Fasting and Exercise in Oncology: Potential Synergism of Combined Interventions
Abstract
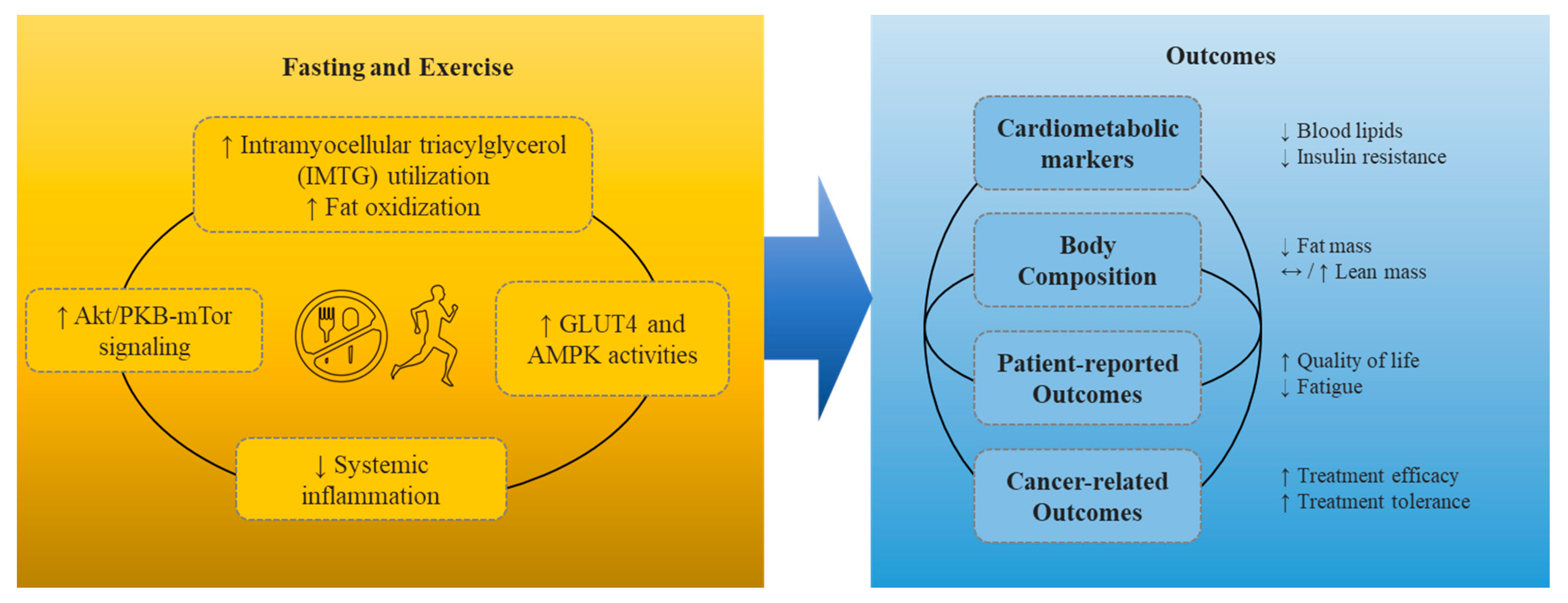
:1. Introduction
2. What Metabolic Changes Occur during Fasting and Exercise?
3. Effect of Fasting, Exercise, and Combined Fasting and Exercise
3.1. Cardiometabolic Biomarkers
3.1.1. Fasting
3.1.2. Exercise
3.1.3. Combined Fasting and Exercise
3.2. Body Composition
3.2.1. Fasting
3.2.2. Exercise
3.2.3. Combined Fasting and Exercise
3.3. Patient-Reported Outcomes
3.3.1. Fasting
3.3.2. Exercise
3.3.3. Combined Fasting and Exercise
3.4. Cancer-Related Outcomes
3.4.1. Fasting
3.4.2. Exercise
3.4.3. Combined Fasting and Exercise
4. Safety with Intervention Implementation
5. Future Research and Key Considerations
5.1. Timing of Intervention Delivery
5.2. Alternative Intervention Modalities
5.3. Treatment and Diagnosis Considerations
5.4. Cultural Relevancy/Religious Considerations
5.5. Age Considerations
5.6. Ongoing Trials
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Buchinger, O., Sr. 40 Years of fasting therapy. Hippokrates 1959, 30, 246–248. [Google Scholar]
- Cespedes Feliciano, E.M.; Chen, W.Y.; Bradshaw, P.T.; Prado, C.M.; Alexeeff, S.; Albers, K.B.; Castillo, A.L.; Caan, B.J. Adipose tissue distribution and cardiovascular disease risk among breast cancer survivors. J. Clin. Oncol. 2019, 37, 2528–2536. [Google Scholar] [CrossRef]
- Keating, N.L.; O’Malley, A.J.; Freedland, S.J.; Smith, M.R. Diabetes and cardiovascular disease during androgen deprivation therapy: Observational study of veterans with prostate cancer. J. Natl. Cancer Inst. 2010, 102, 39–46. [Google Scholar] [CrossRef] [Green Version]
- Felicetti, F.; Fortunati, N.; Brignardello, E. Cancer survivors: An expanding population with an increased cardiometabolic risk. Diabetes Res. Clin. Pract. 2018, 143, 432–442. [Google Scholar] [CrossRef] [PubMed]
- Gupta, D.; Lee Chuy, K.; Yang, J.C.; Bates, M.; Lombardo, M.; Steingart, R.M. Cardiovascular and metabolic effects of androgen-deprivation therapy for prostate cancer. J. Oncol. Pract. 2018, 14, 580–587. [Google Scholar] [CrossRef] [Green Version]
- Corremans, R.; Adao, R.; De Keulenaer, G.W.; Leite-Moreira, A.F.; Bras-Silva, C. Update on pathophysiology and preventive strategies of anthracycline-induced cardiotoxicity. Clin. Exp. Pharmacol. Physiol. 2019, 46, 204–215. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cardinale, D.; Iacopo, F.; Cipolla, C.M. Cardiotoxicity of anthracyclines. Front. Cardiovasc. Med. 2020, 7, 26. [Google Scholar] [CrossRef] [Green Version]
- Wilson, R.L.; Shannon, T.; Calton, E.; Galvao, D.A.; Taaffe, D.R.; Hart, N.H.; Lyons-Wall, P.; Newton, R.U. Efficacy of a weight loss program prior to robot assisted radical prostatectomy in overweight and obese men with prostate cancer. Surg. Oncol. 2020, 35, 182–188. [Google Scholar] [CrossRef]
- Newton, R.U.; Jeffery, E.; Galvao, D.A.; Peddle-McIntyre, C.J.; Spry, N.; Joseph, D.; Denham, J.W.; Taaffe, D.R. Body composition, fatigue and exercise in patients with prostate cancer undergoing androgen-deprivation therapy. BJU Int. 2018, 122, 986–993. [Google Scholar] [CrossRef]
- Baker, A.M.; Smith, K.C.; Coa, K.I.; Helzlsouer, K.J.; Caulfield, L.E.; Peairs, K.S.; Shockney, L.D.; Klassen, A.C. Clinical care providers’ perspectives on body size and weight management among long-term cancer survivors. Integr. Cancer Ther. 2015, 14, 240–248. [Google Scholar] [CrossRef] [Green Version]
- Thomas, R.J.; Holm, M.; Williams, M.; Bowman, E.; Bellamy, P.; Andreyev, J.; Maher, J. Lifestyle factors correlate with the risk of late pelvic symptoms after prostatic radiotherapy. Clin. Oncol. 2013, 25, 246–251. [Google Scholar] [CrossRef]
- Ryan, A.M.; Power, D.G.; Daly, L.; Cushen, S.J.; Ní Bhuachalla, Ē.; Prado, C.M. Cancer-associated malnutrition, cachexia and sarcopenia: The skeleton in the hospital closet 40 years later. Proc. Nutr. Soc. 2016, 75, 199–211. [Google Scholar] [CrossRef] [Green Version]
- Burden, S.; Jones, D.J.; Sremanakova, J.; Sowerbutts, A.M.; Lal, S.; Pilling, M.; Todd, C. Dietary interventions for adult cancer survivors. Cochrane Database Syst. Rev. 2019. [Google Scholar] [CrossRef] [PubMed]
- Christensen, J.F.; Simonsen, C.; Hojman, P. Exercise training in cancer control and treatment. Compr. Physiol. 2018, 9, 165–205. [Google Scholar] [CrossRef] [PubMed]
- Cathcart, P.; Craddock, C.; Stebbing, J. Fasting: Starving cancer. Lancet Oncol. 2017, 18, 431. [Google Scholar] [CrossRef]
- Bauersfeld, S.P.; Kessler, C.S.; Wischnewsky, M.; Jaensch, A.; Steckhan, N.; Stange, R.; Kunz, B.; Bruckner, B.; Sehouli, J.; Michalsen, A. The effects of short-term fasting on quality of life and tolerance to chemotherapy in patients with breast and ovarian cancer: A randomized cross-over pilot study. BMC Cancer 2018, 18, 476. [Google Scholar] [CrossRef]
- de Groot, S.; Lugtenberg, R.T.; Cohen, D.; Welters, M.J.P.; Ehsan, I.; Vreeswijk, M.P.G.; Smit, V.T.H.B.M.; de Graaf, H.; Heijns, J.B.; Portielje, J.E.A.; et al. Fasting mimicking diet as an adjunct to neoadjuvant chemotherapy for breast cancer in the multicentre randomized phase 2 DIRECT trial. Nat. Commun. 2020, 11, 3083. [Google Scholar] [CrossRef]
- Dorff, T.B.; Groshen, S.; Garcia, A.; Shah, M.; Tsao-Wei, D.; Pham, H.; Cheng, C.W.; Brandhorst, S.; Cohen, P.; Wei, M.; et al. Safety and feasibility of fasting in combination with platinum-based chemotherapy. BMC Cancer 2016, 16, 360. [Google Scholar] [CrossRef] [Green Version]
- de Groot, S.; Vreeswijk, M.P.; Welters, M.J.; Gravesteijn, G.; Boei, J.J.; Jochems, A.; Houtsma, D.; Putter, H.; van der Hoeven, J.J.; Nortier, J.W. The effects of short-term fasting on tolerance to (neo) adjuvant chemotherapy in HER2-negative breast cancer patients: A randomized pilot study. BMC Cancer 2015, 15, 652. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Safdie, F.M.; Dorff, T.; Quinn, D.; Fontana, L.; Wei, M.; Lee, C.; Cohen, P.; Longo, V.D. Fasting and cancer treatment in humans: A case series report. Aging 2009, 1, 988. [Google Scholar] [CrossRef] [Green Version]
- De Groot, S.; Pijl, H.; van der Hoeven, J.J.; Kroep, J.R. Effects of short-term fasting on cancer treatment. J. Exp. Clin. Cancer Res. 2019, 38, 1–14. [Google Scholar] [CrossRef] [Green Version]
- Doyle, C.; Kushi, L.H.; Byers, T.; Courneya, K.S.; Demark-Wahnefried, W.; Grant, B.; McTiernan, A.; Rock, C.L.; Thompson, C.; Gansler, T.; et al. Nutrition and physical activity during and after cancer treatment: An American Cancer Society guide for informed choices. CA Cancer J. Clin. 2006, 56, 323–353. [Google Scholar] [CrossRef] [Green Version]
- Wei, M.; Brandhorst, S.; Shelehchi, M.; Mirzaei, H.; Cheng, C.W.; Budniak, J.; Groshen, S.; Mack, W.J.; Guen, E.; Di Biase, S.; et al. Fasting-mimicking diet and markers/risk factors for aging, diabetes, cancer, and cardiovascular disease. Sci. Transl. Med. 2017, 9. [Google Scholar] [CrossRef]
- Di Biase, S.; Lee, C.; Brandhorst, S.; Manes, B.; Buono, R.; Cheng, C.W.; Cacciottolo, M.; Martin-Montalvo, A.; de Cabo, R.; Wei, M.; et al. Fasting-Mimicking Diet Reduces HO-1 to Promote T Cell-Mediated Tumor Cytotoxicity. Cancer Cell 2016, 30, 136–146. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Campbell, K.L.; Winters-Stone, K.M.; Wiskemann, J.; May, A.M.; Schwartz, A.L.; Courneya, K.S.; Zucker, D.S.; Matthews, C.E.; Ligibel, J.A.; Gerber, L.H.; et al. Exercise Guidelines for Cancer Survivors: Consensus Statement from International Multidisciplinary Roundtable. Med. Sci. Sports Exerc. 2019, 51, 2375–2390. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schmitz, K.H. Exercise Oncology: Prescribing Physical Activity before and after a Cancer Diagnosis; Springer International Publishing: Berlin/Heidelberg, Germany, 2020. [Google Scholar]
- Bland, K.A.; Zadravec, K.; Landry, T.; Weller, S.; Meyers, L.; Campbell, K.L. Impact of exercise on chemotherapy completion rate: A systematic review of the evidence and recommendations for future exercise oncology research. Crit. Rev. Oncol. Hematol. 2019, 136, 79–85. [Google Scholar] [CrossRef] [PubMed]
- Morielli, A.R.; Courneya, K.S. Effects of exercise on cancer treatment completion and efficacy. In Exercise Oncology: Prescribing Physical Activity before and after a Cancer Diagnosis; Springer Nature: Cham, Switzerland, 2020; pp. 235–256. [Google Scholar]
- Courneya, K.S.; Segal, R.J.; McKenzie, D.C.; Dong, H.; Gelmon, K.; Friedenreich, C.M.; Yasui, Y.; Reid, R.D.; Crawford, J.J.; Mackey, J.R. Effects of exercise during adjuvant chemotherapy on breast cancer outcomes. Med. Sci. Sports Exerc. 2014, 46, 1744–1751. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rief, H.; Bruckner, T.; Schlampp, I.; Bostel, T.; Welzel, T.; Debus, J.; Förster, R. Resistance training concomitant to radiotherapy of spinal bone metastases—Survival and prognostic factors of a randomized trial. Radiat. Oncol. 2016, 11, 1–7. [Google Scholar] [CrossRef] [Green Version]
- Wiskemann, J.; Kleindienst, N.; Kuehl, R.; Dreger, P.; Schwerdtfeger, R.; Bohus, M. Effects of physical exercise on survival after allogeneic stem cell transplantation. Int. J. Cancer 2015, 137, 2749–2756. [Google Scholar] [CrossRef] [PubMed]
- Caspersen, C.J.; Powell, K.E.; Christenson, G.M. Physical activity, exercise, and physical fitness: Definitions and distinctions for health-related research. Public Health Rep. 1985, 100, 126–131. [Google Scholar]
- Van der Ploeg, H.P.; Hillsdon, M. Is sedentary behaviour just physical inactivity by another name? Int. J. Behav. Nutr. Phys. Act. 2017, 14, 142. [Google Scholar] [CrossRef]
- Jaspers, R.T.; Zillikens, M.C.; Friesema, E.C.; delli Paoli, G.; Bloch, W.; Uitterlinden, A.G.; Goglia, F.; Lanni, A.; de Lange, P. Exercise, fasting, and mimetics: Toward beneficial combinations? FASEB J. 2017, 31, 14–28. [Google Scholar] [CrossRef] [Green Version]
- Wallis, G.A.; Gonzalez, J.T. Is exercise best served on an empty stomach? Proc. Nutr. Soc. 2019, 78, 110–117. [Google Scholar] [CrossRef] [Green Version]
- Hansen, D.; De Strijcker, D.; Calders, P. Impact of endurance exercise training in the fasted state on muscle biochemistry and metabolism in healthy subjects: Can these effects be of particular clinical benefit to type 2 diabetes mellitus and insulin-resistant patients? Sports Med. 2017, 47, 415–428. [Google Scholar] [CrossRef]
- Albrecht, T.A.; Anderson, J.G.; Jones, R.; Bourguignon, C.; Taylor, A.G. A complementary care study combining flaxseed oil, caffeine, fasting, and exercise in women diagnosed with advanced ovarian cancer: Findings from a case study. Holist. Nurs. Pract. 2012, 26, 308–316. [Google Scholar] [CrossRef] [PubMed]
- Rui, L. Energy metabolism in the liver. Compr. Physiol. 2014, 4, 177–197. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stote, K.S.; Baer, D.J.; Spears, K.; Paul, D.R.; Harris, G.K.; Rumpler, W.V.; Strycula, P.; Najjar, S.S.; Ferrucci, L.; Ingram, D.K.; et al. A controlled trial of reduced meal frequency without caloric restriction in healthy, normal-weight, middle-aged adults. Am. J. Clin. Nutr. 2007, 85, 981–988. [Google Scholar] [CrossRef] [PubMed]
- Keenan, S.; Cooke, M.B.; Belski, R. The effects of intermittent fasting combined with resistance training on lean body mass: A systematic review of human studies. Nutrients 2020, 12, 2349. [Google Scholar] [CrossRef] [PubMed]
- Donnelly, R.P.; Finlay, D.K. Glucose, glycolysis and lymphocyte responses. Mol. Immunol. 2015, 68, 513–519. [Google Scholar] [CrossRef]
- Coyle, E.F.; Jeukendrup, A.E.; Wagenmakers, A.J.; Saris, W.H. Fatty acid oxidation is directly regulated by carbohydrate metabolism during exercise. Am. J. Physiol. 1997, 273, E268–E275. [Google Scholar] [CrossRef]
- De Bock, K.; Richter, E.A.; Russell, A.P.; Eijnde, B.O.; Derave, W.; Ramaekers, M.; Koninckx, E.; Leger, B.; Verhaeghe, J.; Hespel, P. Exercise in the fasted state facilitates fibre type-specific intramyocellular lipid breakdown and stimulates glycogen resynthesis in humans. J. Physiol. 2005, 564, 649–660. [Google Scholar] [CrossRef] [PubMed]
- Thomas, D.E.; Brotherhood, J.R.; Brand, J.C. Carbohydrate feeding before exercise: Effect of glycemic index. Int. J. Sports Med. 1991, 12, 180–186. [Google Scholar] [CrossRef]
- Vieira, A.F.; Costa, R.R.; Macedo, R.C.; Coconcelli, L.; Kruel, L.F. Effects of aerobic exercise performed in fasted v. fed state on fat and carbohydrate metabolism in adults: A systematic review and meta-analysis. Br. J. Nutr. 2016, 116, 1153–1164. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics, 2020. CA Cancer J. Clin. 2020, 70, 7–30. [Google Scholar] [CrossRef]
- Weroha, S.J.; Haluska, P. The insulin-like growth factor system in cancer. Endocrinol. Metab. Clin. N. Am. 2012, 41, 335–350. [Google Scholar] [CrossRef] [Green Version]
- Pollak, M.N.; Schernhammer, E.S.; Hankinson, S.E. Insulin-like growth factors and neoplasia. Nat. Rev. Cancer 2004, 4, 505–518. [Google Scholar] [CrossRef]
- Calle, E.E.; Rodriguez, C.; Walker-Thurmond, K.; Thun, M.J. Overweight, obesity, and mortality from cancer in a prospectively studied cohort of U.S. adults. N. Engl. J. Med. 2003, 348, 1625–1638. [Google Scholar] [CrossRef] [Green Version]
- Zorn, S.; Ehret, J.; Schäuble, R.; Rautenberg, B.; Ihorst, G.; Bertz, H.; Urbain, P.; Raynor, A. Impact of modified short-term fasting and its combination with a fasting supportive diet during chemotherapy on the incidence and severity of chemotherapy-induced toxicities in cancer patients-a controlled cross-over pilot study. BMC Cancer 2020, 20, 578. [Google Scholar] [CrossRef]
- Marinac, C.R.; Nelson, S.H.; Breen, C.I.; Hartman, S.J.; Natarajan, L.; Pierce, J.P.; Flatt, S.W.; Sears, D.D.; Patterson, R.E. Prolonged nightly fasting and breast cancer prognosis. JAMA Oncol. 2016, 2, 1049–1055. [Google Scholar] [CrossRef] [PubMed]
- Ferroni, P.; Riondino, S.; Laudisi, A.; Portarena, I.; Formica, V.; Alessandroni, J.; D’Alessandro, R.; Orlandi, A.; Costarelli, L.; Cavaliere, F. Pretreatment insulin levels as a prognostic factor for breast cancer progression. Oncologist 2016, 21, 1041. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Duggan, C.; Wang, C.Y.; Neuhouser, M.L.; Xiao, L.; Smith, A.W.; Reding, K.W.; Baumgartner, R.N.; Baumgartner, K.B.; Bernstein, L.; Ballard-Barbash, R. Associations of insulin-like growth factor and insulin-like growth factor binding protein-3 with mortality in women with breast cancer. Int. J. Cancer 2013, 132, 1191–1200. [Google Scholar] [CrossRef] [PubMed]
- Meyerhardt, J.A.; Catalano, P.J.; Haller, D.G.; Mayer, R.J.; Macdonald, J.S.; Benson, A.B., 3rd; Fuchs, C.S. Impact of diabetes mellitus on outcomes in patients with colon cancer. J. Clin. Oncol. 2003, 21, 433–440. [Google Scholar] [CrossRef]
- Derr, R.L.; Ye, X.; Islas, M.U.; Desideri, S.; Saudek, C.D.; Grossman, S.A. Association between hyperglycemia and survival in patients with newly diagnosed glioblastoma. J. Clin. Oncol. 2009, 27, 1082. [Google Scholar] [CrossRef] [PubMed]
- Kang, D.W.; Fairey, A.S.; Boule, N.G.; Field, C.J.; Courneya, K.S. Effects of exercise on insulin, IGF axis, adipocytokines, and inflammatory markers in breast cancer survivors: A systematic review and meta-analysis. Cancer Epidemiol. Biomark. Prev. 2017, 26, 355–365. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Jin, B.; Paxton, R.J.; Yang, W.; Wang, X.; Jiao, Y.; Yu, C.; Chen, X. The effects of exercise on insulin, glucose, IGF-axis and CRP in cancer survivors: Meta-analysis and meta-regression of randomised controlled trials. Eur. J. Cancer Care 2020, 29, e13186. [Google Scholar] [CrossRef]
- Arcidiacono, B.; Iiritano, S.; Nocera, A.; Possidente, K.; Nevolo, M.T.; Ventura, V.; Foti, D.; Chiefari, E.; Brunetti, A. Insulin resistance and cancer risk: An overview of the pathogenetic mechanisms. Exp. Diabetes Res. 2012, 2012, 789174. [Google Scholar] [CrossRef] [Green Version]
- Butler, L.M.; Perone, Y.; Dehairs, J.; Lupien, L.E.; de Laat, V.; Talebi, A.; Loda, M.; Kinlaw, W.B.; Swinnen, J.V. Lipids and cancer: Emerging roles in pathogenesis, diagnosis and therapeutic intervention. Adv. Drug Deliv. Rev. 2020, 159, 245–293. [Google Scholar] [CrossRef] [PubMed]
- Goodyear, L.J.; Kahn, B.B. Exercise, glucose transport, and insulin sensitivity. Annu. Rev. Med. 1998, 49, 235–261. [Google Scholar] [CrossRef]
- Pesta, D.H.; Goncalves, R.L.S.; Madiraju, A.K.; Strasser, B.; Sparks, L.M. Resistance training to improve type 2 diabetes: Working toward a prescription for the future. Nutr. Metab. 2017, 14, 24. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Keshel, T.E.; Coker, R.H. Exercise Training and Insulin Resistance: A current review. J. Obes. Weight Loss Ther. 2015, 5, S5-003. [Google Scholar] [CrossRef] [PubMed]
- Horowitz, J.F.; Klein, S. Lipid metabolism during endurance exercise. Am. J. Clin. Nutr. 2000, 72, 558S–563S. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gordon, B.; Chen, S.; Durstine, J.L. The effects of exercise training on the traditional lipid profile and beyond. Curr. Sports Med. Rep. 2014, 13, 253–259. [Google Scholar] [CrossRef] [PubMed]
- Mann, S.; Beedie, C.; Jimenez, A. Differential effects of aerobic exercise, resistance training and combined exercise modalities on cholesterol and the lipid profile: Review, synthesis and recommendations. Sports Med. 2014, 44, 211–221. [Google Scholar] [CrossRef] [Green Version]
- Noland, R.C. Chapter Three—Exercise and Regulation of Lipid Metabolism. In Progress in Molecular Biology and Translational Science; Bouchard, C., Ed.; Academic Press: Cambridge, MA, USA, 2015; Volume 135, pp. 39–74. [Google Scholar]
- Haxhi, J.; Scotto di Palumbo, A.; Sacchetti, M. Exercising for metabolic control: Is timing important? Ann. Nutr. Metab. 2013, 62, 14–25. [Google Scholar] [CrossRef]
- Bhutani, S.; Klempel, M.C.; Kroeger, C.M.; Trepanowski, J.F.; Varady, K.A. Alternate day fasting and endurance exercise combine to reduce body weight and favorably alter plasma lipids in obese humans. Obesity 2013, 21, 1370–1379. [Google Scholar] [CrossRef]
- Cho, A.R.; Moon, J.Y.; Kim, S.; An, K.Y.; Oh, M.; Jeon, J.Y.; Jung, D.H.; Choi, M.H.; Lee, J.W. Effects of alternate day fasting and exercise on cholesterol metabolism in overweight or obese adults: A pilot randomized controlled trial. Metabolism 2019, 93, 52–60. [Google Scholar] [CrossRef] [PubMed]
- Galvao, D.A.; Taaffe, D.R.; Spry, N.; Joseph, D.; Newton, R.U. Acute versus chronic exposure to androgen suppression for prostate cancer: Impact on the exercise response. J. Urol. 2011, 186, 1291–1297. [Google Scholar] [CrossRef]
- Kelley, D.E.; Goodpaster, B.H. Skeletal muscle triglyceride. An aspect of regional adiposity and insulin resistance. Diabetes Care 2001, 24, 933–941. [Google Scholar] [CrossRef] [Green Version]
- Collins, K.H.; Herzog, W.; MacDonald, G.Z.; Reimer, R.A.; Rios, J.L.; Smith, I.C.; Zernicke, R.F.; Hart, D.A. Obesity, metabolic syndrome, and musculoskeletal disease: Common inflammatory pathways suggest a central role for loss of muscle integrity. Front. Physiol. 2018, 9, 112. [Google Scholar] [CrossRef]
- Cheung, A.S.; Hoermann, R.; Dupuis, P.; Joon, D.L.; Zajac, J.D.; Grossmann, M. Relationships between insulin resistance and frailty with body composition and testosterone in men undergoing androgen deprivation therapy for prostate cancer. Eur. J. Endocrinol. 2016, 175, 229–237. [Google Scholar] [CrossRef] [Green Version]
- van Loon, L.J.; Koopman, R.; Stegen, J.H.; Wagenmakers, A.J.; Keizer, H.A.; Saris, W.H. Intramyocellular lipids form an important substrate source during moderate intensity exercise in endurance-trained males in a fasted state. J. Physiol. 2003, 553, 611–625. [Google Scholar] [CrossRef] [Green Version]
- Qu, H.-Q.; Li, Q.; Rentfro, A.R.; Fisher-Hoch, S.P.; McCormick, J.B. The definition of insulin resistance using HOMA-IR for Americans of Mexican descent using machine learning. PLoS ONE 2011, 6, e21041. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tang, Q.; Li, X.; Song, P.; Xu, L. Optimal cut-off values for the homeostasis model assessment of insulin resistance (HOMA-IR) and pre-diabetes screening: Developments in research and prospects for the future. Drug Discov. Ther. 2015, 9, 380–385. [Google Scholar] [CrossRef] [Green Version]
- Yamada, C.; Mitsuhashi, T.; Hiratsuka, N.; Inabe, F.; Araida, N.; Takahashi, E. Optimal reference interval for homeostasis model assessment of insulin resistance in a Japanese population. J Diabetes Investig. 2011, 2, 373–376. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, C.H.; Shih, A.Z.; Woo, Y.C.; Fong, C.H.; Leung, O.Y.; Janus, E.; Cheung, B.M.; Lam, K.S. Optimal cut-offs of homeostasis model assessment of insulin resistance (HOMA-IR) to identify dysglycemia and type 2 diabetes mellitus: A 15-year prospective study in Chinese. PLoS ONE 2016, 11, e0163424. [Google Scholar] [CrossRef] [Green Version]
- Van Proeyen, K.; Szlufcik, K.; Nielens, H.; Pelgrim, K.; Deldicque, L.; Hesselink, M.; Van Veldhoven, P.P.; Hespel, P. Training in the fasted state improves glucose tolerance during fat-rich diet. J. Physiol. 2010, 588, 4289–4302. [Google Scholar] [CrossRef] [PubMed]
- Cespedes Feliciano, E.M.; Kroenke, C.H.; Caan, B.J. The obesity paradox in cancer: How important is muscle? Annu. Rev. Nutr. 2018, 38, 357–379. [Google Scholar] [CrossRef]
- Galvao, D.A.; Spry, N.A.; Taaffe, D.R.; Newton, R.U.; Stanley, J.; Shannon, T.; Rowling, C.; Prince, R. Changes in muscle, fat and bone mass after 36 weeks of maximal androgen blockade for prostate cancer. BJU Int. 2008, 102, 44–47. [Google Scholar] [CrossRef]
- Lee, K.; Kruper, L.; Dieli-Conwright, C.M.; Mortimer, J.E. The impact of obesity on breast cancer diagnosis and treatment. Curr. Oncol. Rep. 2019, 21, 41. [Google Scholar] [CrossRef] [Green Version]
- Dickerman, B.A.; Torfadottir, J.E.; Valdimarsdottir, U.A.; Giovannucci, E.; Wilson, K.M.; Aspelund, T.; Tryggvadottir, L.; Sigurdardottir, L.G.; Harris, T.B.; Launer, L.J.; et al. Body fat distribution on computed tomography imaging and prostate cancer risk and mortality in the AGES-Reykjavik study. Cancer 2019, 125, 2877–2885. [Google Scholar] [CrossRef]
- De Nardi, P.; Salandini, M.; Chiari, D.; Pecorelli, N.; Cristel, G.; Damascelli, A.; Ronzoni, M.; Massimino, L.; De Cobelli, F.; Braga, M. Changes in body composition during neoadjuvant therapy can affect prognosis in rectal cancer patients: An exploratory study. Curr. Probl. Cancer 2020, 44, 100510. [Google Scholar] [CrossRef]
- Michalsen, A.; Li, C. Fasting therapy for treating and preventing disease—Current state of evidence. Forsch. Komplement. 2013, 20, 444–453. [Google Scholar] [CrossRef]
- Köppel, M.; Mathis, K.; Schmitz, K.H.; Wiskemann, J. Muscle hypertrophy in cancer patients and survivors via strength training. A meta-analysis and meta-regression. Crit. Rev. Oncol. Hematol. 2021, 163, 103371. [Google Scholar] [CrossRef]
- Haseen, F.; Murray, L.J.; Cardwell, C.R.; O’Sullivan, J.M.; Cantwell, M.M. The effect of androgen deprivation therapy on body composition in men with prostate cancer: Systematic review and meta-analysis. J. Cancer Surviv. 2010, 4, 128–139. [Google Scholar] [CrossRef] [Green Version]
- Chang, D.; Joseph, D.J.; Ebert, M.A.; Galvão, D.A.; Taaffe, D.R.; Denham, J.W.; Newton, R.U.; Spry, N.A. Effect of androgen deprivation therapy on muscle attenuation in men with prostate cancer. J. Med. Imaging Radiat. Oncol. 2014, 58, 223–228. [Google Scholar] [CrossRef] [PubMed]
- Lopez, P.; Taaffe, D.R.; Newton, R.U.; Galvão, D.A. Resistance exercise dosage in men with prostate cancer: Systematic review, meta-analysis, and meta-regression. Med. Sci. Sports Exerc. 2021, 53, 459. [Google Scholar] [CrossRef] [PubMed]
- Zdravkovic, A.; Hasenöhrl, T.; Palma, S.; Crevenna, R. Effects of resistance exercise in prostate cancer patients: A systematic review update as of March 2020. Wien. Klin. Wochenschr. 2020, 132, 452–463. [Google Scholar] [CrossRef]
- McTiernan, A. Obesity and cancer: The risks, science, and potential management strategies. Oncology 2005, 19, 871–881; discussion 881–882, 885–886. [Google Scholar] [PubMed]
- Irwin, M.L.; Alvarez-Reeves, M.; Cadmus, L.; Mierzejewski, E.; Mayne, S.T.; Yu, H.; Chung, G.G.; Jones, B.; Knobf, M.T.; DiPietro, L. Exercise improves body fat, lean mass, and bone mass in breast cancer survivors. Obesity 2009, 17, 1534–1541. [Google Scholar] [CrossRef] [Green Version]
- Guinan, E.M.; Connolly, E.M.; Hussey, J. Exercise training in breast cancer survivors: A review of trials examining anthropometric and obesity-related biomarkers of breast cancer risk. Phys. Ther. Rev. 2013, 18, 79–89. [Google Scholar] [CrossRef]
- Thomas, G.A.; Cartmel, B.; Harrigan, M.; Fiellin, M.; Capozza, S.; Zhou, Y.; Ercolano, E.; Gross, C.P.; Hershman, D.; Ligibel, J.; et al. The effect of exercise on body composition and bone mineral density in breast cancer survivors taking aromatase inhibitors. Obesity 2017, 25, 346–351. [Google Scholar] [CrossRef] [PubMed]
- Brown, J.C.; Zemel, B.S.; Troxel, A.B.; Rickels, M.R.; Damjanov, N.; Ky, B.; Rhim, A.D.; Rustgi, A.K.; Courneya, K.S.; Schmitz, K.H. Dose–response effects of aerobic exercise on body composition among colon cancer survivors: A randomised controlled trial. Br. J. Cancer 2017, 117, 1614–1620. [Google Scholar] [CrossRef] [PubMed]
- Dieli-Conwright, C.M.; Mortimer, J.E.; Schroeder, E.T.; Courneya, K.; Demark-Wahnefried, W.; Buchanan, T.A.; Tripathy, D.; Bernstein, L. Effects of aerobic and resistance exercise on metabolic syndrome, sarcopenic obesity, and circulating biomarkers in overweight or obese survivors of breast cancer: A randomized controlled trial. J. Clin. Oncol. 2018, 36, 875–883. [Google Scholar] [CrossRef] [PubMed]
- Reeves, M.M.; Terranova, C.O.; Eakin, E.G.; Demark-Wahnefried, W. Weight loss intervention trials in women with breast cancer: A systematic review. Obes. Rev. 2014, 15, 749–768. [Google Scholar] [CrossRef] [Green Version]
- LeVasseur, N.; Cheng, W.; Mazzarello, S.; Clemons, M.; Vandermeer, L.; Jones, L.; Joy, A.A.; Barbeau, P.; Wolfe, D.; Ahmadzai, N. Optimising weight-loss interventions in cancer patients—A systematic review and network meta-analysis. PLoS ONE 2021, 16, e0245794. [Google Scholar] [CrossRef]
- Lee, J. The effects of resistance training on muscular strength and hypertrophy in elderly cancer patients: A systematic review and meta-analysis. J. Sport Health Sci. 2021. [Google Scholar] [CrossRef]
- Deng, T.; Lyon, C.J.; Bergin, S.; Caligiuri, M.A.; Hsueh, W.A. Obesity, inflammation, and cancer. Annu. Rev. Pathol. 2016, 11, 421–449. [Google Scholar] [CrossRef] [Green Version]
- Schoenfeld, B.J.; Aragon, A.A.; Wilborn, C.D.; Krieger, J.W.; Sonmez, G.T. Body composition changes associated with fasted versus non-fasted aerobic exercise. J. Int. Soc. Sports Nutr. 2014, 11, 54. [Google Scholar] [CrossRef]
- Stroup, S.P.; Cullen, J.; Auge, B.K.; L’Esperance, J.O.; Kang, S.K. Effect of obesity on prostate-specific antigen recurrence after radiation therapy for localized prostate cancer as measured by the 2006 Radiation Therapy Oncology Group-American Society for Therapeutic Radiation and Oncology (RTOG-ASTRO) Phoenix consensus definition. Cancer 2007, 110, 1003–1009. [Google Scholar] [CrossRef]
- Fearon, K.; Strasser, F.; Anker, S.D.; Bosaeus, I.; Bruera, E.; Fainsinger, R.L.; Jatoi, A.; Loprinzi, C.; MacDonald, N.; Mantovani, G.; et al. Definition and classification of cancer cachexia: An international consensus. Lancet Oncol. 2011, 12, 489–495. [Google Scholar] [CrossRef]
- Hanson, E.D.; Nelson, A.R.; West, D.W.; Violet, J.A.; O’keefe, L.; Phillips, S.M.; Hayes, A. Attenuation of resting but not load-mediated protein synthesis in prostate cancer patients on androgen deprivation. J. Clin. Endocrinol. Metab. 2017, 102, 1076–1083. [Google Scholar] [PubMed] [Green Version]
- Cermak, N.M.; Res, P.T.; de Groot, L.C.; Saris, W.H.; van Loon, L.J. Protein supplementation augments the adaptive response of skeletal muscle to resistance-type exercise training: A meta-analysis. Am. J. Clin. Nutr. 2012, 96, 1454–1464. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Slater, G.J.; Dieter, B.P.; Marsh, D.J.; Helms, E.R.; Shaw, G.; Iraki, J. Is an Energy Surplus Required to Maximize Skeletal Muscle Hypertrophy Associated With Resistance Training. Front. Nutr. 2019, 6, 131. [Google Scholar] [CrossRef] [PubMed]
- Murphy, C.; Koehler, K. Caloric restriction induces anabolic resistance to resistance exercise. Eur. J. Appl. Physiol. 2020, 120, 1155–1164. [Google Scholar] [CrossRef] [PubMed]
- Tinsley, G.M.; Moore, M.L.; Graybeal, A.J.; Paoli, A.; Kim, Y.; Gonzales, J.U.; Harry, J.R.; VanDusseldorp, T.A.; Kennedy, D.N.; Cruz, M.R. Time-restricted feeding plus resistance training in active females: A randomized trial. Am. J. Clin. Nutr. 2019, 110, 628–640. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stark, L.; Tofthagen, C.; Visovsky, C.; McMillan, S.C. The symptom experience of patients with cancer. J. Hosp. Palliat. Nurs. 2012, 14, 61–70. [Google Scholar] [CrossRef]
- Weber, D.; O’Brien, K. Cancer and cancer-related fatigue and the interrelationships with depression, stress, and inflammation. J. Evid. Based Complement. Altern. Med. 2017, 22, 502–512. [Google Scholar] [CrossRef]
- Theobald, D.E. Cancer pain, fatigue, distress, and insomnia in cancer patients. Clin. Cornerstone 2004, 6 (Suppl. S1D), S15–S21. [Google Scholar] [CrossRef]
- Buffart, L.M.; Kalter, J.; Sweegers, M.G.; Courneya, K.S.; Newton, R.U.; Aaronson, N.K.; Jacobsen, P.B.; May, A.M.; Galvão, D.A.; Chinapaw, M.J.; et al. Effects and moderators of exercise on quality of life and physical function in patients with cancer: An individual patient data meta-analysis of 34 RCTs. Cancer Treat. Rev. 2017, 52, 91–104. [Google Scholar] [CrossRef] [Green Version]
- Mishra, S.I.; Scherer, R.W.; Geigle, P.M.; Berlanstein, D.R.; Topaloglu, O.; Gotay, C.C.; Snyder, C. Exercise interventions on health-related quality of life for cancer survivors. Cochrane Database Syst. Rev. 2012. [Google Scholar] [CrossRef]
- Kessels, E.; Husson, O.; Van der Feltz-Cornelis, C.M. The effect of exercise on cancer-related fatigue in cancer survivors: A systematic review and meta-analysis. Neuropsychiatr. Dis. Treat. 2018, 14, 479. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mustian, K.M.; Alfano, C.M.; Heckler, C.; Kleckner, A.S.; Kleckner, I.R.; Leach, C.R.; Mohr, D.; Palesh, O.G.; Peppone, L.J.; Piper, B.F.; et al. Comparison of pharmaceutical, psychological, and exercise treatments for cancer-related fatigue: A meta-analysis. JAMA Oncol. 2017, 3, 961–968. [Google Scholar] [CrossRef]
- Brown, J.C.; Huedo-Medina, T.B.; Pescatello, L.S.; Ryan, S.M.; Pescatello, S.M.; Moker, E.; LaCroix, J.M.; Ferrer, R.A.; Johnson, B.T. The efficacy of exercise in reducing depressive symptoms among cancer survivors: A meta-analysis. PLoS ONE 2012, 7, e30955. [Google Scholar] [CrossRef] [PubMed]
- Kang, D.W.; Fairey, A.S.; Boule, N.G.; Field, C.J.; Courneya, K.S. Exercise duRing Active Surveillance for prostatE cancer-the ERASE trial: A study protocol of a phase II randomised controlled trial. BMJ Open 2019, 9, e026438. [Google Scholar] [CrossRef]
- Cartmel, B.; Hughes, M.; Ercolano, E.A.; Gottlieb, L.; Li, F.; Zhou, Y.; Harrigan, M.; Ligibel, J.A.; von Gruenigen, V.E.; Gogoi, R.; et al. Randomized trial of exercise on depressive symptomatology and brain derived neurotrophic factor (BDNF) in ovarian cancer survivors: The Women’s Activity and Lifestyle Study in Connecticut (WALC). Gynecol. Oncol. 2021, 161, 587–594. [Google Scholar] [CrossRef]
- i Ferrer, B.-C.S.; van Roekel, E.; Lynch, B.M. The role of physical activity in managing fatigue in cancer survivors. Curr. Nutr. Rep. 2018, 7, 59–69. [Google Scholar] [CrossRef]
- Bourke, L.; Gilbert, S.; Hooper, R.; Steed, L.A.; Joshi, M.; Catto, J.W.; Saxton, J.M.; Rosario, D.J. Lifestyle changes for improving disease-specific quality of life in sedentary men on long-term androgen-deprivation therapy for advanced prostate cancer: A randomised controlled trial. Eur. Urol. 2014, 65, 865–872. [Google Scholar] [CrossRef]
- Kohler, L.N.; Garcia, D.O.; Harris, R.B.; Oren, E.; Roe, D.J.; Jacobs, E.T. Adherence to diet and physical activity cancer prevention guidelines and cancer outcomes: A systematic review. Cancer Epidemiol. Biomark. Prev. 2016, 25, 1018–1028. [Google Scholar] [CrossRef] [Green Version]
- Warburg, O. On the origin of cancer cells. Science 1956, 123, 309–314. [Google Scholar] [CrossRef] [PubMed]
- Romijn, J.; Godfried, M.; Hommes, M.; Endert, E.; Sauerwein, H. Decreased glucose oxidation during short-term starvation. Metabolism 1990, 39, 525–530. [Google Scholar] [CrossRef]
- Pedersen, L.; Christensen, J.F.; Hojman, P. Effects of exercise on tumor physiology and metabolism. Cancer J. 2015, 21, 111–116. [Google Scholar] [CrossRef]
- Fontana, L.; Weiss, E.P.; Villareal, D.T.; Klein, S.; Holloszy, J.O. Long-term effects of calorie or protein restriction on serum IGF-1 and IGFBP-3 concentration in humans. Aging Cell 2008, 7, 681–687. [Google Scholar] [CrossRef] [Green Version]
- Renehan, A.G.; Zwahlen, M.; Minder, C.; T O’Dwyer, S.; Shalet, S.M.; Egger, M. Insulin-like growth factor (IGF)-I, IGF binding protein-3, and cancer risk: Systematic review and meta-regression analysis. Lancet 2004, 363, 1346–1353. [Google Scholar] [CrossRef]
- Walford, R.L.; Mock, D.; Verdery, R.; MacCallum, T. Calorie restriction in biosphere 2: Alterations in physiologic, hematologic, hormonal, and biochemical parameters in humans restricted for a 2-year period. J. Gerontol. Ser. A Biol. Sci. Med. Sci. 2002, 57, B211–B224. [Google Scholar] [CrossRef] [PubMed]
- Raffaghello, L.; Lee, C.; Safdie, F.M.; Wei, M.; Madia, F.; Bianchi, G.; Longo, V.D. Starvation-dependent differential stress resistance protects normal but not cancer cells against high-dose chemotherapy. Proc. Natl. Acad. Sci. USA 2008, 105, 8215–8220. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, C.; Raffaghello, L.; Brandhorst, S.; Safdie, F.M.; Bianchi, G.; Martin-Montalvo, A.; Pistoia, V.; Wei, M.; Hwang, S.; Merlino, A.; et al. Fasting cycles retard growth of tumors and sensitize a range of cancer cell types to chemotherapy. Sci. Transl. Med. 2012, 4, 124ra27. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Safdie, F.; Brandhorst, S.; Wei, M.; Wang, W.; Lee, C.; Hwang, S.; Conti, P.S.; Chen, T.C.; Longo, V.D. Fasting enhances the response of glioma to chemo- and radiotherapy. PLoS ONE 2012, 7, e44603. [Google Scholar] [CrossRef] [Green Version]
- Laviano, A.; Rossi Fanelli, F. Toxicity in chemotherapy—When less is more. N. Engl. J. Med. 2012, 366, 2319–2320. [Google Scholar] [CrossRef]
- Longo, V.D.; Mattson, M.P. Fasting: Molecular mechanisms and clinical applications. Cell Metab. 2014, 19, 181–192. [Google Scholar] [CrossRef] [Green Version]
- Fontana, L.; Partridge, L.; Longo, V.D. Extending healthy life span—From yeast to humans. Science 2010, 328, 321–326. [Google Scholar] [CrossRef] [Green Version]
- Bishop, N.A.; Guarente, L. Genetic links between diet and lifespan: Shared mechanisms from yeast to humans. Nat. Rev. Genet. 2007, 8, 835–844. [Google Scholar] [CrossRef]
- Cheng, C.W.; Adams, G.B.; Perin, L.; Wei, M.; Zhou, X.; Lam, B.S.; Da Sacco, S.; Mirisola, M.; Quinn, D.I.; Dorff, T.B.; et al. Prolonged fasting reduces IGF-1/PKA to promote hematopoietic-stem-cell-based regeneration and reverse immunosuppression. Cell Stem Cell 2014, 14, 810–823. [Google Scholar] [CrossRef] [Green Version]
- Sun, P.; Wang, H.; He, Z.; Chen, X.; Wu, Q.; Chen, W.; Sun, Z.; Weng, M.; Zhu, M.; Ma, D.; et al. Fasting inhibits colorectal cancer growth by reducing M2 polarization of tumor-associated macrophages. Oncotarget 2017, 8, 74649–74660. [Google Scholar] [CrossRef] [Green Version]
- Koelwyn, G.J.; Zhuang, X.; Tammela, T.; Schietinger, A.; Jones, L.W. Exercise and immunometabolic regulation in cancer. Nat. Metab. 2020, 2, 849–857. [Google Scholar] [CrossRef]
- Hojman, P.; Gehl, J.; Christensen, J.F.; Pedersen, B.K. Molecular Mechanisms Linking Exercise to Cancer Prevention and Treatment. Cell Metab. 2018, 27, 10–21. [Google Scholar] [CrossRef] [Green Version]
- Pedersen, L.; Idorn, M.; Olofsson, G.H.; Lauenborg, B.; Nookaew, I.; Hansen, R.H.; Johannesen, H.H.; Becker, J.C.; Pedersen, K.S.; Dethlefsen, C.; et al. Voluntary Running Suppresses Tumor Growth through Epinephrine- and IL-6-Dependent NK Cell Mobilization and Redistribution. Cell Metab. 2016, 23, 554–562. [Google Scholar] [CrossRef] [Green Version]
- Idorn, M.; Hojman, P. Exercise-Dependent Regulation of NK Cells in Cancer Protection. Trends Mol. Med. 2016, 22, 565–577. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Betof, A.S.; Lascola, C.D.; Weitzel, D.; Landon, C.; Scarbrough, P.M.; Devi, G.R.; Palmer, G.; Jones, L.W.; Dewhirst, M.W. Modulation of murine breast tumor vascularity, hypoxia, and chemotherapeutic response by exercise. JNCI J. Natl. Cancer Inst. 2015, 107. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Morrell, M.B.G.; Alvarez-Florez, C.; Zhang, A.; Kleinerman, E.S.; Savage, H.; Marmonti, E.; Park, M.; Shaw, A.; Schadler, K.L. Vascular modulation through exercise improves chemotherapy efficacy in Ewing sarcoma. Pediatr. Blood Cancer 2019, 66, e27835. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, L.; Morielli, A.R.; Heer, E.; Kirkham, A.A.; Cheung, W.Y.; Usmani, N.; Friedenreich, C.M.; Courneya, K.S. Effects of Exercise on Cancer Treatment Efficacy: A Systematic Review of Preclinical and Clinical Studies. Cancer Res. 2021. [Google Scholar] [CrossRef] [PubMed]
- Pin, F.; Couch, M.E.; Bonetto, A. Preservation of muscle mass as a strategy to reduce the toxic effects of cancer chemotherapy on body composition. Curr. Opin. Support. Palliat. Care 2018, 12, 420–426. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cespedes Feliciano, E.M.; Chen, W.Y.; Lee, V.; Albers, K.B.; Prado, C.M.; Alexeeff, S.; Xiao, J.; Shachar, S.S.; Caan, B.J. Body composition, adherence to anthracycline and taxane-based chemotherapy, and survival after nonmetastatic breast cancer. JAMA Oncol. 2020, 6, 264–270. [Google Scholar] [CrossRef] [PubMed]
- Prado, C.M.; Lieffers, J.R.; McCargar, L.J.; Reiman, T.; Sawyer, M.B.; Martin, L.; Baracos, V.E. Prevalence and clinical implications of sarcopenic obesity in patients with solid tumours of the respiratory and gastrointestinal tracts: A population-based study. Lancet Oncol. 2008, 9, 629–635. [Google Scholar] [CrossRef]
- Dupertuis, Y.M.; Meguid, M.M.; Pichard, C. Colon cancer therapy: New perspectives of nutritional manipulations using polyunsaturated fatty acids. Curr. Opin. Clin. Nutr. Metab. Care 2007, 10, 427–432. [Google Scholar] [CrossRef]
- Scheim, D.E. Cytotoxicity of unsaturated fatty acids in fresh human tumor explants: Concentration thresholds and implications for clinical efficacy. Lipids Health Dis. 2009, 8, 54. [Google Scholar] [CrossRef] [Green Version]
- Comba, A.; Lin, Y.H.; Eynard, A.R.; Valentich, M.A.; Fernandez-Zapico, M.E.; Pasqualini, M.E. Basic aspects of tumor cell fatty acid-regulated signaling and transcription factors. Cancer Metastasis Rev. 2011, 30, 325–342. [Google Scholar] [CrossRef] [Green Version]
- Horne, B.D.; Muhlestein, J.B.; Anderson, J.L. Health effects of intermittent fasting: Hormesis or harm? A systematic review. Am. J. Clin. Nutr. 2015, 102, 464–470. [Google Scholar] [CrossRef] [Green Version]
- Piercy, K.L.; Troiano, R.P.; Ballard, R.M.; Carlson, S.A.; Fulton, J.E.; Galuska, D.A.; George, S.M.; Olson, R.D. The Physical Activity Guidelines for Americans. JAMA 2018, 320, 2020–2028. [Google Scholar] [CrossRef]
- Ligibel, J. Pilot Study of the Impact of a Combined Intermittent Fasting and Exercise Intervention on Metabolic Markers in Patients with Advanced, Hormone Receptor Positive Breast Cancer. Available online: https://clinicaltrials.gov/ct2/show/NCT04708860 (accessed on 3 September 2021).
- Varady, K. Alternate Day Fasting Combined with Exercise for the Treatment of Non-Alcoholic Fatty Liver Disease (NAFLD). Available online: https://clinicaltrials.gov/ct2/show/NCT04004403 (accessed on 3 September 2021).
- Ryan, A. Promotion of Successful Weight Management in Overweight and Obese Veterans. Available online: https://clinicaltrials.gov/ct2/show/NCT04131647 (accessed on 3 September 2021).
- Risa, Ø. Before the Beginning: Preconception Lifestyle Interventions to Improve Future Metabolic Health. Available online: https://clinicaltrials.gov/ct2/show/NCT04585581 (accessed on 3 September 2021).
- Sungkarat, S. A Randomized Controlled Trial Investigating the Effects of Combined Physical-Cognitive Exercise and Dietary Intervention on Cognitive Performance and Changes in Blood Biomarkers of Postmenopausal Obese Women. Available online: https://clinicaltrials.gov/ct2/show/NCT04768725 (accessed on 3 September 2021).
- Zhu, Y. Effects of Diet and Exercise Interventions on Cardiometabolic Risk Markers, Executive Function, and Intestinal Flora in Undergraduate Students: A Randomized Controlled Trial. Available online: https://clinicaltrials.gov/ct2/show/NCT04834687 (accessed on 3 September 2021).
- Gabel, K. Time Restricted Eating with Physical Activity for Weight Management. Available online: https://clinicaltrials.gov/ct2/show/NCT04978376 (accessed on 3 September 2021).
| Type/Concept | Definition |
|---|---|
| Fasting-related | |
| Intermittent energy restriction | Restricting energy intake to approximately 60–75% below energy requirements for short periods, followed by periods with normal energy intake. One example is the 5:2 diet, consisting of approximately 5 days of eucaloric (a diet that provides the number of calories to maintain your body weight) feeding and approximately 2 days of a very-low-calorie diet per week. |
| Long-term fasting | Temporarily fasting, typically for a period >72 h. |
| Short-term fasting | Temporarily fasting, typically for a period between 12 and 72 h. An example of this type of fasting is alternate day fasting. |
| Time-restricted feeding | Reducing food intake to a set number of hours each day (e.g., eating in a <10 h daily period). One method of time restricted feeding is Prolonged overnight fasting whereby time-restricted feeding occurs overnight. |
| (Alternate definition) the practice of consuming ad libitum energy within a restricted window of time and fasting thereafter (upwards of 12–16 h). | |
| Religious fasting | Intermittent fasting exists in some religious practices. These include the Black Fast of Christianity most often practiced during Lent, Varta (Hinduism), Ramadan (Islam), Yom Kippur and other fasts (Judaism), Fast Sunday (Latter-day Saints), Jain (Buddhist) fasting. Religious fasting practices may only require abstinence from certain foods or last for a short period of time and cause negligible effects. |
| Fasting-mimicking diet | Maintaining a fasting-like state by periodically consuming a very-low-calorie, low-protein diet (not necessarily fasting) |
| Exercise-related | |
| Exercise | Planned and structured, and repetitive bodily movement in order to improve or maintain physical health outcomes [32]. |
| Physical activity | Any bodily movement produced by skeletal muscles that results in energy expenditure [32]. |
| Physical inactivity | Not performing sufficient amounts of moderate- and vigorous-intensity activity (MVPA), i.e., not meeting specified physical activity guidelines [33]. |
| Sedentary behavior | Any waking behavior characterized by an energy expenditure ≤1.5 metabolic equivalent tasks (METs) while in a sitting, reclining or lying posture [33]. |
| Identifier | Study Design | Population | Experimental Groups | Intervention Characteristics | Outcomes of Interest |
|---|---|---|---|---|---|
| Cancer Populations | |||||
| NCT04708860 [152] | Single-arm trial | Women ages 18 and older with Metastatic Breast Cancer | Combined POF and exercise | 12-week trial POF: Restriction of caloric food/drink after 8 pm, wait 13 h after last meal before eating, fasting 6 days/week Exercise: Moderate-intensity aerobic and strength training, 2 times/week of 30–45 min strength classes, 120 min aerobic activity per week |
|
| Other Populations | |||||
| NCT04004403 [153] | Randomized Clinical Trail | Obese prediabetic adults ages 18–64 with NAFLD |
| 24-week trial ADF: Fast day: 25% energy intake (~500 kcal), Feed day: ad libitum fed Exercise: Aerobic exercise training, 5 sessions/week |
|
| NCT04131647 [154] | Randomized Clinical Trail | Overweight and obese older adult (ages 50–70) veterans |
| 24-week program Weight Maintenance Program: Nutrition advice, walking, and resistance training, 12-week program IF + Exercise: One day of IF per week, consisting of 2 small meals/day; the combined program continues for 24-week program, following completion of the 12-week weight maintenance program; walking and resistance exercises |
|
| NCT04585581 [155] | Randomized Clinical Trail | Overweight women getting pregnant in next 6 months |
| Duration of pregnancy (min. 28 weeks) TRE + Exercise: Minimum of 14 h/day HIIT exercise: 2–3 days/week |
|
| NCT04768725 [156] | Randomized Clinical Trail | Obese, postmenopausal women ages 45–59, sedentary lifestyle |
| 12-week trial IF: Self-selected diet with 25–75% of estimated baseline energy requirements for 2 days/week (fast day) along with ad libitum for 5 days/week (feed day) Exercise: Moderate-Vigorous intensity [60–70% of heart rate maximum for aerobic and 60–70% of 1 repetition maximum, 8–12 repetitions/set, 3 sets of each exercise for resistance exercise, 60 min/session, 3 sessions/week (36 total sessions)] |
|
| NCT04834687 [157] | Randomized Clinical Trail | Healthy 17–24-year-old young adults |
| Trial length not reported Exercise: Aerobic rope-skipping, 3 days/week, 90 min/session TRE: 14 h fast, 10 h eating window, high-fiber diet |
|
| NCT04978376 [158] | Non-Randomized Clinical Trial | Overweight, older adults 50–70 years old with pre-diabetes |
| 10-week trial TRE is restricted eating with ad libitum eating between 12:00–20:00 Endurance Exercise: 3–5 days/week of supervised exercise Resistance Exercise: 3–5 days/week of supervised exercise |
|
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wilson, R.L.; Kang, D.-W.; Christopher, C.N.; Crane, T.E.; Dieli-Conwright, C.M. Fasting and Exercise in Oncology: Potential Synergism of Combined Interventions. Nutrients 2021, 13, 3421. https://doi.org/10.3390/nu13103421
Wilson RL, Kang D-W, Christopher CN, Crane TE, Dieli-Conwright CM. Fasting and Exercise in Oncology: Potential Synergism of Combined Interventions. Nutrients. 2021; 13(10):3421. https://doi.org/10.3390/nu13103421
Chicago/Turabian StyleWilson, Rebekah L., Dong-Woo Kang, Cami N. Christopher, Tracy E. Crane, and Christina M. Dieli-Conwright. 2021. "Fasting and Exercise in Oncology: Potential Synergism of Combined Interventions" Nutrients 13, no. 10: 3421. https://doi.org/10.3390/nu13103421
APA StyleWilson, R. L., Kang, D.-W., Christopher, C. N., Crane, T. E., & Dieli-Conwright, C. M. (2021). Fasting and Exercise in Oncology: Potential Synergism of Combined Interventions. Nutrients, 13(10), 3421. https://doi.org/10.3390/nu13103421







