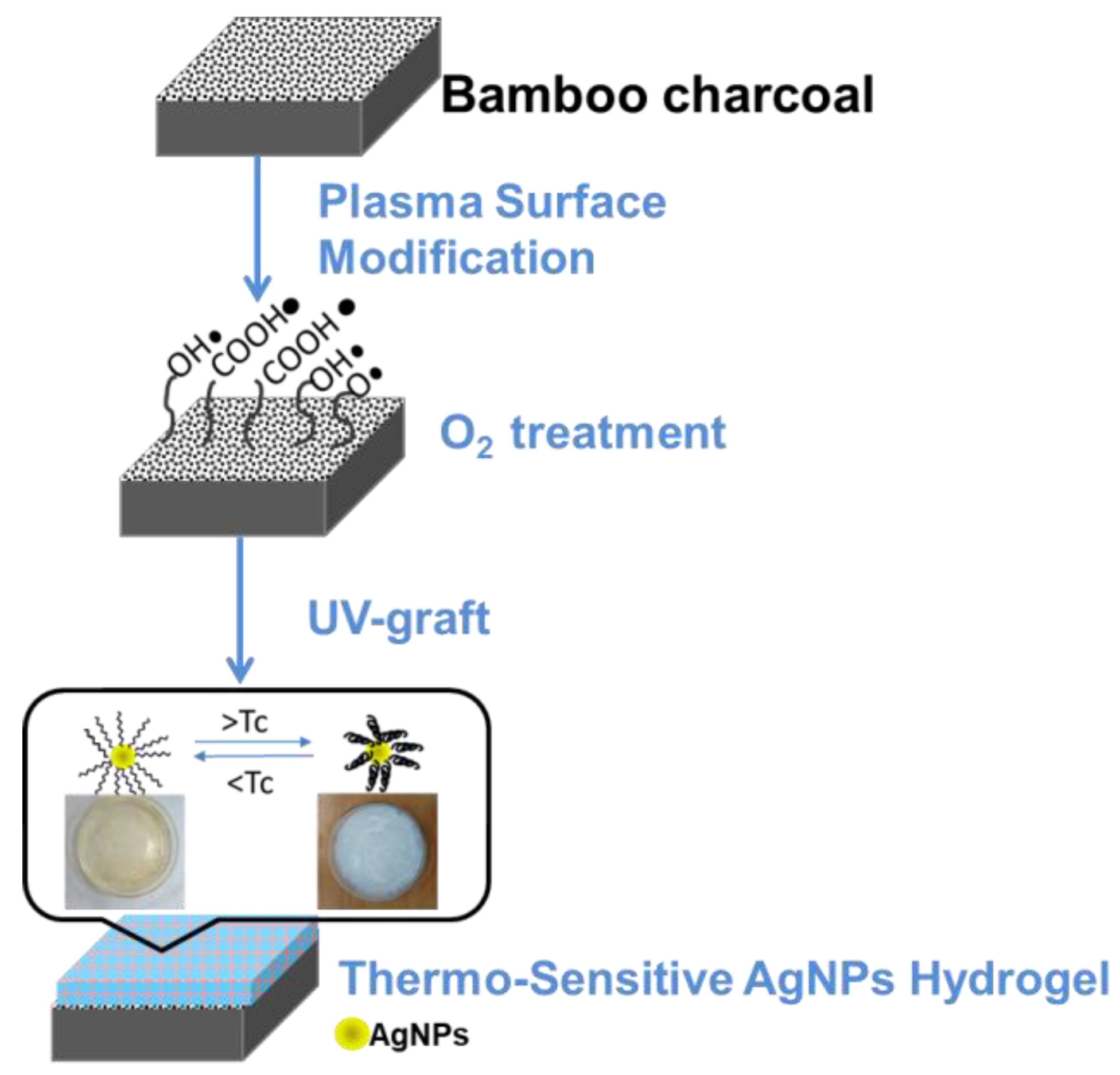
Surface Modification of Bamboo Charcoal by O2 Plasma Treatment and UV-Grafted Thermo-Sensitive AgNPs Hydrogel to Improve Antibacterial Properties in Biomedical Application
Abstract
:1. Introduction
2. Materials and Methods
2.1. Pretreatment of Materials
2.2. Synthesis of Silver Nanoparticles (AgNPs)
2.3. O2 Plasma Activation Pre-Treatment
2.4. Thermo-Sensitive AgNPs Hydrogel by UV Light Surface Graft Polymerization
2.5. Characterization Analysis
2.5.1. UV-VIS Spectra
2.5.2. Wettability (Surface Hydrophobicity/Hydrophilicity) Test
2.5.3. Surface Characterization
2.5.4. Swelling Studies of the Treatment BC
2.6. Cytotoxicity Test of Treatment BC
2.7. Antibacterial Efficacy Test
3. Results and Discussion
3.1. UV-Visible Spectroscopy Characterization of the AgNPs and Thermo-Sensitive AgNPs Hydrogels
3.2. XRD Characterization of Thermo-Sensitive AgNPs Hydrogels
3.3. Wettability of Surface-Modified Bamboo Charcoal
3.4. Swelling Ratio of Surface-Modified Bamboo Charcoal
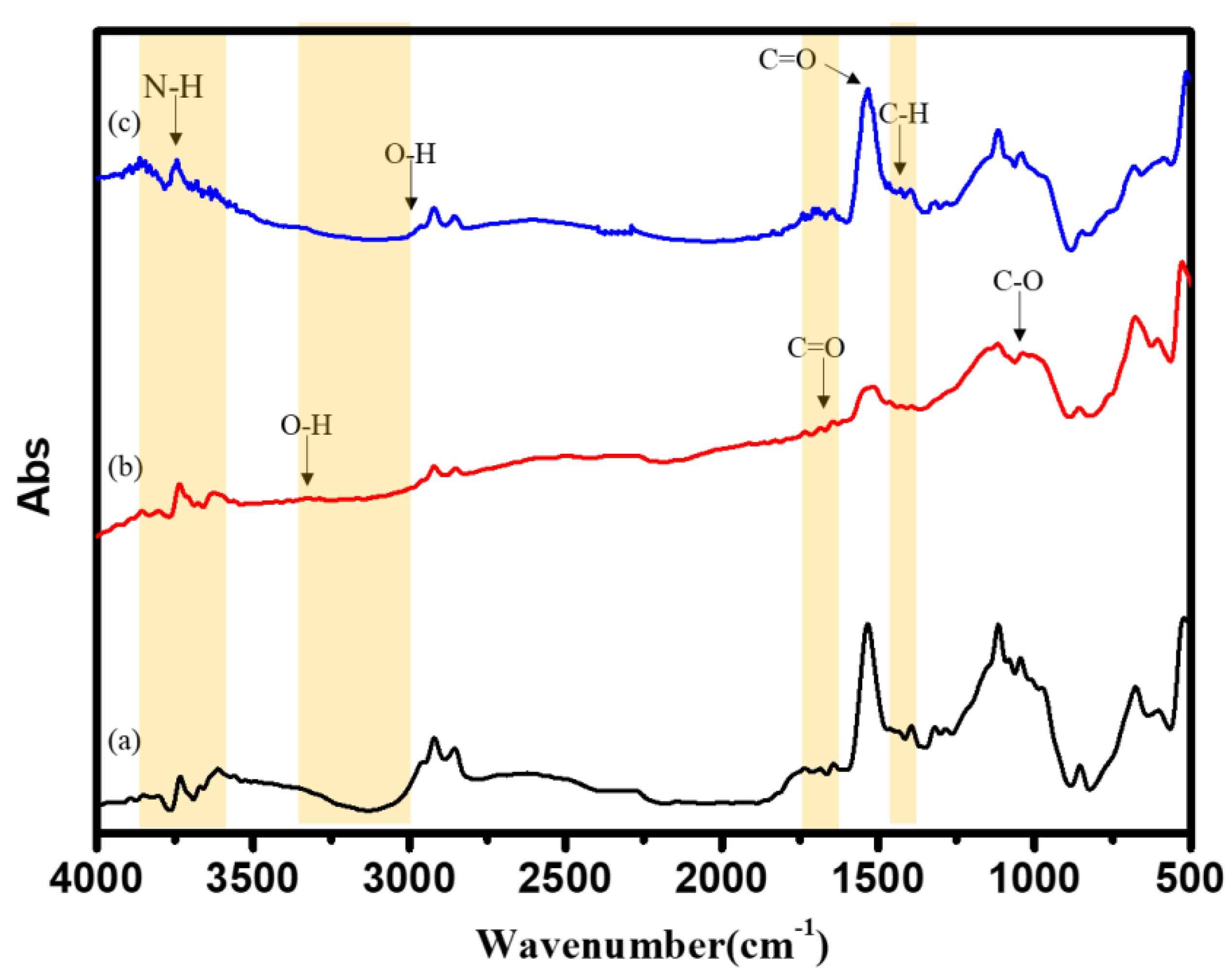
3.5. FTIR Characterization of Surface-Modified Bamboo Charcoal
3.6. Chemical Composition Analysis of Surface-Modified Bamboo Charcoal
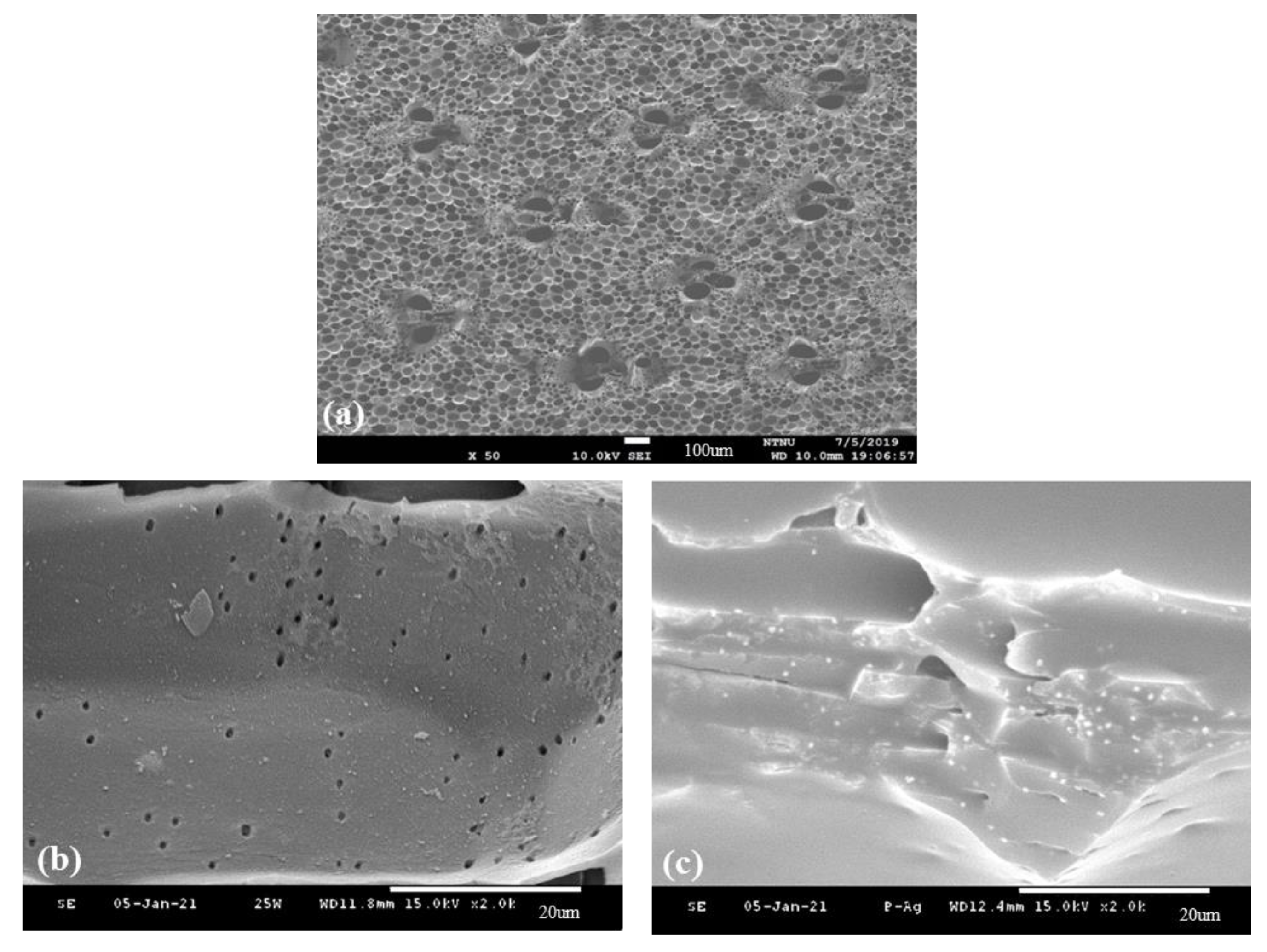
3.7. Surface Morphology of Surface-Modified Bamboo Charcoal
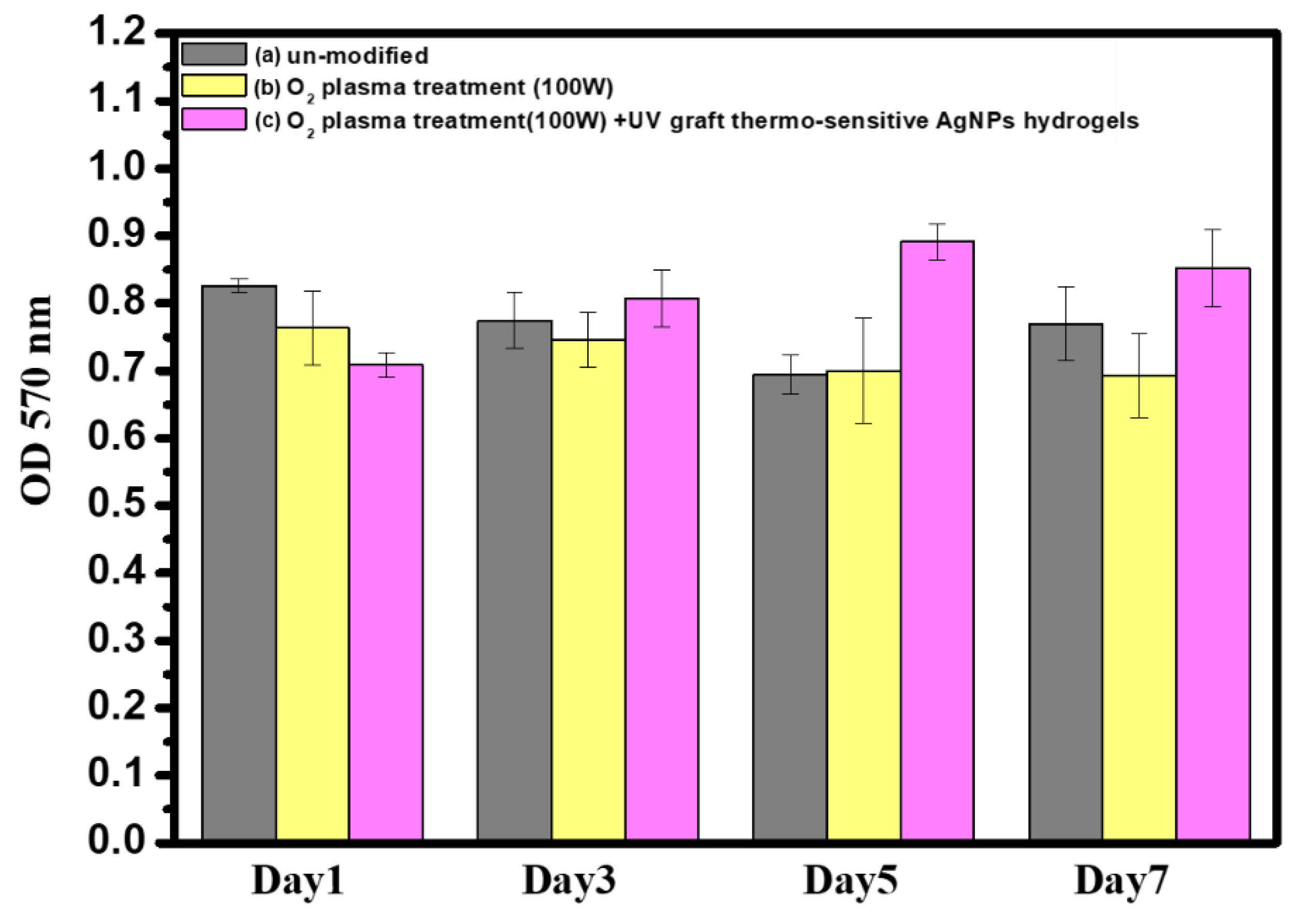
3.8. In Vitro Cytocompatibility Assay of Surface-Modified Bamboo Charcoal
3.9. Antibacterial Characteristics of Surface-Modified Bamboo Charcoal
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Isa, S.; Ramli, M.; Hambali, N.; Kasjoo, S.; Isa, M.; Nor, N.; Khalid, N.; Ahmad, N. Adsorption Properties and Potential Applications of Bamboo Charcoal: A Review. In MATEC Web of Conferences; EDP Sciences: Les Ulis, France, 2016; Volume 78, p. 01097. [Google Scholar]
- Ye, Y.; Zhang, Z. Research Progress of the Properties and Application of Bamboo Charcoal. Appl. Mech. Mater. 2013, 395, 646–649. [Google Scholar] [CrossRef]
- Yang, F.; Wu, K.; Lin, W.; Hu, M.; Sheu, T.; Horng, D. Preparation and Antibacterial Effect of Bamboo Charcoal/Ag on Staphylococcus Aureusand Pseudomonas Aeruginosa. J. Chin. Chem. Soc. 2009, 56, 327–334. [Google Scholar] [CrossRef]
- Wang, R.; Amano, Y.; Machida, M. Surface properties and water vapor adsorption–desorption characteristics of bamboo-based activated carbon. J. Anal. Appl. Pyrolysis 2013, 104, 667–674. [Google Scholar] [CrossRef]
- Yang, F.; Wu, K.; Liu, M.; Lin, W.; Hu, M. Evaluation of the antibacterial efficacy of bamboo charcoal/silver biological protective material. Mater. Chem. Phys. 2009, 113, 474–479. [Google Scholar] [CrossRef]
- Li, G.; Li, N.; Wu, S. Preparation of Bamboo Charcoal/Polyvinyl Formal Foam as Biological Support Material. Adv. Mater. Res. 2012, 466, 106–110. [Google Scholar] [CrossRef]
- Lin, C.; Lin, J. Evaluation of the Function and Manufacturing Technique of Bamboo Charcoal Complex Yarns and Knitted Fabrics. J. Eng. Fibers Fabr. 2012, 7, 155892501200700. [Google Scholar] [CrossRef] [Green Version]
- Wang, S.; Zhang, L.; Semple, K.; Zhang, M.; Zhang, W.; Dai, C. Development of Biodegradable Flame-Retardant Bamboo Charcoal Composites, Part I: Thermal and Elemental Analyses. Polymers 2020, 12, 2217. [Google Scholar] [CrossRef]
- Hsieh, M.; Wen, H.; Shyu, C.; Chen, S.; Li, W.; Wang, W.; Chen, W. Synthesis, in vitro macrophage response and detoxification of bamboo charcoal beads for purifying blood. J. Biomed. Mater. Res. Part A 2010, 94, 1133–1140. [Google Scholar]
- Nitayaphat, W.; Jiratumnukul, N.; Charuchinda, S.; Kittinaovarat, S. Mechanical properties of chitosan/bamboo charcoal composite films made with normal and surface oxidized charcoal. Carbohydr. Polym. 2009, 78, 444–448. [Google Scholar] [CrossRef]
- Lin, C.; Ni, M.; Chang, Y.; Yeh, H.; Lin, F. A cell sorter with modified bamboo charcoal for the efficient selection of specific antibody-producing hybridomas. Biomaterials 2010, 31, 8445–8453. [Google Scholar] [CrossRef] [PubMed]
- Iravani, S. Green synthesis of metal nanoparticles using plants. Green Chem. 2011, 13, 2638. [Google Scholar] [CrossRef]
- Khan, I.; Saeed, K.; Khan, I. Nanoparticles: Properties, applications and toxicities. Arab. J. Chem. 2019, 12, 908–931. [Google Scholar] [CrossRef]
- Jeevanandam, J.; Barhoum, A.; Chan, Y.; Dufresne, A.; Danquah, M. Review on nanoparticles and nanostructured materials: History, sources, toxicity and regulations. Beilstein J. Nanotechnol. 2018, 9, 1050–1074. [Google Scholar] [CrossRef] [Green Version]
- Kim, J.; Kuk, E.; Yu, K.; Kim, J.; Park, S.; Lee, H.; Kim, S.H.; Park, Y.K.; Park, Y.H.; Hwang, C.Y.; et al. Antimicrobial effects of silver nanoparticles. Nanomed. Nanotechnol. Biol. Med. 2007, 3, 95–101. [Google Scholar] [CrossRef] [PubMed]
- Franci, G.; Falanga, A.; Galdiero, S.; Palomba, L.; Rai, M.; Morelli, G.; Galdiero, M. Silver Nanoparticles as Potential Antibacterial Agents. Molecules 2015, 20, 8856–8874. [Google Scholar] [CrossRef] [Green Version]
- Thakkar, K.; Mhatre, S.; Parikh, R. Biological synthesis of metallic nanoparticles. Nanomed. Nanotechnol. Biol. Med. 2010, 6, 257–262. [Google Scholar] [CrossRef]
- Rai, M.; Yadav, A.; Gade, A. Silver nanoparticles as a new generation of antimicrobials. Biotechnol. Adv. 2009, 27, 76–83. [Google Scholar] [CrossRef]
- Singh, A.; Gaud, B.; Jaybhaye, S. Optimization of Synthesis Parameters of Silver Nanoparticles and Its Antimicrobial Activity. Mater. Sci. Energy Technol. 2019, 3, 232–236. [Google Scholar] [CrossRef]
- Panáček, A.; Kvítek, L.; Smékalová, M.; Večeřová, R.; Kolář, M.; Röderová, M.; Dyčka, F.; Šebela, M.; Prucek, R.; Tomanec, O.; et al. Bacterial resistance to silver nanoparticles and how to overcome it. Nat. Nanotechnol. 2017, 13, 65–71. [Google Scholar] [CrossRef]
- Sood, R.; Chopra, D. Optimization of reaction conditions to fabricate Ocimum sanctum synthesized silver nanoparticles and its application to nano-gel systems for burn wounds. Mater. Sci. Eng. C 2018, 92, 575–589. [Google Scholar] [CrossRef]
- Guzman, M.; Dille, J.; Godet, S. Synthesis and antibacterial activity of silver nanoparticles against gram-positive and gram-negative bacteria. Nanomed. Nanotechnol. Biol. Med. 2012, 8, 37–45. [Google Scholar] [CrossRef]
- Siddiqui, A.; Shakoor, A.; Zohra, R.; Ahmad, M.; Zohra, R. Green Synthesis of Antibacterial Silver Nanoparticles against Drug-Resistant Bacteria. Anti-Infect. Agents 2021, 19. [Google Scholar] [CrossRef]
- Chang, B.; Pan, L.; Lin, H.; Chang, H. Nanodiamond-supported silver nanoparticles as potent and safe antibacterial agents. Sci. Rep. 2019, 9, 13164. [Google Scholar] [CrossRef] [Green Version]
- Miyan, M.N.; Durg, V. Study of Silver Complexes as Potential Antibacterial and Antifungal Agents. Strad Res. 2020, 7, 710–716. [Google Scholar]
- Hyeon, T. Designed chemical synthesis and assembly of uniform-sized nanoparticles for medical applications. Nanomed. Nanotechnol. Biol. Med. 2018, 14, 1800–1801. [Google Scholar] [CrossRef]
- Sun, S. Monodisperse magnetic nanoparticles for biomedical applications. Nanomed. Nanotechnol. Biol. Med. 2006, 2, 288. [Google Scholar] [CrossRef]
- Jain, J.; Arora, S.; Rajwade, J.; Omray, P.; Khandelwal, S.; Paknikar, K. Silver Nanoparticles in Therapeutics: Development of an Antimicrobial Gel Formulation for Topical Use. Mol. Pharm. 2009, 6, 1388–1401. [Google Scholar] [CrossRef] [PubMed]
- Arshadi, E. Green synthesis and characterization of silver nanoparticles using fructose. Asian J. Green Chem. 2017, 3, 41–50. [Google Scholar] [CrossRef]
- Zhong, Z.; Luo, S.; Yang, K.; Wu, X.; Ren, T. High-performance anionic waterborne polyurethane/Ag nanocomposites with excellent antibacterial property via in situ synthesis of Ag nanoparticles. RSC Adv. 2017, 7, 42296–42304. [Google Scholar] [CrossRef] [Green Version]
- Xu, L.; Wang, Y.; Huang, J.; Chen, C.; Wang, Z.; Xie, H. Silver nanoparticles: Synthesis, medical applications and biosafety. Theranostics 2020, 10, 8996–9031. [Google Scholar] [CrossRef] [PubMed]
- El Badawy, A.; Silva, R.; Morris, B.; Scheckel, K.; Suidan, M.; Tolaymat, T. Surface Charge-Dependent Toxicity of Silver Nanoparticles. Environ. Sci. Technol. 2011, 45, 283–287. [Google Scholar] [CrossRef]
- Raza, M.; Kanwal, Z.; Rauf, A.; Sabri, A.; Riaz, S.; Naseem, S. Size- and Shape-Dependent Antibacterial Studies of Silver Nanoparticles Synthesized by Wet Chemical Routes. Nanomaterials 2016, 6, 74. [Google Scholar] [CrossRef] [Green Version]
- Ganguly, S.; Das, P.; Das, T.; Ghosh, S.; Das, S.; Bose, M.; Mondal, M.; Das, A.; Das, N. Acoustic cavitation assisted destratified clay tactoid reinforced in situ elastomer mimetic semi-IPN hydrogel for catalytic and bactericidal application. Ultrason. Sonochem. 2020, 60, 104797. [Google Scholar] [CrossRef] [PubMed]
- Griessner, H.; Hodgkin, J. Biopolymer surface modification using plasma grafting. Biomaterials 1988, 9, 292. [Google Scholar] [CrossRef]
- Steckelmacher, W. Film deposition by plasma techniques. Vacuum 1993, 44, 1069. [Google Scholar] [CrossRef]
- Inagaki, N. Plasma Surface Modification and Plasma Polymerization; Technomic Pub. Co.: Lancaster, UK, 1996. [Google Scholar]
- Liao, S.; Chen, K.; Chen, W.; Chou, C.; Wai, K. Surface Graft Polymerization of Acrylamide onto Plasma Activated Nylon Microfiber Artificial Leather for Improving Dyeing Properties. Int. J. Chem. Eng. Appl. 2013, 4, 78–81. [Google Scholar] [CrossRef] [Green Version]
- Wolf, R.; Sparavigna, A. Role of Plasma Surface Treatments on Wetting and Adhesion. Engineering 2010, 2, 397–402. [Google Scholar] [CrossRef] [Green Version]
- Chaiwong, C.; Rachtanapun, P.; Wongchaiya, P.; Auras, R.; Boonyawan, D. Effect of plasma treatment on hydrophobicity and barrier property of polylactic acid. Surf. Coat. Technol. 2010, 204, 2933–2939. [Google Scholar] [CrossRef]
- Luque-Agudo, V.; Hierro-Oliva, M.; Gallardo-Moreno, A.; González-Martín, M. Effect of plasma treatment on the surface properties of polylactic acid films. Polym. Test. 2021, 96, 107097. [Google Scholar] [CrossRef]
- Liao, S.C.; Chen, K.S.; Chien, J.L.; Chen, S.C.; Lin, W.L. Acetic-Acid Plasma-Polymerization on Polymeric Substrates for Biomedical Application. Nanomaterials 2019, 9, 941. [Google Scholar] [CrossRef] [Green Version]
- Razavi, A. Plasma Surface Modification of Polymeric Materials. In MRS Proceedings; 2005; Volume 890. [Google Scholar]
- Tabares, F.; Junkar, I. Cold Plasma Systems and Their Application in Surface Treatments for Medicine. Molecules 2021, 26, 1903. [Google Scholar] [CrossRef]
- Vesel, A.; Primc, G.; Zaplotnik, R.; Mozetič, M. Applications of highly non-equilibrium low-pressure oxygen plasma for treatment of polymers and polymer composites on an industrial scale. Plasma Phys. Control. Fusion 2020, 62, 024008. [Google Scholar] [CrossRef]
- Kariž, M.; Tomec, D.; Dahle, S.; Kuzman, M.; Šernek, M.; Žigon, J. Effect of Sanding and Plasma Treatment of 3D-Printed Parts on Bonding to Wood with PVAc Adhesive. Polymers 2021, 13, 1211. [Google Scholar] [CrossRef] [PubMed]
- Pankaj, S.; Bueno-Ferrer, C.; Misra, N.; Milosavljević, V.; O’Donnell, C.; Bourke, P.; Keener, K.; Cullen, P. Applications of cold plasma technology in food packaging. Trends Food Sci. Technol. 2014, 35, 5–17. [Google Scholar] [CrossRef]
- Shishoo, R. Plasma Treatment—Industrial Applications and Its Impact on the C&L Industry. J. Coat. Fabr. 1996, 26, 26–35. [Google Scholar]
- Tiede, R.; Hirschberg, J.; Daeschlein, G.; von Woedtke, T.; Vioel, W.; Emmert, S. Plasma Applications: A Dermatological View. Contrib. Plasma Phys. 2014, 54, 118–130. [Google Scholar] [CrossRef]
- Virtanen, J.; Tenhu, H. Thermal Properties of poly(N-isopropylacrylamide)-g-poly (ethylene oxide) in Aqueous Solutions: Influence of the Number and Distribution of the Grafts. Macromolecules 2000, 33, 5970–5975. [Google Scholar] [CrossRef]
- Naddaf, A.; Bart, H. Diffusion Coefficients in Thermosensitive Poly (NIPAAm) Hydrogels. Macromol. Symp. 2011, 306, 150–165. [Google Scholar] [CrossRef]
- Chou, C.; Chen, K.; Lin, W.; Ye, Y.; Liao, S. Plasma Polymerization of SnOxCy Organic-Like Films and Grafted PNIPAAm Composite Hydrogel with Nanogold Particles for Promotion of Thermal Resistive Properties. Micromachines 2016, 8, 5. [Google Scholar] [CrossRef] [Green Version]
- Frazar, E.; Shah, R.; Dziubla, T.; Hilt, J. Multifunctional temperature-responsive polymers as advanced biomaterials and beyond. J. Appl. Polym. Sci. 2020, 137, 48770. [Google Scholar] [CrossRef] [Green Version]
- Haq, M.; Su, Y.; Wang, D. Mechanical properties of PNIPAM based hydrogels: A review. Mater. Sci. Eng. C 2017, 70, 842–855. [Google Scholar] [CrossRef]
- Kim, S.; Park, S.; Kim, S. Synthesis and characteristics of interpenetrating polymer network hydrogels composed of poly (vinyl alcohol) and poly(N-isopropylacrylamide). React. Funct. Polym. 2003, 55, 61–67. [Google Scholar] [CrossRef]
- Chen, K.; Chang, S.; Feng, C.; Lin, W.; Liao, S. Plasma Deposition and UV Light Induced Surface Grafting Polymerization of NIPAAm on Stainless Steel for Enhancing Corrosion Resistance and Its Drug Delivery Property. Polymers 2018, 10, 1009. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ankareddi, I.; Brazel, C. Development of a thermosensitive grafted drug delivery system-synthesis and characterization of NIPAAm-based grafts and hydrogel structure. J. Appl. Polym. Sci. 2010, 120, 1597–1606. [Google Scholar] [CrossRef]
- Suradi, S.; Naemuddin, N.; Hashim, S.; Adrus, N. Impact of carboxylation and hydrolysis functionalisations on the anti-oil staining behaviour of textiles grafted with poly(N-isopropylacrylamide) hydrogel. RSC Adv. 2018, 8, 13423–13432. [Google Scholar] [CrossRef] [Green Version]
- Conzatti, G.; Cavalie, S.; Combes, C.; Torrisani, J.; Carrere, N.; Tourrette, A. PNIPAM grafted surfaces through ATRP and RAFT polymerization: Chemistry and bioadhesion. Colloids Surf. B Biointerfaces 2017, 151, 143–155. [Google Scholar] [CrossRef] [Green Version]
- Li, Z.; Guan, J. Thermosensitive hydrogels for drug delivery. Expert Opin. Drug Deliv. 2011, 8, 991–1007. [Google Scholar] [CrossRef]
- Chen, K.; Chou, C.; Hsu, S.; Lin, H. Surface Grafting Polymerization and Crosslinking of Thermosensitive NIPAAm Hydrogels onto Plasma Pretreated Substrates and Drug Delivery Properties. Mater. Sci. Forum 2003, 426, 3267–3272. [Google Scholar] [CrossRef]
- Lanzalaco, S.; Armelin, E. Poly(N-isopropylacrylamide) and Copolymers: A Review on Recent Progresses in Biomedical Applications. Gels 2017, 3, 36. [Google Scholar] [CrossRef]
- Ohya, S.; Nakayama, Y.; Matsuda, T. Thermoresponsive Artificial Extracellular Matrix for Tissue Engineering: Hyaluronic Acid Bioconjugated with poly(N-isopropylacrylamide) Grafts. Biomacromolecules 2001, 2, 856–863. [Google Scholar] [CrossRef]
- Yang, L.; Fan, X.; Zhang, J.; Ju, J. Preparation and Characterization of Thermoresponsive poly(N-Isopropylacrylamide) for Cell Culture Applications. Polymers 2020, 12, 389. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, Y.; Tang, X.; Chen, B.; Qiu, G. Low temperature plasma vapor treatment of thermo-sensitive poly(N-isopropylacrylamide) and its application. Appl. Surf. Sci. 2013, 268, 332–336. [Google Scholar] [CrossRef]
- Cui, Y.; Xing, Z.; Yan, J.; Lu, Y.; Xiong, X.; Zheng, L. Thermosensitive Behavior and Super-Antibacterial Properties of Cotton Fabrics Modified with a Sercin-NIPAAm-AgNPs Interpenetrating Polymer Network Hydrogel. Polymers 2018, 10, 818. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Byth, H.; Mchunu, B.; Dubery, I.; Bornman, L. Assessment of a simple, non-toxic alamar blue cell survival assay to monitor tomato cell viability. Phytochem. Anal. 2001, 12, 340–346. [Google Scholar] [CrossRef] [PubMed]
- Liao, S.; Chang, C.; Chen, C.; Lee, C.; Lin, W. Functionalization of pure titanium MAO coatings by surface modifications for biomedical applications. Surf. Coat. Technol. 2020, 394, 125812. [Google Scholar] [CrossRef]
- Paladini, F.; Di Franco, C.; Panico, A.; Scamarcio, G.; Sannino, A.; Pollini, M. In Vitro Assessment of the Antibacterial Potential of Silver Nano-Coatings on Cotton Gauzes for Prevention of Wound Infections. Materials 2016, 9, 411. [Google Scholar] [CrossRef] [Green Version]
- Pollini, M.; Russo, M.; Licciulli, A.; Sannino, A.; Maffezzoli, A. Characterization of antibacterial silver coated yarns. J. Mater. Sci. Mater. Med. 2009, 20, 2361–2366. [Google Scholar] [CrossRef]
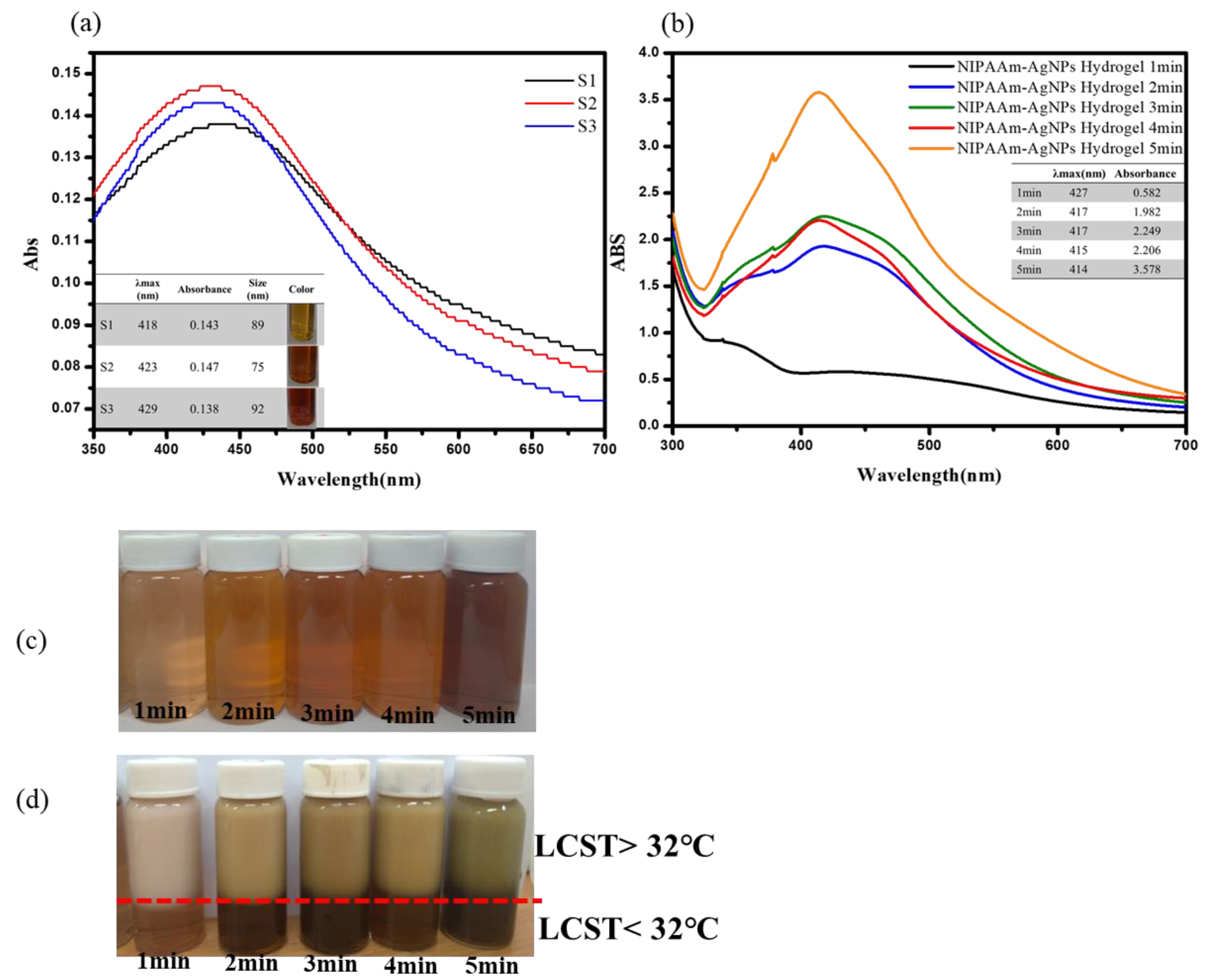



| Type | AgNO3 (mM) | Na3C6H5O7 (mM) | PVP (μM) |
|---|---|---|---|
| S1 | 10 | 10 | 75 |
| S2 | 10 | 50 | 75 |
| S3 | 10 | 100 | 75 |
| Untreated | Treatment A | Treatment B | Treatment C | Treatment D | |
|---|---|---|---|---|---|
| θH2O | 63.5 ± 7.8° | <0° | <0° | 32.1 ± 1.1° | 34 ± 1.5° |
| Temperature (°C) | ||||
| 25 | 37 | 42 | ||
| RO water | Untreated | 103.2 | 103.9 | 106.9 |
| A | 91.5 | 69.8 | 72.6 | |
| B | 134.8 | 51.2 | 54.8 | |
| Temperature (°C) | ||||
| 25 | 37 | 42 | ||
| SBF solution | Untreated | 39.6 | 33.7 | 33.0 |
| A | 64.7 | 59.8 | 54.3 | |
| B | 87.8 | 71.1 | 67.8 | |
| Test Organism | Diameter Zone (mm), Mean (n = 3) | |
|---|---|---|
| Un-Modified | O2 Plasma Treatment (100 W) +UV Graft Thermo-Sensitive AgNPs Hydrogels | |
| E. coli | 0 | 15.7 ± 0.2 (mm) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, S.-J.; Liao, S.-C. Surface Modification of Bamboo Charcoal by O2 Plasma Treatment and UV-Grafted Thermo-Sensitive AgNPs Hydrogel to Improve Antibacterial Properties in Biomedical Application. Nanomaterials 2021, 11, 2697. https://doi.org/10.3390/nano11102697
Liu S-J, Liao S-C. Surface Modification of Bamboo Charcoal by O2 Plasma Treatment and UV-Grafted Thermo-Sensitive AgNPs Hydrogel to Improve Antibacterial Properties in Biomedical Application. Nanomaterials. 2021; 11(10):2697. https://doi.org/10.3390/nano11102697
Chicago/Turabian StyleLiu, Shih-Ju, and Shu-Chuan Liao. 2021. "Surface Modification of Bamboo Charcoal by O2 Plasma Treatment and UV-Grafted Thermo-Sensitive AgNPs Hydrogel to Improve Antibacterial Properties in Biomedical Application" Nanomaterials 11, no. 10: 2697. https://doi.org/10.3390/nano11102697





