Design, Synthesis, and Antipoliferative Activities of Novel Substituted Imidazole-Thione Linked Benzotriazole Derivatives
Abstract
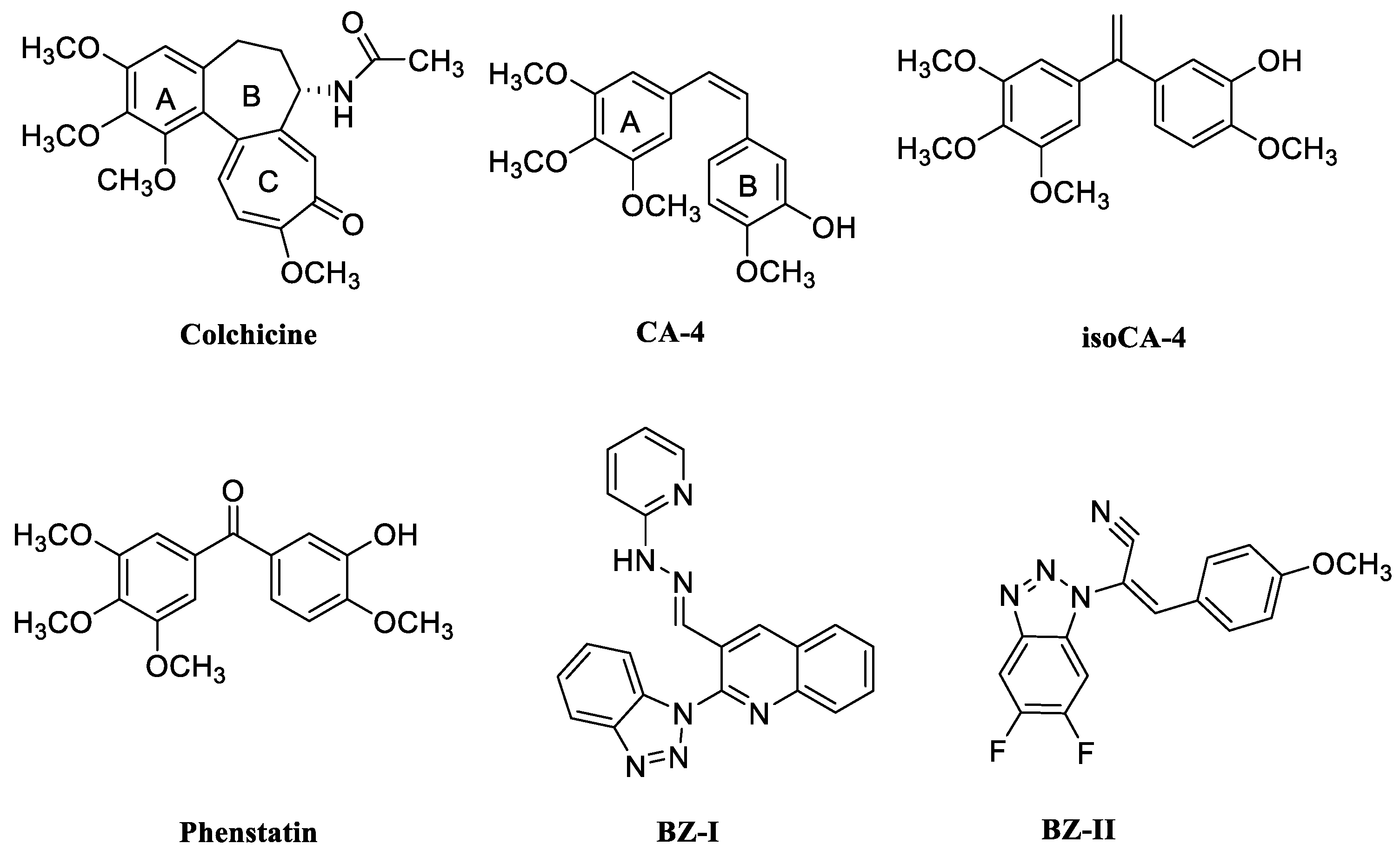
:1. Introduction
2. Results and Discussion
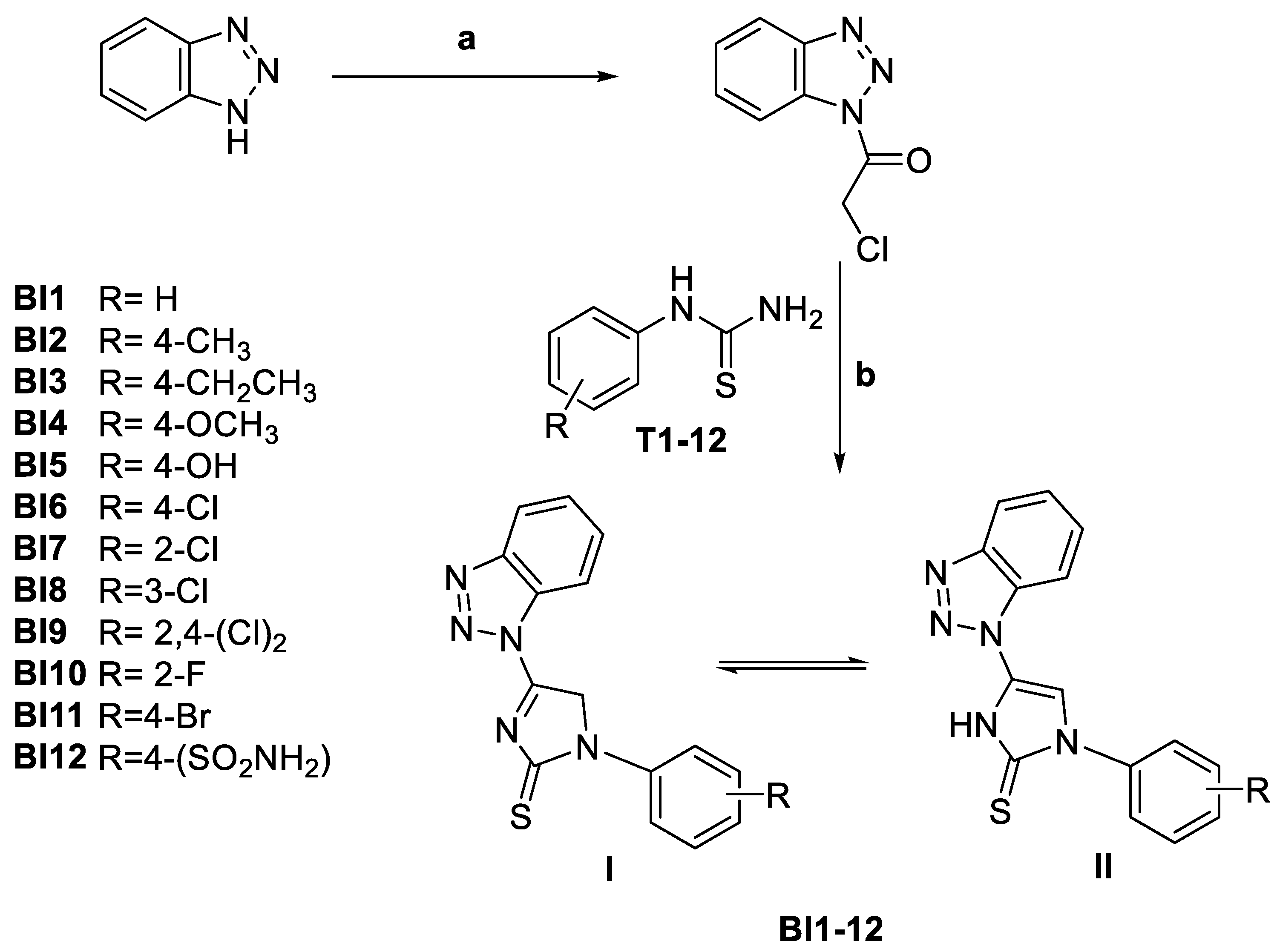
2.1. Design and Chemistry
2.2. Biological Results and Discussion
2.2.1. In Vitro Cell Growth Inhibitory Activity
2.2.2. In Vitro Inhibition of Tubulin Polymerization and Colchicine Binding
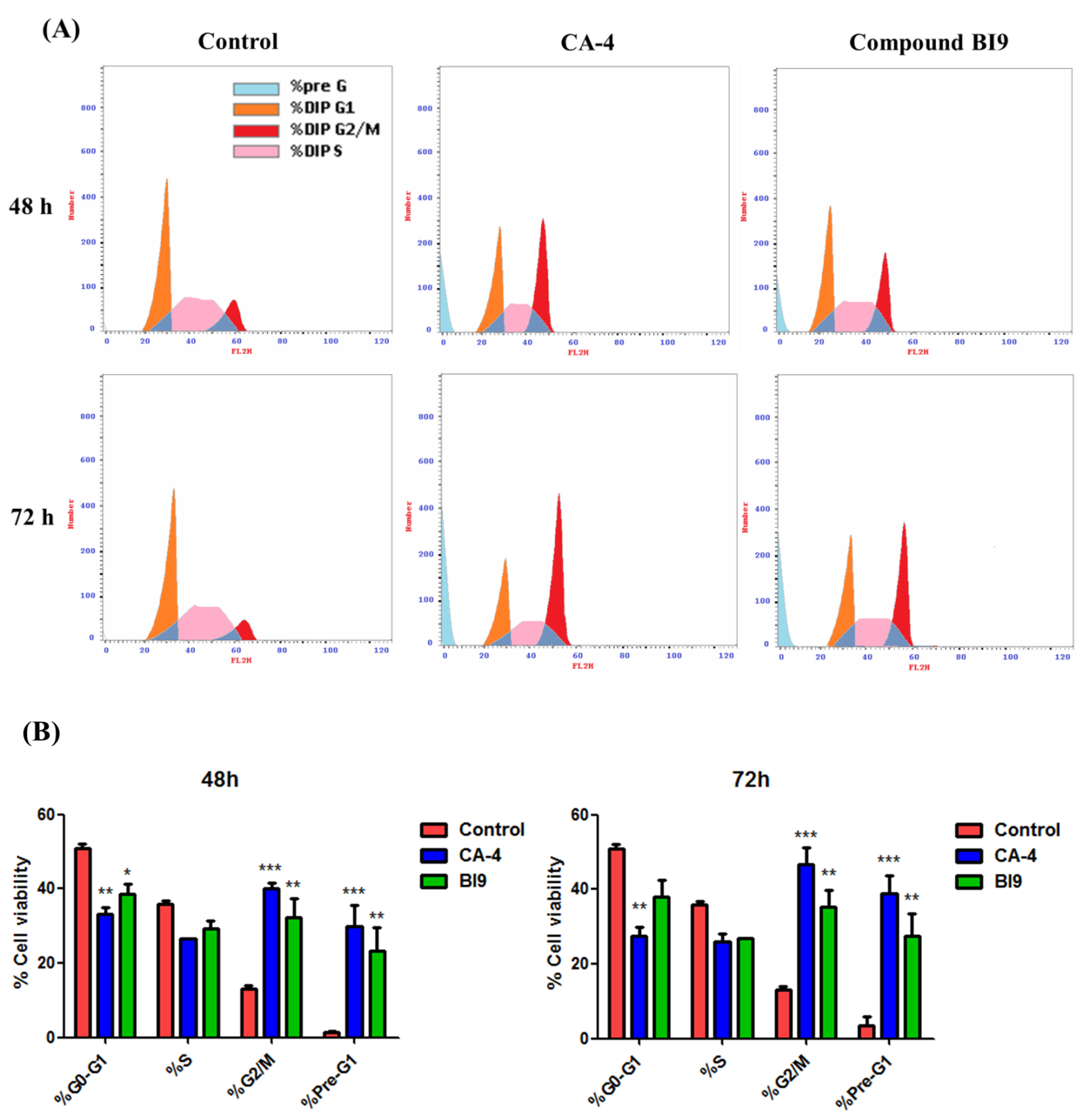
2.2.3. HL-60 Cell Cycle Arrest
2.2.4. HL-60 Cell Apoptosis along with Alteration of Apoptosis Checkpoints Proteins
3. Materials and Methods
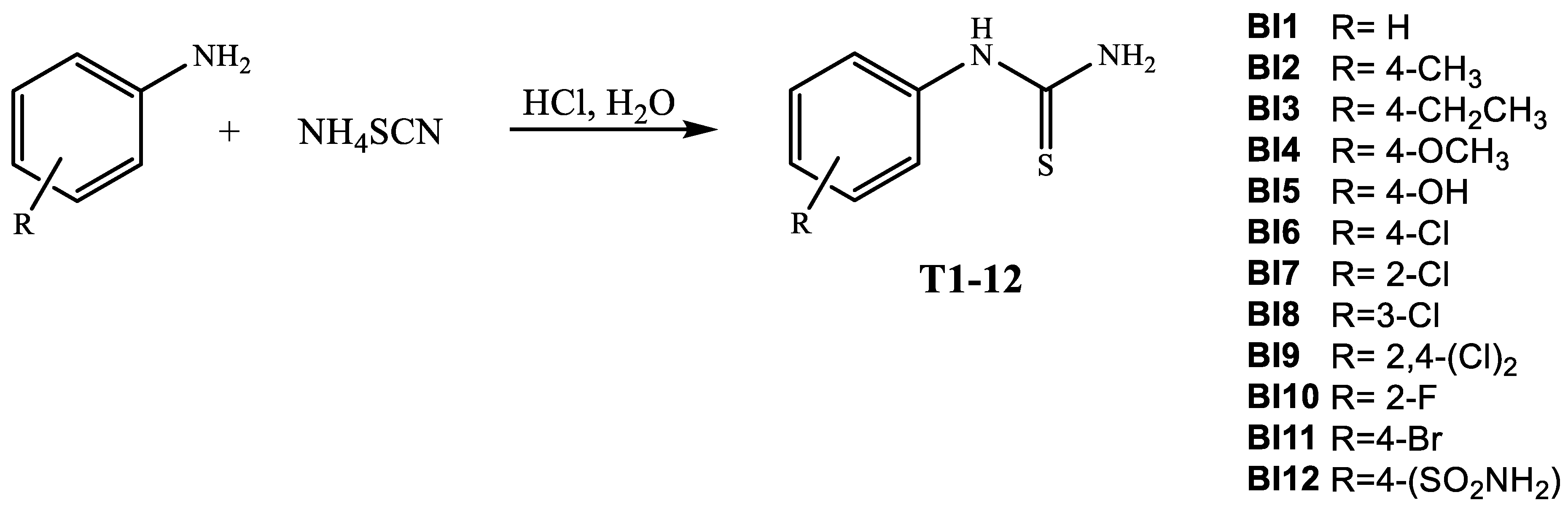
3.1. Chemistry
3.1.1. Synthesis of 1-(1H-Benzo[d][1,2,3]triazol-1-yl)-2-chloroethanone
3.1.2. General Procedure for the Synthesis of 4-(1H-Benzo[d][1,2,3]triazol-1-yl)-1-aryl-1H-imidazole-2(3H)-thione (BI1-12)
3.2. Biochemical Evaluation of Activity
3.2.1. Cell Culture
3.2.2. Cell Viability Assay
3.2.3. Tubulin Polymerization Assay
3.2.4. Colchicine Site Competitive Binding Assay
3.2.5. Cell Cycle Analysis
3.2.6. Annexin V/PI Apoptotic Assay
3.2.7. Evaluation of Expression Levels of Anti-Apoptotic Proteins Bcl-2, Pro-Apoptotic Proteins Bcl-2, Bax and PARP Cleavage
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Jordan, M.A.; Wilson, L. Microtubules as a target for anticancer drugs. Nat. Rev. Cancer 2004, 4, 253–265. [Google Scholar] [CrossRef] [PubMed]
- Muroyama, A.; Lechler, T. Microtubule organization, dynamics and functions in differentiated cells. Development 2017, 144, 3012–3021. [Google Scholar] [CrossRef] [Green Version]
- Cao, Y.-N.; Zheng, L.-L.; Wang, D.; Liang, X.-X.; Gao, F.; Zhou, X.-L. Recent advances in microtubule-stabilizing agents. Eur. J. Med. Chem. 2018, 143, 806–828. [Google Scholar] [CrossRef] [Green Version]
- Honore, S.; Pasquier, E.; Braguer, D. Understanding microtubule dynamics for improved cancer therapy. Cell. Mol. Life Sci. 2005, 62, 3039–3056. [Google Scholar] [CrossRef]
- McLoughlin, E.C.; O’Boyle, N.M. Colchicine-binding site inhibitors from chemistry to clinic: A review. Pharmaceuticals 2020, 13, 8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gracheva, I.A.; Shchegravina, E.S.; Schmalz, H.-G.; Beletskaya, I.P.; Fedorov, A.Y. Colchicine Alkaloids and Synthetic Analogues: Current Progress and Perspectives. J. Med. Chem. 2020, 63, 10618–10651. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Sun, H.; Xu, S.; Zhu, Z.; Xu, J. Tubulin inhibitors targeting the colchicine binding site: A perspective of privileged structures. Futur. Med. Chem. 2017, 9, 1765–1794. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xia, L.-Y.; Zhang, Y.-L.; Yang, R.; Wang, Z.-C.; Lu, Y.-D.; Wang, B.-Z.; Zhu, H.-L. Tubulin Inhibitors Binding to Colchicine-Site: A Review from 2015 to 2019. Curr. Med. Chem. 2020, 27, 6787–6814. [Google Scholar] [CrossRef]
- Riu, F.; Sanna, L.; Ibba, R.; Piras, S.; Bordoni, V.; Scorciapino, M.A.; Lai, M.; Sestito, S.; Bagella, L.; Carta, A. A comprehensive assessment of a new series of 5′,6′-difluorobenzotriazole-acrylonitrile derivatives as microtubule targeting agents (MTAs). Eur. J. Med. Chem. 2021, 222, 113590. [Google Scholar] [CrossRef]
- Lin, M.-S.; Hong, T.-M.; Chou, T.-H.; Yang, S.-C.; Chung, W.-C.; Weng, C.-W.; Tsai, M.-L.; Cheng, T.-J.R.; Chen, J.J.; Lee, T.-C.; et al. 4(1H)-quinolone derivatives overcome acquired resistance to anti-microtubule agents by targeting the colchicine site of β-tubulin. Eur. J. Med. Chem. 2019, 181, 111584. [Google Scholar] [CrossRef]
- Bukhari, S.N.A.; Kumar, G.B.; Revankar, H.M.; Qin, H.-L. Development of combretastatins as potent tubulin polymerization inhibitors. Bioorg. Chem. 2017, 72, 130–147. [Google Scholar] [CrossRef] [PubMed]
- Greene, M.L.; Meegan, M.J.; Zisterer, D.M. Combretastatins: More than just vascular targeting agents? J. Pharmacol. Exp. Ther. 2015, 355, 212–227. [Google Scholar] [CrossRef] [PubMed]
- Shuai, W.; Li, X.; Li, W.; Xu, F.; Lu, L.; Yao, H.; Yang, L.; Zhu, H.; Xu, S.; Zhu, Z.; et al. Design, synthesis and anticancer properties of isocombretapyridines as potent colchicine binding site inhibitors. Eur. J. Med. Chem. 2020, 197, 112308. [Google Scholar] [CrossRef] [PubMed]
- Chaudhary, A.; Pandeya, S.N.; Kumar, P.; Sharma, P.P.; Gupta, S.; Soni, N.; Verma, K.K.; Bhardwaj, G. Combretastatin A-4 Analogs as Anticancer Agents. Mini-Rev. Med. Chem. 2007, 7, 1186–1205. [Google Scholar] [CrossRef]
- Seddigi, Z.S.; Malik, M.S.; Saraswati, A.P.; Ahmed, S.A.; Babalghith, A.O.; Lamfon, H.A.; Kamal, A. Recent advances in combretastatin based derivatives and prodrugs as antimitotic agents. MedChemComm 2017, 8, 1592–1603. [Google Scholar] [CrossRef]
- Dhiman, N.; Kaur, K.; Jaitak, V. Tetrazoles as anticancer agents: A review on synthetic strategies, mechanism of action and SAR studies. Bioorg. Med. Chem. 2020, 28, 115599. [Google Scholar] [CrossRef]
- Jian, X.E.; Yang, F.; Jiang, C.S.; You, W.W.; Zhao, P.L. Synthesis and biological evaluation of novel pyrazolo [3, 4-b] pyridines as cis-restricted combretastatin A-4 analogues. Bioorg. Med. Chem. Lett. 2020, 30, 127025. [Google Scholar] [CrossRef] [PubMed]
- Qi, Z.-Y.; Hao, S.-Y.; Tian, H.-Z.; Bian, H.-L.; Hui, L.; Chen, S.-W. Synthesis and biological evaluation of 1-(benzofuran-3-yl)-4-(3,4,5-trimethoxyphenyl)-1H-1,2,3-triazole derivatives as tubulin polymerization inhibitors. Bioorg. Chem. 2019, 94, 103392. [Google Scholar] [CrossRef]
- Romagnoli, R.; Baraldi, P.G.; Salvador, M.K.; Preti, D.; Aghazadeh Tabrizi, M.; Brancale, A.; Fu, X.-H.; Li, J.; Zhang, S.-Z.; Hamel, E.; et al. Discovery and optimization of a series of 2-aryl-4-amino-5-(3′,4′,5′-trimethoxybenzoyl) thiazoles as novel anticancer agents. J. Med. Chem. 2012, 55, 5433–5445. [Google Scholar] [CrossRef]
- Kumari, A.; Srivastava, S.; Manne, R.K.; Sisodiya, S.; Santra, M.K.; Guchhait, S.K.; Panda, D. C12, a combretastatin-A4 analog, exerts anticancer activity by targeting microtubules. Biochem. Pharmacol. 2019, 170, 113663. [Google Scholar] [CrossRef]
- Banimustafa, M.; Kheirollahi, A.; Safavi, M.; Ardestani, S.K.; Aryapour, H.; Foroumadi, A.; Emami, S. Synthesis and biological evaluation of 3-(trimethoxyphenyl)-2(3H)-thiazole thiones as combretastatin analogs. Eur. J. Med. Chem. 2013, 70, 692–702. [Google Scholar] [CrossRef] [PubMed]
- Ansari, M.; Shokrzadeh, M.; Karima, S.; Rajaei, S.; Fallah, M.; Ghassemi-Barghi, N.; Ghasemian, M.; Emami, S. New thiazole-2(3H)-thiones containing 4-(3,4,5-trimethoxyphenyl) moiety as anticancer agents. Eur. J. Med. Chem. 2019, 185, 111784. [Google Scholar] [CrossRef]
- Salehi, M.; Amini, M.; Ostad, S.N.; Riazi, G.H.; Assadieskandar, A.; Shafiei, B.; Shafiee, A. Synthesis, cytotoxic evaluation and molecular docking study of 2-alkylthio-4-(2,3,4-trimethoxyphenyl)-5-aryl-thiazoles as tubulin polymerization inhibitors. Bioorg. Med. Chem. 2013, 21, 7648–7654. [Google Scholar] [CrossRef]
- Abdel-Aziz, M.; Metwally, A.K.; Gamal-Eldeen, A.M.; Aly, M.O. 1,3,4-oxadiazole-2-thione Derivatives; Novel Approach for Anticancer and Tubulin Polymerization Inhibitory Activities. Anti-Cancer Agents Med. Chem. 2016, 16, 269–277. [Google Scholar] [CrossRef]
- Sharma, S.; Kumar Gupta, M.; Kumar Saxena, A.; Singh Bedi, P.M. Thiazolidinone Constraint Combretastatin Analogs as Novel Antitubulin Agents: Design, Synthesis, Biological Evaluation and Docking Studies. Anti-Cancer Agents Med. Chem. 2017, 17, 230–240. [Google Scholar] [CrossRef]
- Tantak, M.P.; Malik, M.; Klingler, L.; Olson, Z.; Kumar, A.; Sadana, R.; Kumar, D. Indolyl-α-keto-1,3,4-oxadiazoles: Synthesis, anti-cell proliferation activity, and inhibition of tubulin polymerization. Bioorg. Med. Chem. Lett. 2021, 37, 127842. [Google Scholar] [CrossRef]
- Alraqa, S.Y.; Alharbi, K.; Aljuhani, A.; Rezki, N.; Aouad, M.R.; Ali, I. Design, click conventional and microwave syntheses, DNA binding, docking and anticancer studies of benzotriazole-1,2,3-triazole molecular hybrids with different pharmacophores. J. Mol. Struct. 2020, 1225, 129192. [Google Scholar] [CrossRef]
- Briguglio, I.; Piras, S.; Corona, P.; Gavini, E.; Nieddu, M.; Boatto, G.; Carta, A. Benzotriazole: An overview on its versatile biological behavior. Eur. J. Med. Chem. 2014, 97, 612–648. [Google Scholar] [CrossRef] [PubMed]
- Carta, A.; Palomba, M.; Boatto, G.; Busonera, B.; Murreddu, M.; Loddo, R. Synthesis and antiproliferative activity of 3-aryl-2-[1H(2H)-benzotriazol-1(2)-yl]acrylonitriles variously substituted: Part 4. Il Farm. 2004, 59, 637–644. [Google Scholar] [CrossRef] [PubMed]
- Carta, A.; Briguglio, I.; Piras, S.; Boatto, G.; La Colla, P.; Loddo, R.; Tolomeo, M.; Grimaudo, S.; Di Cristina, A.; Pipitone, R.M.; et al. 3-Aryl-2-[1H-benzotriazol-1-yl]acrylonitriles: A novel class of potent tubulin inhibitors. Eur. J. Med. Chem. 2011, 46, 4151–4167. [Google Scholar] [CrossRef]
- Korcz, M.; Sączewski, F.; Bednarski, P.J.; Kornicka, A. Synthesis, Structure, Chemical Stability, and In Vitro Cytotoxic Properties of Novel Quinoline-3-Carbaldehyde Hydrazones Bearing a 1,2,4-Triazole or Benzotriazole Moiety. Molecules 2018, 23, 1497. [Google Scholar] [CrossRef] [Green Version]
- Ren, Y.; Zhang, H.Z.; Zhang, S.L.; Luo, Y.L.; Zhang, L.; Zhou, C.H.; Geng, R.X. Synthesis and bioactive evaluations of novel benzotriazole compounds as potential antimicrobial agents and the interaction with calf thymus DNA. J. Chem. Sci. 2015, 127, 2251–2260. [Google Scholar] [CrossRef]
- Wan, J.; Lv, P.-C.; Tian, N.-N.; Zhu, H.-L. Facile synthesis of novel benzotriazole derivatives and their antibacterial activities. J. Chem. Sci. 2010, 122, 597–606. [Google Scholar] [CrossRef] [PubMed]
- Loddo, R.; Novelli, F.; Sparatore, A.; Tasso, B.; Tonelli, M.; Boido, V.; Sparatore, F.; Collu, G.; Delogu, I.; Giliberti, G.; et al. Antiviral activity of benzotriazole derivatives. 5-[4-(Benzotriazol-2-yl)phenoxy]-2,2-dimethylpentanoic acids potently and selectively inhibit Coxsackie Virus B. Bioorg. Med. Chem. 2015, 23, 7024–7034. [Google Scholar] [CrossRef]
- Sanna, G.; Piras, S.; Madeddu, S.; Busonera, B.; Klempa, B.; Corona, P.; Ibba, R.; Murineddu, G.; Carta, A.; Loddo, R. 5,6-Dichloro-2-Phenyl-Benzotriazoles: New Potent Inhibitors of Orthohantavirus. Viruses 2020, 12, 122. [Google Scholar] [CrossRef] [Green Version]
- Dawood, K.M.; Abdel-Gawad, H.; Rageb, E.A.; Ellithey, M.; Mohamed, H. Synthesis, anticonvulsant, and anti-inflammatory evaluation of some new benzotriazole and benzofuran-based heterocycles. Bioorg. Med. Chem. 2006, 14, 3672–3680. [Google Scholar] [CrossRef]
- Ren, Y.; Zhang, L.; Zhou, C.-H.; Geng, R.-X. Recent Development of Benzotriazole-based Medicinal Drugs. Med. Chem. 2014, 4, 640–662. [Google Scholar] [CrossRef]
- Soussi, M.A.; Provot, O.; Bernadat, G.; Bignon, J.; Desravines, D.; Dubois, J.; Brion, J.-D.; Messaoudi, S.; Alami, M. IsoCombretaQuinazolines: Potent Cytotoxic Agents with Antitubulin Activity. ChemMedChem 2015, 10, 1392–1402. [Google Scholar] [CrossRef]
- Khelifi, I.; Naret, T.; Renko, D.; Hamze, A.; Bernadat, G.; Bignon, J.; Lenoir, C.; Dubois, J.; Brion, J.-D.; Provot, O.; et al. Design, synthesis and anticancer properties of Iso Combreta Quinolines as potent tubulin assembly inhibitors. Eur. J. Med. Chem. 2017, 127, 1025–1034. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, T.S.; Hawwas, M.M.; Malebari, A.M.; Taher, E.S.; Omar, A.M.; Neamatallah, T.; Abdel-Samii, Z.K.; Safo, M.K.; Elshaier, Y.A.M.M. Discovery of novel quinoline-based analogues of combretastatin A-4 as tubulin polymerisation inhibitors with apoptosis inducing activity and potent anticancer effect. J. Enzym. Inhib. Med. Chem. 2021, 36, 802–818. [Google Scholar] [CrossRef]
- Ibrahim, T.S.; Hawwas, M.M.; Malebari, A.M.; Taher, E.S.; Omar, A.M.; O’Boyle, N.M.; McLoughlin, E.; Abdel-Samii, Z.K.; Elshaier, Y.A.M.M. Potent Quinoline-Containing Combretastatin A-4 Analogues: Design, Synthesis, Antiproliferative, and Anti-Tubulin Activity. Pharmaceuticals 2020, 13, 393. [Google Scholar] [CrossRef]
- Mohammed, S.J.; Salih, A.K.; Rashid, M.A.M.; Omer, K.M.; Abdalkarim, K.A. Synthesis, Spectroscopic Studies and Keto-Enol Tautomerism of Novel 1,3,4-Thiadiazole Derivative Containing 3-Mercaptobutan-2-one and Quinazolin-4-one Moieties. Molecules 2020, 25, 5441. [Google Scholar] [CrossRef]
- Helali, A.Y.H.; Sarg, M.T.M.; Koraa, M.M.S.; El-Zoghbi, M.S.F. Utility of 2-Methyl-quinazolin-4(3H)-one in the Synthesis of Heterocyclic Compounds with Anticancer Activity. Open J. Med. Chem. 2014, 04, 12–37. [Google Scholar] [CrossRef] [Green Version]
- Assadieskandar, A.; Amini, M.; Salehi, M.; Sadeghian, H.; Alimardani, M.; Sakhteman, A.; Nadri, H.; Shafiee, A. Synthesis and SAR study of 4, 5-diaryl-1H-imidazole-2 (3H)-thione derivatives, as potent 15-lipoxygenase inhibitors. Bioorg. Med. Chem. 2012, 20, 7160–7166. [Google Scholar] [CrossRef] [PubMed]
- Shaker, M.; Davoodnia, A.; Vahedi, H.; Lari, J.; Roshani, M.; Mallaeke, H. Synthesis of Some New 1, 3, 4, 5-tetrasubstituted-1H-imidazole-2 (3H)-thiones Via a Facile One-Pot Three-Component Reaction in the Presence of Solvent and Heteropolyacids. J. Heterocycl. Chem. 2017, 54, 313–317. [Google Scholar] [CrossRef]
- Florian, S.; Mitchison, T.J. Anti-Microtubule Drugs. Mitotic Spindl. 2016, 1413, 403–421. [Google Scholar] [CrossRef]
- Agut, R.; Falomir, E.; Murga, J.; Martín-Beltrán, C.; Gil-Edo, R.; Pla, A.; Carda, M.; Marco, J.A. Synthesis of Combretastatin A-4 and 3′-Aminocombretastatin A-4 derivatives with Aminoacid Containing Pendants and Study of their Interaction with Tubulin and as Downregulators of the VEGF, hTERT and c-Myc Gene Expression. Molecules 2020, 25, 660. [Google Scholar] [CrossRef] [Green Version]
- Pérez-Pérez, M.J.; Priego, E.M.; Bueno, O.; Martins, M.S.; Canela, M.D.; Liekens, S. Blocking blood flow to solid tumors by destabilizing tubulin: An approach to targeting tumor growth. J. Med. Chem. 2016, 59, 8685–8711. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cui, Y.-J.; Ma, C.; Zhang, C.-M.; Tang, L.-Q.; Liu, Z.-P. The discovery of novel indazole derivatives as tubulin colchicine site binding agents that displayed potent antitumor activity both in vitro and in vivo. Eur. J. Med. Chem. 2019, 187, 111968. [Google Scholar] [CrossRef]
- Tangutur, A.D.; Kumar, D.; Krishna, K.V.; Kantevari, S. Microtubule Targeting Agents as Cancer Chemotherapeutics: An Overview of Molecular Hybrids as Stabilizing and Destabilizing Agents. Curr. Top. Med. Chem. 2017, 17, 2523–2537. [Google Scholar] [CrossRef] [PubMed]
- Kalhor, M.; Salehifar, M.; Nikokar, I. Synthesis, characterization, and antibacterial activities of some novel N,N′-disubstituted thiourea, 2-amino thiazole, and imidazole-2-thione derivatives. Med. Chem. Res. 2013, 23, 2947–2954. [Google Scholar] [CrossRef]
- Al-Muamin, T.; Lami, N.A.; Rahman, S.; Ali, R. Synthesis, Characterization and Antimicrobial Activity of New Nucleoside Analogues from Benzotriazole. Chem. Chem. Technol. 2016, 10, 271–278. [Google Scholar] [CrossRef]
- Abu Almaaty, A.H.; Toson, E.E.; El-Sayed, E.S.H.; Tantawy, M.A.; Fayad, E.; Abu Ali, O.A.; Zaki, I. 5-Aryl-1-Arylideneamino-1H-Imidazole-2 (3H)-Thiones: Synthesis and In Vitro Anticancer Evaluation. Molecules 2021, 26, 1706. [Google Scholar] [CrossRef]
- Kapoor, S.; Srivastava, S.; Panda, D. Indibulin dampens microtubule dynamics and produces synergistic antiproliferative effect with vinblastine in MCF-7 cells: Implications in cancer chemotherapy. Sci. Rep. 2018, 8, 12363. [Google Scholar] [CrossRef]
- Lai, M.-J.; Ojha, R.; Lin, M.-H.; Liu, Y.-M.; Lee, H.-Y.; Lin, T.E.; Hsu, K.-C.; Chang, C.-Y.; Chen, M.-C.; Nepali, K.; et al. 1-Arylsulfonyl indoline-benzamides as a new antitubulin agents, with inhibition of histone deacetylase. Eur. J. Med. Chem. 2018, 162, 612–630. [Google Scholar] [CrossRef]
- Du, T.; Lin, S.; Ji, M.; Xue, N.; Liu, Y.; Zhang, Z.; Zhang, K.; Zhang, J.; Zhang, Y.; Wang, Q.; et al. A novel orally active microtubule destabilizing agent S-40 targets the colchicine-binding site and shows potent antitumor activity. Cancer Lett. 2020, 495, 22–32. [Google Scholar] [CrossRef]


| Compound No. | R | Antiproliferative Activities IC50 µM a | |||
|---|---|---|---|---|---|
| MCF-7 b | HL-60 c | HCT-116 d | HUVEC e | ||
| BI1 | H | 6.4 ± 0.18 | 5.23 ± 0.36 | 17.5 ± 1.13 | 63 ± 3.02 |
| BI2 | 4-CH3 | 12.5 ± 0.36 | 37.1 ± 2.54 | 14.8 ± 0.95 | 12.2 ± 0.58 |
| BI3 | 4-CH2CH3 | 10.6 ± 0.31 | 3.91 ± 0.27 | 2.75 ± 0.18 | 135 ± 6.49 |
| BI4 | 4-OCH3 | 2.29 ± 0.07 | 22.1 ± 1.52 | 1.48 ± 0.0.03 | 117 ± 5.62 |
| BI5 | 4-OH | 4.45 ± 0.13 | 1.18 ± 0.08 | 2.15 ± 0.14 | 4.74 ± 0.23 |
| BI6 | 4-Cl | 7.29 ± 0.21 | 3.28 ± 0.22 | 1.51 ± 0.12 | 27.4 ± 1.31 |
| BI7 | 2-Cl | 15.5 ± 0.44 | 3.42 ± 0.23 | 7.38 ± 0.48 | 17.33 ± 0.35 |
| BI8 | 3-Cl | 2.66 ± 0.08 | 5.12 ± 0.35 | 2.1 ± 0.14 | 39.8 ± 1.91 |
| BI9 | 2,4-Cl | 3.57 ± 0.16 | 0.4 ± 0.03 | 2.63 ± 0.17 | 118.9 ± 5.91 |
| BI10 | 2-F | 21.5 ± 0.61 | 8.41 ± 0.58 | 7.02 ± 0.45 | 10.8 ± 0.52 |
| BI11 | 4-Br | 38.2 ± 1.09 | 11.5 ± 0.11 | 17.4 ± 1.12 | 47.8 ± 2.29 |
| BI12 | 4-SO2NH3 | 6.1 ± 0.75 | 0.9 ± 0.06 | 7.83 ± 0.5 | 25.1 ± 1.2 |
| CA-4 | 0.58 ± 0.02 | 0.77 ± 0.05 | 0.24 ± 0.02 | 13.6 ± 0.65 | |
| Compound | Anti-Tubulin Activity a IC50 ± SD (µM) | Colchicine Binding b % ± SD | |
|---|---|---|---|
| 1 µM Drug | 5 µM Drug | ||
| BI3 | 0.928 ± 0.04 | 65.38 ± 2 | 86.85 ± 2 |
| BI5 | 0.497 ± 0.03 | 61.78 ± 2 | 81.34 ± 2 |
| BI6 | 0.928 ±0.03 | 63.23 ± 2 | 77.63 ± 2 |
| BI7 | 0.840 ± 0.02 | 61.18 ± 2 | 79.95 ± 2 |
| BI8 | 3.057 ± 9.69 | 59.74± 2 | 82.13 ± 2 |
| BI9 | 0.520 ±0.02 | 49.38 ± 2 | 66.57 ± 2 |
| BI12 | 0.784 ± 0.02 | 64.67 ± 2 | 80.26 ± 2 |
| CA-4 | 0.579 ± 0.01 | 64.23 ± 0.9 | 85.63 ± 2 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khayyat, A.N.; Mohamed, K.O.; Malebari, A.M.; El-Malah, A. Design, Synthesis, and Antipoliferative Activities of Novel Substituted Imidazole-Thione Linked Benzotriazole Derivatives. Molecules 2021, 26, 5983. https://doi.org/10.3390/molecules26195983
Khayyat AN, Mohamed KO, Malebari AM, El-Malah A. Design, Synthesis, and Antipoliferative Activities of Novel Substituted Imidazole-Thione Linked Benzotriazole Derivatives. Molecules. 2021; 26(19):5983. https://doi.org/10.3390/molecules26195983
Chicago/Turabian StyleKhayyat, Ahdab N., Khaled O. Mohamed, Azizah M. Malebari, and Afaf El-Malah. 2021. "Design, Synthesis, and Antipoliferative Activities of Novel Substituted Imidazole-Thione Linked Benzotriazole Derivatives" Molecules 26, no. 19: 5983. https://doi.org/10.3390/molecules26195983






