Genetic and Epigenetic Aspects of Skin Collagen Fiber Turnover and Functioning
Abstract
1. Introduction
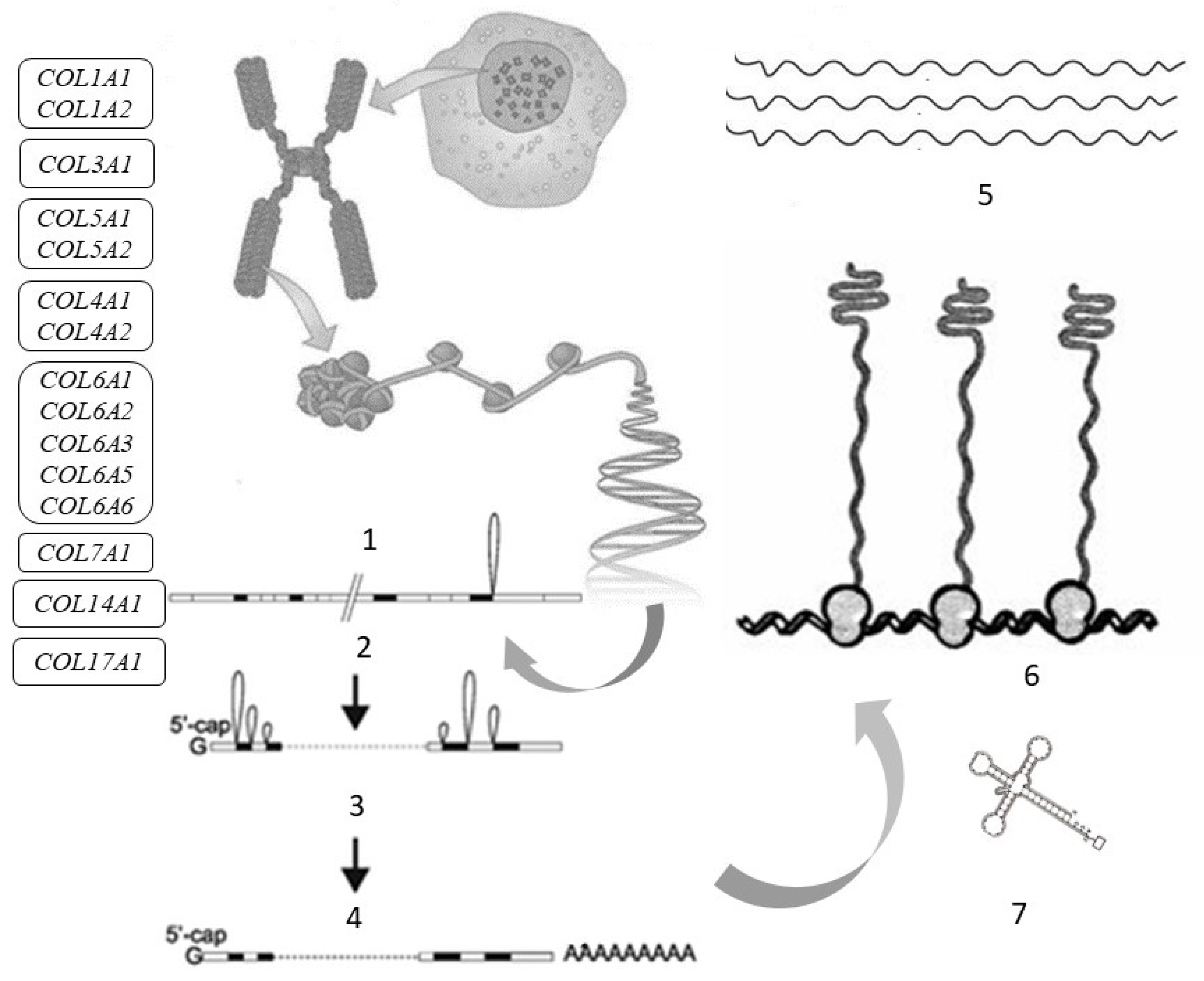
2. Collagen Fibers in the Skin
| Gene (Protein) | Chromosome Localization | Clinical Manifestations of Mutation/Polymorphism | Expression in the Skin (RPKM) |
|---|---|---|---|
| Fibrillar Collagens | |||
| COL1A1 (α1 chain of collagen type I) | 17q21.33 (51 exons) | Osteogenesis imperfecta, classic type of Ehlers-Danlos syndrome, Caffey disease, idiopathic osteoporosis | 164.508 ± 48.747 |
| COL1A2 (α2 chain of collagen type I) | 17q21.3 (52 exons) | Osteogenesis imperfecta, type VII B of Ehlers-Danlos syndrome, idiopathic osteoporosis, atypical Marfan syndrome | 190.333 ± 32.009 |
| COL3A1 (α1 chain of collagen type III) | 2q32.2 (51 exons) | Ehlers-Danlos syndrome type IV, aortic and arterial aneurysms | 168.586 ± 46.57 |
| COL5A1 (α1 chain of collagen type V) | 9q34.3 (67 exons) | Ehlers-Danlos syndrome types I and II | 7.679 ± 0.808 |
| COL5A2 (α2 chain of collagen type V) | 2q32.2 (55 exons) | Ehlers-Danlos syndrome types I and II | 6.217 ± 1.778 |
| Networking Collagens | |||
| COL4A1 (α1 chain of collagen type IV) | 13q34 (54 exons) | Cerebrovascular diseases, kidney and muscle pathology | 1.798 ± 0.45 |
| COL4A2 (6 subunits of collagen type IV) | 13q34 (48 exons) | Cerebrovascular diseases, kidney and muscle pathology | 5.245 ± 1.325 |
| Collagens Forming Filament Beads | |||
| COL6A1 (α1 chain of collagen type VI) | 21q22.3 (35 exons) | Bethlem myopathy | 65.872 ± 35.541 |
| COL6A2 (α2 chain of collagen type VI) | 21q22.3 (30 exons) | Bethlem myopathy, Ullrich scleroatonic muscular dystrophy Bethlem myopathy | 68.022 ± 43.357 |
| COL6A3 (α3 chain of collagen type VI) | 2q37.3 (50 exons) | Ullrich scleroatonic muscular dystrophy, autosomal dominant proximal myopathy | 68.022 ± 43.357 |
| COL6A5 (a protein that can interact with the α1 and α2 chains of type VI collagen to form a trimer) | 3q22.1 (44 exons) | Eczema | 1.896 ± 0.958 |
| COL6A6 (a protein that regulates the interaction of epithelial cells with fibronectin) | 3q22.1 (44 exons) | Dermatoses (eczema) | 0.183 ± 0.082 |
| Collagens Forming Anchor Fibrils | |||
| COL7A1 (α3 chain of collagen type VII) | 3q21.1 (120 exons) | Dystrophic epidermolysis bullosa | 62.83 ± 31.474 |
| Fibril-Associated Collagens | |||
| COL14A1 (α-chain of collagen type XIV) | 8q24.12 (50 exons) | Increased risk of carcinogenesis | 3.173 ± 1.431 |
| Transmembrane Collagens | |||
| COL17A1 (α1 chain of collagen type XVII) | 10q25.1 (56 exons) | Generalized atrophic epidermolysis and epidermolysis bullosa | 284.358 ± 48.16 |
3. Collagen Molecule Structure
4. Collagen Synthesis
5. Regulation of Collagen Synthesis
6. Epigenetic Regulation of Collagen Synthesis
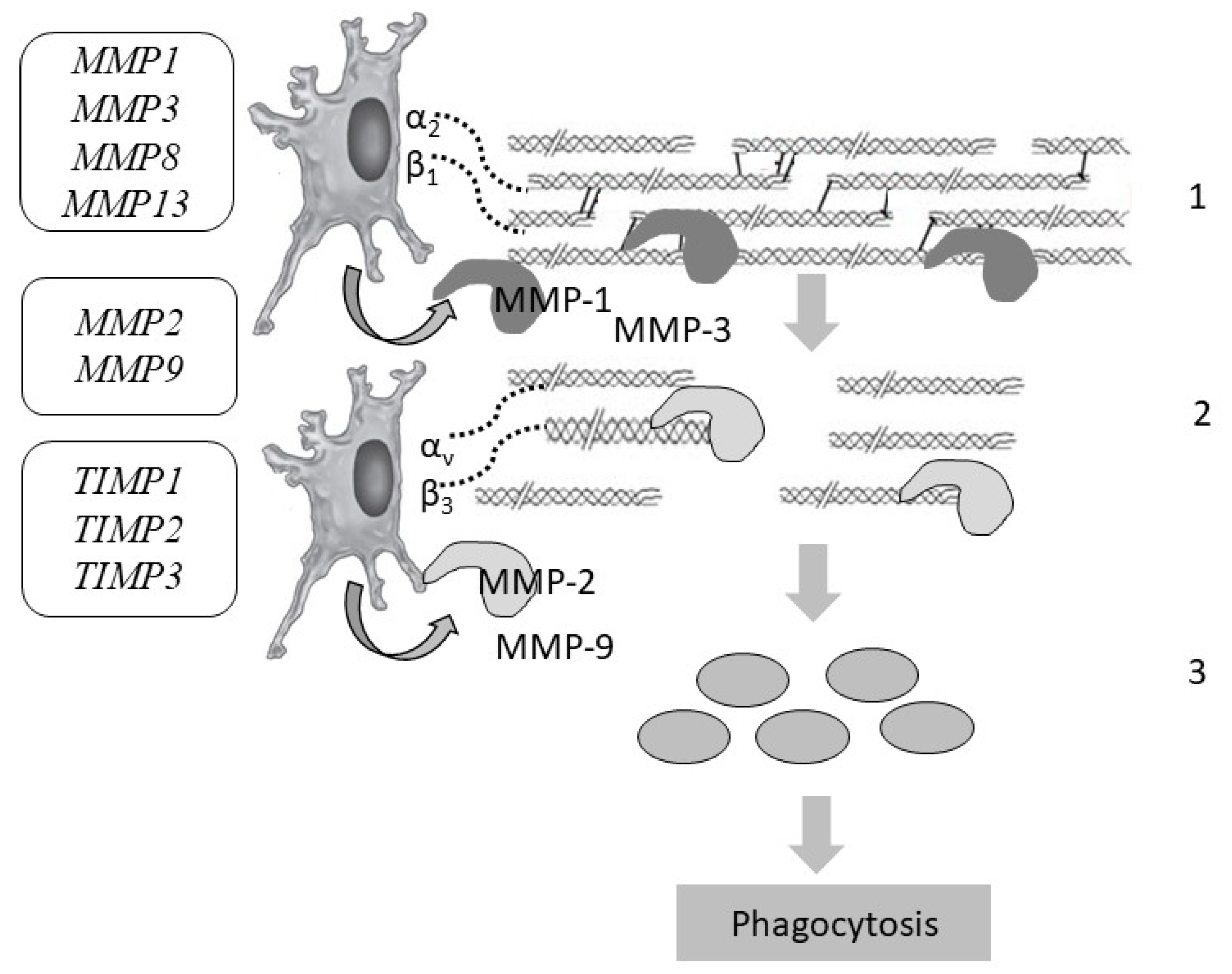
7. Collagen Degradation
8. Disruption of Collagen Fiber Degradation
9. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Kapuler, O.; Selskaya, B.; Galeeva, A.; Kamilo, F. Metabolism of collagen fibers in the presence of age-related changes. Vrach 2015, 8, 64–69. [Google Scholar]
- Antoine, E.E.; Vlachos, P.P.; Rylander, M.N. Review of collagen I hydrogels for bioengineered tissue microenvironments: Characterization of mechanics, structure, and transport. Tissue Eng. Part B Rev. 2014, 20, 683–696. [Google Scholar] [CrossRef] [PubMed]
- Manturova, N.E.; Stenko, A.G.; Petinati, Y.A.; Chaikovskaya, E.A.; Bolgarina, A.A. Injectable collagen in correction of age-related skin changes: Experimental and clinical parallels. Bull. RSMU 2019, 1, 79–85. [Google Scholar] [CrossRef]
- Blair, M.J.; Jones, J.D.; Woessner, A.E.; Quinn, K.P. Skin structure-function relationships and the wound healing response to intrinsic aging. Adv. Wound Care (New Rochelle) 2020, 9, 127–143. [Google Scholar] [CrossRef]
- Zhang, S.; Duan, E. Fighting against skin aging: The way from bench to bedside. Cell Transplant. 2018, 27, 729–738. [Google Scholar] [CrossRef] [PubMed]
- Goh, K.L.; Holmes, D.F. Collagenous extracellular matrix biomaterials for tissue engineering: Lessons from the common sea urchin tissue. Int. J. Mol. Sci. 2017, 18, 901. [Google Scholar] [CrossRef] [PubMed]
- Seo, B.R.; Chen, X.; Ling, L.; Song, Y.H.; Shimpi, A.A.; Choi, S.; Gonzalez, J.; Sapudom, J.; Wang, K.; Andresen Eguiluz, R.C.; et al. Collagen microarchitecture mechanically controls myofibroblast differentiation. Proc. Natl. Acad. Sci. USA 2020, 117, 11387–11398. [Google Scholar] [CrossRef] [PubMed]
- Park, S.; Jung, W.H.; Pittman, M.; Chen, J.; Chen, Y. The effects of stiffness, viscosity, and geometry of microenvironment in Homeostasis, Aging and Diseases. J. Biomech. Eng. 2020, 142, 100804. [Google Scholar] [CrossRef] [PubMed]
- Roy, B.; Yuan, L.; Lee, Y.; Bharti, A.; Mitra, A.; Shivashankar, G.V. Fibroblast rejuvenation by mechanical reprogramming and redifferentiation. Proc. Natl. Acad. Sci. USA 2020, 117, 10131–10141. [Google Scholar] [CrossRef]
- Litman, T. Personalized medicine-concepts, technologies, and applications in inflammatory skin diseases. APMIS 2019, 127, 386–424. [Google Scholar] [CrossRef]
- Pawlaczyk, M.; Lelonkiewicz, M.; Wieczorowski, M. Age-dependent biomechanical properties of the skin. Postepy Dermatol. Alergol. 2013, 30, 302–306. [Google Scholar] [CrossRef]
- Vashi, N.A.; de Castro Maymone, M.B.; Kundu, R.V. Aging differences in ethnic skin. J. Clin. Aesthetic Dermatol. 2016, 9, 31–38. [Google Scholar]
- Lynch, B.; Bonod-Bidaud, C.; Ducourthial, G.; Affagard, J.S.; Bancelin, S.; Psilodimitrakopoulos, S.; Ruggiero, F.; Allain, J.M.; Schanne-Klein, M.C. How aging impacts skin biomechanics: A multiscale study in mice. Sci. Rep. 2017, 7, 13750. [Google Scholar] [CrossRef] [PubMed]
- Shao, Y.; Qin, Z.; Alexander Wilks, J.; Balimunkwe, R.M.; Fisher, G.J.; Voorhees, J.J.; Quan, T. Physical properties of the photodamaged human skin dermis: Rougher collagen surface and stiffer/harder mechanical properties. Exp. Dermatol. 2019, 28, 914–921. [Google Scholar] [CrossRef] [PubMed]
- Chattopadhyay, S.; Raines, R.T. Review collagen-based biomaterials for wound healing. Biopolymers 2014, 101, 821–833. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.; Sherman, V.R.; Gludovatz, B.; Schaible, E.; Stewart, P.; Ritchie, R.O.; Meyers, M.A. On the tear resistance of skin. Nat. Commun. 2015, 6, 6649. [Google Scholar] [CrossRef]
- Mostaço-Guidolin, L.; Rosin, N.L.; Hackett, T.L. Imaging Collagen in Scar Tissue: Developments in Second Harmonic Generation Microscopy for Biomedical Applications. Int. J. Mol. Sci. 2017, 18, 1772. [Google Scholar] [CrossRef]
- de Wild, M.; Pomp, W.; Koenderink, G.H. Thermal memory in self-assembled collagen fibril networks. Biophys. J. 2013, 105, 200–210. [Google Scholar] [CrossRef]
- Terzi, A.; Storelli, E.; Bettini, S.; Sibillano, T.; Altamura, D.; Salvatore, L.; Madaghiele, M.; Romano, A.; Siliqi, D.; Ladisa, M.; et al. Effects of processing on structural, mechanical and biological properties of collagen-based substrates for regenerative medicine. Sci. Rep. 2018, 8, 1429. [Google Scholar] [CrossRef]
- Kang, M.C.; Yumnam, S.; Kim, S.Y. Oral intake of collagen peptide attenuates ultraviolet B irradiation-induced skin dehydration in vivo by regulating hyaluronic acid synthesis. Int. J. Mol. Sci. 2018, 19, 3551. [Google Scholar] [CrossRef]
- Söderhäll, C.; Marenholz, I.; Kerscher, T.; Rüschendorf, F.; Esparza-Gordillo, J.; Worm, M.; Gruber, C.; Mayr, G.; Albrecht, M.; Rohde, K.; et al. Variants in a novel epidermal collagen gene (COL29A1) are associated with atopic dermatitis. PLoS Biol. 2007, 5, e242. [Google Scholar] [CrossRef]
- Manka, S.W.; Bihan, D.; Farndale, R.W. Structural studies of the MMP-3 interaction with triple-helical collagen introduce new roles for the enzyme in tissue remodeling. Sci. Rep. 2019, 9, 18785. [Google Scholar] [CrossRef]
- Ouyang, M.; Yu, J.Y.; Chen, Y.; Deng, L.; Guo, C.L. Cell-extracellular matrix interactions in the fluidic phase direct the topology and polarity of self-organized epithelial structures. Cell Prolif. 2021, 54, e13014. [Google Scholar] [CrossRef] [PubMed]
- Musiime, M.; Chang, J.; Hansen, U.; Kadler, K.E.; Zeltz, C.; Gullberg, D. Collagen Assembly at the Cell Surface: Dogmas Revisited. Cells 2021, 10, 662. [Google Scholar] [CrossRef] [PubMed]
- Kuivaniemi, H.; Tromp, G. Type III collagen (COL3A1): Gene and protein structure, tissue distribution, and associated diseases. Gene 2019, 707, 151–171. [Google Scholar] [CrossRef] [PubMed]
- Wulandari, E.; Jusman, S.W.; Moenadjat, Y.; Jusuf, A.A.; Sadikin, M. Expressions of collagen I and III in hypoxic keloid tissue. Kobe J. Med. Sci. 2016, 62, E58–E69. [Google Scholar] [PubMed]
- Kehlet, S.N.; Willumsen, N.; Armbrecht, G.; Dietzel, R.; Brix, S.; Henriksen, K.; Karsdal, M.A. Age-related collagen turnover of the interstitial matrix and basement membrane: Implications of age- and sex-dependent remodeling of the extracellular matrix. PLoS ONE 2018, 13, e0194458. [Google Scholar] [CrossRef]
- Niu, K.; Chen, X.; Lu, Y. COL3A1 rs1800255 polymorphism is associated with pelvic organ prolapse susceptibility in Caucasian individuals: Evidence from a meta-analysis. PLoS ONE 2021, 16, e0250943. [Google Scholar] [CrossRef]
- Xu, X.; Wang, Z.; Zan, T. A case of Ehlers-Danlos syndrome presenting with widened atrophic scars of forehead, elbow, knee, and pretibial area: A case report. Medicine (Baltimore) 2019, 98, e17138. [Google Scholar] [CrossRef] [PubMed]
- Costa, A.; Naranjo, J.D.; Londono, R.; Badylak, S.F. Biologic Scaffolds. Cold Spring Harb. Perspect. Med. 2017, 7, a025676. [Google Scholar] [CrossRef]
- Brown, K.L.; Cummings, C.F.; Vanacore, R.M.; Hudson, B.G. Building collagen IV smart scaffolds on the outside of cells. Protein Sci. 2017, 26, 2151–2161. [Google Scholar] [CrossRef]
- Fidler, A.L.; Boudko, S.P.; Rokas, A.; Hudson, B.G. The triple helix of collagens—An ancient protein structure that enabled animal multicellularity and tissue evolution. J. Cell Sci. 2018, 131, jcs203950. [Google Scholar] [CrossRef]
- Gatseva, A.; Sin, Y.Y.; Brezzo, G.; Van Agtmael, T. Basement membrane collagens and disease mechanisms. Essays Biochem. 2019, 63, 297–312. [Google Scholar] [CrossRef]
- Li, L.; Sun, Z.; Chen, J.; Zhang, Y.; Shi, H.; Zhu, L. Genetic polymorphisms in collagen-related genes are associated with pelvic organ prolapse. Menopause 2020, 27, 223–229. [Google Scholar] [CrossRef] [PubMed]
- Liu, N.; Matsumura, H.; Kato, T.; Ichinose, S.; Takada, A.; Namiki, T.; Asakawa, K.; Morinaga, H.; Mohri, Y.; De Arcangelis, A.; et al. Stem cell competition orchestrates skin homeostasis and ageing. Nature 2019, 568, 344–350. [Google Scholar] [CrossRef]
- NCBI. Genes & Expression. Gene. Available online: https://www.ncbi.nlm.nih.gov/gene/ (accessed on 23 April 2021).
- Wietecha, M.S.; Pensalfini, M.; Cangkrama, M.; Müller, B.; Jin, J.; Brinckmann, J.; Mazza, E.; Werner, S. Activin-mediated alterations of the fibroblast transcriptome and matrisome control the biomechanical properties of skin wounds. Nat. Commun. 2020, 11, 2604. [Google Scholar] [CrossRef] [PubMed]
- Demina, O.M.; Karpova, E.I.; Borzykh, O.B. Modern aspects of medical genetics. Russ. J. Clin. Dermatol. Venereol. 2021, 20, 124–134. [Google Scholar] [CrossRef]
- Borzykh, O.B.; Petrova, M.M.; Shnayder, N.A.; Nasyrova, R.F. Problems of implementation of personalized medicine in medical cosmetology in Russia. Sib. Med. Rev. 2021, 2, 12–22. [Google Scholar] [CrossRef]
- Yeo, J.; Jung, G.; Tarakanova, A.; Martín-Martínez, F.J.; Qin, Z.; Cheng, Y.; Zhang, Y.W.; Buehler, M.J. Multiscale modeling of keratin, collagen, elastin and related human diseases: Perspectives from atomistic to coarse-grained molecular dynamics simulations. Extreme Mech. Lett. 2018, 20, 112–124. [Google Scholar] [CrossRef]
- Arseni, L.; Lombardi, A.; Orioli, D. From structure to phenotype: Impact of collagen alterations on human health. Int. J. Mol. Sci. 2018, 19, 1407. [Google Scholar] [CrossRef]
- Limandjaja, G.C.; Niessen, F.B.; Scheper, R.J.; Gibbs, S. Hypertrophic scars and keloids: Overview of the evidence and practical guide for differentiating between these abnormal scars. Exp. Dermatol. 2021, 30, 146–161. [Google Scholar] [CrossRef]
- Fertala, A. Three Decades of research on recombinant collagens: Reinventing the wheel or developing new biomedical products? Bioengineering (Basel) 2020, 7, 155. [Google Scholar] [CrossRef]
- Sharma, U.; Carrique, L.; Vadon-Le Goff, S.; Mariano, N.; Georges, R.N.; Delolme, F.; Koivunen, P.; Myllyharju, J.; Moali, C.; Aghajari, N.; et al. Structural basis of homo- and heterotrimerization of collagen I. Nat. Commun. 2017, 8, 14671. [Google Scholar] [CrossRef]
- Lu, Y.; Zhang, S.; Wang, Y.; Ren, X.; Han, J. Molecular mechanisms and clinical manifestations of rare genetic disorders associated with type I collagen. Intractable Rare Dis. Res. 2019, 8, 98–107. [Google Scholar] [CrossRef]
- Asgari, M.; Latifi, N.; Heris, H.K.; Vali, H.; Mongeau, L. In vitro fibrillogenesis of tropocollagen type III in collagen type I affects its relative fibrillar topology and mechanics. Sci. Rep. 2017, 7, 1392. [Google Scholar] [CrossRef]
- Rowley, A.T.; Nagalla, R.R.; Wang, S.W.; Liu, W.F. Extracellular matrix-based strategies for immunomodulatory biomaterials engineering. Adv. Healthc. Mater. 2019, 8, e1801578. [Google Scholar] [CrossRef] [PubMed]
- San Antonio, J.D.; Jacenko, O.; Fertala, A.; Orgel, J.P.R.O. Collagen Structure-Function Mapping Informs Applications for Regenerative Medicine. Bioengineering (Basel) 2020, 8, 3. [Google Scholar] [CrossRef] [PubMed]
- Jafari, H.; Lista, A.; Siekapen, M.M.; Ghaffari-Bohlouli, P.; Nie, L.; Alimoradi, H.; Shavandi, A. Fish collagen: Extraction, characterization, and applications for biomaterials engineering. Polymers (Basel) 2020, 12, 2230. [Google Scholar] [CrossRef]
- Wan, T.; Ye, J.; Wu, P.; Cheng, M.; Jiang, B.; Wang, H.; Li, J.; Ma, J.; Wang, L.; Huang, X. Recurrent pneumothorax and intrapulmonary cavitary lesions in a male patient with vascular Ehlers-Danlos syndrome and a novel missense mutation in the COL3A1 gene: A case report. BMC Pulm. Med. 2020, 20, 149. [Google Scholar] [CrossRef] [PubMed]
- Sakai, K.; Toda, M.; Kyoyama, H.; Nishimura, H.; Kojima, A.; Kuwabara, Y.; Kobayashi, Y.; Kikuchi, S.; Hirata, Y.; Moriyama, G.; et al. Vascular Ehlers-Danlos syndrome with a novel missense mutation in COL3A1: A man in His 50s with aortic dissection after interventional treatment for hemothorax as the first manifestation. Intern. Med. 2019, 58, 3441–3447. [Google Scholar] [CrossRef]
- Rajan, A.M.; Ma, R.C.; Kocha, K.M.; Zhang, D.J.; Huang, P. Dual function of perivascular fibroblasts in vascular stabilization in zebrafish. PLoS Genet. 2020, 16, e1008800. [Google Scholar] [CrossRef]
- Merl-Pham, J.; Basak, T.; Knüppel, L.; Ramanujam, D.; Athanason, M.; Behr, J.; Engelhardt, S.; Eickelberg, O.; Hauck, S.M.; Vanacore, R.; et al. Quantitative proteomic profiling of extracellular matrix and site-specific collagen post-translational modifications in an in vitro model of lung fibrosis. Matrix Biol. Plus 2019, 1, 100005. [Google Scholar] [CrossRef]
- Wong, M.Y.; Shoulders, M.D. Targeting defective proteostasis in the collagenopathies. Curr. Opin. Chem. Biol. 2019, 50, 80–88. [Google Scholar] [CrossRef] [PubMed]
- Sipilä, K.H.; Drushinin, K.; Rappu, P.; Jokinen, J.; Salminen, T.A.; Salo, A.M.; Käpylä, J.; Myllyharju, J.; Heino, J. Proline hydroxylation in collagen supports integrin binding by two distinct mechanisms. J. Biol. Chem. 2018, 293, 7645–7658. [Google Scholar] [CrossRef] [PubMed]
- Qi, Y.; Xu, R. Roles of PLODs in Collagen Synthesis and Cancer Progression. Front. Cell Dev. Biol. 2018, 6, 66. [Google Scholar] [CrossRef] [PubMed]
- Karna, E.; Szoka, L.; Huynh, T.Y.L.; Palka, J.A. Proline-dependent regulation of collagen metabolism. Cell. Mol. Life Sci. 2020, 77, 1911–1918. [Google Scholar] [CrossRef] [PubMed]
- Lim, P.J.; Lindert, U.; Opitz, L.; Hausser, I.; Rohrbach, M.; Giunta, C. Transcriptome profiling of primary skin fibroblasts reveal distinct molecular features between PLOD1- and FKBP14-kyphoscoliotic Ehlers-Danlos syndrome. Genes (Basel) 2019, 10, 517. [Google Scholar] [CrossRef] [PubMed]
- Pullar, J.M.; Carr, A.C.; Vissers, M.C.M. The roles of vitamin C in skin health. Nutrients 2017, 9, 866. [Google Scholar] [CrossRef]
- Castori, M. Ehlers-Danlos syndrome, hypermobility type: An underdiagnosed hereditary connective tissue disorder with mucocutaneous, articular, and systemic manifestations. ISRN Dermatol. 2012, 2012, 751768. [Google Scholar] [CrossRef] [PubMed]
- DiChiara, A.S.; Li, R.C.; Suen, P.H.; Hosseini, A.S.; Taylor, R.J.; Weickhardt, A.F.; Malhotra, D.; McCaslin, D.R.; Shoulders, M.D. A cysteine-based molecular code informs collagen C-propeptide assembly. Nat. Commun. 2018, 9, 4206. [Google Scholar] [CrossRef]
- Caviness, P.; Bauer, R.; Tanaka, K.; Janowska, K.; Roeser, J.R.; Harter, D.; Sanders, J.; Ruth, C.; Matsushita, O.; Sakon, J. Ca2+-induced orientation of tandem collagen binding domains from clostridial collagenase ColG permits two opposing functions of collagen fibril formation and retardation. FEBS J. 2018, 285, 3254–3269. [Google Scholar] [CrossRef]
- Heumüller, S.E.; Talantikite, M.; Napoli, M.; Armengaud, J.; Mörgelin, M.; Hartmann, U.; Sengle, G.; Paulsson, M.; Moali, C.; Wagener, R. C-terminal proteolysis of the collagen VI α3 chain by BMP-1 and proprotein convertase (s) releases endotrophin in fragments of different sizes. J. Biol. Chem. 2019, 294, 13769–13780. [Google Scholar] [CrossRef]
- Graham, J.; Raghunath, M.; Vogel, V. Fibrillar fibronectin plays a key role as nucleator of collagen I polymerization during macromolecular crowding-enhanced matrix assembly. Biomater. Sci. 2019, 7, 4519–4535. [Google Scholar] [CrossRef]
- Goldbloom-Helzner, L.; Hao, D.; Wang, A. Developing regenerative treatments for developmental defects, injuries, and diseases using extracellular matrix collagen-targeting peptides. Int. J. Mol. Sci. 2019, 20, 4072. [Google Scholar] [CrossRef]
- Hoop, C.L.; Zhu, J.; Nunes, A.M.; Case, D.A.; Baum, J. Revealing accessibility of cryptic protein binding sites within the functional collagen fibril. Biomolecules 2017, 7, 76. [Google Scholar] [CrossRef]
- McKay, T.B.; Priyadarsini, S.; Karamichos, D. Mechanisms of collagen crosslinking in diabetes and keratoconus. Cells 2019, 8, 1239. [Google Scholar] [CrossRef] [PubMed]
- Rosell-Garcia, T.; Rodriguez-Pascual, F. Enhancement of collagen deposition and cross-linking by coupling lysyl oxidase with bone morphogenetic protein-1 and its application in tissue engineering. Sci. Rep. 2018, 8, 10780. [Google Scholar] [CrossRef] [PubMed]
- Rosell-García, T.; Paradela, A.; Bravo, G.; Dupont, L.; Bekhouche, M.; Colige, A.; Rodriguez-Pascual, F. Differential cleavage of lysyl oxidase by the metalloproteinases BMP1 and ADAMTS2/14 regulates collagen binding through a tyrosine sulfate domain. J. Biol. Chem. 2019, 294, 11087–11100. [Google Scholar] [CrossRef] [PubMed]
- Harlow, C.R.; Wu, X.; van Deemter, M.; Gardiner, F.; Poland, C.; Green, R.; Sarvi, S.; Brown, P.; Kadler, K.E.; Lu, Y.; et al. Targeting lysyl oxidase reduces peritoneal fibrosis. PLoS ONE 2017, 12, e0183013. [Google Scholar] [CrossRef] [PubMed]
- Cai, L.; Xiong, X.; Kong, X.; Xie, J. The role of the lysyl oxidases in tissue repair and remodeling: A concise review. Tissue Eng. Regen. Med. 2017, 14, 15–30. [Google Scholar] [CrossRef]
- Petersen, J.W.; Douglas, J.Y. Tenascin-X, collagen, and Ehlers-Danlos syndrome: Tenascin-X gene defects can protect against adverse cardiovascular events. Med. Hypotheses 2013, 81, 443–447. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Hielscher, A.; Ellis, K.; Qiu, C.; Porterfield, J.; Gerecht, S. Fibronectin deposition participates in extracellular matrix assembly and vascular morphogenesis. PLoS ONE 2016, 11, e0147600. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.; Kim, J.S.; Choi, E.K.; Kim, J.; Kim, K.M.; Seo, H.R. TGF-β-independent CTGF induction regulates cell adhesion mediated drug resistance by increasing collagen I in HCC. Oncotarget 2017, 8, 21650–21662. [Google Scholar] [CrossRef] [PubMed]
- Spada, S.; Tocci, A.; Di Modugno, F.; Nisticò, P. Fibronectin as a multiregulatory molecule crucial in tumor matrisome: From structural and functional features to clinical practice in oncology. J. Exp. Clin. Cancer Res. 2021, 40, 102. [Google Scholar] [CrossRef]
- Duong, T.E.; Hagood, J.S. Epigenetic regulation of myofibroblast phenotypes in fibrosis. Curr. Pathobiol. Rep. 2018, 6, 79–96. [Google Scholar] [CrossRef]
- Wang, P.; Shu, B.; Xu, Y.; Zhu, J.; Liu, J.; Zhou, Z.; Chen, L.; Zhao, J.; Liu, X.; Qi, S.; et al. Basic fibroblast growth factor reduces scar by inhibiting the differentiation of epidermal stem cells to myofibroblasts via the Notch1/Jagged1 pathway. Stem Cell Res. Ther. 2017, 8, 114. [Google Scholar] [CrossRef]
- Gómez-Leduc, T.; Desancé, M.; Hervieu, M.; Legendre, F.; Ollitrault, D.; de Vienne, C.; Herlicoviez, M.; Galéra, P.; Demoor, M. Hypoxia is a critical parameter for chondrogenic differentiation of human umbilical cord blood mesenchymal stem cells in type I/III collagen sponges. Int. J. Mol. Sci. 2017, 18, 1933. [Google Scholar] [CrossRef]
- Zeng, F.; Harris, R.C. Epidermal growth factor, from gene organization to bedside. Semin Cell Dev. Biol. 2014, 28, 2–11. [Google Scholar] [CrossRef]
- Yang, I.V. Epigenomics of idiopathic pulmonary fibrosis. Epigenomics 2012, 4, 195–203. [Google Scholar] [CrossRef]
- Perdigoto, C.N.; Valdes, V.J.; Bardot, E.S.; Ezhkova, E. Epigenetic regulation of epidermal differentiation. Cold Spring Harb. Perspect. Med. 2014, 4, a015263. [Google Scholar] [CrossRef]
- Stoll, S.; Wang, C.; Qiu, H. DNA methylation and histone modification in hypertension. Int. J. Mol. Sci. 2018, 19, 1174. [Google Scholar] [CrossRef]
- Vandiver, A.R.; Irizarry, R.A.; Hansen, K.D.; Garza, L.A.; Runarsson, A.; Li, X.; Chien, A.L.; Wang, T.S.; Leung, S.G.; Kang, S.; et al. Age and sun exposure-related widespread genomic blocks of hypomethylation in nonmalignant skin. Genome Biol. 2015, 16, 80. [Google Scholar] [CrossRef]
- Moulin, L.; Cenizo, V.; Antu, A.N.; André, V.; Pain, S.; Sommer, P.; Debret, R. Methylation of LOXL1 promoter by DNMT3A in aged human skin fibroblasts. Rejuvenation Res. 2017, 20, 103–110. [Google Scholar] [CrossRef]
- Ghosh, K.; O’Neil, K.; Capell, B.C. Histone modifiers: Dynamic regulators of the cutaneous transcriptome. J. Dermatol. Sci. 2018, 89, 226–232. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Peterson, L.M.; Wilking-Busch, M.J.; Ndiaye, M.A.; Philippe, C.G.A.; Setaluri, V.; Ahmad, N. Sirtuins in skin and skin cancers. Skin Pharmacol. Physiol. 2017, 30, 216–224. [Google Scholar] [CrossRef] [PubMed]
- Carlomosti, F.; D’Agostino, M.; Beji, S.; Torcinaro, A.; Rizzi, R.; Zaccagnini, G.; Maimone, B.; Di Stefano, V.; De Santa, F.; Cordisco, S.; et al. Oxidative stress-induced miR-200c disrupts the regulatory loop among SIRT1, FOXO1, and eNOS. Antioxid. Redox Signal. 2017, 27, 328–344. [Google Scholar] [CrossRef]
- Laronha, H.; Caldeira, J. Structure and function of human matrix metalloproteinases. Cells 2020, 9, 1076. [Google Scholar] [CrossRef]
- Li, X.; Jin, L.; Tan, Y. Different roles of matrix metalloproteinase 2 in osteolysis of skeletal dysplasia and bone metastasis (Review). Mol. Med. Rep. 2021, 23, 70. [Google Scholar] [CrossRef] [PubMed]
- Cui, N.; Hu, M.; Khalil, R.A. Biochemical and biological attributes of matrix metalloproteinases. Prog. Mol. Biol. Transl. Sci. 2017, 147, 1–73. [Google Scholar] [CrossRef]
- Amar, S.; Smith, L.; Fields, G.B. Matrix metalloproteinase collagenolysis in health and disease. Biochim. Biophys. Acta Mol. Cell Res. 2017, 1864 Pt A, 1940–1951. [Google Scholar] [CrossRef]
- Durmanova, V.; Javor, J.; Parnicka, Z.; Minarik, G.; Ocenasova, A.; Vaseckova, B.; Reznakova, V.; Kralova, M.; Hromadka, T.; Shawkatova, I. Impact of MMP2 rs243865 and MMP3 rs3025058 Polymorphisms on Clinical Findings in Alzheimer’s Disease Patients. Mediat. Inflamm. 2021, 2021, 5573642. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Zhou, Y.; Huang, W.; Zeng, Y.; Li, X. Association between matrix metalloproteinase-1 (MMP-1) protein level and the risk of rheumatoid arthritis and osteoarthritis: A meta-analysis. Braz. J. Med. Biol. Res. 2020, 54, e10366. [Google Scholar] [CrossRef] [PubMed]
- Lago, J.C.; Puzzi, M.B. The effect of aging in primary human dermal fibroblasts. PLoS ONE 2019, 14, e0219165. [Google Scholar] [CrossRef]
- Loffek, S.; Schilling, O.; Franzke, C.W. Biological role of matrix metalloproteinases: A critical balance. Eur. Respir. J. 2011, 38, 191–208. [Google Scholar] [CrossRef]
- Mirastschijski, U.; Lupše, B.; Maedler, K.; Sarma, B.; Radtke, A.; Belge, G.; Dorsch, M.; Wedekind, D.; McCawley, L.J.; Boehm, G.; et al. Matrix metalloproteinase-3 is key effector of TNF-α-induced collagen degradation in skin. Int. J. Mol. Sci. 2019, 20, 5234. [Google Scholar] [CrossRef]
- Ren, X.; Lamb, G.D.; Murphy, R.M. Distribution and activation of matrix metalloproteinase-2 in skeletal muscle fibers. Am. J. Physiol.-Cell Physiol. 2019, 317, 613–625. [Google Scholar] [CrossRef]
- Liu, J.; Khalil, R.A. Matrix metalloproteinase inhibitors as investigational and therapeutic tools in unrestrained tissue remodeling and pathological disorders. Prog. Mol. Biol. Transl. Sci. 2017, 148, 355–420. [Google Scholar] [CrossRef]
- Tracy, L.E.; Minasian, R.A.; Caterson, E.J. Extracellular matrix and dermal fibroblast function in the healing wound. Adv. Wound Care (New Rochelle) 2016, 5, 119–136. [Google Scholar] [CrossRef] [PubMed]
- Velez, D.O.; Ranamukhaarachchi, S.K.; Kumar, A.; Modi, R.N.; Lim, E.W.; Engler, A.J.; Metallo, C.M.; Fraley, S.I. 3D collagen architecture regulates cell adhesion through degradability, thereby controlling metabolic and oxidative stress. Integr. Biol. 2019, 11, 221–234. [Google Scholar] [CrossRef] [PubMed]
- Reily, C.; Stewart, T.J.; Renfrow, M.B.; Novak, J. Glycosylation in health and disease. Nat. Rev. Nephrol. 2019, 15, 346–366. [Google Scholar] [CrossRef]
- Gardelli, C.; Russo, L.; Cipolla, L.; Moro, M.; Andriani, F.; Rondinone, O.; Nicotra, F.; Sozzi, G.; Bertolini, G.; Roz, L. Differential glycosylation of collagen modulates lung cancer stem cell subsets through β1 integrin-mediated interactions. Cancer Sci. 2021, 112, 217–230. [Google Scholar] [CrossRef]
- Hoy, R.C.; D’Erminio, D.N.; Krishnamoorthy, D.; Natelson, D.M.; Laudier, D.M.; Illien-Jünger, S.; Iatridis, J.C. Advanced glycation end products cause RAGE-dependent annulus fibrosus collagen disruption and loss identified using in situ second harmonic generation imaging in mice intervertebral disk in vivo and in organ culture models. JOR Spine 2020, 3, e1126. [Google Scholar] [CrossRef]
- Bansode, S.; Bashtanova, U.; Li, R.; Clark, J.; Müller, K.H.; Puszkarska, A.; Goldberga, I.; Chetwood, H.H.; Reid, D.G.; Colwell, L.J.; et al. Glycation changes molecular organization and charge distribution in type I collagen fibrils. Sci. Rep. 2020, 10, 3397. [Google Scholar] [CrossRef]
- Pennacchi, P.C.; de Almeida, M.E.; Gomes, O.L.; Faião-Flores, F.; de Araújo Crepaldi, M.C.; Dos Santos, M.F.; de Moraes Barros, S.B.; Maria-Engler, S.S. Glycated reconstructed human skin as a platform to study the pathogenesis of skin aging. Tissue Eng. Part A 2015, 21, 2417–2425. [Google Scholar] [CrossRef]
- Tsamis, A.; Krawiec, J.T.; Vorp, D.A. Elastin and collagen fiber microstructure of the human aorta in aging and disease: A review. J. R. Soc. Interface 2013, 10, 20121004. [Google Scholar] [CrossRef]
- Zhang, W.; Fan, J.; Chen, Q.; Lei, C.; Qiao, B.; Liu, Q. SPP1 and AGER as potential prognostic biomarkers for lung adenocarcinoma. Oncol. Lett. 2018, 15, 7028–7036. [Google Scholar] [CrossRef]
- Nyström, A.; Thriene, K.; Mittapalli, V.; Kern, J.S.; Kiritsi, D.; Dengjel, J.; Bruckner-Tuderman, L. Losartan ameliorates dystrophic epidermolysis bullosa and uncovers new disease mechanisms. EMBO Mol. Med. 2015, 7, 1211–1228. [Google Scholar] [CrossRef] [PubMed]
- Sotiropoulou, G.; Zingkou, E.; Pampalakis, G. Redirecting drug repositioning to discover innovative cosmeceuticals. Exp. Dermatol. 2021, 30, 628–644. [Google Scholar] [CrossRef] [PubMed]
- Shnayder, N.A.; Dyuzhakova, A.V.; Vaiman, E.E.; Nikitina, E.I.; Borzykh, O.B.; Nasyrova, R.F. The role of genetic factors of endogenous hyaluronic acid metabolism in maintaining skin homeostasis. Bull. Dermatol. Venerol. 2021, 97, 24–38. [Google Scholar] [CrossRef]

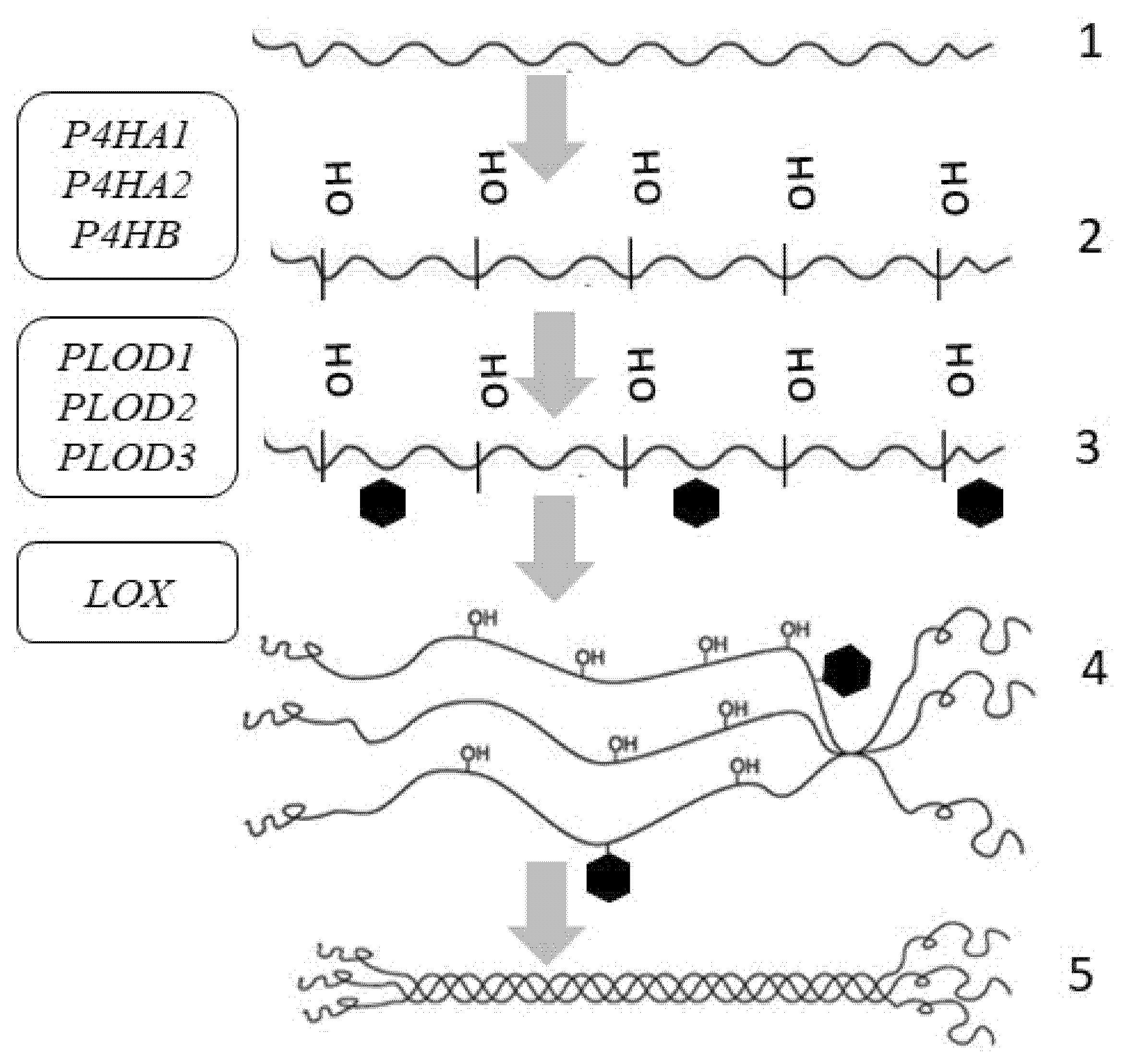
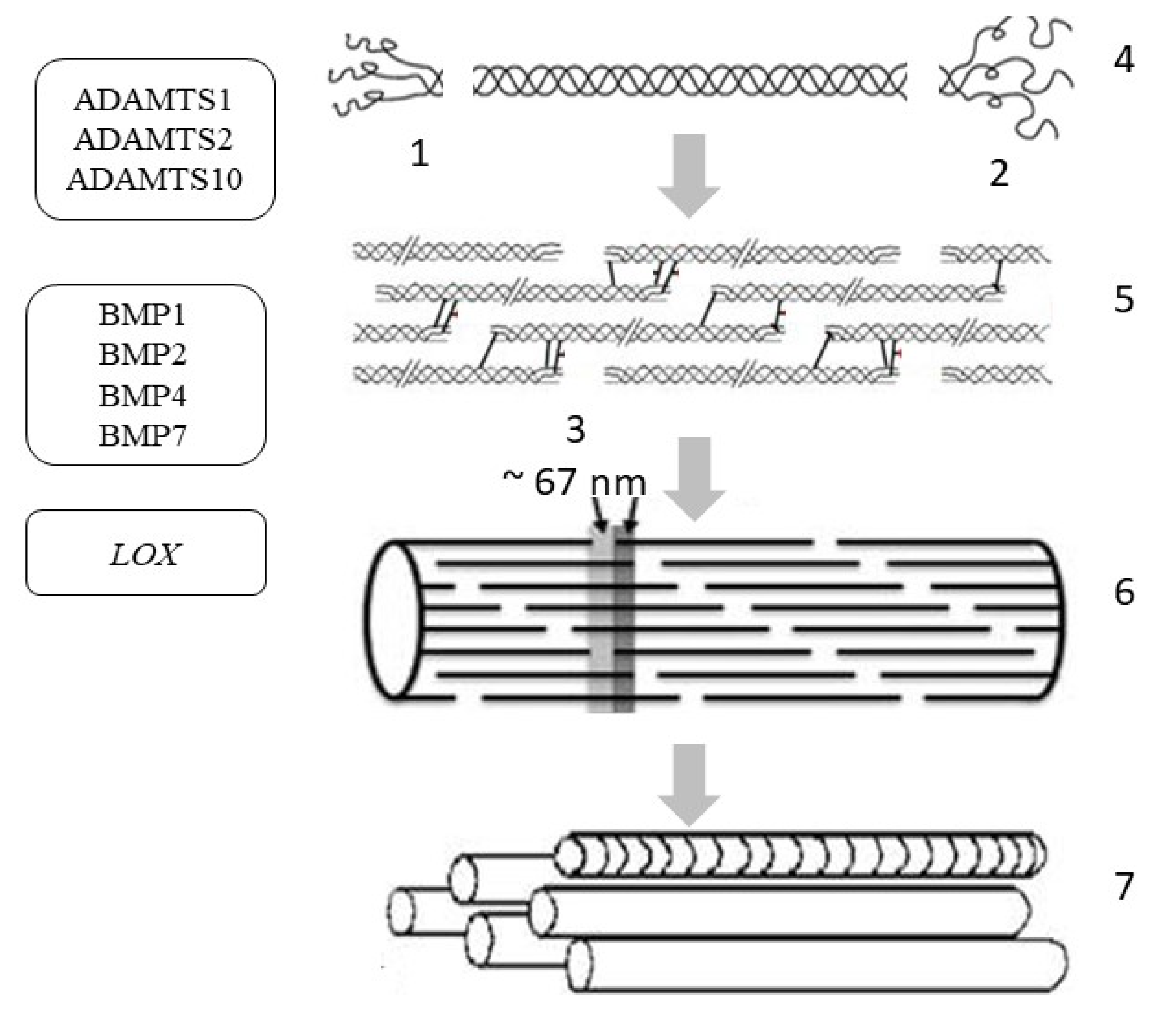
| Gene (Protein/Enzyme) | Chromosome Localization | Clinical Manifestations of Mutation/Polymorphism | Expression in the Skin (RPKM) |
|---|---|---|---|
| P4HA1 (α-subunit of the 4-hydroxylase) | 10q22.1 (17 exons) | Poor prognosis in malignant neoplasms | 7.129 ± 2.121 |
| P4HA2 (α-subunit of the 4-hydroxylase) | 5q31.1 (17 exons) | Poor prognosis in malignant neoplasms, risk of myopia | 4.6 ± 0.816 |
| P4HB (β-subunit of the 4-hydroxylase) | 17q25.3 (10 exons) | Poor prognosis in malignant neoplasms | 89.377 ± 8.824 |
| PLOD1 (LH1) (lysyl hydroxylase (procollagen lysine, 2-oxoglutarate 5-dioxygenase 1)) | 1p36.22 (20 exons) | Ehlers-Danlos syndrome type VI | 11.061 ± 2.249 |
| PLOD2 (LH2) (lysyl hydroxylase (procollagen lysine, 2-oxoglutarate 5-dioxygenase type 2)) | 3q24 (23 exons) | Ehlers-Danlos syndrome type VIB, Brook’s syndrome | 0.988 ± 0.202 |
| PLOD3 (LH3) (lysyl hydroxylase (procollagen lysine, 2-oxoglutarate 5-dioxygenase type 3)) | 7q22.1 (19 exons) | Ehlers-Danlos syndrome type VIB, Stickler-like syndrome | 7.062 ± 2.361 |
| LOX (lysyl oxidase) | 5q23.1 (8 exons) | Aortic aneurysms, vascular disorders | 4.234 ± 1.207 |
| ADAMTS (enzyme disintegrin and metalloprotease with thrombospondin motif 1) | 21q21.3, includes 9 exons | Impaired growth, fertility and organ morphology | 2.796 ± 0.682 |
| ADAMTS2 (enzyme disintegrin and metalloprotease with thrombospondin motif 2) | 5q35.3 (23 exons) | Ehlers-Danlos syndrome type VIIC | 1.395 ± 0.248 |
| ADAMTS10 (enzyme disintegrin and metalloprotease with thrombospondin motif 10) | 5q35.3 (23 exons) | Disruption of the growth and development of the skin, lens and heart, Weill Marchesani syndrome | 1.485 ± 0.952 |
| BMP1 (bone morphogenetic protein 1/tolloid-like proteinase) | 8p21.3 (21 exons) | Osteogenesis imperfecta, disruption of morphogenesis, regeneration of various tissues | 5.382 ± 1.39 |
| BMP2 (bone morphogenetic protein 2/tolloid-like proteinase) | 20p12.3 (3 exons) | Disruption of the development of bone and cartilage tissue | 2.551 ± 0.444 |
| BMP4 (bone morphogenetic protein 4/tolloid-like proteinase) | 14q22.2 (6 exons) | Dental system pathology, orofacial cleft, microphthalmia, cardiovascular pathology | 2.207 ± 0.446 |
| BMP7 (bone morphogenetic protein 7/tolloid-like proteinase) | 20q13.31 (7 exons) | Pathology of the skeletal system, kidneys, and brown fat | 7.722 ± 0.536 |
| Gene (Protein/Enzyme) | Chromosome Localization | Clinical Manifestations of Mutation/Polymorphism | Expression in the Skin (RPKM) |
|---|---|---|---|
| Matrix Metalloprotease | |||
| MMP1 (matrix metalloproteinase 1) (peptidase) | 11q22.2 (10 exons) | Pathology of the osteoarticular system, chronic obstructive pulmonary disease | Low-level |
| MMP2 (matrix metalloproteinase 2) (gelatinase A) | 16q12.2 (17 exons) | Winchester syndrome, nodular arthropathy syndrome | 63.804 ± 22.347 |
| MMP3 (matrix metalloproteinase 3) (stromelysin) | 11q22.2 (10 exons) | Disruption of tissue remodeling, the development of atherosclerosis, malignant neoplasms | Low-level |
| MMP8 (matrix metalloproteinase 8) | 11q22.2 (12 exons) | Disruption of tissue remodeling, progression of arthritis and metastasis | Low-level |
| MMP9 (matrix metalloproteinase 9) (gelatinase) | 20q13.12 (13 exons) | Disruption of tissue remodeling, progression of arthritis and metastasis | 1.322 ± 0.782 |
| MMP13 (matrix metalloproteinase 13) (peptidase) | 11q22.2 (10 exons) | Disruption of tissue remodeling, progression of arthritis and metastasis | Low-level |
| Matrix Metalloprotease Inhibitor | |||
| TIMP1 (matrix metalloproteinase 1 inhibitor) | Xp13.3 (5 exons) | Disruption of tissue remodeling, progression of metastasis | 12.523 ± 2.84 |
| TIMP2 (matrix metalloproteinase 2 inhibitor) | 17q25.3 (5 exons) | Disruption of tissue homeostasis, proliferation, tissue remodeling, progression of metastasis | 61.764 ± 15.275 |
| TIMP3 (encoding matrix metalloproteinase 3 inhibitor) | 22q12.3 (5 exons) | Disruption of tissue remodeling, progression of metastasis, Sorsby fundus dystrophy | 20.537 ± 6.783 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Potekaev, N.N.; Borzykh, O.B.; Medvedev, G.V.; Petrova, M.M.; Gavrilyuk, O.A.; Karpova, E.I.; Trefilova, V.V.; Demina, O.M.; Popova, T.E.; Shnayder, N.A. Genetic and Epigenetic Aspects of Skin Collagen Fiber Turnover and Functioning. Cosmetics 2021, 8, 92. https://doi.org/10.3390/cosmetics8040092
Potekaev NN, Borzykh OB, Medvedev GV, Petrova MM, Gavrilyuk OA, Karpova EI, Trefilova VV, Demina OM, Popova TE, Shnayder NA. Genetic and Epigenetic Aspects of Skin Collagen Fiber Turnover and Functioning. Cosmetics. 2021; 8(4):92. https://doi.org/10.3390/cosmetics8040092
Chicago/Turabian StylePotekaev, Nikolay N., Olga B. Borzykh, German V. Medvedev, Marina M. Petrova, Oksana A. Gavrilyuk, Elena I. Karpova, Vera V. Trefilova, Olga M. Demina, Tatiana E. Popova, and Natalia A. Shnayder. 2021. "Genetic and Epigenetic Aspects of Skin Collagen Fiber Turnover and Functioning" Cosmetics 8, no. 4: 92. https://doi.org/10.3390/cosmetics8040092
APA StylePotekaev, N. N., Borzykh, O. B., Medvedev, G. V., Petrova, M. M., Gavrilyuk, O. A., Karpova, E. I., Trefilova, V. V., Demina, O. M., Popova, T. E., & Shnayder, N. A. (2021). Genetic and Epigenetic Aspects of Skin Collagen Fiber Turnover and Functioning. Cosmetics, 8(4), 92. https://doi.org/10.3390/cosmetics8040092







