly space velocity |
| GPP | Gas processing plant |
| JSC | Joint-stock company |
| k | Rate constant |
| LAS | Lewis acid sites |
| LLC | Limited liability company |
| MEA | Monoethanolamine |
| Nm3 | Normal cubic meters |
| OAG | Oil-associated gases |
| ppmv | Part per million by volume |
| PJSC | Public joint-stock company |
| SB RAS | Siberian Branch of the Russian Academy of Sciences |
| W | Reaction rate |
| WHSV | Weight hourly space velocity |
| WHB | Waste heat boiler |
References
- Hendrickson, R.G.; Chang, A.; Hamilton, R.J. Fatalities from Hydrogen Sulfide. Am. J. Ind. Med. 2004, 45, 346–350. [Google Scholar] [CrossRef] [PubMed]
- Chou, C.H.S.J. Hydrogen Sulfide: Human Health Aspects; World Health Organization: Geneva, Switzerland, 2003. [Google Scholar]
- Ministry of Justice of the Russian Federation. Hygienic Standard 2.2.5.3532-18; Ministry of Justice of the Russian Federatio: Moscow, Russia, 2018.
- Chief State Sanitary Doctor of the Russian Federation. Hygienic Standard 2.1.6.3492-17; Chief State Sanitary Doctor of the Russian Federation: Moscow, Russia, 2017. [Google Scholar]
- Lallemand, F.; Lecomte, F.; Streicher, C. Highly sour gas processing: h2s bulk removal with the sprex process. In Proceedings of the International Petroleum Technology Conference, Doha, Qatar, 21 November 2005. [Google Scholar]
- Faramawy, S.; Zaki, T.; Sakr, A.A.-E. Natural Gas Origin, Composition, and Processing: A Review. J. Nat. Gas Sci. Eng. 2016, 34, 34–54. [Google Scholar] [CrossRef]
- Ponkratov, V.V.; Pozdnyaev, A.S. Tax and Regulatory Incentives to Improve Utilization of Associated Petroleum Gas (APG). Russ. Econ. Taxes Law 2014, 5, 88–94. [Google Scholar]
- World Energy Outlook. Available online: http://www.worldenergyoutlook.org/ (accessed on 9 September 2021).
- Mokhatab, S.; Poe, W.A. Handbook of Natural Gas Transmission and Processing, 2nd ed.; Gulf Professional Publishing: Houston, TX, USA, 2012. [Google Scholar]
- Merichem Company. LO-CAT® Process for Cost Effective Desulfurization of All Types of Gas Streams; Merichem Company: Houston, TX, USA, 2013. [Google Scholar]
- Hydrocarbon Processing. Available online: http://hydrocarbon.processengineer.info/sulferox-process-by-shell.html (accessed on 9 September 2021).
- Mazgarov, A.M. Liquid Phase Oxidations of Mercaptans and Hydrogen Sulfide with Metal Phthalocyanine Catalysts and Development of Pro-Cesses for Desulfurization of Hydrocarbon Feed. Ph.D. Thesis, Kazan University of Chemical Technology, Kazan, Russia, 2004. [Google Scholar]
- Mokhatab, S.; Mak, J.Y. Gas Processing Plant Automation. In Handbook of Natural Gas Transmission and Processing, 2nd ed.; Elsevier Inc.: Amsterdam, The Netherlands, 2019. [Google Scholar]
- Sulfur. Available online: https://pubs.usgs.gov/periodicals/mcs2021/mcs2021-sulfur.pdf (accessed on 9 September 2021).
- Westbrook, C.K.; Dryer, F.L. Simplified Reaction Mechanisms for the Oxidation of Hydrocarbon Fuels in Flames. Combust. Sci. Technol. 1981, 27, 31–43. [Google Scholar] [CrossRef]
- Zarei, S. Life Cycle Assessment and Optimization of Claus Reaction Furnace through Kinetic Modeling. Chem. Eng. Res. Des. 2019, 148, 75–85. [Google Scholar] [CrossRef]
- Zarei, S. Exergetic, Energetic and Life Cycle Assessments of the Modified Claus Process. Energy 2020, 191, 116584. [Google Scholar] [CrossRef]
- Zare Nezhad, A.B.; Hosseinpour, N. Evaluation of Different Alternatives for Increasing the Reaction Furnace Temperature of Claus SRU by Chemical Equilibrium Calculations. Appl. Therm. Eng. 2008, 28, 738–744. [Google Scholar] [CrossRef]
- Tong, S.; Dalla Lana, I.G.; Chuang, K.T. Kinetic Modelling of the Hydrolysis of Carbonyl Sulfide Catalyzed by Either Titania or Alumina. Can. J. Chem. Eng. 1993, 71, 392–400. [Google Scholar] [CrossRef]
- Tong, S.; Dalla Lana, I.G.; Chuang, K.T. EFfect of Catalyst Shape on the Hydrolysis of COS and CS2 in a Simulated Claus Converter. Ind. Eng. Chem. Res. 1997, 36, 4087–4093. [Google Scholar] [CrossRef]
- Tong, S.; Dalla Lana, I.G.; Chuang, K.T. Kinetic Modeling of the Hydrolysis of Carbon Disulfide Catalyzed by Either Titania or Alumina. Can. J. Chem. Eng. 1995, 73, 220–227. [Google Scholar] [CrossRef]
- Mendioroz, S.; Munoz, V.; Alvarez, E.; Palacios, J.M. Kinetic Study of the Claus Reaction at Low Temperature Using γ-Alumina as Catalyst. Appl. Catal. Gen. 1995, 132, 111–126. [Google Scholar] [CrossRef]
- Claus Catalysts and Tail Gas Treatment Solutions for Sulfur Recovery. Available online: https://catalysts.basf.com/files/literature-library/BASF_CAT-001731_SRU_Bruschuere_AS-viewing.pdf (accessed on 9 September 2021).
- Scirè, S.; Fiorenza, R.; Bellardita, M.; Palmisano, L. Titanium Dioxide (TiO2) and Its Applications Catalytic Applications of TiO2. Met. Oxides 2021, 21, 637–679. [Google Scholar]
- Claus Catalysts. Available online: https://www.eurosupport.com/media/cache/20170925050616BrochureClaus.pdf (accessed on 9 September 2021).
- Available online: https://www.axens.net/solutions/catalysts-adsorbents-grading-supply/claus-catalysts (accessed on 9 September 2021).
- Ismagilov, Z.R.; Kuznetsov, V.V.; Okhlopova, L.B.; Tsickza, L.T.; Yashnik, S.A. Oxides of Titanium, Cerium, Zirconium, Yttrium and Aluminum. Properties, Applications and Methods of Production; Publishing House of SB RAS: Novosibirsk, Russia, 2010. [Google Scholar]
- Marshnyova, V.I.; Mokrinsky, V.V. Catalytic Activity of Metal Oxides in Hydrogen Sulfide Reactions with Oxygen and Sulfur Dioxide. Kinet. Catal. 1988, 29, 989–993. [Google Scholar]
- Bukhtiyarova, G.A. Development of a Polyfunctional V-Mg-Ti-Ca Catalyst for the Claus Process. Ph.D. Thesis, Boreskov Institute of Catalysis, Novosibirsk, Russia, 1999. [Google Scholar]
- Khudenko, B.; Gitman, G.M.; Wechsler, T. Oxygen Based Claus Process for Recovery of Sulfur from H2S Gases. J. Environ. Eng. 1993, 119, 1233–1251. [Google Scholar] [CrossRef]
- HeGarly, W.P.; Davis, R.; Kammiller, R. Claus Plant Capacity Boosted by Oxygen Enrichment Process. Technol. Oil Gas J. 1985, 30, 39–41. [Google Scholar]
- El-Bishtawi, R.; Haimour, N. Claus Recycle with Double Combustion Process. Fuel Process. Technol. 2004, 86, 245–260. [Google Scholar] [CrossRef]
- Kwong, K.V. Process for Direct Reduction of Sulfur Compounds to Elemental Sulfur in Combination with the Claus Process. U.S. Patent US6214311B1, 4 October 2001. [Google Scholar]
- Rameshni, M.; Street, R. PROClaus: The New Standard for Claus Performance. In Sulfur Recovery Symposium; Brimstone Engineering Services: Calgary, AB, Canada, 2001. [Google Scholar]
- Jin, Y.; Yu, Q.; Chang, S. Catalyst for the Reduction of Sulfur Dioxide to Elemental Sulfur. U.S. Patent 5494879, 27 February 1996. [Google Scholar]
- Alkhazov, T.; Meissner, R.E., III. Catalysts and Process for Selective Oxidation of Hydrogen Sulfide to Elemental Sulfur. U.S. Patent 5603913, 18 February 1997. [Google Scholar] [CrossRef]
- Alkhazov, T.G.; Amirgulyan, N.S. Catalytic Oxidation of Hydrogen Sulfide on Iron Oxides. Kinet. Catal. 1982, 23, 1130–1134. [Google Scholar]
- Borsboom, J.; Van, N.P.F. Process for the Removal of Sulphur Compounds from Gases. U.S. Patent 6800261, 5 October 2004. [Google Scholar]
- Berben, P.H.; Borsboom, J.; Geus, J.W.; Lagas, J.A. Process for Recovering Sulfur from Sulfur-Containing Gases. U.S. Patent 4988494, 29 January 1991. [Google Scholar]
- Geus, J.W.; Teroerde, R.J.A. Catalyst for the Selective Oxidation of Sulfur Compounds to Elemental Sulfur. U.S. Patent 6919296, 19 January 2005. [Google Scholar]
- Borsboom, J.A.; Lagas, J.A.; Berben, P.H. The Superclaus Process Increases Sulfur Recovery. In AT ACHEMA-88; Frankfurt Am Main: Frankfurt, Germany, 1988. [Google Scholar]
- Van Nisselrooy, P.F.M.T.; Lagas, J.A. Superclaus Reduced SO2 Emission by the Use of a Selective Oxidation Catalyst. Catal. Today 1993, 16, 263–271. [Google Scholar] [CrossRef]
- Besher, E.M.; Meisen, A. Low-Temperature Fluidized Bed Claus Reactor Performance. Chem. Eng. Sci. 1990, 45, 3035–3045. [Google Scholar] [CrossRef]
- Santo, S.; Rameshni, M. The Challenges of Designing Grass Root Sulphur Recovery Units with a Wide Range of H2S Concentration from Natural Gas. J. Nat. Gas Sci. Eng. 2014, 18, 137–148. [Google Scholar] [CrossRef]
- Chekalov, L.V.; Sanaev, J.I. Electric Precipitator. R.U. Patent 2563481, 20 September 2015. [Google Scholar]
- Bassani, A.; Pirola, C.; Maggio, E.; Pettinau, A.; Frau, C.; Bozzano, G.; Pierucci, S.; Ranzi, E.; Manenti, F. Acid Gas to Syngas (AG2STM) Technology Applied to Solid Fuel Gasification: Cutting H2S and CO2 Emissions by Improving Syngas Production. Appl. Energy 2016, 184, 1284–1291. [Google Scholar] [CrossRef]
- Pirola, C.; Ranzi, E.; Manenti, F. Technical Feasibility of AG2S™ Process Revamping. Computer. Aided Chem. Eng. 2017, 40, 385–390. [Google Scholar]
- Ismagilov, Z.R.; Khairulin, S.R.; Ismagilov, F.R. Russian Refiner Tests New one-stage H2S Removal process. Oil Gas J. 1994, 7, 81–82. [Google Scholar]
- Ismagilov, Z.R.; Khairulin, S.R.; Barannik, G.B.; Kerzhentsev, M.A.; Nemkov, V.V.; Parmon, V.N. A Method for Cleaning the Blow-Off Gases of Wells from Hydrogen Sulfide. USSR A. S. 1608109, 9 February 1988. [Google Scholar]
- Clark, P.D. Partial Oxidation of Hydrogen Sulfide in the Manufacture of Hydrogen, Sulfur, Ethylene and Propylene. In SULPHUR; Alberta Sulphur Research Ltd.: Caglary, AB, Canada, 2003. [Google Scholar]
- Gamson, B.W.; Elkins, R.H. Sulfur from Hydrogen Sulfide. Chem. Eng. Progress. 1953, 49, 203. [Google Scholar]
- Zagoruiko, A.N. Development of the Process of Obtaining Elementary Sulfur Method of Claus in Non-Stationary Mode. Ph.D. Thesis, Boreskov Institute of Catalysis, Novosibirsk, Russia, 1991. [Google Scholar]
- Klein, J.; Henning, K.-D. Catalytic Oxidation of Hydrogen Sulphide on Activated Carbons. Fuel 1984, 63, 1064–1067. [Google Scholar] [CrossRef]
- Pan, Z.; Shan, W.H.; Feng, H.; Smith, J.M. Kinetics of the Self-Fouling Oxidation of Hydrogen Sulfide on Activated Carbon. AIChE J. 1984, 30, 1021–1025. [Google Scholar]
- Prokopenko, V.S.; Zemlyansky, N.N.; Vasilenko, V.A.; Artyushenko, G.V. Purification of Natural Gas from Hydrogen Sulfide by the Method of Direct Oxidation. Gas Ind. 1977, 9, 44–46. [Google Scholar]
- Meeyoo, V.; Trimm, D.L.; Cant, N.W.J. Adsorption-Reaction Processes for the Removal of Hydrogen Sulphide from Gas Streams. Chem. Technol. Biotechnol. 1997, 68, 411–416. [Google Scholar] [CrossRef]
- Sun, Y.; He, J.; Wang, Y.; Yang, G.; Sun, G.; Sage, V. Experimental and CFD study of H2S oxidation by activated carbon prepared from cotton pulp black liquor. Process Saf. Environ. Prot. 2020, 134, 131–139. [Google Scholar] [CrossRef]
- Adib, F.; Bagreev, A.; Bandosz, T.J. Effect of pH and Surface Chemistry on the Mechanism of H2S Removal by Activated Carbons. J. Colloid Interface Sci. 1999, 216, 360–369. [Google Scholar] [CrossRef]
- Liu, Y.; Song, C.; Wang, Y.; Cao, W.; Lei, Y.; Feng, Q.; Chen, Z.; Liang, S.; Xu, L.; Jiang, L. Rational designed Co@N-doped carbon catalyst for high-efficient H2S selective oxidation by regulating electronic structures. Chem. Eng. J. 2020, 401, 1–10. [Google Scholar] [CrossRef]
- Sun, M.; Wang, X.; Pan, X.; Liu, L.; Li, Y.; Zhao, Z.; Qiu, J. Nitrogen-rich hierarchical porous carbon nanofibers for selective oxidation of hydrogen sulfide. Fuel Process. Technol. 2019, 191, 121–128. [Google Scholar] [CrossRef]
- Wu, X.X.; Schwartz, V.; Overbury, S.H.; Armstrong, T.R. Desulfurization of Gaseous Fuels Using Activated Carbons as Catalysts for the Selective Oxidation of Hydrogen Sulfide. Energy Fuels 2005, 19, 1774–1782. [Google Scholar] [CrossRef]
- Fang, H.B.; Zhao, J.T.; Fang, Y.T.; Huang, J.J.; Wang, Y. Selective Oxidation of Hydrogen Sulfide to Sulfur Over Activated Carbon-Supported Metal Oxides. Fuel 2013, 108, 143–148. [Google Scholar] [CrossRef]
- Tsai, J.H.; Jeng, F.T.; Chiang, H.L. Removal of H2S from Exhaust Gas by Use of Alkaline Activated Carbon. Adsorpt. J. Int. Adsorpt. Soc. 2001, 7, 357–366. [Google Scholar] [CrossRef]
- Xiao, Y.; Wang, S.; Wu, D.; Yuan, Q. Catalytic Oxidation of Hydrogen Sulfide Over Unmodified and Impregnated Activated Carbon. Sep. Purif. Technol. 2008, 59, 326–332. [Google Scholar] [CrossRef]
- Abatzoglou, N.; Boivin, S. A Review of Biogas Purification Processes. Biofuels Bioprod. Bioref. 2009, 3, 42–71. [Google Scholar] [CrossRef]
- Seredych, M.; Bandosz, T.J. Adsorption of Hydrogen Sulfide on Graphite Derived Materials Modified by Incorporation of Nitrogen. Mater. Chem. Phys. 2009, 113, 946–952. [Google Scholar] [CrossRef]
- Bashkova, S.; Armstrong, T.R.; Schwartz, V. Selective Catalytic Oxidation of Hydrogen Sulfide on Activated Carbons Impregnated with Sodium Hydroxide. Energy Fuels 2009, 23, 1674–1682. [Google Scholar] [CrossRef]
- Bashkova, S.; Baker, F.S.; Wu, X.; Armstrong, T.R.; Schwartz, V. Activated Carbon Catalyst for Selective Oxidation of Hydrogen Sulphide: On the Influence of Pore Structure, Surface Character-Istics, and Catalytically-Active Nitrogen. Carbon 2007, 45, 1354–1363. [Google Scholar] [CrossRef]
- Primavera, A.; Trovarelli, A.; Andreussi, P.; Dolcetti, G. The Effect of Water in the Low-Temperature Catalytic Oxidation of Hydrogen Sulfide to Sulfur Over Activated Carbon. Appl. Catal. A 1998, 173, 185–192. [Google Scholar] [CrossRef]
- Bashkova, S.; Bagreev, A.; Locke, D.C.; Bandosz, T.J. Adsorption of SO2 on Sewage Sludge-Derived Materials. Environ. Sci. Technol. 2001, 35, 3263–3269. [Google Scholar] [CrossRef]
- Chiang, H.L.; Tsai, J.H.; Tsai, C.L.; Hsu, Y.C. Adsorption Characteristics of Alkaline Activated Carbon Exemplified by Water Vapor, H2S, and CH3SH Gas. Sep. Sci. Technol. 2000, 35, 903–918. [Google Scholar] [CrossRef]
- Harry, W. Regeneration of Zeolites Used for Sulfur Removal. Oil Gas J. 1961, 167, 99–116. [Google Scholar]
- Bandosz, T.J. (Ed.) Desulfurization on Activated. In Carbons in Activated Carbon Surfaces in Environmental Remediation; Elsevier Ltd.: New York, NY, USA, 2006. [Google Scholar]
- Cal, M.P.; Strickler, B.W.; Lizzio, A.A.; Gangwal, S.K. High Temperature Hydrogen Sulfide Adsorption on Activated Carbon: II. Effects of Gas Temperature, Gas Pressure and Sorbent Regeneration. Carbon 2000, 38, 1767–1774. [Google Scholar] [CrossRef]
- Feng, W.; Kwon, S.; Borguet, E.; Vidic, R. Adsorption of Hydrogen Sulfide onto Activated Carbon Fibers: Effect of Pore Structure and Surface Chemistry. Environ. Sci. Technol. 2005, 39, 9744–9749. [Google Scholar] [CrossRef]
- Bagreev, A.; Bandosz, T.J. A Role of Sodium Hydroxide in the Process of Hydrogen Sulfide Adsorption/Oxidation on Caustic-Impregnated Activated Carbons. Ind. Eng. Chem. Res. 2002, 41, 672–679. [Google Scholar] [CrossRef]
- Mikhalovsky, S.V.; Zaitsev, Y.P. Catalytic Properties of Activated Carbons I. Gas-Phase Oxidation of Hydrogen Sulphide. Carbon 1997, 35, 1367–1374. [Google Scholar] [CrossRef]
- Gardner, T.H.; Berry, D.A.; David Lyons, K.; Beer, S.K.; Freed, A.D. Fuel Processor Integrated H2S Catalytic Partial Oxidation Technology for Sulfur Removal in Fuel Cell Power Plants. Fuel 2002, 81, 2157–2166. [Google Scholar] [CrossRef]
- Wu, X.; Kercher, A.K.; Schwartz, V.; Overbury, S.H.; Armstrong, T.R. Activated Carbons for Selective Catalytic Oxidation of Hydrogen Sulfide to Sulfur. Carbon 2005, 43, 1087–1090. [Google Scholar] [CrossRef]
- Bandosz, T.J.; Bagreev, A.; Adib, F.; Turk, A. Unmodified versus Caustics-Impregnated Carbons for Control of Hydrogen Sulfide Emissions from Sewage Treatment Plants. Environ. Sci. Technol. 2000, 34, 1069–1074. [Google Scholar] [CrossRef]
- Bagreev, A.; Bandosz, T.J. On the Mechanism of Hydrogen Sulfide Removal from Moist Air on Catalytic Carbonaceous Adsorbents. Ind. Eng. Chem. Res. 2005, 44, 530–538. [Google Scholar] [CrossRef]
- Rhodes, C.; Riddel, S.A.; West, J.; Williams, B.P.; Hutchings, G.J. The Low-Temperature Hydrolysis of Carbonyl Sulfide and Carbon Disulfide: A Review. Catal. Today 2000, 59, 443–464. [Google Scholar] [CrossRef]
- Sun, F.; Liu, J.; Chen, H.; Zhang, Z.; Qiao, W.; Long, D.; Ling, L. Nitrogen-Rich Mesoporous Carbons: Highly Efficient, Regenerable Metal-Free Catalysts for Low-Temperature Oxidation of H2S. ACS Catal. 2013, 3, 862–870. [Google Scholar] [CrossRef]
- Ledoux, M.J.; Vieira, R.; Pham-Huu, C.; Keller, N. New Catalytic Phenomena on Nanostructured (Fibers and Tubes) Catalysts. J. Catal. 2003, 216, 333–342. [Google Scholar] [CrossRef]
- Ismagilov, Z.R.; Shalagina, A.E.; Podyacheva, O.Y.; Kvon, R.I.; Ismagilov, I.Z.; Kerzhentsev, M.A.; Barnakov, C.N.; Kozlov, A.P. Synthesis of Nitrogen-Containing Carbon Materials for Solid Polymer Fuel Cell Cathodes. Kinet. Catal. 2007, 48, 581–588. [Google Scholar] [CrossRef]
- Ismagilov, Z.R.; Shalagina, A.E.; Podyacheva, O.Y.; Ischenko, A.V.; Kibis, L.S.; Boronin, A.I.; Chesalov, Y.A.; Kochubey, D.I.; Romanenko, A.I.; Anikeeva, O.B.; et al. Structure and Electrical Conductivity of Nitrogen-Doped Carbon Nanofibers. Carbon 2009, 47, 1922–1929. [Google Scholar] [CrossRef]
- Nhut, J.-M.; Nguyen, P.; Pham-Huu, C.; Keller, N.; Ledoux, M.-J. Carbon Nanotubes as Nanosized Reactor for the Selective Oxidation of H2S Into Elemental Sulfur. Catal. Today 2004, 91, 91–97. [Google Scholar] [CrossRef]
- Nhut, J.M.; Pesant, L.; Tessonnier, J.P.; Winé, G.; Guille, J.; Pham Huu, C.; Ledoux, M.J. Mesoporous Carbon Nanotubes for Use as Support in Catalysis and as Nanosized Reactors for One-Dimensional Inorganic Material Synthesis. Appl. Catal. A 2003, 254, 345–363. [Google Scholar] [CrossRef]
- Chen, Q.; Wang, J.; Liu, X.; Zhao, X.; Qiao, W.; Long, D.; Ling, L. Alkaline Carbon Nanotubes As Effective Catalysts for H2S Oxidation. Carbon 2011, 49, 3773–3780. [Google Scholar] [CrossRef]
- Mohamadalizadeh, A.; Towfighi, J.; Adinehnia, M.; Bozorgzadeh, H.R. H2S Oxidation by Multi-Wall Carbon Nanotubes Decorated with Tungsten Sulfide. Korean J. Chem. Eng. 2013, 30, 871–877. [Google Scholar] [CrossRef]
- Ba, H.; Duong-Viet, C.; Liu, Y.; Nhut, J.-M.; Granger, P.; Ledoux, M.J.; Pham-Huu, C. Nitrogen-Doped Carbon Nanotube Spheres As Metal-Free Catalysts for the Partial Oxidation of H2S. Comptes Rendus Chim. 2016, 19, 1303–1309. [Google Scholar] [CrossRef] [Green Version]
- Chizari, K.; Deneuve, A.; Ersen, O.; Florea, I.; Liu, Y.; Edouard, D.; Janowska, I.; Begin, D.; Pham-Huu, C. Nitrogen-Doped Carbon Nanotubes as a Highly Active Metal-Free Catalyst for Selective Oxidation. Chem. Sus. Chem. 2012, 5, 102–108. [Google Scholar] [CrossRef]
- De Jong, K.P.; Geus, J.W. Carbon Nanofibers: Catalytic Synthesis and Applications. Catal. Rev.-Sci. Eng. 2000, 42, 481–510. [Google Scholar] [CrossRef]
- Kuvshinov, G.G.; Shinkarev, V.V.; Glushenkov, A.M.; Boyko, M.N.; Kuvshinov, D.G. Catalytic Properties of Nanofibrous Carbon in Selective Oxidation of Hydrogen Sulfide. China Particuol. 2006, 4, 70–72. [Google Scholar] [CrossRef]
- Shinkarev, V.V.; Glushenkov, A.M.; Kuvshinov, D.G.; Kuvshinov, G.G. New Effective Catalysts Based on Mesoporous Nanofibrous Carbon for Selective Oxidation of Hydrogen Sulfide. Appl. Catal. B 2009, 85, 180–191. [Google Scholar] [CrossRef]
- Shinkarev, V.V.; Kuvshinov, G.G.; Zagoruiko, A.N. Kinetics of H2S Selective Oxidation by Oxygen at the Carbon Nanofibrous Catalyst. Reac. Kinet. Mech. Cat. 2018, 123, 625–639. [Google Scholar] [CrossRef]
- Chen, Q.; Wang, Z.; Long, D.; Liu, X.; Zhan, L.; Liang, X.; Qiao, W.; Ling, L. Role of Pore Structure of Activated Carbon Fibers in the Catalytic Oxidation of H2S. Ind. Eng. Chem. Res. 2010, 49, 3152–3159. [Google Scholar] [CrossRef]
- Coelho, N.M.A.; da Cruz, G.M.; Vieira, R. Effect of Temperature and Water on the Selective Oxidation of H2S to Elemental Sulfur on a Macroscopic Carbon Nanofiber Based Catalyst. Catal. Lett. 2012, 142, 108–111. [Google Scholar] [CrossRef]
- Liu, B.-T.; Ke, Y.-X. Enhanced Selective Catalytic Oxidation of H2S Over Ce-Fe/AC Catalysts at Ambient Temperature. J. Taiwan Inst. Chem. Eng. 2020, 110, 28–33. [Google Scholar] [CrossRef]
- Puri, B.R. Studies of Catalytic Reactions on Activated Carbons. Carbon 1982, 20, 139. [Google Scholar] [CrossRef]
- Bouzaza, A.; Laplanche, A.; Marsteau, S. Adsorption-Oxidation of Hydrogen Sulfide on Activated Carbon Fibers: Effect of the Composition and the Relative Humidity of the Gas Phase. Chemosphere 2004, 54, 481–488. [Google Scholar] [CrossRef] [PubMed]
- Ziółek, M.; Dudzik, Z. Structural Changes of the NaX Zeolite During H2S + O2 Reaction. React. Kinet. Catal. Lett. 1980, 14, 213–217. [Google Scholar] [CrossRef]
- Ziółek, M.; Dudzik, Z. Catalytically Active Centres in the H2S + O2 Reaction on Faujasites. Zeolites 1981, 1, 117–122. [Google Scholar] [CrossRef]
- Lee, J.D.; Park, N.-K.; Han, K.B.; Ryu, S.O.; Lee, T.J. Influence of Reducing Power on Selective Oxidation of H2S Over V2O5 Catalyst in IGCC System. Stud. Surf. Sci. Catal. 2006, 159, 425–428. [Google Scholar]
- Nguyen, P.; Edouard, D.; Nhut, J.-M.; Ledoux, M.J.; Pham, C.; Pham-Huu, C. High Thermal Conductive β-SiC for Selective Oxidation of H2S: A New Support for Exothermal Reactions. Appl. Catal. B Environ. 2007, 76, 300–310. [Google Scholar] [CrossRef]
- Keller, N.; Pham-Huu, C.; Estournes, C.; Ledoux, M.J. Low Temperature Use of SiC-Supported NiS2-Based Catalysts for Selective H2S Oxidation Role of SiC Surface Heterogeneity and Nature of the Active Phase. Appl. Catal. A Gen. 2002, 234, 191–205. [Google Scholar] [CrossRef]
- Keller, N.; Pham-Huu, C.; Estournes, C.; Ledoux, M.J. Continuous Process for Selective Oxidation of H2S Over SiC-Supported Iron Catalysts into Elemental Sulfur Above Its Dewpoint. Appl. Catal. A Gen. 2001, 217, 205–217. [Google Scholar] [CrossRef]
- Zhang, X.; Tang, Y.; Qu, S.; Da, J.; Hao, Z. H2S-Selective Catalytic Oxidation: Catalysts and Processes. ACS Catal. 2015, 5, 1053–1067. [Google Scholar] [CrossRef]
- Alkhazov, T.G.; Bagirov, R.A.; Dovlatova, S.M.; Filatova, O.E.; Kuliev, A.M.; Vartanov, A.A. Catalyst for Oxidizing Hydrogen Sulphide in Gaseous Phase to Elementary Sulphur. USSR Certiificate of Authorship 665939, 5 June 1979. [Google Scholar]
- Alkhazov, T.G.; Bagdasaryan, B.V.; Mamedova, R.I.; Vartanov, A.A. Catalyst for Oxidizing Hydrogen Sulphide to Sulphur. USSR Certiificate of Authorship 882589, 23 November 1981. [Google Scholar]
- Davydov, A.A.; Marshneva, V.I.; Shepotko, M.L. Metal Oxides in Hydrogen Sulfide Oxidation by Oxygen and Sulfur Dioxide I. the Comparison Study of the Catalytic Activity. Mechanism of the Interactions Between H2S and SO2 on Some Oxides. Appl. Catal. A Gen. 2003, 244, 93–100. [Google Scholar] [CrossRef]
- Alkhazov, T.G.H.; Vartanov, A.A. Prjamoe gheteroghenno-katalytytcheskoe okyslenye serovodoroda v ehlementarnuju seru. Yzvestyja VUZov. Neftj Y Ghaz. 1981, 3, 45–49. [Google Scholar]
- Batygina, M.V.; Dobrynkin, N.M.; Kirichenko, O.A.; Khairulin, S.R.; Ismagilov, Z.R. Studies of Supported Oxide Catalysts in the Direct Selective Oxidation of Hydrogen Sulfide. React. Kinet. Catal. Lett. 1992, 48, 55–63. [Google Scholar] [CrossRef]
- Zheng, X.; Li, Y.; Zhang, L.; Shen, L.; Xiao, Y.; Zhang, Y.; Au, C.; Jiang, L. Insight Into the Effect of Morphology on Catalytic Performance of Porous CeO2 Nanocrystals for H2S Selective Oxidation. Appl. Catal. B Environ. 2019, 252, 98–110. [Google Scholar] [CrossRef]
- Ismagilov, Z.R.; Shkrabina, R.A.; Barannik, G.B.; Kerzhentsev, M.A. New Catalysts and Processes for Environment Protection. React. Kinet. Catal. Lett. 1995, 55, 489–499. [Google Scholar] [CrossRef]
- Alkhazov, T.G.; Balyberdina, I.T.; Filatova, O.E.; Khendro, M.; Korotaev, Y.P.; Vartanov, A.A. Method of Producing Elemental sulphur. USSR A.s. 856974, 23 August 1981. [Google Scholar]
- Amyrghuljan, N.S. Materyalyh Pjatoj Respublykanskoj Konferentsyy Po Okyslyteljnomu Gheteroghennomu Katalyzu; Sociology and Law of Azerbaijan National Academy of Sciences: Baku, Azerbaijan, 1981. [Google Scholar]
- Geus, J.W. Preparation and Properties of Iron Oxide and Metallic Iron Catalysts. Appl. Catal. 1986, 25, 313–333. [Google Scholar] [CrossRef]
- Terorde, R.J.A.M.; van den Brink, P.J.; Visser, L.M.; van Dillen, A.J.; Geus, J.W. Selective oxidation of hydrogen sulfide to elemental sulfur using iron oxide catalysts on various supports. Catal. Today 1993, 17, 217–224. [Google Scholar] [CrossRef]
- Terorde, R.J.A.M.; de Jong, M.C.; Crombag, M.J.D.; van den Brink, P.J.; van Dillen, A.J.; Geus, J.W. Selective oxidation of hydrogen sulfide on a sodium promoted iron oxide on silica catalyst V. New Dev. Sel. Oxid. II 1994, 861–868. [Google Scholar]
- Khairulin, S.R.; Parmon, V.N.; Kuznetsov, V.V.; Batuev, R.A.; Tryasunov, B.G.; Teryaev, T.N.; Mazgarov, A.M.; Vildanov, A.F.; Golovanov, A.N.; Garaiev, A.M.; et al. Methods of purification of coke gas from hydrogen sulfide. H2S recycling processes. Direct catalytic oxidation. Developments of the Institute of Catalysis SB RAS (review). Altern. Energy Ecol. 2014, 19, 86–106. [Google Scholar]
- Ismagilov, Z.R.; Khairulin, S.R.; Ismagilov, F.R. The Technology of Catalytic Purification of Hydrogen Sulfide Containing Gases Over Honeycomb Monolith Catalysts. Proc. First Int. Semin. Monolith. Honeycomb Supports Catal. 1995, 2, 206–207. [Google Scholar]
- Barannik, G.B.; Dobrynkin, N.M.; Ismagilov, F.R.; Ismagilov, Z.R.; Khajrulin, S.R.; Navalikhin, P.G.; Podshivalin, A.V. Method of Cleaning Gases from Sulfurous Compounds. R.U. Patent 2144495, 20 January 2000. [Google Scholar]
- Khairulin, S.R.; Ismagilov, Z.R.; Kerzhentsev, M.A. Direct Selective Oxidation of Hydrogen Sulfide to Elementary Sulfur. In Process for Geothermal Steam Purification 2nd World Congress on Environmental Catalysis; Extended Abstracts: Miami, FL, USA, 1998. [Google Scholar]
- Palma, V.; Barba, D.; Gerardi, V. Honeycomb-Structured Catalysts for the Selective Partial Oxidation of H2S. J. Clean. Prod. 2016, 111, 69–75. [Google Scholar] [CrossRef]
- Eom, H.; Jangb, Y.; Choib, S.Y.; Leea, S.M.; Kim, S.S. Application and Regeneration of Honeycomb-Type Catalysts for the Selective Catalytic Oxidation of H2S to Sulfur from Landfill Gas. Appl. Catal. A Gen. 2020, 590, 117365. [Google Scholar] [CrossRef]
- Broecker, F.J.; Gettert, H.A.; Kaempfer, K. Desulfurization of H2S-Containing Gases. U.S. Patent 4507274, 26 March 1985. [Google Scholar]
- Miles, J.R.; Zhou, T. Forward Models for Gamma Ray Measurement Analysis of Subterranean Formations. International Patent Application 201002791, 7 January 2011. [Google Scholar]
- Jategaonkar, S.; Kay, B.; Braga, T.; Srinivas, G.; Gebhard, S. SulfaTreat-DO: Direct Oxidation for Hydrogen Sulfide Removal. In Proceedings of the 84th Annual GPA Convention, San Antonio, TX, USA, 13–15 March 2005. [Google Scholar]
- Royan, T.; Wichert, E. Options for Small-Scale Sulfur Recovery. SPE Prod. Fac. 1997, 12, 267–272. [Google Scholar] [CrossRef]
- Kettner, R.; Liermann, N. New Claus Tail-Gas Process Proved in German Operation. Oil Gas J. 1982, 11, 63–66. [Google Scholar]
- Borsboom, J.; Lagas, J.A.; Berben, P.H. Sulphur-88 Conference Proceedings; British Sulphur Corp.: London, UK, 1988. [Google Scholar]
- Lagas, J.A.; Borsboom, J.; Johannes, B.; Geus, P.H.; John, W. Process for Recovering Sulfur from Sulfur-Containing Gases. U.S. Patent 4988494, 13 April 1987. [Google Scholar]
- Julian, R.H. Chapter 10-Catalysis in the Production of Energy Carriers from Oil. Ross Contemporary Catalysis. Fundam. Curr. Appl. 2019, 233–249. [Google Scholar]
- Avdzhiev, G.R.; Dobrynkin, N.M.; Ismagilov, Z.R.; Khajrulin, S.R.; Koryabkina, N.A.; Krasilnikova, V.A.; Lygalova, A.S.; Ryabchenko, P.V.; Shkrabina, R.A. Method of Catalyst Preparing for Sulfur Production from Hydrogen Sulfide. R.U. Patent 1829182, 20 November 1990. [Google Scholar]
- Dobrynkin, N.M.; Ismagilov, Z.R.; Khajrulin, S.R.; Koryabkina, N.A.; Shcherbilin, V.B.; Shkrabina, R.A. Method for Preparation of Catalyst for Production of Sulfur from Hydrogen Sulfide. R.U. Patent 2035221, 20 May 1992. [Google Scholar]
- Ismagilov, Z.R. Monolithic Catalyst Design, Engineering and Prospects of Application for Environmental Protection in Russia Reaction. Kinet. Catal. Lett. 1997, 60, 215–218. [Google Scholar] [CrossRef]
- Barannik, G.B.; Dobrynin, G.F.; Dobrynkin, N.M.; Ismagilov, F.R.; Ismagilov, Z.R.; Khajrulin, S.R.; Kulikovskaya, N.A. Method of Preparing Catalyst. R.U. Patent 2069586, 27 November 1996. [Google Scholar]
- Ismagilov, Z.R.; Yashnik, S.A.; Shikina, N.V.; Kuznetsov, V.V.; Babich, I.V.; Moulijn, J.A. Development of the monolithic catalyst for deep recovery of elemental sulfur from technological off-gases of metallurgical coke plants and chemical refineries of crude oil II. Synthesis, characterization and testing of monolithic impregnated and wash coated catalysts for direct H2S oxidation. In Proceedings of the 1st Nordic Symposium on Catalysis, Oulu, Finland, 16–18 August 2004. [Google Scholar]
- Khairulin, S.R. The Study of Reaction of Direct Catalytic Hydrogen Sulfide Oxidation and Development the Technologies of Gas Purification from Hydrogen Sulfide. Ph.D. Thesis, Institute of Technical Chemistry, Perm, Russia, 1998. [Google Scholar]
- Ismagilov, Z.R.; Khairulin, S.R.; Kerzhentsev, M.A.; Mazgarov, A.M.; Vildanov, A.F. Development of Catalytic Technologies of Gases Purification from Hydrogen Sulfide Based on Direct Selective catalytic Oxidation of H2S to Elemental Sulfur. Euro-Asian. J. Chem. Technol. 1999, 1, 49–56. [Google Scholar] [CrossRef] [Green Version]
- Ismagilov, Z.R.; Kuznetsov, V.V.; Arendarskii, D.A.; Khairulin, S.R.; Kerzhentsev, M.A. Investigation of the Reaction of Hydrogen Sulphide Oxidation by Optical and Kinetic Methods. In Proceedings of the 11th ICC, Baltimore, MD, USA, 30 June–5 July 1996. [Google Scholar]
- Yashnik, S.A.; Kuznetsov, V.V.; Ismagilov, Z.R.; Babich, I.V.; Moulijn, J.A. Development of the monolithic catalyst for deep recovery of elemental sulfur from technological off-gases of metallurgical coke plants and chemical refineries of crude oil I. FTIR study of surface acidity of aluminas and their activity in H2S oxidation. In Proceedings of the 11th Nordic Symposium on Catalysis, Oulu, Finland, 16–18 August 2004. [Google Scholar]
- Ismagilov, Z.R.; Kerzhentsev, M.; Kuznetsov, V.; Golovanov, A.; Garifullin, R.; Zakiev, F.; Takhautdinov, S. The process of H2S selective catalytic oxidation for on-site purification of hydrocarbon gaseous feedstock; technology demonstration. In Proceedings of the 10th Natural Gas Conversion Symposium, Doha, Qatar, 2–7 March 2013. [Google Scholar]
- Ismagilov, Z.R. Catalytic Fuel Combustion-A Way of Reducing Emissions of Nitrogen Oxides. Catal. Rev. Sci. Eng. 1990, 32, 51–103. [Google Scholar] [CrossRef]
- Ismagilov, Z.R. Catalysis for Energy Production Chemistry for 21st Century Monograph; Thomas, J.M., Zamaraev, K.I., Eds.; Blackwell Scientific Publication: Oxford, UK, 1992; pp. 337–357. [Google Scholar]
- Ismagilov, Z.R.; Kerzhentsev, M.A.; Shikina, N.V.; Khairulin, S.R. Catalytic processes for the treatment of mixed organic waste containing radionuclides. Chem. Sustain. Dev. 2021, in press. [Google Scholar] [CrossRef]
- Ismagilov, Z.R.; Kerzhentsev, M.A. Fluidized Bed Catalytic Combustion. Catal. Today 1999, 47, 339–346. [Google Scholar] [CrossRef]
- Alkhazov, T.G.; Barannik, G.B.; Ismagilov, F.R.; Ismagilov, Z.R.; Ivanov, A.A.; Kerzhentsev, M.A.; Khairulin, S.R.; Nemkov, V.V.; Parmon, V.N.; Zamaraev, K.I. Method for the Purification of Hydrogen Sulfide-Containing Gases. U.S. Patent 4886649, 12 December 1989. [Google Scholar]
- Ismagilov, Z.R.; Khairulin, S.R.; Ismagilov, F.R.; Kerzhetsev, M.A. Direct oxidation of hydrogen sulphide. Hydrocarbon Technology International. Winter 1995, 1994, 59–64. [Google Scholar]
- Khairulin, S.R.; Ismagilov, Z.R.; Kerzhentsev, M.A. Direct Heterogeneous-Catalytic H2S Oxidation to Elemental Sulfur. Khim. Prom. 1996, 4, 265–268. [Google Scholar]
- Ismagilov, Z.R.; Kerzhentsev, M.A.; Khairulin, S.R.; Kuznetsov, V.V. One-Stage Catalytic Methods of Acid Gases Purification from Hydrogen Sulfide. Chem. Sustain. Dev. 1999, 7, 375–396. [Google Scholar]
- Ismagilov, Z.R.; Kerzhentsev, M.A.; Khairulin, S.R. Catalytic Purification of Geothermal Steam from Hydrogen Sulfide. Chem. Sustain. Dev. 1999, 7, 443–449. [Google Scholar]
- Ismagilov, Z.R.; Yashnik, S.A.; Moulijn, J.A.; Startsev, A.N.; Boronin, A.I.; Stadnichenko, A.I.; Kriventsov, V.V.; Kasztelan, S.; Guillaume, D.; Makkee, M. Deep Desulfurization of Diesel Fuel on Bifunctional Monolithic Nanostructured Pt-Zeolite Catalysts. Catal. Today 2009, 144, 235–250. [Google Scholar] [CrossRef]
- Ismagilov, Z.R.; Khairulin, S.R.; Parmon, V.N.; Sadykov, A.F.; Golovanov, A.A.; Yarullin, R.S.; Gibadukov, M.M.; Mazgarov, A.M.; Takhautdinov, S.F.; Zakiev, F.A.; et al. Direct Catalytic Oxidation of Hydrogen Sulfide as a Process for the Purification of Oil-Associated Gases. Experience of the Operation of the First Industrial Installation. Gazokhimiya 2011, 3, 57–60. [Google Scholar]
- Ismagilov, Z.; Khairulin, S.; Kuznetsov, V.; Golovanov, A.; Garifullin, R.; Zakiev, F.; Takhautdinov, S. Field testing of the process of H2S selective oxidation for purification of oil-associated gases. In Proceedings of the 7th International Conference on Environmental Catalysis (ICEC 2012), Lyon, France, 2–6 September 2012. [Google Scholar]
- Ismagilov, Z.; Parmon, V.; Yarullin, R.; Mazgarov, A.; Khairulin, S.; Kerzhentsev, M.; Golovanov, A.; Vildanov, A.; Garifullin, R. The Process of H2S Selective Catalytic Oxidation for on-site. Purification of Hydrocarbon Gaseous Feedstock-Technology. Demonstration at Bavly Oil Field in Republic of Tatarstan. In Proceedings of the XII European Congress on Catalysis EuropaCat XII, Kazan, Russia, 30 August–4 September 2015. [Google Scholar]
- Vildanov, A.F.; Golovanov, A.A.; Ismagilov, Z.R.; Kerzhentsev, M.A.; Mazgarov, A.M.; Filippov, A.G.; Khairullin, S.R. Installation for the Processing of Hydrogen Sulfide-Containing Gases. R.U. Patent 149826, 20 January 2015. [Google Scholar]
- Khairulin, S.R.; Ismagilov, Z.R.; Kerzhentsev, M.A.; Salnikov, A.V.; Loginov, R.I.; Philippov, A.G.; Vildanov, A.F.; Mazgarov, A.M. Carbon Materials for Gas Purification from Hydrogen Sulphide and Prospects of Their Use in Base Technologies for Associated Petroleum Gas Treatment. Chem. Sustain. Dev. 2018, 26, 679–689. [Google Scholar]
- Ismagilov, Z.R.; Khairulin, S.R.; Filippov, A.G.; Mazgarov, A.M.; Vildanov, A.F. Direct Heterogeneous Catalytic Oxidation of Hydrogen Sulphide for Associated Petroleum Gas Treatment. Chem. Sustain. Dev. 2017, 25, 535–543. [Google Scholar]
- Khairulin, S.R.; Ismagilov, Z.R.; Shabalin, O.N.; Komarov, F.F. Direct heterogeneous-catalytic oxidation of hydrogen sulfide for the purification of associated petroleum gases. In Proceedings of the III Russian Congress on Catalysis “Roskataliz”, Nizhny Novgorod, Russia, 22–26 May 2017. [Google Scholar]
- Burov, V.V.; Golovanov, A.A.; Ismagilov, Z.R.; Khajrulin, S.R.; Komarov, F.F.; Lotfullin, N.N.; Parmon, V.N.; Shabalin, O.N. Plant for Hydrogen-Sulphide-Containing Gas Cleaning Process. R.U. Patent 2639912, 11 November 2016. [Google Scholar]
- Golubeva, I.A.; Khairullina, G.R.; Starynin, A.Y.; Karatun, O.N. The Production Analysis of Sulfur Using the Claus Method at Oil and Gas Industry of Russia, Unsolved Problems. Oil Gas Chem. 2017, 3, 5–12. [Google Scholar]
- Khairulin, S.R.; Kerzhentsev, M.A.; Salnikov, A.V.; Ismagilov, Z.R. Basic technologies of direct catalytic oxidation of H2S to sulfur. In Proceedings of the XXI Mendeleev Congress on General and Applied Chemistry, St. Petersburg, Russia, 9–13 September 2019. [Google Scholar]



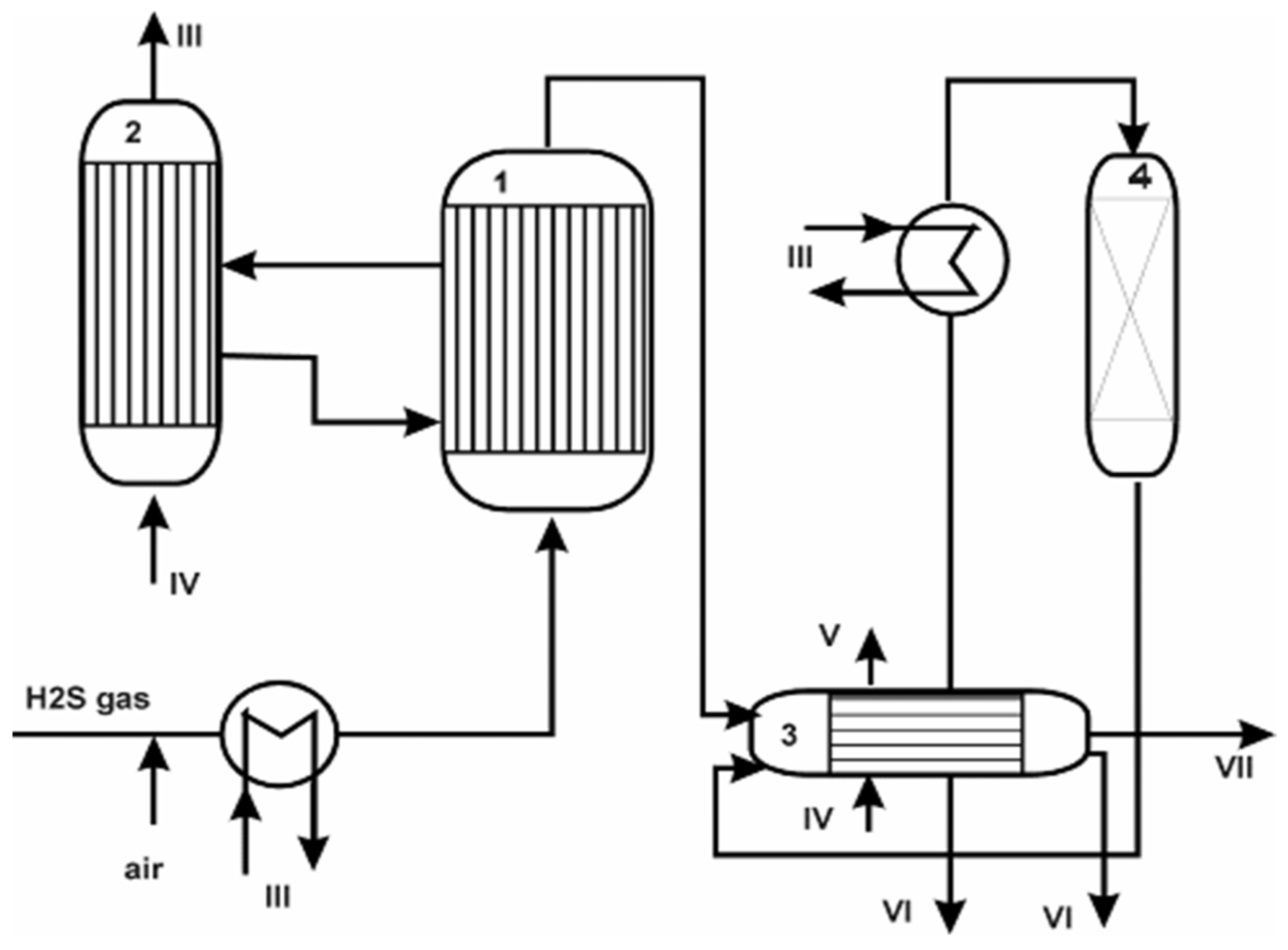
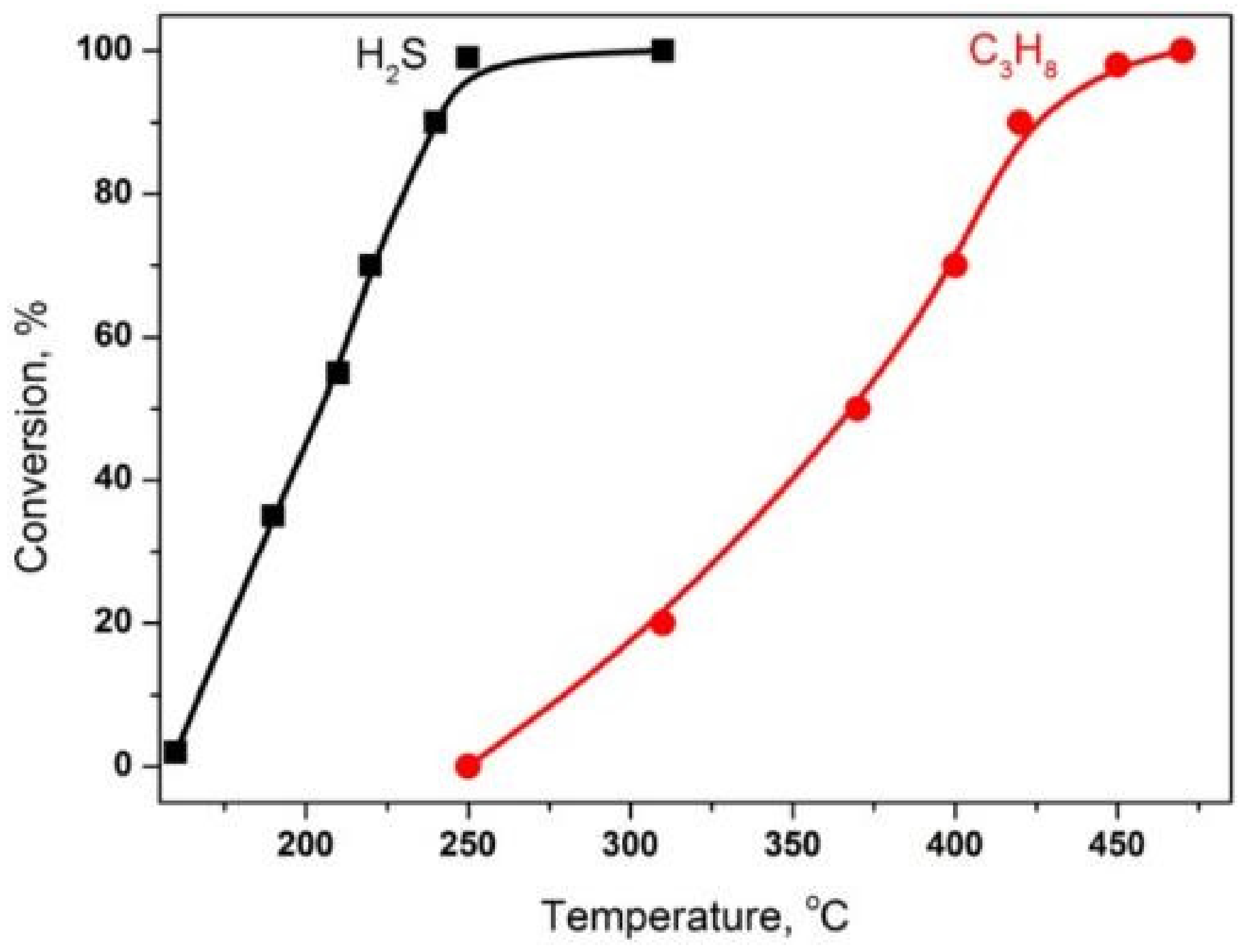
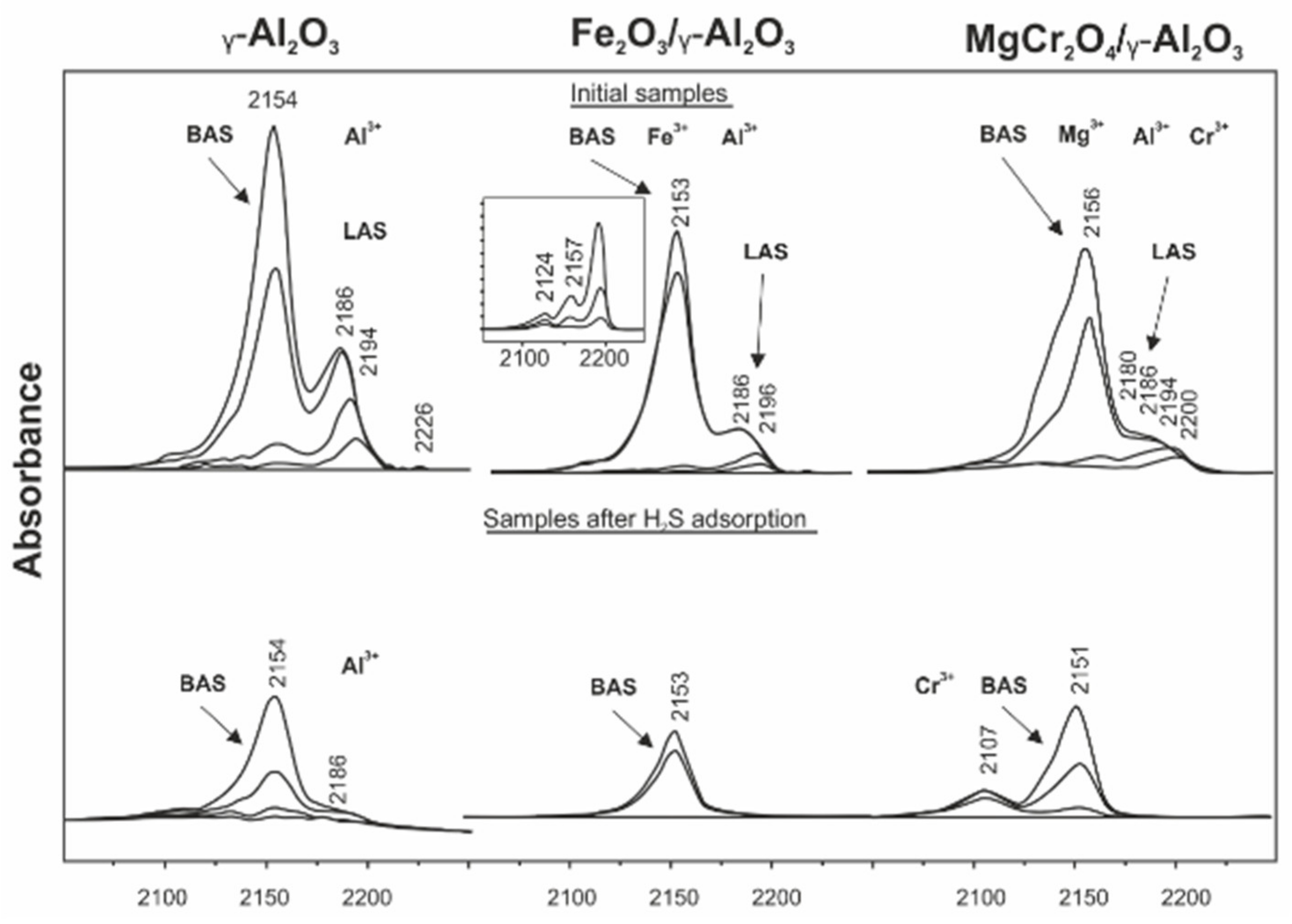
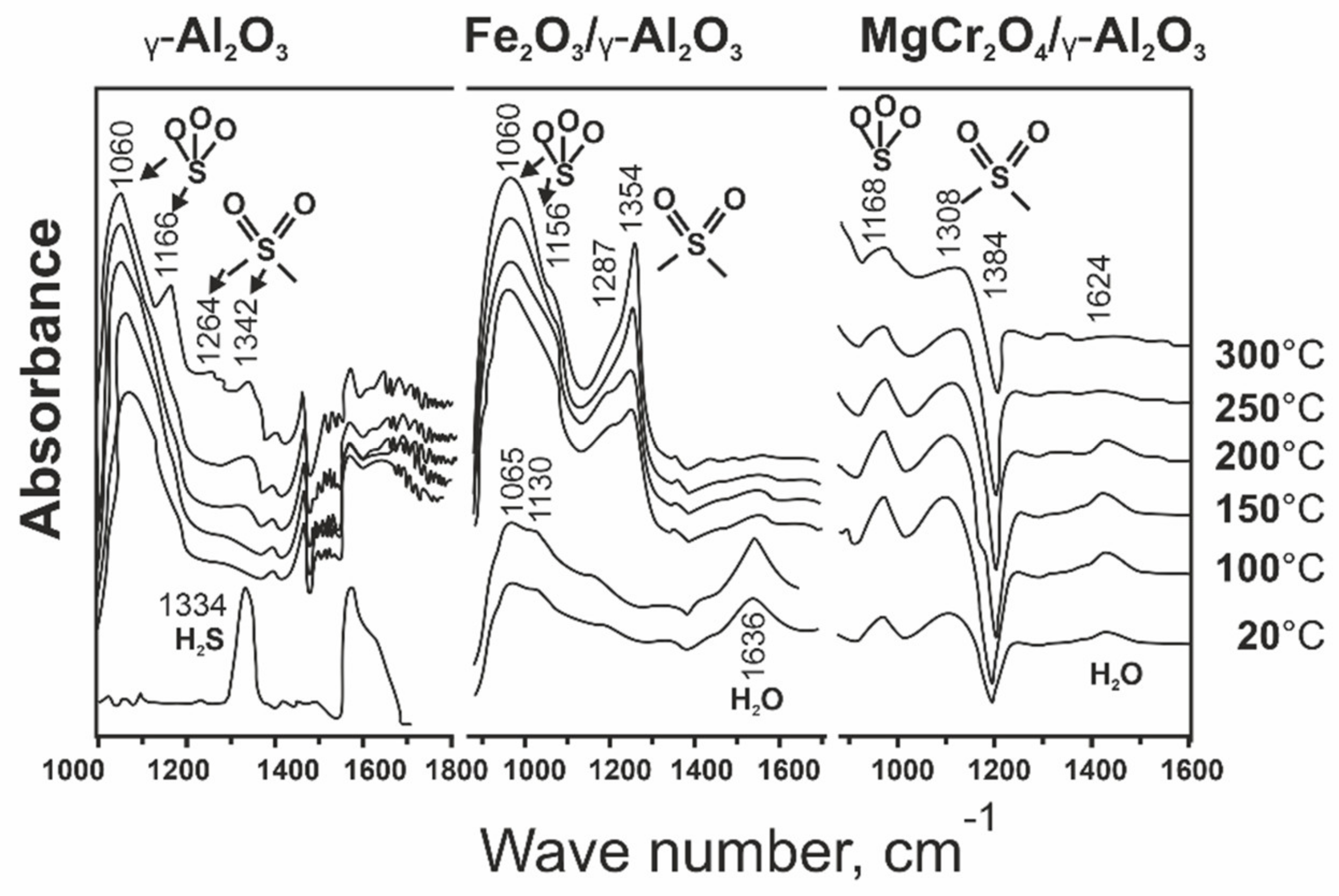
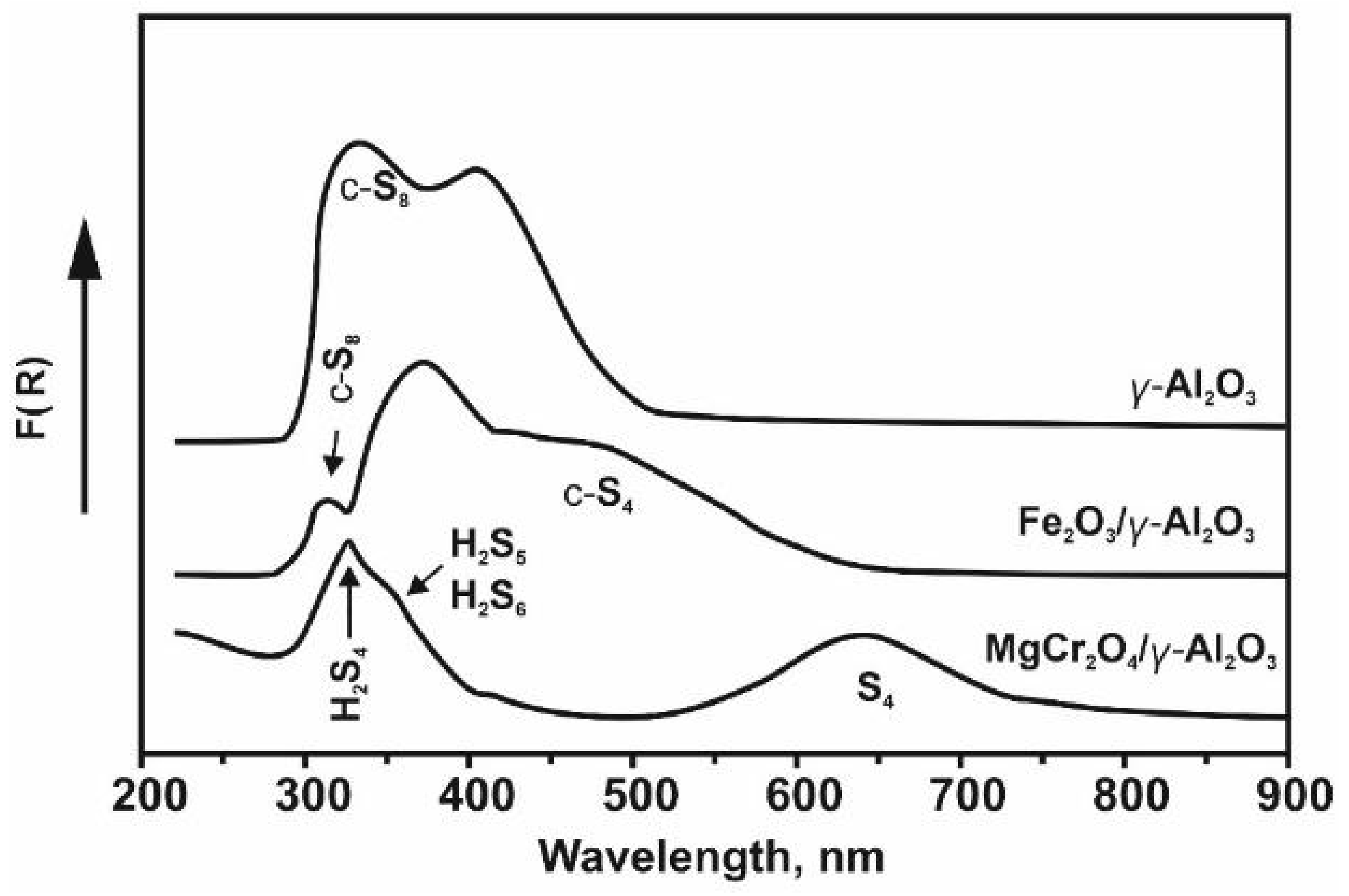
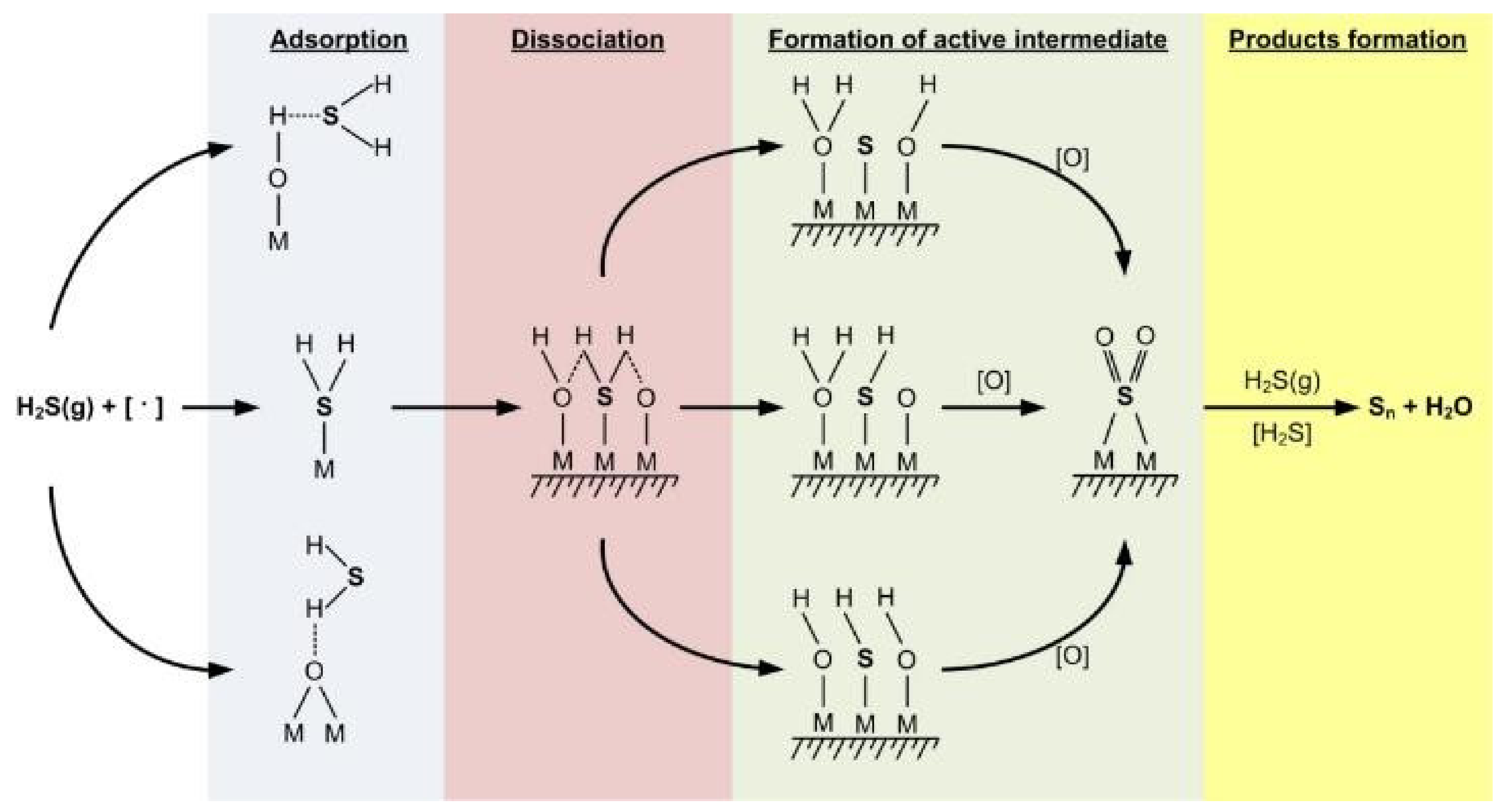
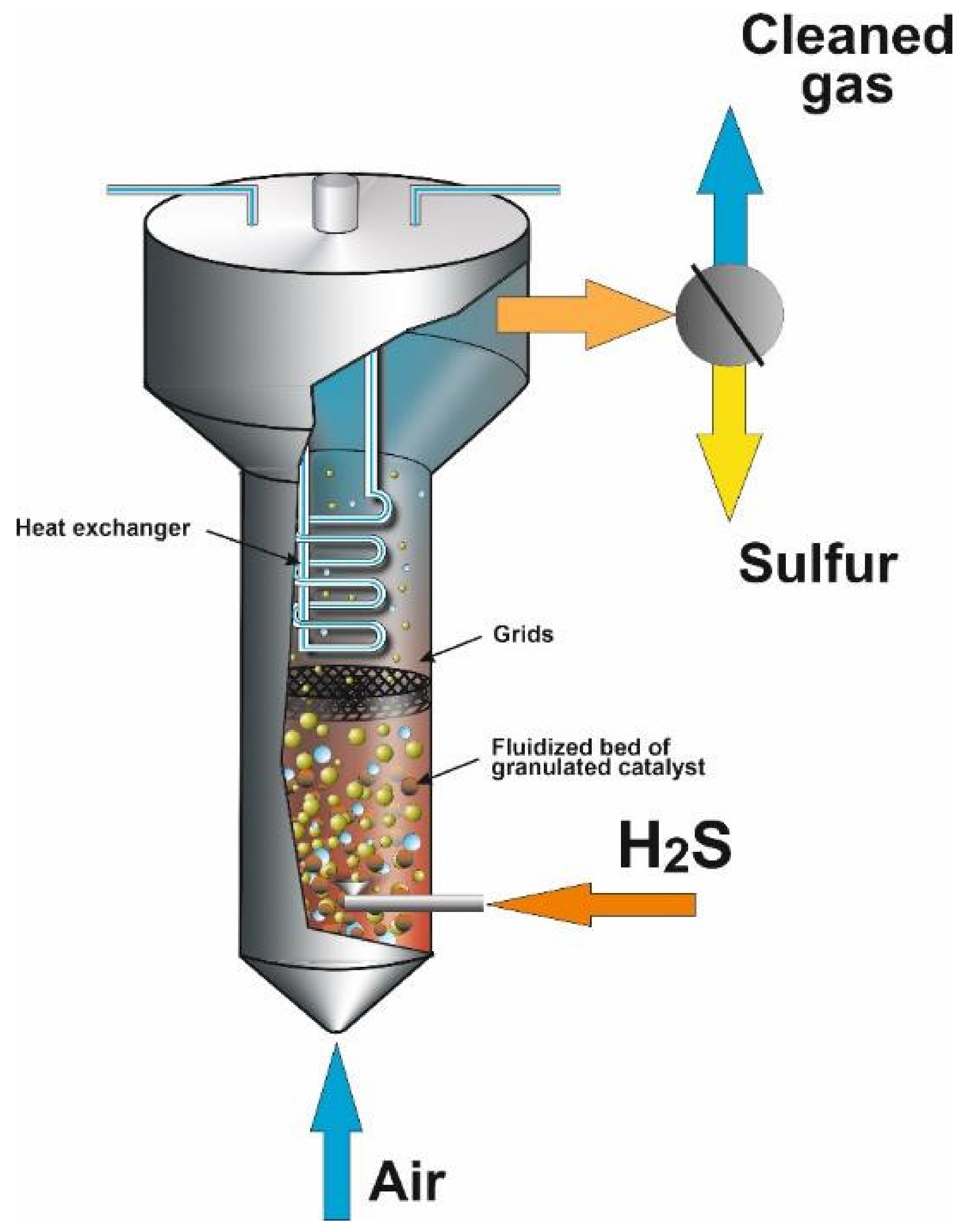
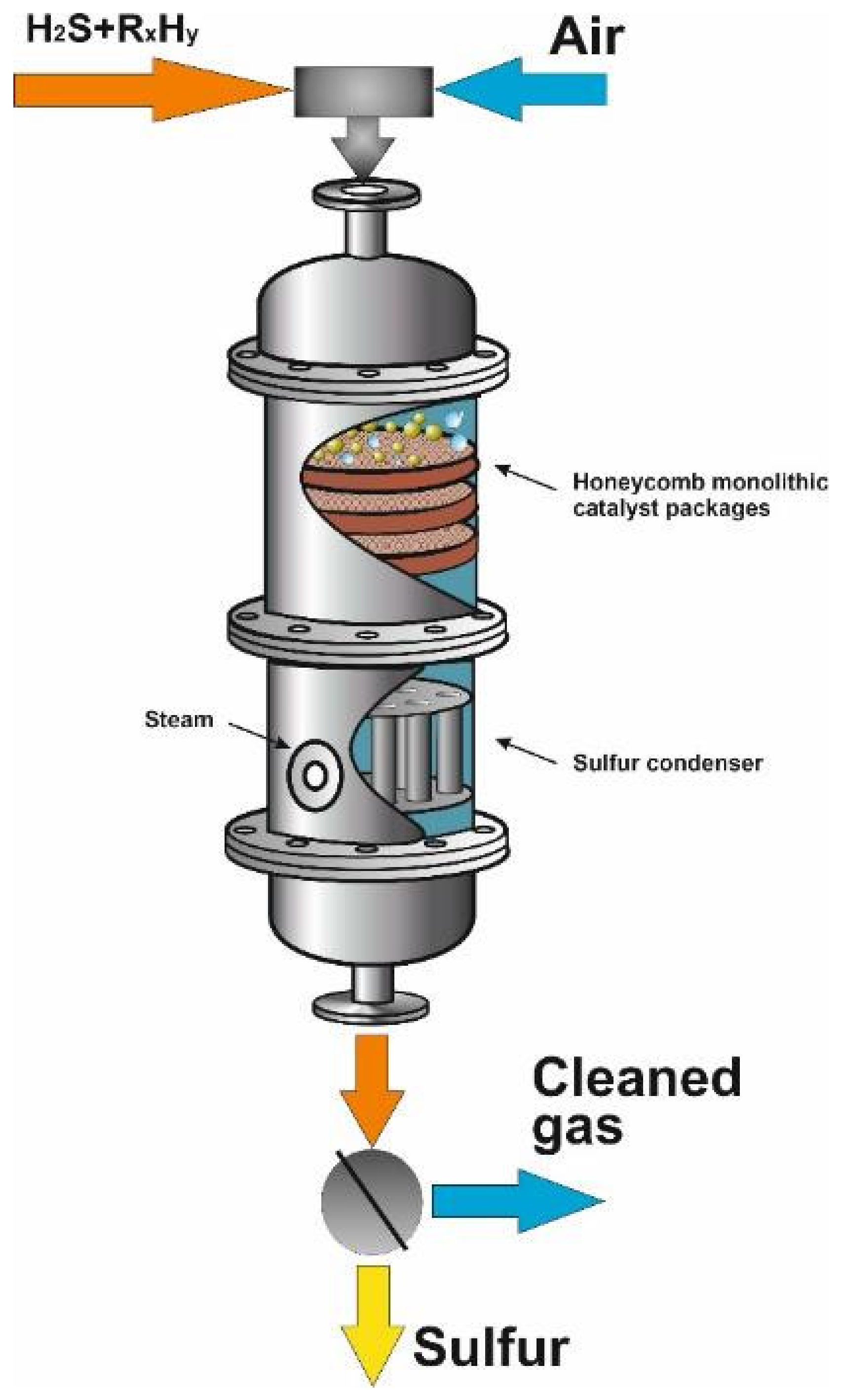


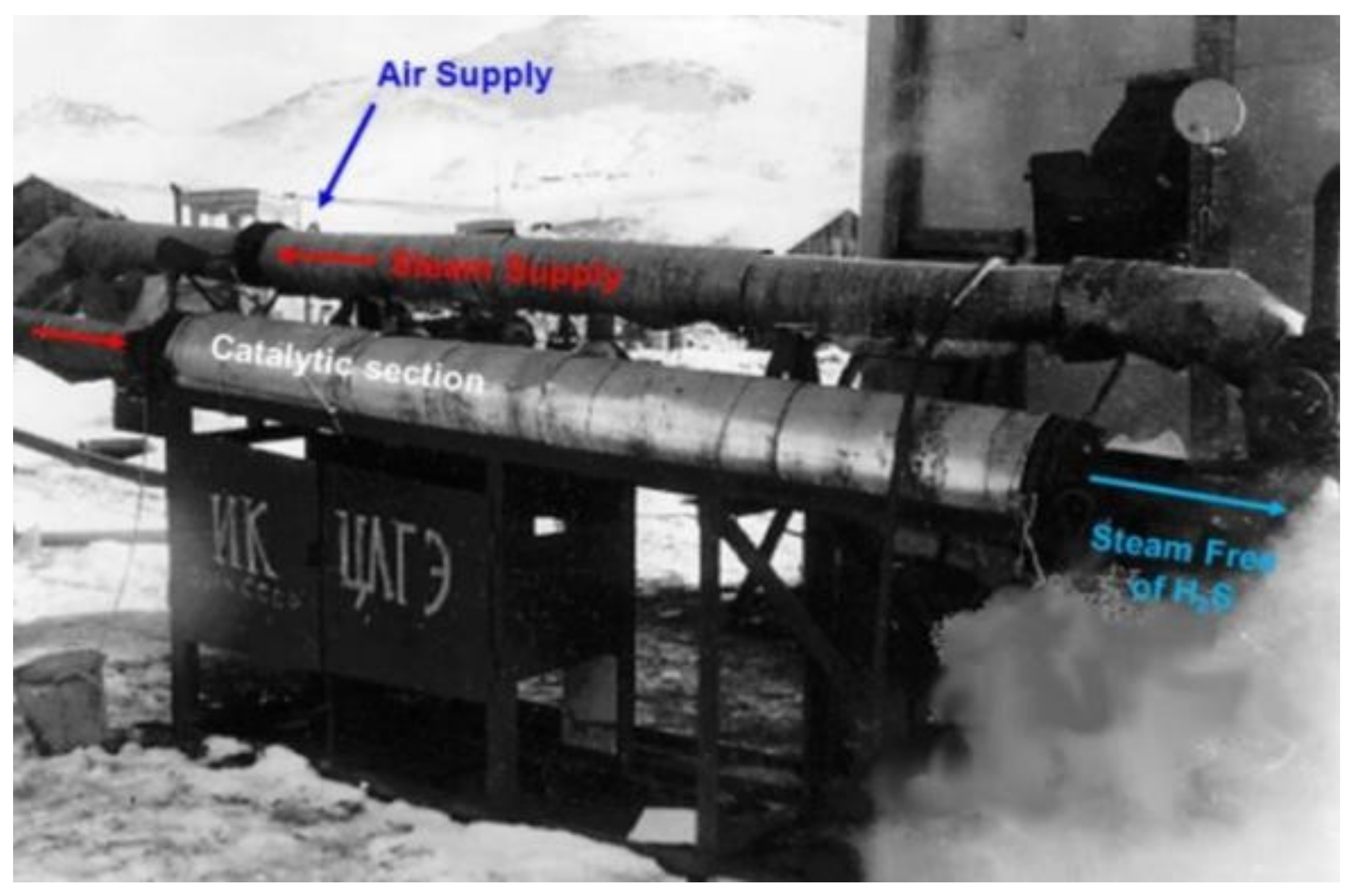
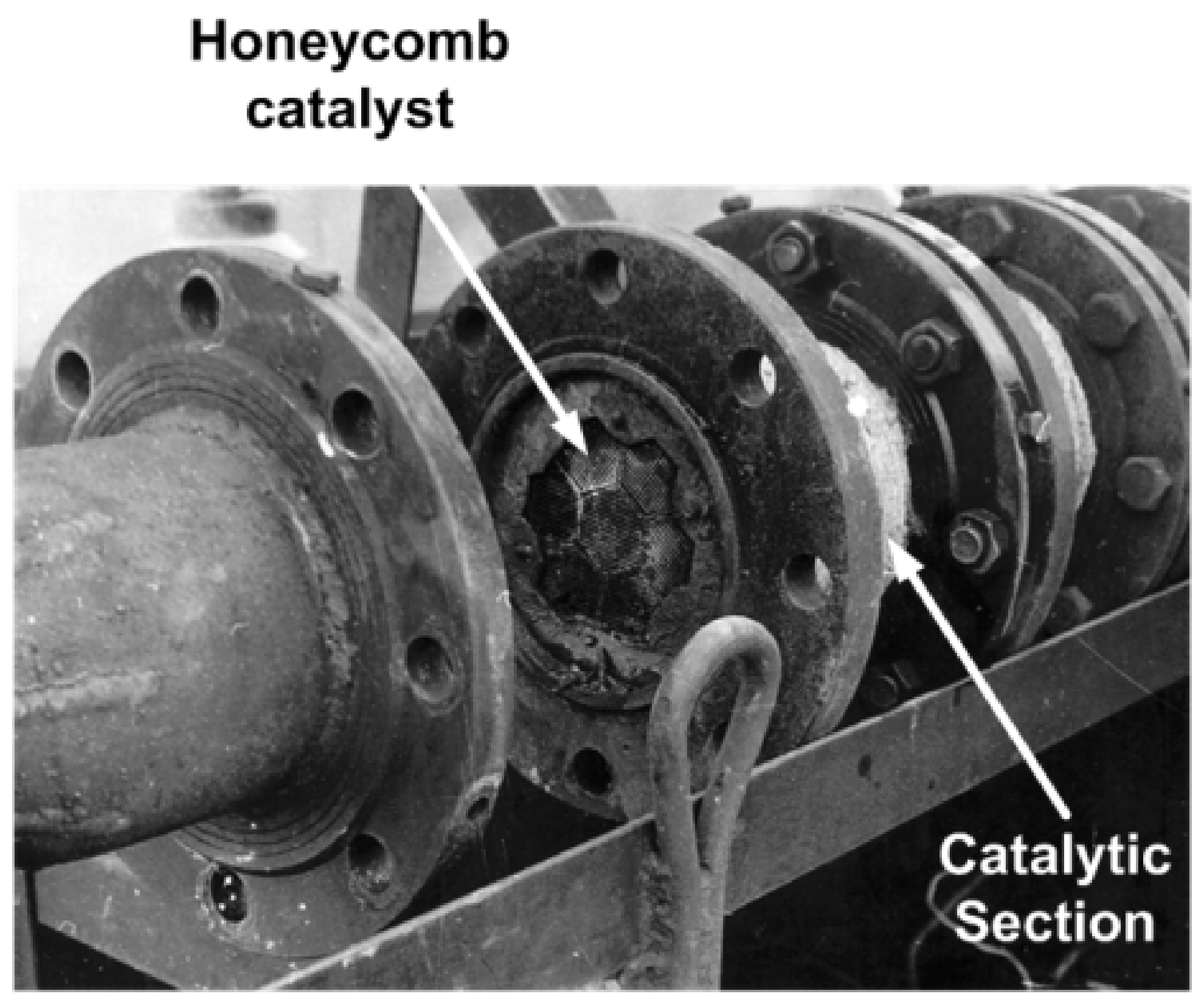

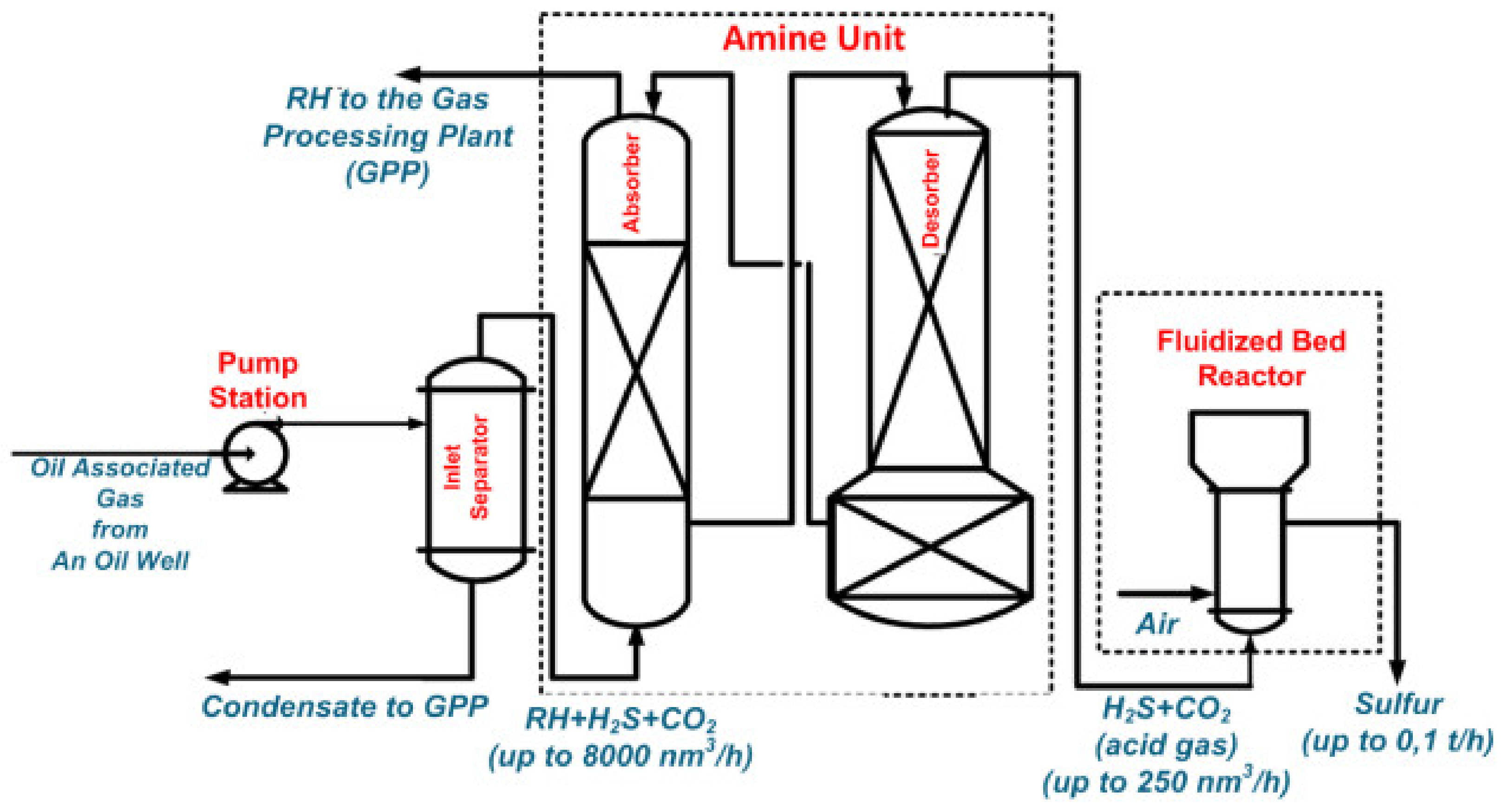
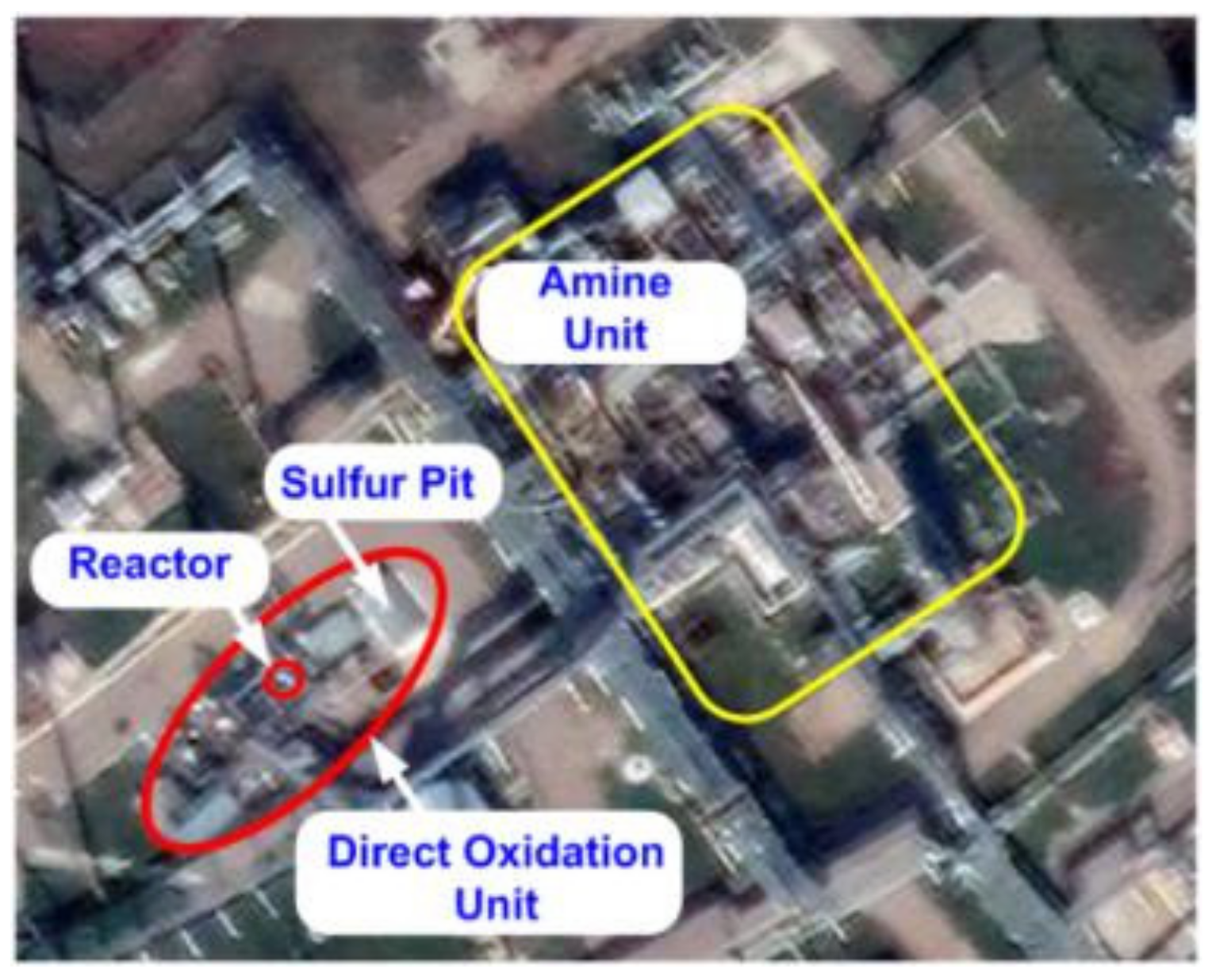

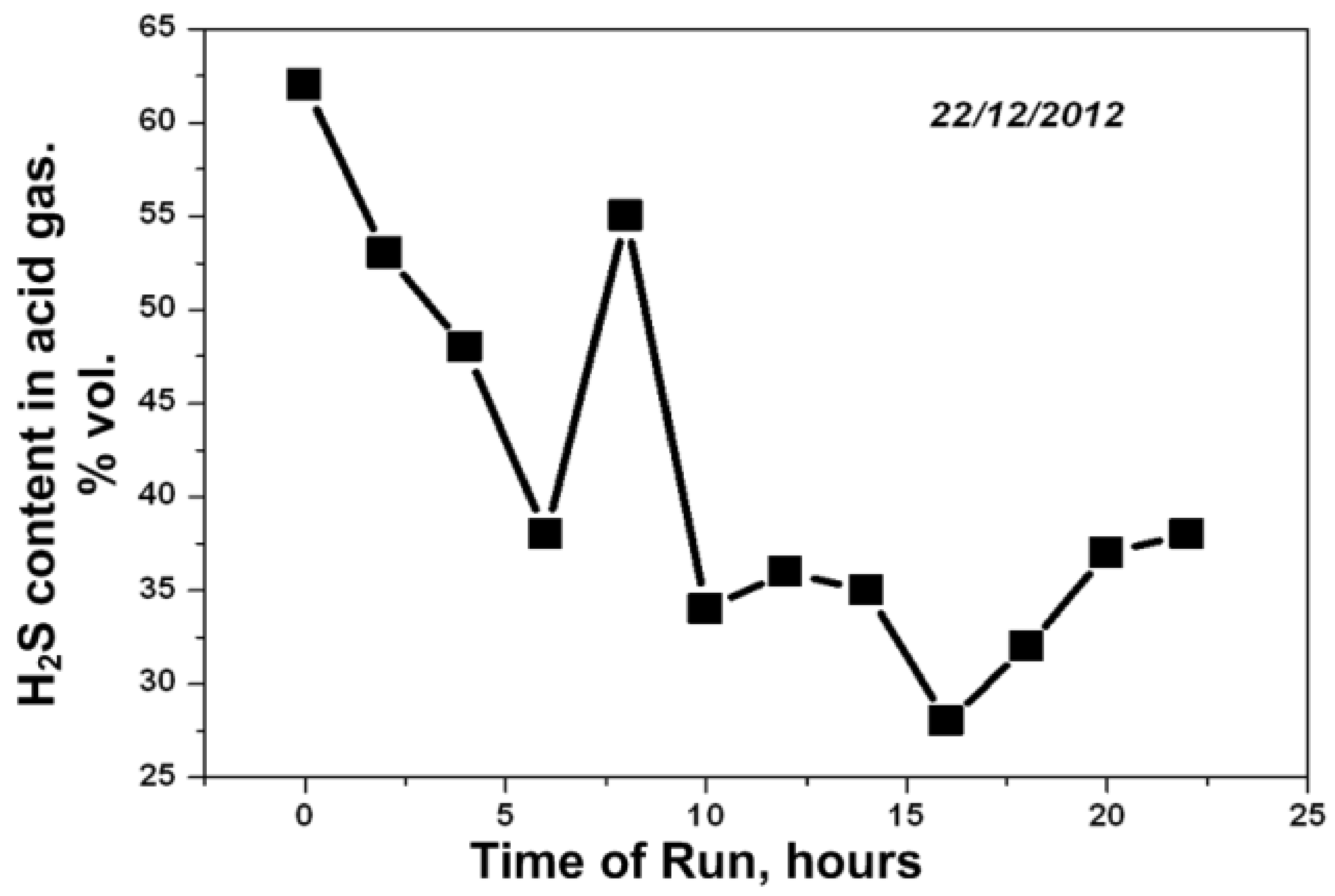



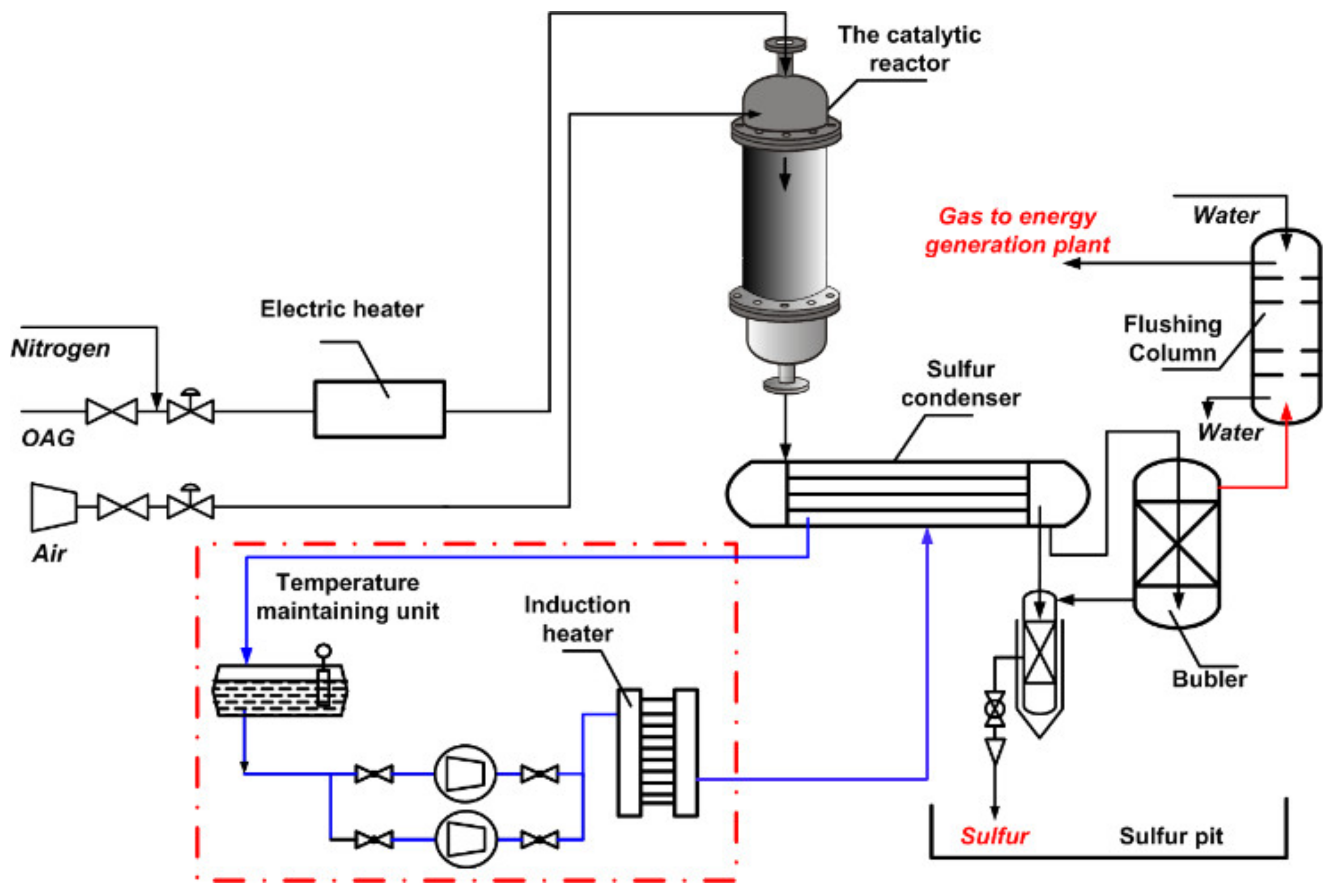

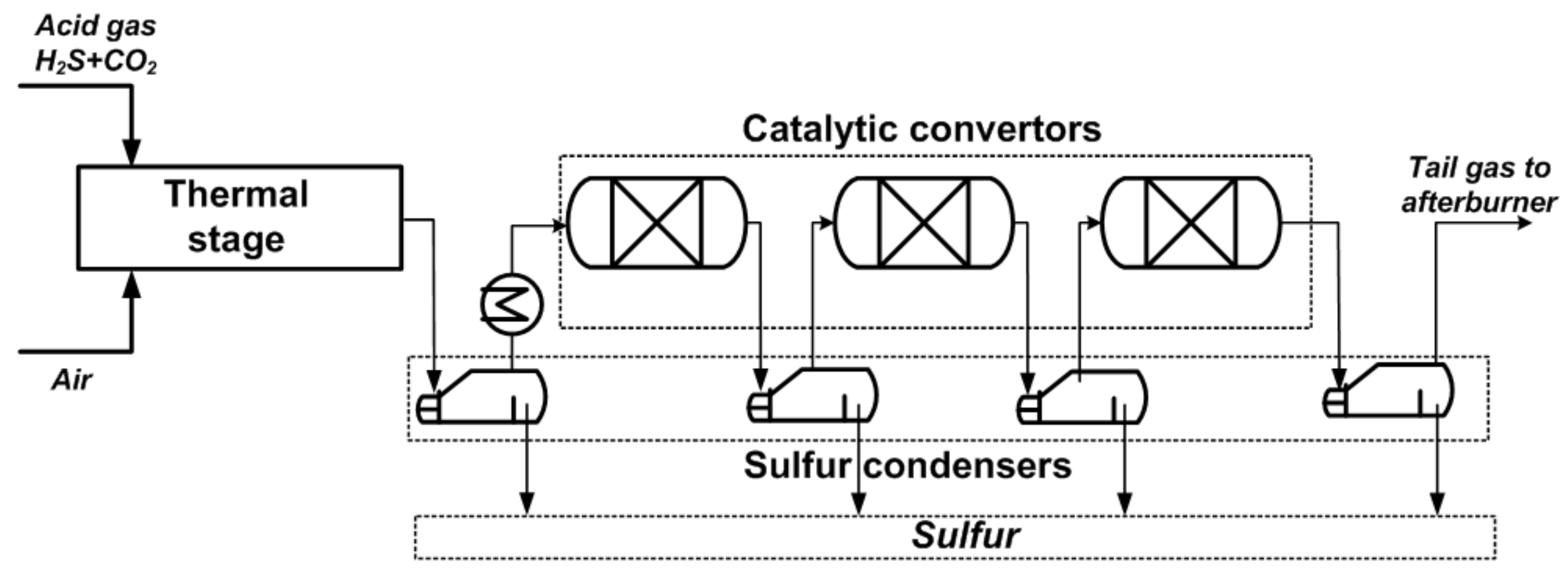
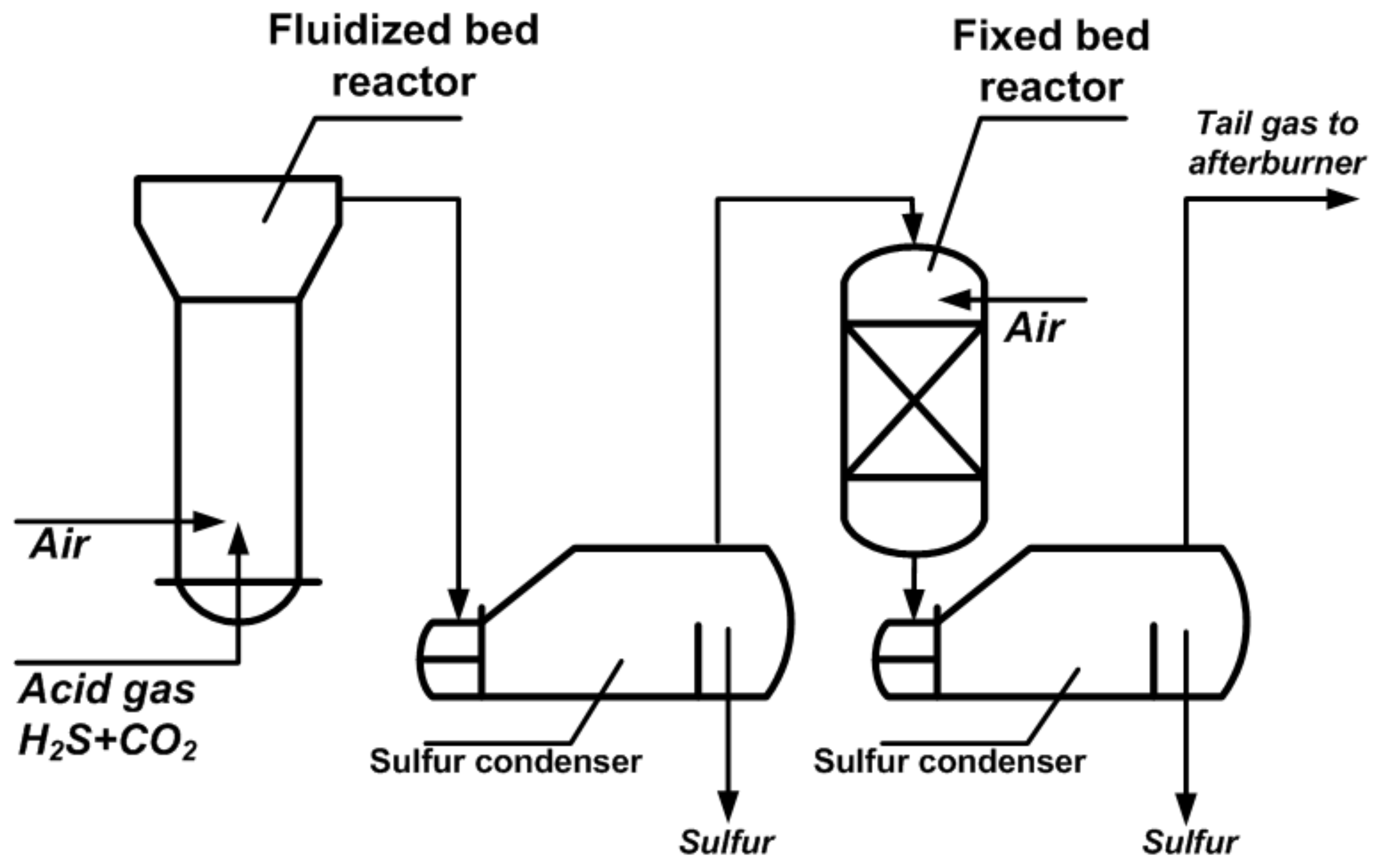
| Process | H2S Content, Vol.% | Other Gas Components |
|---|---|---|
| Purification of gases from oil processing (MEA treatment) | 90–98 | Carbon dioxide, hydrogen, methane |
| Purification of natural and oil-associated gas (MEA or DEA treatment) | 10–70 | Carbon dioxide, water vapor, hydrocarbons C1–C6 |
| # | Location Object H2S Content | Operation Conditions | Year | H2S, % | |
|---|---|---|---|---|---|
| Scale | Gas Supply | ||||
| 1 | Astrakhan sour gas field Natural gas C(H2S) = 27 vol.% | Pilot | up to 50 nm3/h | 1987 | 98 |
| 2 | Astrakhan sour gas field Natural gas C(H2S) = 27 vol.% | Pilot | up to 50 nm3/h | 1988 | 98 |
| A3 | Astrakhan sour gas field Natural gas C(H2S) = 27 vol.% | Pilot | up to 20 nm3/h | 1991 | 98 |
| 4 | Ufa Refinery Hydrodesulfurization gas C(H2S) = 70 vol.% | Pilot | up to 50 nm3/h | 1990 | 98 |
| 5 | Shkapovo GPP Acid gas from amine unit C(H2S) = 65 vol.% | Semi-industrial | up to 350 nm3/h | 1995 | 98 |
| 6 | Bavly oil field Acid gas from amine unit C(H2S) = 65 vol.% | Semi-industrial | up to 70 nm3/h of acid gas | 2004–2009 | 99.5 |
| # | Location Object H2S Content | Operation Conditions | Year | H2S, % | |
|---|---|---|---|---|---|
| Scale | Gas Supply | ||||
| 1 | Novo-Ufimsky Refinery Tail gas of Claus process C(H2S) = 2 vol.% | Pilot | up to 20 nm3/h | 1989-1990 | 98 |
| 2 | Astrakhan GPP Tail gas of Claus process C(H2S) = 2 vol.% | Pilot | up to 20 nm3/h | 1991 | 98 |
| 3 | Orenburg GPP Gases of zeolites regeneration C(H2S) = 2 vol.% C(RSH) = 5 vol.% | Pilot | up to 20 nm3/h P up to 0.5 MPa | 1990 | 98 |
| 4 | Kamchatka peninsula Geothermal steam C(H2S) < 1 vol.% C(H2O) > 99 vol.% | Fixed bed Pilot | up to 0.5 tn. steam/h P up to 1.0 MPa | 1989-1990 | 99.9 2500 h of continuous operation |
| 5 | Novo-Ufimsky Refinery Tail gas of Claus process C(H2S) = 2 vol. % | Semi-industrial | up to 7000 nm3/h | 1994 | 98 |
| # | Parameters | Value |
|---|---|---|
| 1 | Acid gas flow rate after amine unite to the direct oxidation unit, nm3/hour | to 110 |
| 2 | H2S concentration in acid gas, vol. % | 75–90 |
| 3 | Diameter of the fluidized bed reactor, m | 0.52 |
| 4 | Catalyst loading, kg | 185 |
| 5 | Sulfur yield, tons/hour | 0.13 |
| # | Compound | Initial Feedstock Gas, %Vol. | Purified Gas, %Vol. |
|---|---|---|---|
| 1 | H2S | 1.50 | <50 ppmv |
| 2 | Water | 0.69 | 2.030 |
| 3 | He | 0.05 | 0.04 |
| 4 | Hydrogen | 0.006 | 0.004 |
| 5 | Oxygen | 0.04 | 0.92 |
| 6 | CO2 | 4.70 | 4.56 |
| 7 | Nitrogen | 39.82 | 41.00 |
| 8 | Ethane | 9.60 | 9.60 |
| 9 | Methane | 25.60 | 24.22 |
| 10 | Propane | 9.96 | 9.80 |
| 11 | iso-Butane | 2.02 | 1.96 |
| 12 | n-Butane | 3.45 | 3.34 |
| 13 | neo-Pentane | 0.003 | 0.003 |
| 14 | iso-Pentane | 1.23 | 1.19 |
| 15 | n-Pentane | 0.85 | 0.81 |
| 16 | Hexanes | 0.32 | 0.31 |
| 17 | Heptanes | 0.07 | 0.07 |
| 18 | Octanes | 0.10 | 0.09 |
| # | Parameters | Direct Oxidation Unit | Three Stage Claus Unit Minibay Gas Processing Plant |
|---|---|---|---|
| 1 | Acid gas (H2S+CO2) supply, nm3/h | 1050 | 1050 |
| 2 | H2S content, %vol. | 80 | 80 |
| 3 | Air supply, nm3/h | 2000 | 2000 |
| 4 | Sulfur production Annually, ton | 10.000 | 10.000 |
| 5 | Dimensions of the main units | Calculation Fluidized bed reactor: Diameter = 1.5 m Height = 6 m Fixed bed reactor Diameter = 2.5 m Height = 6 m | Direct data Thermal stage furnace Diameter = 2.5 m Length = 7 m Catalytic converters (3 pieces) Diameter = 2.5 m Length = 4 m |
| 6 | Catalyst load, ton | Calculation Fluidized bed reactor-2 Fixed bed reactor-5 | Direct data Total: 18 |
| 7 | Sulfur cost Arbitrary units, estimation | 1 | 2.5 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khairulin, S.; Kerzhentsev, M.; Salnikov, A.; Ismagilov, Z.R. Direct Selective Oxidation of Hydrogen Sulfide: Laboratory, Pilot and Industrial Tests. Catalysts 2021, 11, 1109. https://doi.org/10.3390/catal11091109
Khairulin S, Kerzhentsev M, Salnikov A, Ismagilov ZR. Direct Selective Oxidation of Hydrogen Sulfide: Laboratory, Pilot and Industrial Tests. Catalysts. 2021; 11(9):1109. https://doi.org/10.3390/catal11091109
Chicago/Turabian StyleKhairulin, Sergei, Mikhail Kerzhentsev, Anton Salnikov, and Zinfer R. Ismagilov. 2021. "Direct Selective Oxidation of Hydrogen Sulfide: Laboratory, Pilot and Industrial Tests" Catalysts 11, no. 9: 1109. https://doi.org/10.3390/catal11091109
APA StyleKhairulin, S., Kerzhentsev, M., Salnikov, A., & Ismagilov, Z. R. (2021). Direct Selective Oxidation of Hydrogen Sulfide: Laboratory, Pilot and Industrial Tests. Catalysts, 11(9), 1109. https://doi.org/10.3390/catal11091109






