Incidence and Risk Factors of Thromboembolism with Multiple Myeloma in the Presence of Death as a Competing Risk: An Empirical Comparison of Statistical Methodologies
Abstract
:1. Introduction
2. Materials and Methods
2.1. Data Source
2.2. Patient Inclusion
2.3. Subject Characteristics
2.4. Outcome Events
2.5. Survival Analysis
3. Results
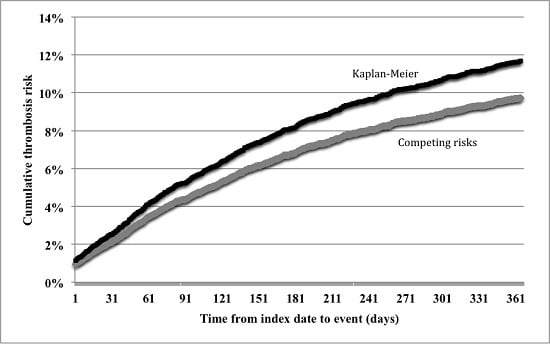
3.1. Incidence of Thrombosis
3.2. Survival Model Results
4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| VTE | venous thromboembolism |
| MM | multiple myeloma |
| IMID | immunomodulating drugs |
| PI | proteasome inhibitors |
| CVC | central venous catheters |
| LMWH | low-molecular weight heparin |
| RCT | randomized controlled trial |
| ICD-9-CM | International classification of diseases, 9th revision, clinical modification |
| DVT | deep vein thrombosis |
| PE | pulmonary embolism |
| HR | hazard ratio |
| PH | proportional hazard |
| CI | confidence interval |
| PVT | portal vein thrombosis |
| AT | arterial thrombosis |
| SD | standard deviation |
References
- Timp, J.F.; Braekkan, S.K.; Versteeg, H.H.; Cannegieter, S.C. Epidemiology of cancer-associated venous thrombosis. Blood 2013, 122, 1712–1723. [Google Scholar] [CrossRef] [PubMed]
- Blom, J.W.; Doggen, C.J.; Osanto, S.; Rosendaal, F.R. Malignancies, prothrombotic mutations, and the risk of venous thrombosis. JAMA 2005, 293, 715–722. [Google Scholar] [CrossRef] [PubMed]
- Cronin-Fenton, D.P.; Sondergaard, F.; Pedersen, L.A.; Fryzek, J.P.; Cetin, K.; Acquavella, J.; Baron, J.A.; Sørensen, H.T. Hospitalisation for venous thromboembolism in cancer patients and the general population: A population-based cohort study in Denmark, 1997–2006. Br. J. Cancer 2010, 103, 947–953. [Google Scholar] [CrossRef] [PubMed]
- Heit, J.A.; Silverstein, M.D.; Mohr, D.N.; Petterson, T.M.; O’Fallon, W.M.; Melton, L.J. Risk factors for deep vein thrombosis and pulmonary embolism: A population-based case-control study. Arch. Intern. Med. 2000, 160, 809–815. [Google Scholar] [CrossRef] [PubMed]
- Lip, G.Y.; Chin, B.S.; Blann, A.D. Cancer and the prothrombotic state. Lancet Oncol. 2002, 3, 27–34. [Google Scholar] [CrossRef]
- Khorana, A.A.; Dalal, M.; Lin, J.; Connolly, G.C. Incidence and predictors of venous thromboembolism (VTE) among ambulatory high-risk cancer patients undergoing chemotherapy in the United States. Cancer 2013, 119, 648–655. [Google Scholar] [CrossRef] [PubMed]
- Zamagni, E.; Brioli, A.; Tacchetti, P.; Zannetti, B.; Pantani, L.; Cavo, M. Multiple myeloma, venous thromboembolism, and treatment-related risk of thrombosis. Semin. Thromb. Hemost. 2011, 37, 209–219. [Google Scholar] [CrossRef] [PubMed]
- Horsted, F.; West, J.; Grainge, M.J. Risk of venous thromboembolism in patients with cancer: A systematic review and meta-analysis. PLoS Med. 2012, 9, e1001275. [Google Scholar] [CrossRef] [PubMed]
- Khorana, A.A.; Kuderer, N.M.; Culakova, E.; Lyman, G.H.; Francis, C.W. Development and validation of a predictive model for chemotherapy-associated thrombosis. Blood 2008, 111, 4902–4907. [Google Scholar] [CrossRef] [PubMed]
- Wun, T.; White, R.H. Venous thromboembolism (VTE) in patients with cancer: Epidemiology and risk factors. Cancer Investig. 2009, 27, 63–74. [Google Scholar] [CrossRef] [PubMed]
- Frere, C.; Debourdeau, P.; Hij, A.; Cajfinger, F.; Onan, M.N.; Panicot-Dubois, L.; Dubois, C.; Farge, D. Therapy for cancer-related thromboembolism. Semin. Oncol. 2014, 41, 319–338. [Google Scholar] [CrossRef] [PubMed]
- Khorana, A.A.; Francis, C.W.; Culakova, E.; Kuderer, N.M.; Lyman, G.H. Thromboembolism is a leading cause of death in cancer patients receiving outpatient chemotherapy. J. Thromb. Haemost. 2007, 5, 632–634. [Google Scholar] [CrossRef] [PubMed]
- Khorana, A.A. Venous thromboembolism and prognosis in cancer. Thromb. Res. 2010, 125, 490–493. [Google Scholar] [CrossRef] [PubMed]
- Srkalovic, G.; Cameron, M.G.; Rybicki, L.; Deitcher, S.R.; Kattke-Marchant, K.; Hussein, M.A. Monoclonal gammopathy of undetermined significance and multiple myeloma are associated with an increased incidence of venothromboembolic disease. Cancer 2004, 101, 558–566. [Google Scholar] [CrossRef] [PubMed]
- Zangari, M.; Anaissie, E.; Barlogie, B.; Badros, A.; Desikan, R.; Gopal, A.V.; Morris, C.; Toor, A.; Siegel, E.; Fink, L.; et al. Increased risk of deep-vein thrombosis in patients with multiple myeloma receiving thalidomide and chemotherapy. Blood 2001, 98, 1614–1615. [Google Scholar] [CrossRef] [PubMed]
- Zangari, M.; Siegel, E.; Barlogie, B.; Anaissie, E.; Saghafifar, F.; Fassas, A.; Morris, C.; Fink, L.; Tricot, G. Thrombogenic activity of doxorubicin in myeloma patients receiving thalidomide: Implications for therapy. Blood 2002, 100, 1168–1171. [Google Scholar] [CrossRef] [PubMed]
- Bennett, C.L.; Angelotta, C.; Yarnold, P.R.; Evens, A.M.; Zonder, J.A.; Raisch, D.W.; Richardson, P. Thalidomide- and lenalidomide-associated thromboembolism among patients with cancer. JAMA 2006, 296, 2558–2560. [Google Scholar] [CrossRef] [PubMed]
- Leleu, X.; Rodon, P.; Hulin, C.; Daley, L.; Dauriac, C.; Hacini, M.; Decaux, O.; Eisemann, J.C.; Fitoussi, O.; Lioure, B.; et al. MELISSE, a large multicentric observational study to determine risk factors of venous thromboembolism in patients with multiple myeloma treated with immunomodulatory drugs. Thromb. Haemost. 2013, 110, 844–851. [Google Scholar] [CrossRef] [PubMed]
- Carrier, M.; Le Gal, G.; Tay, J.; Wu, C.; Lee, A.Y. Rates of venous thromboembolism in multiple myeloma patients undergoing immunomodulatory therapy with thalidomide or lenalidomide: A systematic review and meta-analysis. J. Thromb. Haemost. 2011, 9, 653–663. [Google Scholar] [CrossRef] [PubMed]
- Zangari, M.; Fink, L.; Zhan, F.; Tricot, G. Low venous thromboembolic risk with bortezomib in multiple myeloma and potential protective effect with thalidomide/lenalidomide-based therapy: Review of data from phase 3 trials and studies of novel combination regimens. Clin. Lymphoma Myeloma Leuk. 2011, 11, 228–236. [Google Scholar] [CrossRef] [PubMed]
- Bennett, C.L.; Silver, S.M.; Djulbegovic, B.; Samaras, A.T.; Blau, C.A.; Gleason, K.J.; Barnato, S.E.; Elverman, K.M.; Courtney, D.M.; McKoy, J.M.; et al. Venous thromboembolism and mortality associated with recombinant erythropoietin and darbepoetin administration for the treatment of cancer-associated anemia. JAMA 2008, 299, 914–924. [Google Scholar] [CrossRef] [PubMed]
- Khorana, A.A.; Francis, C.W.; Culakova, E.; Lyman, G.H. Risk factors for chemotherapy-associated venous thromboembolism in a prospective observational study. Cancer 2005, 104, 2822–2829. [Google Scholar] [CrossRef] [PubMed]
- Lippi, G.; Franchini, M.; Favaloro, E.J. Thrombotic complications of erythropoiesis-stimulating agents. Semin. Thromb. Hemost. 2010, 36, 537–549. [Google Scholar] [CrossRef] [PubMed]
- Beckers, M.M.; Ruven, H.J.; Seldenrijk, C.A.; Prins, M.H.; Biesma, D.H. Risk of thrombosis and infections of central venous catheters and totally implanted access ports in patients treated for cancer. Thromb. Res. 2010, 125, 318–321. [Google Scholar] [CrossRef] [PubMed]
- Shivakumar, S.P.; Anderson, D.R.; Couban, S. Catheter-associated thrombosis in patients with malignancy. J. Clin. Oncol. 2009, 27, 4858–4864. [Google Scholar] [CrossRef] [PubMed]
- Esmon, C.T. Does inflammation contribute to thrombotic events? Haemostasis 2000, 30, 34–40. [Google Scholar] [CrossRef] [PubMed]
- Lyman, G.H.; Khorana, A.A.; Kuderer, N.M.; Lee, A.Y.; Arcelus, J.I.; Balaban, E.P.; Clarke, J.M.; Flowers, C.R.; Francis, C.W.; Gates, L.E.; et al. Venous thromboembolism prophylaxis and treatment in patients with cancer: American Society of Clinical Oncology clinical practice guideline update. J. Clin. Oncol. 2013, 31, 2189–2204. [Google Scholar] [CrossRef] [PubMed]
- Anderson, K.C.; Alsina, M.; Atanackovic, D.; Biermann, J.S.; Chandler, J.C.; Costello, C.; Djulbegovic, B.; Fung, H.C.; Gasparetto, C.; Godby, K.; et al. Multiple Myeloma, Version 2.2016. J. Natl. Compr. Cancer Netw. 2015, 13, 1398–1435. [Google Scholar]
- Ay, C.; Posch, F.; Kaider, A.; Zielinski, C.; Pabinger, I. Estimating risk of venous thromboembolism in patients with cancer in the presence of competing mortality. J. Thromb. Haemost. 2015, 13, 390–397. [Google Scholar] [CrossRef] [PubMed]
- Quan, H.; Sundararajan, V.; Halfon, P.; Fong, A.; Burnand, B.; Luthi, J.C.; Saunders, L.D.; Beck, C.A.; Feasby, T.E.; Ghali, W.A. Coding algorithms for defining comorbidities in ICD-9-CM and ICD-10 administrative data. Med. Care 2005, 43, 1130–1139. [Google Scholar] [CrossRef] [PubMed]
- Sanfilippo, K.M.; Wang, T.; Gage, B.F.; Liu, W.; Carson, K.R. Improving accuracy of International Classification of Diseases codes for venous thromboembolism in administrative data. Thromb. Res. 2015, 135, 616–620. [Google Scholar] [CrossRef] [PubMed]
- White, R.H.; Garcia, M.; Sadeghi, B.; Tancredi, D.J.; Zrelak, P.; Cuny, J.; Sama, P.; Gammon, H.; Schmaltz, S.; Romano, P.S. Evaluation of the predictive value of ICD-9-CM coded administrative data for venous thromboembolism in the United States. Thromb. Res. 2010, 126, 61–67. [Google Scholar] [CrossRef] [PubMed]
- Zhan, C.; Battles, J.; Chiang, Y.P.; Hunt, D. The validity of ICD-9-CM codes in identifying postoperative deep vein thrombosis and pulmonary embolism. Jt. Comm. J. Qual. Patient Saf. 2007, 33, 326–331. [Google Scholar] [PubMed]
- Mahindra, A.; Laubach, J.; Raje, N.; Munshi, N.; Richardson, P.G.; Anderson, K. Latest advances and current challenges in the treatment of multiple myeloma. Nat. Rev. Clin. Oncol. 2012, 9, 135–143. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.K.; Rajkumar, S.V.; Dispenzieri, A.; Lacy, M.Q.; Hayman, S.R.; Buadi, F.K.; Zeldenrust, S.R.; Dingli, D.; Russell, S.J.; Lust, J.A.; et al. Improved survival in multiple myeloma and the impact of novel therapies. Blood 2008, 111, 2516–2520. [Google Scholar] [CrossRef] [PubMed]
- Palumbo, A.; Cavo, M.; Bringhen, S.; Zamagni, E.; Romano, A.; Patriarca, F.; Rossi, D.; Gentilini, F.; Crippa, C.; Galli, M.; et al. Aspirin, warfarin, or enoxaparin thromboprophylaxis in patients with multiple myeloma treated with thalidomide: A phase III, open-label, randomized trial. J. Clin. Oncol. 2011, 29, 986–993. [Google Scholar] [CrossRef] [PubMed]
- Larocca, A.; Cavallo, F.; Bringhen, S.; Di Raimondo, F.; Falanga, A.; Evangelista, A.; Cavalli, M.; Stanevsky, A.; Corradini, P.; Pezzatti, S.; et al. Aspirin or enoxaparin thromboprophylaxis for patients with newly diagnosed multiple myeloma treated with lenalidomide. Blood 2012, 119, 933–939. [Google Scholar] [CrossRef] [PubMed]
- Khorana, A.A.; Dalal, M.R.; Lin, J.; Connolly, G.C. Health care costs associated with venous thromboembolism in selected high-risk ambulatory patients with solid tumors undergoing chemotherapy in the United States. Clinicoecon. Outcomes Res. 2013, 5, 101–108. [Google Scholar] [CrossRef] [PubMed]
- Lee, A.Y. Anticoagulation in the treatment of established venous thromboembolism in patients with cancer. J. Clin. Oncol. 2009, 27, 4895–4901. [Google Scholar] [CrossRef] [PubMed]
- Lyman, G.H.; Bohlke, K.; Khorana, A.A.; Kuderer, N.M.; Lee, A.Y.; Arcelus, J.I.; Balaban, E.P.; Clarke, J.M.; Flowers, C.R.; Francis, C.W.; et al. Venous thromboembolism prophylaxis and treatment in patients with cancer: American Society of Clinical Oncology clinical practice guideline update 2014. J. Clin. Oncol. 2015, 33, 654–656. [Google Scholar] [CrossRef] [PubMed]
- Blom, J.W.; Vanderschoot, J.P.; Oostindier, M.J.; Osanto, S.; van der Meer, F.J.; Rosendaal, F.R. Incidence of venous thrombosis in a large cohort of 66,329 cancer patients: Results of a record linkage study. J. Thromb. Haemost. 2006, 4, 529–535. [Google Scholar] [CrossRef] [PubMed]
- Menon, S.P.; Rajkumar, S.V.; Lacy, M.; Falco, P.; Palumbo, A. Thromboembolic events with lenalidomide-based therapy for multiple myeloma. Cancer 2008, 112, 1522–1528. [Google Scholar] [CrossRef] [PubMed]
- Schneeweiss, S.; Avorn, J. A review of uses of health care utilization databases for epidemiologic research on therapeutics. J. Clin. Epidemiol. 2005, 58, 323–337. [Google Scholar] [CrossRef] [PubMed]
- Zhan, C.; Miller, M.R. Administrative data based patient safety research: A critical review. Qual. Saf. Health Care 2003, 12, ii58–ii63. [Google Scholar] [CrossRef] [PubMed]
- Tamariz, L.; Harkins, T.; Nair, V. A systematic review of validated methods for identifying venous thromboembolism using administrative and claims data. Pharmacoepidemiol. Drug Saf. 2012, 21, 154–162. [Google Scholar] [CrossRef] [PubMed]
- Pauly, N.J.; Brown, J.D. Prevalence of low-cost generic program use in a nationally representative cohort of privately insured adults. J. Manag. Care Spec. Pharm. 2015, 21, 1162–1170. [Google Scholar] [CrossRef] [PubMed]

| Overall Cohort | ||
|---|---|---|
| N = 13,700 | ||
| Age | Mean 63.9 | SD 13.7 |
| 18–34 | 283 | 2.1 |
| 35–64 | 7389 | 53.9 |
| 65–74 | 2648 | 19.3 |
| 75+ | 3380 | 24.7 |
| Gender | ||
| Male | 6625 | 48.4 |
| Female | 7075 | 51.6 |
| Charlson Comorbidity Index | Mean 1.1 | SD 1.5 |
| 0 | 6892 | 50.3 |
| 1–2 | 4758 | 34.7 |
| 3–4 | 1476 | 10.8 |
| 5+ | 574 | 4.2 |
| Comorbidity | ||
| MI | 251 | 1.8 |
| CHF | 933 | 6.8 |
| PVD | 841 | 6.1 |
| Dementia | 123 | 0.9 |
| COPD | 1817 | 13.3 |
| Rheumatism | 734 | 5.4 |
| PUD | 134 | 1.0 |
| Mild liver disease | 551 | 4.0 |
| Diabetes | 2814 | 20.5 |
| Diabetes with complications | 755 | 5.5 |
| Paralysis | 68 | 0.5 |
| Renal disease | 1826 | 13.3 |
| Severe liver disease | 45 | 0.3 |
| CVD | 910 | 6.6 |
| HIV/AIDS | 30 | 0.2 |
| Hypertension | 6466 | 47.2 |
| CHD | 1725 | 12.6 |
| Lipids | 4260 | 31.1 |
| High platelets | 70 | 0.5 |
| High white cell | 270 | 2.0 |
| Anemia | 3727 | 27.2 |
| Obesity | 491 | 3.6 |
| Hypocoagulopathies | 544 | 4.0 |
| Thrombocytopenia | 363 | 2.7 |
| Low white cell | 237 | 1.7 |
| Year of Diagnosis | ||
| 2009 | 2469 | 18.0 |
| 2010 | 2476 | 18.1 |
| 2011 | 3153 | 23.0 |
| 2012 | 3053 | 22.3 |
| 2013 | 2549 | 18.6 |
| Competing Risks | Cox PH | ||||||
|---|---|---|---|---|---|---|---|
| Covariate | sHR | 95% CI | HR | 95% CI | % Bias | ||
| Age | |||||||
| 18–34 | Ref. | Ref. | Ref. | Ref. | Ref. | Ref. | |
| * 35–64 | 1.7 | 1.0 | 3.0 | 2.2 | 1.3 | 3.7 | 29.4% |
| * 65–74 | 1.9 | 1.1 | 3.2 | 2.4 | 1.4 | 4.1 | 26.3% |
| 75+ | 1.6 | 0.9 | 2.7 | 2.8 | 1.6 | 4.8 | 75.0% |
| Gender | |||||||
| Male | Ref. | Ref. | Ref. | Ref. | Ref. | Ref. | |
| * Female | 0.7 | 0.7 | 0.8 | 0.7 | 0.7 | 0.8 | 0.0% |
| Charlson Comorbidity Index | |||||||
| 0 | Ref. | Ref. | Ref. | Ref. | Ref. | Ref. | |
| 1–2 | 1.0 | 0.5 | 2.1 | 1.0 | 0.8 | 1.2 | 0.0% |
| 3–4 | 0.8 | 0.5 | 1.3 | 0.8 | 0.6 | 1.2 | 0.0% |
| 5+ | 1.0 | 0.8 | 1.2 | 0.9 | 0.5 | 1.7 | −10.0% |
| Comorbidities | |||||||
| MI | 0.9 | 0.6 | 1.4 | 1.1 | 0.8 | 1.5 | 22.2% |
| * CHF | 1.7 | 1.4 | 2.1 | 1.9 | 1.6 | 2.3 | 11.8% |
| PVD | 1.2 | 0.9 | 1.5 | 1.2 | 1.0 | 1.4 | 0.0% |
| Dementia | 1.2 | 0.7 | 2.1 | 1.5 | 0.9 | 2.3 | 25.0% |
| COPD | 0.9 | 0.8 | 1.1 | 1.1 | 0.9 | 1.3 | 22.2% |
| Rheumatism | 0.9 | 0.6 | 1.2 | 0.7 | 0.5 | 1.0 | −22.2% |
| PUD | 0.8 | 0.5 | 1.5 | 1.0 | 0.7 | 1.6 | 25.0% |
| Mild liver disease | 0.8 | 0.5 | 1.1 | 0.9 | 0.7 | 1.2 | 12.5% |
| Diabetes | 1.0 | 0.8 | 1.2 | 1.1 | 0.9 | 1.2 | 10.0% |
| Diabetes with complications | 1.1 | 0.8 | 1.5 | 1.0 | 0.7 | 1.3 | −9.1% |
| Paralysis | 1.4 | 0.8 | 2.5 | 1.2 | 0.7 | 2.0 | −14.3% |
| Renal disease | 1.0 | 0.8 | 1.3 | 1.0 | 0.8 | 1.3 | 0.0% |
| Severe liver disease | 1.1 | 0.4 | 3.0 | 1.7 | 0.9 | 3.4 | 54.5% |
| CVD | 1.0 | 0.8 | 1.3 | 1.1 | 0.9 | 1.4 | 10.0% |
| * Hypertension | 1.2 | 1.0 | 1.3 | 1.1 | 1.0 | 1.3 | −8.3% |
| CHD | 1.0 | 0.8 | 1.1 | 1.0 | 0.9 | 1.1 | 0.0% |
| Lipids | 1.0 | 0.9 | 1.1 | 1.0 | 0.9 | 1.1 | 0.0% |
| High platelets | 1.0 | 0.5 | 2.1 | 1.0 | 0.5 | 1.8 | 0.0% |
| * High white cell | 1.3 | 1.0 | 1.9 | 1.2 | 0.9 | 1.6 | −7.7% |
| Anemia | 0.9 | 0.8 | 1.1 | 1.0 | 0.9 | 1.6 | 11.1% |
| Obesity | 1.2 | 0.9 | 1.6 | 1.1 | 0.9 | 1.4 | −8.3% |
| Hypocoagulopathies | 0.9 | 0.6 | 1.4 | 0.8 | 0.6 | 1.3 | −11.1% |
| Thrombocytopenia | 1.0 | 0.6 | 1.8 | 1.2 | 0.8 | 2.0 | 20.0% |
| * Low white cell | 1.6 | 1.1 | 2.2 | 1.4 | 1.0 | 1.9 | −12.5% |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Brown, J.D.; Adams, V.R. Incidence and Risk Factors of Thromboembolism with Multiple Myeloma in the Presence of Death as a Competing Risk: An Empirical Comparison of Statistical Methodologies. Healthcare 2016, 4, 16. https://doi.org/10.3390/healthcare4010016
Brown JD, Adams VR. Incidence and Risk Factors of Thromboembolism with Multiple Myeloma in the Presence of Death as a Competing Risk: An Empirical Comparison of Statistical Methodologies. Healthcare. 2016; 4(1):16. https://doi.org/10.3390/healthcare4010016
Chicago/Turabian StyleBrown, Joshua D., and Val R. Adams. 2016. "Incidence and Risk Factors of Thromboembolism with Multiple Myeloma in the Presence of Death as a Competing Risk: An Empirical Comparison of Statistical Methodologies" Healthcare 4, no. 1: 16. https://doi.org/10.3390/healthcare4010016
APA StyleBrown, J. D., & Adams, V. R. (2016). Incidence and Risk Factors of Thromboembolism with Multiple Myeloma in the Presence of Death as a Competing Risk: An Empirical Comparison of Statistical Methodologies. Healthcare, 4(1), 16. https://doi.org/10.3390/healthcare4010016