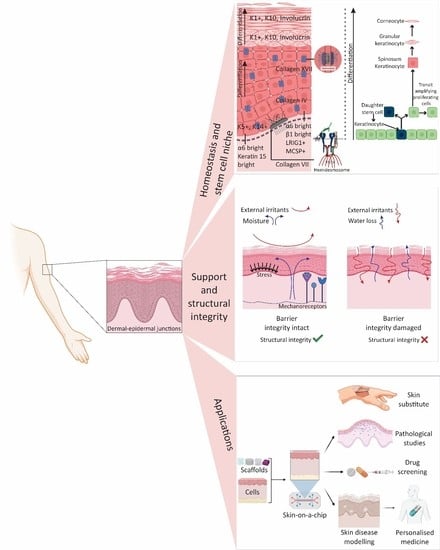
The Importance of Mimicking Dermal-Epidermal Junction for Skin Tissue Engineering: A Review
Abstract
1. Introduction
2. Ultrastructure
2.1. Epidermis
2.2. Dermal-Epidermal Junctions (DEJ)
2.3. Dermis
3. Proteins
Proteins and Their Roles in DEJ
4. Stem Cells
Stem Cells in Epidermis and DEJ
5. Conventional Techniques to Preserve the DEJ in Clinical Grafts
6. Tissue Engineering Strategies: From Basic Concepts to Developing a DEJ
6.1. Photolithography
6.2. Laser Structuring
6.3. Electrospinning
6.3.1. Electrospinning on Templates Designed by Stereolithography
6.3.2. Laser Structuring of Electrospun Mats
6.4. Additive Manufacturing and Bioprinting
7. Conclusions and Future Perspective
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Groeber, F.; Holeiter, M.; Hampel, M.; Hinderer, S.; Schenke-Layland, K. Skin tissue engineering—In vivo and in vitro applications. Adv. Drug Deliv. Rev. 2011, 63, 352–366. [Google Scholar] [CrossRef] [PubMed]
- Shevchenko, R.V.; James, S.L.; James, S.E. A review of tissue-engineered skin bioconstructs available for skin reconstruction. J. R. Soc. Interface 2010, 7, 229–258. [Google Scholar] [CrossRef] [PubMed]
- Zaidi, Z.; Lanigan, S.W. Skin: Structure and function. In Dermatology in Clinical Practice; Springer: London, UK, 2010; pp. 1–15. [Google Scholar]
- Montagna, W. The Structure and Function of Skin; Elsevier: London, UK, 2012; ISBN 0323138691. [Google Scholar]
- Vig, K.; Chaudhari, A.; Tripathi, S.; Dixit, S.; Sahu, R.; Pillai, S.; Dennis, V.A.; Singh, S.R. Advances in skin regeneration using tissue engineering. Int. J. Mol. Sci. 2017, 18, 789. [Google Scholar] [CrossRef] [PubMed]
- Langton, A.K.; Graham, H.K.; Griffiths, C.E.M.; Watson, R.E.B. Ageing significantly impacts the biomechanical function and structural composition of skin. Exp. Dermatol. 2019, 28, 981–984. [Google Scholar] [CrossRef]
- Blanpain, C. Skin regeneration and repair. Nature 2010, 464, 686–687. [Google Scholar] [CrossRef]
- Takeo, M.; Lee, W.; Ito, M. Wound healing and skin regeneration. Cold Spring Harb. Perspect. Med. 2015, 5, a023267. [Google Scholar] [CrossRef]
- Pereira, R.F.; Sousa, A.; Barrias, C.C.; Bayat, A.; Granja, P.L.; Bártolo, P.J. Advances in bioprinted cell-laden hydrogels for skin tissue engineering. Biomanufacturing Rev. 2017, 2, 1. [Google Scholar] [CrossRef]
- Kim, B.S.; Gao, G.; Kim, J.Y.; Cho, D. 3D cell printing of perfusable vascularized human skin equivalent composed of epidermis, dermis, and hypodermis for better structural recapitulation of native skin. Adv. Healthc. Mater. 2019, 8, 1801019. [Google Scholar] [CrossRef]
- Wong, R.; Geyer, S.; Weninger, W.; Guimberteau, J.; Wong, J.K. The dynamic anatomy and patterning of skin. Exp. Dermatol. 2016, 25, 92–98. [Google Scholar] [CrossRef]
- Kolarsick, P.A.J.; Kolarsick, M.A.; Goodwin, C. Anatomy and physiology of the skin. J. Dermatol. Nurses Assoc. 2011, 3, 203–213. [Google Scholar] [CrossRef]
- Clement, A.L.; Moutinho, T.J., Jr.; Pins, G.D. Micropatterned dermal–epidermal regeneration matrices create functional niches that enhance epidermal morphogenesis. Acta Biomater. 2013, 9, 9474–9484. [Google Scholar] [CrossRef]
- Asencio, I.O.; Mittar, S.; Sherborne, C.; Raza, A.; Claeyssens, F.; MacNeil, S. A methodology for the production of microfabricated electrospun membranes for the creation of new skin regeneration models. J. Tissue Eng. 2018, 9, 2041731418799851. [Google Scholar] [CrossRef]
- Blackstone, B.N.; Malara, M.M.; Baumann, M.E.; McFarland, K.L.; Supp, D.M.; Powell, H.M. Fractional CO2 laser micropatterning of cell-seeded electrospun collagen scaffolds enables rete ridge formation in 3D engineered skin. Acta Biomater. 2020, 102, 287–297. [Google Scholar] [CrossRef]
- Wei, J.C.J.; Edwards, G.A.; Martin, D.J.; Huang, H.; Crichton, M.L.; Kendall, M.A.F. Allometric scaling of skin thickness, elasticity, viscoelasticity to mass for micro-medical device translation: From mice, rats, rabbits, pigs to humans. Sci. Rep. 2017, 7, 1–16. [Google Scholar] [CrossRef]
- Langton, A.K.; Halai, P.; Griffiths, C.E.M.; Sherratt, M.J.; Watson, R.E.B. The impact of intrinsic ageing on the protein composition of the dermal-epidermal junction. Mech. Ageing Dev. 2016, 156, 14–16. [Google Scholar] [CrossRef]
- Bruckner-Tuderman, L.; Has, C. Disorders of the cutaneous basement membrane zone—The paradigm of epidermolysis bullosa. Matrix Biol. 2014, 33, 29–34. [Google Scholar] [CrossRef]
- Woodley, D.T.; Peterson, H.D.; Herzog, S.R.; Stricklin, G.P.; Burgeson, R.E.; Briggaman, R.A.; Cronce, D.J.; O’Keefe, E.J. Burn wounds resurfaced by cultured epidermal autografts show abnormal reconstitution of anchoring fibrils. JAMA 1988, 259, 2566–2571. [Google Scholar] [CrossRef]
- Giangreco, A.; Goldie, S.J.; Failla, V.; Saintigny, G.; Watt, F.M. Human skin aging is associated with reduced expression of the stem cell markers β1 integrin and MCSP. J. Investig. Dermatol. 2010, 130, 604–608. [Google Scholar] [CrossRef]
- Murphy, M.; Kerr, P.; Grant-Kels, J.M. The histopathologic spectrum of psoriasis. Clin. Dermatol. 2007, 25, 524–528. [Google Scholar] [CrossRef]
- Cash, S.H.; Dever, T.T.; Hyde, P.; Lee, J.B. Epidermolysis bullosa nevus: An exception to the clinical and dermoscopic criteria for melanoma. Arch. Dermatol. 2007, 143, 1164–1167. [Google Scholar] [CrossRef][Green Version]
- Hamdoon, Z.; Jerjes, W.; Rashed, D.; Kawas, S.; abdul Sattar, A.; Samsudin, R.; Hopper, C. In vivo optical coherence tomography-guided photodynamic therapy for skin pre-cancer and cancer. Photodiagnosis Photodyn. Ther. 2021, 36, 102520. [Google Scholar] [CrossRef] [PubMed]
- Jones, P.H.; Harper, S.; Watt, F.M. Stem cell patterning and fate in human epidermis. Cell 1995, 80, 83–93. [Google Scholar] [CrossRef]
- Topczewska, J.M.; Ledwon, J.K.; Vaca, E.E.; Gosain, A.K. Mechanical stretching stimulates growth of the basal layer and rete ridges in the epidermis. J. Tissue Eng. Regen. Med. 2019, 13, 2121–2125. [Google Scholar] [CrossRef] [PubMed]
- El Genedy-Kalyoncu, M.; Richter, C.; Surber, C.; Blume-Peytavi, U.; Kottner, J. The effect of a basic skin care product on the structural strength of the dermo-epidermal junction: An exploratory, randomised, controlled split-body trial. Int. Wound J. 2021, 1–10. [Google Scholar] [CrossRef]
- Watt, F.M. Keratinocyte integrins. Keratinocyte Handb. 1994, 83, 957–968. [Google Scholar]
- Levy, L.; Broad, S.; Diekmann, D.; Evans, R.D.; Watt, F.M. β1 integrins regulate keratinocyte adhesion and differentiation by distinct mechanisms. Mol. Biol. Cell 2000, 11, 453–466. [Google Scholar] [CrossRef]
- Quan, C.; Cho, M.K.; Shao, Y.; Mianecki, L.E.; Liao, E.; Perry, D.; Quan, T. Dermal fibroblast expression of stromal cell-derived factor-1 (SDF-1) promotes epidermal keratinocyte proliferation in normal and diseased skin. Protein Cell 2015, 6, 890–903. [Google Scholar] [CrossRef]
- Aumailley, M.; Niemann, C. Epidermal stem cells and dermal–epidermal junction. Cutan. Photoaging 2019, 19, 167. [Google Scholar]
- Bush, K.A.; Pins, G.D. Development of microfabricated dermal epidermal regenerative matrices to evaluate the role of cellular microenvironments on epidermal morphogenesis. Tissue Eng. Part A 2012, 18, 2343–2353. [Google Scholar] [CrossRef]
- Black, A.F.; Berthod, F.; L’heureux, N.; Germain, L.; Auger, F.A. In vitro reconstruction of a human capillary-like network in a tissue-engineered skin equivalent. FASEB J. 1998, 12, 1331–1340. [Google Scholar] [CrossRef]
- Malara, M.M.; Blackstone, B.N.; Baumann, M.E.; Bailey, J.K.; Supp, D.M.; Powell, H.M. Cultured epithelial autograft combined with micropatterned dermal template forms rete ridges in vivo. Tissue Eng. 2020, 6, 21–22. [Google Scholar] [CrossRef]
- Höfer, T.; Gerner, I.; Gundert-Remy, U.; Liebsch, M.; Schulte, A.; Spielmann, H.; Vogel, R.; Wettig, K. Animal testing and alternative approaches for the human health risk assessment under the proposed new European chemicals regulation. Arch. Toxicol. 2004, 78, 549–564. [Google Scholar] [CrossRef]
- Doke, S.K.; Dhawale, S.C. Alternatives to animal testing: A review. Saudi. Pharm. J. 2015, 23, 223–229. [Google Scholar] [CrossRef]
- Ng, W.L.; Wang, S.; Yeong, W.Y.; Naing, M.W. Skin bioprinting: Impending reality or fantasy? Trends Biotechnol. 2016, 34, 689–699. [Google Scholar] [CrossRef]
- Kamel, R.A.; Ong, J.F.; Eriksson, E.; Junker, J.P.E.; Caterson, E.J. Tissue engineering of skin. J. Am. Coll. Surg. 2013, 217, 533–555. [Google Scholar] [CrossRef]
- Yudovsky, D.; Pilon, L. Rapid and accurate estimation of blood saturation, melanin content, and epidermis thickness from spectral diffuse reflectance. Appl. Opt. 2010, 49, 1707–1719. [Google Scholar] [CrossRef]
- Senoo, M. Epidermal stem cells in homeostasis and wound repair of the skin. Adv. Wound Care 2013, 2, 273–282. [Google Scholar] [CrossRef]
- Niemann, C.; Watt, F.M. Designer skin: Lineage commitment in postnatal epidermis. Trends Cell Biol. 2002, 12, 185–192. [Google Scholar] [CrossRef]
- Findlay, M.W.; Gurtner, G.C. Engineering niches for skin and wound healing. In Biology and Engineering of Stem Cell Niches; Elsevier: London, UK, 2017; pp. 559–579. [Google Scholar]
- Fenner, J.; Clark, R.A. Anatomy, physiology, histology, and immunohistochemistry of human skin. Skin Tissue Eng. Regen. Med. 2016, 1. [Google Scholar]
- Xiong, X.; Wu, T.; He, S. Physical forces make rete ridges in oral mucosa. Med. Hypotheses 2013, 81, 883–886. [Google Scholar] [CrossRef]
- Simpson, C.L.; Patel, D.M.; Green, K.J. Deconstructing the skin: Cytoarchitectural determinants of epidermal morphogenesis. Nat. Rev. Mol. Cell Biol. 2011, 12, 565–580. [Google Scholar] [CrossRef] [PubMed]
- Lavker, R.M.; Sun, T.-T. Heterogeneity in epidermal basal keratinocytes: Morphological and functional correlations. Science 1982, 215, 1239–1241. [Google Scholar] [CrossRef] [PubMed]
- Watt, F.M. Epidermal stem cells: Markers, patterning and the control of stem cell fate. Philos. Trans. R. Soc. London Ser. B Biol. Sci. 1998, 353, 831–837. [Google Scholar] [CrossRef] [PubMed]
- Ha, M.; Lim, S.; Cho, S.; Lee, Y.; Na, S.; Baig, C.; Ko, H. Skin-inspired hierarchical polymer architectures with gradient stiffness for spacer-free, ultrathin, and highly sensitive triboelectric sensors. ACS Nano 2018, 12, 3964–3974. [Google Scholar] [CrossRef]
- Aumailley, M. Laminins and interaction partners in the architecture of the basement membrane at the dermal-epidermal junction. Exp. Dermatol. 2020, 30, 17–24. [Google Scholar] [CrossRef]
- Burgeson, R.E.; Christiano, A.M. The dermal—Epidermal junction. Curr. Opin. Cell Biol. 1997, 9, 651–658. [Google Scholar] [CrossRef]
- Aumailley, M.; Has, C.; Tunggal, L.; Bruckner-Tuderman, L. Molecular basis of inherited skin-blistering disorders, and therapeutic implications. Expert Rev. Mol. Med. 2006, 8, 1–21. [Google Scholar] [CrossRef]
- Okajima, M. Development of dermal ridges in the fetus. J. Med. Genet. 1975, 12, 243–250. [Google Scholar] [CrossRef]
- Babler, W. Embryologic development of epidermal ridges and their configurations. Birth Defects Orig. Artic. Ser. 1991, 27, 95–112. [Google Scholar]
- Pellacani, G.; Cesinaro, A.M.; Longo, C.; Grana, C.; Seidenari, S. Microscopic in vivo description of cellular architecture of dermoscopic pigment network in nevi and melanomas. Arch. Dermatol. 2005, 141, 147–154. [Google Scholar] [CrossRef][Green Version]
- Hirobe, T.; Enami, H.; Nakayama, A. The human melanocyte and melanoblast populations per unit area of epidermis in the rete ridge are greater than in the inter-rete ridge. Int. J. Cosmet. Sci. 2020, 43, 211–217. [Google Scholar] [CrossRef]
- Solanas, G.; Benitah, S.A. Regenerating the skin: A task for the heterogeneous stem cell pool and surrounding niche. Nat. Rev. Mol. Cell Biol. 2013, 14, 737–748. [Google Scholar] [CrossRef]
- Korosec, A.; Frech, S.; Gesslbauer, B.; Vierhapper, M.; Radtke, C.; Petzelbauer, P.; Lichtenberger, B.M. Lineage identity and location within the dermis determine the function of papillary and reticular fibroblasts in human skin. J. Investig. Dermatol. 2019, 139, 342–351. [Google Scholar] [CrossRef]
- Wertheim, K.; Maceo, A. The critical stage of friction ridge and pattern formation. J. Forensic Identif. 2002, 52, 35–85. [Google Scholar]
- Wolf, K.; Alexander, S.; Schacht, V.; Coussens, L.M.; von Andrian, U.H.; van Rheenen, J.; Deryugina, E.; Friedl, P. Collagen-based cell migration models in vitro and in vivo. Semin. Cell Dev. Biol. 2009, 20, 931–941. [Google Scholar] [CrossRef]
- Driskell, R.R.; Watt, F.M. Understanding fibroblast heterogeneity in the skin. Trends Cell Biol. 2015, 25, 92–99. [Google Scholar] [CrossRef]
- Mayet, N.; Choonara, Y.E.; Kumar, P.; Tomar, L.K.; Tyagi, C.; Du Toit, L.C.; Pillay, V. A comprehensive review of advanced biopolymeric wound healing systems. J. Pharm. Sci. 2014, 103, 2211–2230. [Google Scholar] [CrossRef]
- Margadant, C.; Charafeddine, R.A.; Sonnenberg, A. Unique and redundant functions of integrins in the epidermis. FASEB J. 2010, 24, 4133–4152. [Google Scholar] [CrossRef]
- Goletz, S.; Zillikens, D.; Schmidt, E. Structural proteins of the dermal-epidermal junction targeted by autoantibodies in pemphigoid diseases. Exp. Dermatol. 2017, 26, 1154–1162. [Google Scholar] [CrossRef]
- Breitkreutz, D.; Koxholt, I.; Thiemann, K.; Nischt, R. Skin basement membrane: The foundation of epidermal integrity—BM functions and diverse roles of bridging molecules nidogen and perlecan. Biomed. Res. Int. 2013, 2013, 179784. [Google Scholar] [CrossRef]
- Ko, M.S.; Marinkovich, M.P. Role of dermal-epidermal basement membrane zone in skin, cancer, and developmental disorders. Dermatol. Clin. 2010, 28, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Lane, S.W.; Williams, D.A.; Watt, F.M. Modulating the stem cell niche for tissue regeneration. Nat. Biotechnol. 2014, 32, 795–803. [Google Scholar] [CrossRef] [PubMed]
- Guvendiren, M.; Burdick, J.A. The control of stem cell morphology and differentiation by hydrogel surface wrinkles. Biomaterials 2010, 31, 6511–6518. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.-H.; Lipke, E.A.; Kim, P.; Cheong, R.; Thompson, S.; Delannoy, M.; Suh, K.-Y.; Tung, L.; Levchenko, A. Nanoscale cues regulate the structure and function of macroscopic cardiac tissue constructs. Proc. Natl. Acad. Sci. USA 2010, 107, 565–570. [Google Scholar] [CrossRef]
- Yim, E.K.F.; Darling, E.M.; Kulangara, K.; Guilak, F.; Leong, K.W. Nanotopography-induced changes in focal adhesions, cytoskeletal organization, and mechanical properties of human mesenchymal stem cells. Biomaterials 2010, 31, 1299–1306. [Google Scholar] [CrossRef]
- Connelly, J.T.; Gautrot, J.E.; Trappmann, B.; Tan, D.W.-M.; Donati, G.; Huck, W.T.S.; Watt, F.M. Actin and serum response factor transduce physical cues from the microenvironment to regulate epidermal stem cell fate decisions. Nat. Cell Biol. 2010, 12, 711–718. [Google Scholar] [CrossRef]
- Gautrot, J.E.; Wang, C.; Liu, X.; Goldie, S.J.; Trappmann, B.; Huck, W.T.S.; Watt, F.M. Mimicking normal tissue architecture and perturbation in cancer with engineered micro-epidermis. Biomaterials 2012, 33, 5221–5229. [Google Scholar] [CrossRef]
- Jaks, V.; Barker, N.; Kasper, M.; Van Es, J.H.; Snippert, H.J.; Clevers, H.; Toftgård, R. Lgr5 marks cycling, yet long-lived, hair follicle stem cells. Nat. Genet. 2008, 40, 1291. [Google Scholar] [CrossRef]
- Hsu, Y.-C.; Li, L.; Fuchs, E. Emerging interactions between skin stem cells and their niches. Nat. Med. 2014, 20, 847–856. [Google Scholar] [CrossRef]
- Goldstein, J.; Horsley, V. Home sweet home: Skin stem cell niches. Cell. Mol. Life Sci. 2012, 69, 2573–2582. [Google Scholar] [CrossRef]
- Niemann, C. Controlling the stem cell niche: Right time, right place, right strength. Bioessays 2006, 28, 1–5. [Google Scholar] [CrossRef]
- Mascré, G.; Dekoninck, S.; Drogat, B.; Youssef, K.K.; Brohée, S.; Sotiropoulou, P.A.; Simons, B.D.; Blanpain, C. Distinct contribution of stem and progenitor cells to epidermal maintenance. Nature 2012, 489, 257–262. [Google Scholar] [CrossRef]
- Cheung, T.H.; Rando, T.A. Molecular regulation of stem cell quiescence. Nat. Rev. Mol. Cell Biol. 2013, 14, 329–340. [Google Scholar] [CrossRef]
- Li, A.; Simmons, P.J.; Kaur, P. Identification and isolation of candidate human keratinocyte stem cells based on cell surface phenotype. Proc. Natl. Acad. Sci. USA 1998, 95, 3902–3907. [Google Scholar] [CrossRef]
- Schlüter, H.; Paquet-Fifield, S.; Gangatirkar, P.; Li, J.; Kaur, P. Functional characterization of quiescent keratinocyte stem cells and their progeny reveals a hierarchical organization in human skin epidermis. Stem Cells 2011, 29, 1256–1268. [Google Scholar] [CrossRef]
- Ovadia, J.; Nie, Q. Stem cell niche structure as an inherent cause of undulating epithelial morphologies. Biophys. J. 2013, 104, 237–246. [Google Scholar] [CrossRef]
- Mackenzie, I.C. Retroviral transduction of murine epidermal stem cells demonstrates clonal units of epidermal structure. J. Invest. Dermatol. 1997, 109, 377–383. [Google Scholar] [CrossRef]
- Liu, S.; Zhang, H.; Duan, E. Epidermal development in mammals: Key regulators, signals from beneath, and stem cells. Int. J. Mol. Sci. 2013, 14, 10869–10895. [Google Scholar] [CrossRef]
- Lechler, T.; Fuchs, E. Asymmetric cell divisions promote stratification and differentiation of mammalian skin. Nature 2005, 437, 275–280. [Google Scholar] [CrossRef]
- Doupé, D.P.; Klein, A.M.; Simons, B.D.; Jones, P.H. The ordered architecture of murine ear epidermis is maintained by progenitor cells with random fate. Dev. Cell 2010, 18, 317–323. [Google Scholar] [CrossRef]
- Fuchs, E. Skin stem cells: Rising to the surface. J. Cell Biol. 2008, 180, 273–284. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Upadhyay, A.K.; Bullock, A.J.; Dicolandrea, T.; Xu, J.; Binder, R.L.; Robinson, M.K.; Finlay, D.R.; Mills, K.J.; Bascom, C.C. Skin stem cell hypotheses and long term clone survival–explored using agent-based modelling. Sci. Rep. 2013, 3, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Farage, M.A.; Miller, K.W.; Maibach, H.I. Textbook of Aging Skin; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2009; ISBN 3540896554. [Google Scholar]
- Watanabe, M.; Natsuga, K.; Nishie, W.; Kobayashi, Y.; Donati, G.; Suzuki, S.; Fujimura, Y.; Tsukiyama, T.; Ujiie, H.; Shinkuma, S. Type XVII collagen coordinates proliferation in the interfollicular epidermis. eLife 2017, 6, e26635. [Google Scholar] [CrossRef] [PubMed]
- Liu, N.; Matsumura, H.; Kato, T.; Ichinose, S.; Takada, A.; Namiki, T.; Asakawa, K.; Morinaga, H.; Mohri, Y.; De Arcangelis, A. Stem cell competition orchestrates skin homeostasis and ageing. Nature 2019, 568, 344–350. [Google Scholar] [CrossRef]
- Stine, R.R.; Matunis, E.L. Stem cell competition: Finding balance in the niche. Trends Cell Biol. 2013, 23, 357–364. [Google Scholar] [CrossRef]
- Merino, M.M.; Levayer, R.; Moreno, E. Survival of the fittest: Essential roles of cell competition in development, aging, and cancer. Trends Cell Biol. 2016, 26, 776–788. [Google Scholar] [CrossRef]
- Matsumura, H.; Mohri, Y.; Binh, N.T.; Morinaga, H.; Fukuda, M.; Ito, M.; Kurata, S.; Hoeijmakers, J.; Nishimura, E.K. Hair follicle aging is driven by transepidermal elimination of stem cells via COL17A1 proteolysis. Science 2016, 351, ad4395:1–10. [Google Scholar] [CrossRef]
- Victorelli, S.; Lagnado, A.; Halim, J.; Moore, W.; Talbot, D.; Barrett, K.; Chapman, J.; Birch, J.; Ogrodnik, M.; Meves, A. Senescent human melanocytes drive skin ageing via paracrine telomere dysfunction. EMBO J. 2019, 38, e101982. [Google Scholar] [CrossRef]
- Shimizu, R.; Kishi, K. Skin graft. Plast. Surg. Int. 2012, 2012, 1–5. [Google Scholar] [CrossRef]
- Iida, T.; Takami, Y.; Yamaguchi, R.; Shimazaki, S.; Harii, K. Development of a tissue-engineered human oral mucosa equivalent based on an acellular allogeneic dermal matrix: A preliminary report of clinical application to burn wounds. Scand. J. Plast. Reconstr. Surg. Hand Surg. 2005, 39, 138–146. [Google Scholar] [CrossRef]
- Yim, H.; Cho, Y.S.; Seo, C.H.; Lee, B.C.; Ko, J.H.; Kim, D.; Hur, J.; Chun, W.; Kim, J.H. The use of AlloDerm on major burn patients: AlloDerm prevents post-burn joint contracture. Burns 2010, 36, 322–328. [Google Scholar] [CrossRef]
- Bannasch, H.; Stark, G.B.; Knam, F.; Horch, R.E.; Föhn, M. Decellularized dermis in combination with cultivated keratinocytes in a short-and long-term animal experimental investigation. J. Eur. Acad. Dermatol. Venereol. 2008, 22, 41–49. [Google Scholar] [CrossRef]
- Estrach, S.; Legg, J.; Watt, F.M. Syntenin mediates Delta1-induced cohesiveness of epidermal stem cells in culture. J. Cell Sci. 2007, 120, 2944–2952. [Google Scholar] [CrossRef]
- Osborne, S.N.; Schmidt, M.A.; Derrick, K.; Harper, J.R. Epidermal micrografts produced via an automated and minimally invasive tool form at the dermal/epidermal junction and contain proliferative cells that secrete wound healing growth factors. Adv. Skin Wound Care 2015, 28, 397. [Google Scholar] [CrossRef]
- Zare, P.; Aleemardani, M.; Seifalian, A.; Bagher, Z.; Seifalian, A.M. Graphene oxide: Opportunities and challenges in biomedicine. Nanomaterials 2021, 11, 1083. [Google Scholar] [CrossRef]
- Sun, T.; Jackson, S.; Haycock, J.W.; MacNeil, S. Culture of skin cells in 3D rather than 2D improves their ability to survive exposure to cytotoxic agents. J. Biotechnol. 2006, 122, 372–381. [Google Scholar] [CrossRef]
- Gangatirkar, P.; Paquet-Fifield, S.; Li, A.; Rossi, R.; Kaur, P. Establishment of 3D organotypic cultures using human neonatal epidermal cells. Nat. Protoc. 2007, 2, 178. [Google Scholar] [CrossRef]
- Gura, T. Systems for identifying new drugs are often faulty. Science 1997, 278, 1041–1042. [Google Scholar] [CrossRef]
- Hansen, K.; Khanna, C. Spontaneous and genetically engineered animal models: Use in preclinical cancer drug development. Eur. J. Cancer 2004, 40, 858–880. [Google Scholar] [CrossRef]
- Zhao, X.; Lang, Q.; Yildirimer, L.; Lin, Z.Y.; Cui, W.; Annabi, N.; Ng, K.W.; Dokmeci, M.R.; Ghaemmaghami, A.M.; Khademhosseini, A. Photocrosslinkable gelatin hydrogel for epidermal tissue engineering. Adv. Healthc. Mater. 2016, 5, 108–118. [Google Scholar] [CrossRef]
- McCullough, J.L.; Kelly, K.M. Prevention and treatment of skin aging. In Aging Interventions and Therapies; World Scientific: Singapore, 2005; pp. 29–50. [Google Scholar]
- MacNeil, S. Progress and opportunities for tissue-engineered skin. Nature 2007, 445, 874–880. [Google Scholar] [CrossRef] [PubMed]
- Aleemardani, M.; Bagher, Z.; Farhadi, M.; Chahsetareh, H.; Najafi, R.; Eftekhari, B.; Seifalian, A. Can tissue engineering bring hope to the development of human tympanic membrane? Tissue Eng. Part B Rev. 2021. [Google Scholar] [CrossRef] [PubMed]
- Marino, D.; Luginbühl, J.; Scola, S.; Meuli, M.; Reichmann, E. Bioengineering dermo-epidermal skin grafts with blood and lymphatic capillaries. Sci. Transl. Med. 2014, 6, 221ra14–221ra41. [Google Scholar] [CrossRef] [PubMed]
- Meuli, M.; Hartmann-Fritsch, F.; Hüging, M.; Marino, D.; Saglini, M.; Hynes, S.; Neuhaus, K.; Manuel, E.; Middelkoop, E.; Reichmann, E. A cultured autologous dermo-epidermal skin substitute for full-thickness skin defects: A phase i, open, prospective clinical trial in children. Plast. Reconstr. Surg. 2019, 144, 188–198. [Google Scholar] [CrossRef]
- Casale, C.; Imparato, G.; Urciuolo, F.; Netti, P.A. Endogenous human skin equivalent promotes in vitro morphogenesis of follicle-like structures. Biomaterials 2016, 101, 86–95. [Google Scholar] [CrossRef]
- Farokhi, M.; Aleemardani, M.; Solouk, A.; Mirzadeh, H.; Teuschl, A.H.; Redl, H. Crosslinking strategies for silk fibroin hydrogels: Promising biomedical materials. Biomed. Mater. 2021, 16, 22004. [Google Scholar] [CrossRef]
- Viswanathan, P.; Guvendiren, M.; Chua, W.; Telerman, S.B.; Liakath-Ali, K.; Burdick, J.A.; Watt, F.M. Mimicking the topography of the epidermal–dermal interface with elastomer substrates. Integr. Biol. 2016, 8, 21–29. [Google Scholar] [CrossRef]
- Mobasseri, S.A.; Zijl, S.; Salameti, V.; Walko, G.; Stannard, A.; Garcia-Manyes, S.; Watt, F.M. Patterning of human epidermal stem cells on undulating elastomer substrates reflects differences in cell stiffness. Acta Biomater. 2019, 87, 256–264. [Google Scholar] [CrossRef]
- Helling, A.L.; Viswanathan, P.; Cheliotis, K.S.; Mobasseri, S.A.; Yang, Y.; El Haj, A.J.; Watt, F.M. Dynamic culture substrates that mimic the topography of the epidermal–dermal junction. Tissue Eng. Part A. 2019, 25, 214–223. [Google Scholar] [CrossRef]
- Ramos-Rodriguez, D.H.; MacNeil, S.; Claeyssens, F.; Ortega Asencio, I. Fabrication of topographically controlled electrospun scaffolds to mimic the stem cell microenvironment in the dermal-epidermal junction. ACS Biomater. Sci. Eng. 2021, 7, 2803–2813. [Google Scholar] [CrossRef]
- Admane, P.; Gupta, A.C.; Jois, P.; Roy, S.; Lakshmanan, C.C.; Kalsi, G.; Bandyopadhyay, B.; Ghosh, S. Direct 3D bioprinted full-thickness skin constructs recapitulate regulatory signaling pathways and physiology of human skin. Bioprinting 2019, 15, e00051. [Google Scholar] [CrossRef]
- Jiang, T.; Deng, M.; James, R.; Nair, L.S.; Laurencin, C.T. Micro-and nanofabrication of chitosan structures for regenerative engineering. Acta Biomater. 2014, 10, 1632–1645. [Google Scholar] [CrossRef]
- Danilevičius, P.; Rekštytė, S.; Balčiūnas, E.; Kraniauskas, A.; Širmenis, R.; Baltriukienė, D.; Bukelskienė, V.; Gadonas, R.; Sirvydis, V.; Piskarskas, A. Laser 3D micro/nanofabrication of polymers for tissue engineering applications. Opt. Laser Technol. 2013, 45, 518–524. [Google Scholar] [CrossRef]
- Ramos-Rodriguez, D.H.; MacNeil, S.; Claeyssens, F.; Ortega Asencio, I. Delivery of bioactive compounds to improve skin cell responses on microfabricated electrospun microenvironments. Bioengineering 2021, 8, 105. [Google Scholar] [CrossRef]
- Aleemardani, M.; Solouk, A.; Akbari, S.; Dehghan, M.M.; Moeini, M. Silk-derived oxygen-generating electrospun patches for enhancing tissue regeneration: Investigation of calcium peroxide role and its effects in controlled oxygen delivery. Materialia 2020, 100877. [Google Scholar] [CrossRef]
- Newton, V.L.; Bradley, R.S.; Seroul, P.; Cherel, M.; Griffiths, C.E.M.; Rawlings, A.V.; Voegeli, R.; Watson, R.E.B.; Sherratt, M.J. Novel approaches to characterize age-related remodelling of the dermal-epidermal junction in 2D, 3D and in vivo. Skin Res. Technol. 2017, 23, 131–148. [Google Scholar] [CrossRef]
- Girardeau-Hubert, S.; Pageon, H.; Asselineau, D. In vivo and in vitro approaches in understanding the differences between Caucasian and African skin types: Specific involvement of the papillary dermis. Int. J. Dermatol. 2012, 51, 1–4. [Google Scholar]
- Hirsch, T.; Rothoeft, T.; Teig, N.; Bauer, J.W.; Pellegrini, G.; De Rosa, L.; Scaglione, D.; Reichelt, J.; Klausegger, A.; Kneisz, D. Regeneration of the entire human epidermis using transgenic stem cells. Nature 2017, 551, 327–332. [Google Scholar] [CrossRef]
- Del Amor, R.; Morales, S.; Colomer, A.; Mogensen, M.; Jensen, M.; Israelsen, N.M.; Bang, O.; Naranjo, V. Automatic segmentation of epidermis and hair follicles in optical coherence tomography images of normal skin by convolutional neural networks. Front. Med. 2020, 7, 220. [Google Scholar] [CrossRef]
- Oh, B.-H.; Kim, K.H.; Chung, K.-Y. Skin imaging using ultrasound imaging, optical coherence tomography, confocal microscopy, and two-photon microscopy in cutaneous oncology. Front. Med. 2019, 6, 274. [Google Scholar] [CrossRef]
- Kurugol, S.; Kose, K.; Park, B.; Dy, J.G.; Brooks, D.H.; Rajadhyaksha, M. Automated delineation of dermal–epidermal junction in reflectance confocal microscopy image stacks of human skin. J. Investig. Dermatol. 2015, 135, 710–717. [Google Scholar] [CrossRef] [PubMed]
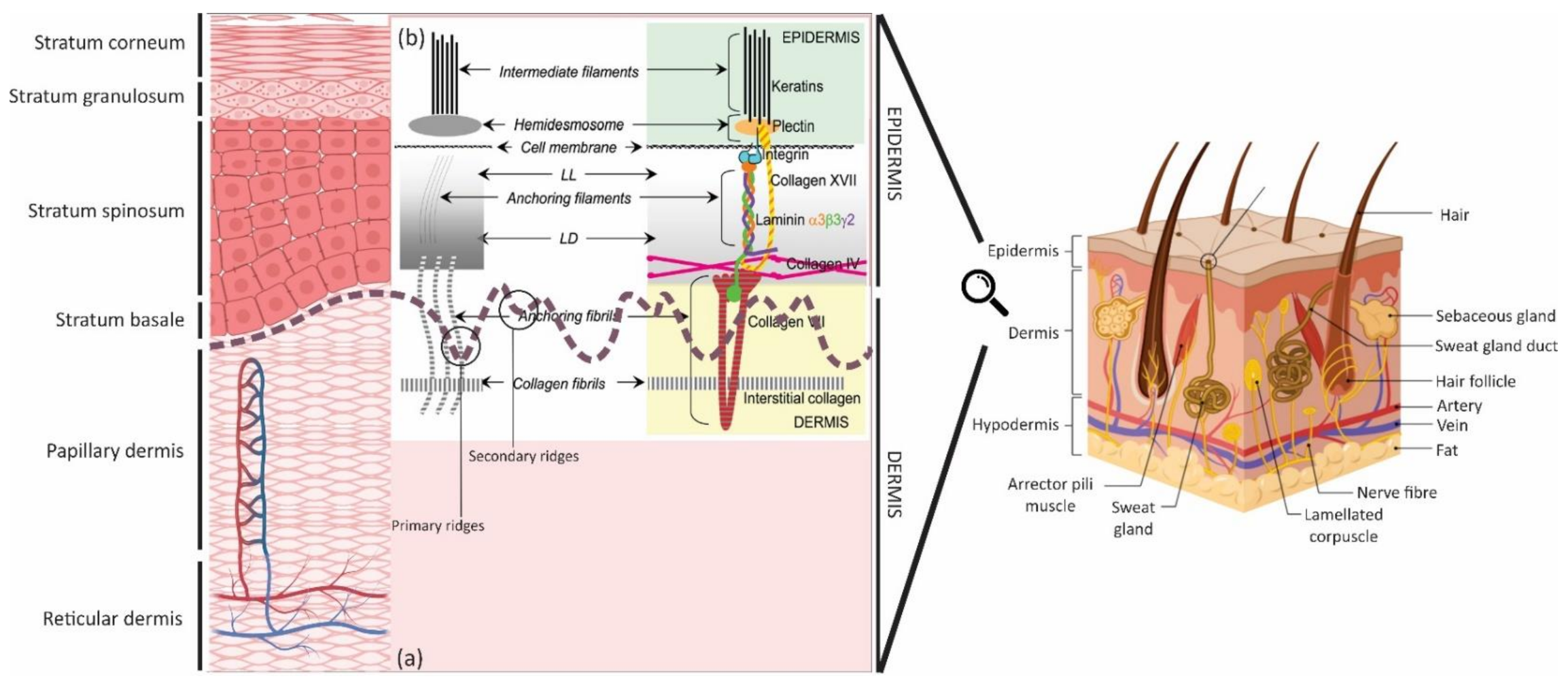
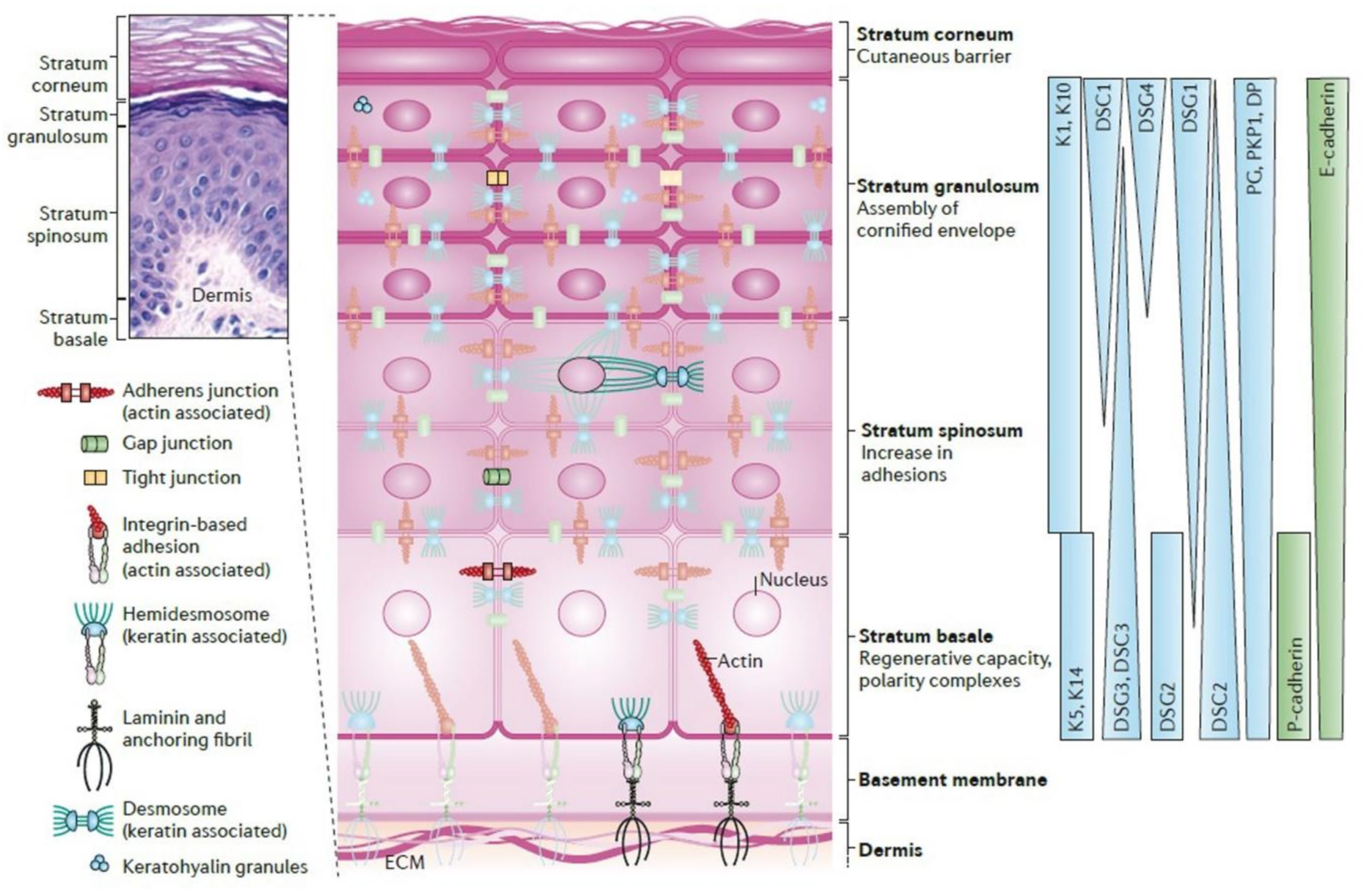



| Name and Type | MW (kDa) | Located at | Main Function | Characteristics | ||
|---|---|---|---|---|---|---|
| IF | Keratins (cytokeratins) | 1 & 10 | 50–100 | Suprabasal layers | • Formation of the backbone of the IF network connecting to HDs • Connecting to desmosomes • Providing an extensive mechanical framework | • Express by basal Ks (Interfollicular epidermis) |
| 5 & 14 | 50 & 58 | Basal layers | • Attaching intracellular cytoskeleton to HD | • Express by basal Ks | ||
| HD | HD components | HD1* | 450–500 | Inner HD plaque |
• Anchoring epidermis steadily to LD • Attaching keratin filaments to the basolateral epidermal surface | • Plectin molecule • Associating with cytoskeleton • Distributed throughout epidermis (stratified squamous epithelium) |
| HD2 | 230 | Plasma membrane of basal Ks | • Identical to BPAG1 | |||
| HD3 | 200 | • Correspond to β4 (subunit of α6β4 integrin) | ||||
| HD4 | 180 | • Identical to BPAG2 | ||||
| HD5 | 120 | • Correspond to α6 (subunit of α6β4 integrin) | ||||
| Dystonin (BP230 or BPAG1*) | 230 | Inner HD plaque | • Attaching intracellular cytoskeleton to HD (cell-matrix adhesion by keratins) | • Intracellular non-COL protein | ||
| BPAG2 | 180 | HD complex | • Facilitating HD assembly through aiding to cluster BPAG1 and plectin Not fully known | • COL transmembrane protein; extracellular domain • COL-like repeats: GXY, X represents any amino acid, known as COL XVII (COL17A1) • Anchoring complex within the LL | ||
| Integrin | β4 & α6 | 205 & 160 | • Cell-matrix or cell-cell adhesion • Transducing signals to regulate gene expression and cell growth | • Transmembrane glycoprotein receptors • Heterodimeric molecules • Genetically distinct α and β subunits | ||
| Integrin: α and β polypeptides complexes | α2β1 | Lateral surface of basal Ks | • Cell-cell interactions | • Ligand-binding | ||
| α3β1 | Both locations of α2β1 & α6β4 & exclusively in the mature epithelium | • Contributing to basal Ks anchoring to BM (specific to epithelial structures) • Keeping DEJ integrity |
• Ligand-binding Ligand: Ln α3 chain (located within Ln 5 & 6 complex) | |||
| α6β4 | Ventral surface (opposed to BM zone) |
• Cell-matrix stable adhesion (basal Ks to BM) |
• Ligand-binding • Promoting the assembly of stable anchoring contacts | |||
| αv: αvβ5, αvβ6 & αvβ8 | αvβ5 & αvβ8: adult epidermis (very low levels). αvβ6: SCs in the HF & in Ks in culture |
• αvβ5 & αvβ8: Binding mainly to vitronectin. • αvβ6: Binding mainly to fibronectin & hyperproliferation under circumstances | • Binding to RGD motifs | |||
| AF | Laminin (Ln) | 5; subunits: α3, β3 & γ2 | 190/165, 140& 155/105 | LL, surround sweat, eccrine glands & hair follicles | • BM assembly, connecting HD from LD (primary link between HD integrin α6β4 & LD of BM) • InterHD BM formation • Focal adhesions | • BM glycoproteins • Thin & threadlike structures (2–4 nm diameter) • Secreted by Ks • Results from truncation of all 3 constituent chains • Encoded by the genes LAMA3, LAMB3 & LAMC2 • Binding directly with the COL XVII & amino-terminal end (NC1) domain of COL VII |
| 6; Subunits: α3, β1 & γ1 | Within DEJ | • Regulating cellular adhesion & migration (differently from Ln 332) • Dictating the response of epithelial cells to mechanical stimulation | • Formation: Ln 5 associate with intracellular Ln β1γ1 dimer • Product of Ks & other epithelial cells, particularly amniotic epithelium | |||
| 10; subunits: α5, β1 & γ1 | Interfollicular epidermis & blood vessels in the dermis | • Promoting the proliferation and migration of epidermal Ks • Maintaining the dermal papilla • Regulating the T cells level Not fully known | • Product of human dermal microvascular endothelial cells | |||
| AP | Collagen (COL) | IV (mostly α5 & α6) | 540 (trimer) | Mostly in LD, sweat glands & blood vessels | • Network-forming COL • Forming the backbone of BM | • Synthesized by both Ks & Fs |
| AFib | COL | VII | 900 (trimer) | LD & extend into dermis (matrix, anchoring plaques (electron-dense structures), or LD) | • Intertwining between interstitial COL fibrils • Attaching the LD to the papillary dermis | • Cross-banded, fibrillar structures • A nonfibrillar COL composing of 3 identical α1 (VII) chains • Binding to: COL VII (NC1 domain) to COL IV (in LD) & Ln 5 (in LL) • Synthesized by both Ks & Fs COL |
| Biomaterial | Characterization Methods | Key Results, [Ref] | |
| Photolithography | CI (microfabricated portion), COL–GAG (sponges) & FN (conjugated on surface) | 1. H&E 2. NHKs; A/L interface; 3 or 7 days 3. Ki67 biomarker & IHC | Well-differentiated epidermal layers. ↑ Epithelialization for the narrow-width than the wider channels. Epithelialization like the natural process. A heterogeneous population of basal Ks. Providing an environment for SC niche. Detecting β1 integrin in µDERM channels [31]. |
| Same biomaterials (CI, COL-GAG & FN), but modified process by adding Fs (sponge) & reducing the CI’s thickness. | 1. H&E. 2. NHKs & NHFs co-culture; A/L interface; 3 or 7 days 3. IHC | ↑ Stratification in the graft regions containing microtopographies. ↑ Ks proliferative phenotype (in narrower channels). ↑ Synthesis of BM protein & Ln 332 (in wider channels). Detecting the β1brip63 + Ks within the base of narrow channels & the corners of wider channels [13]. | |
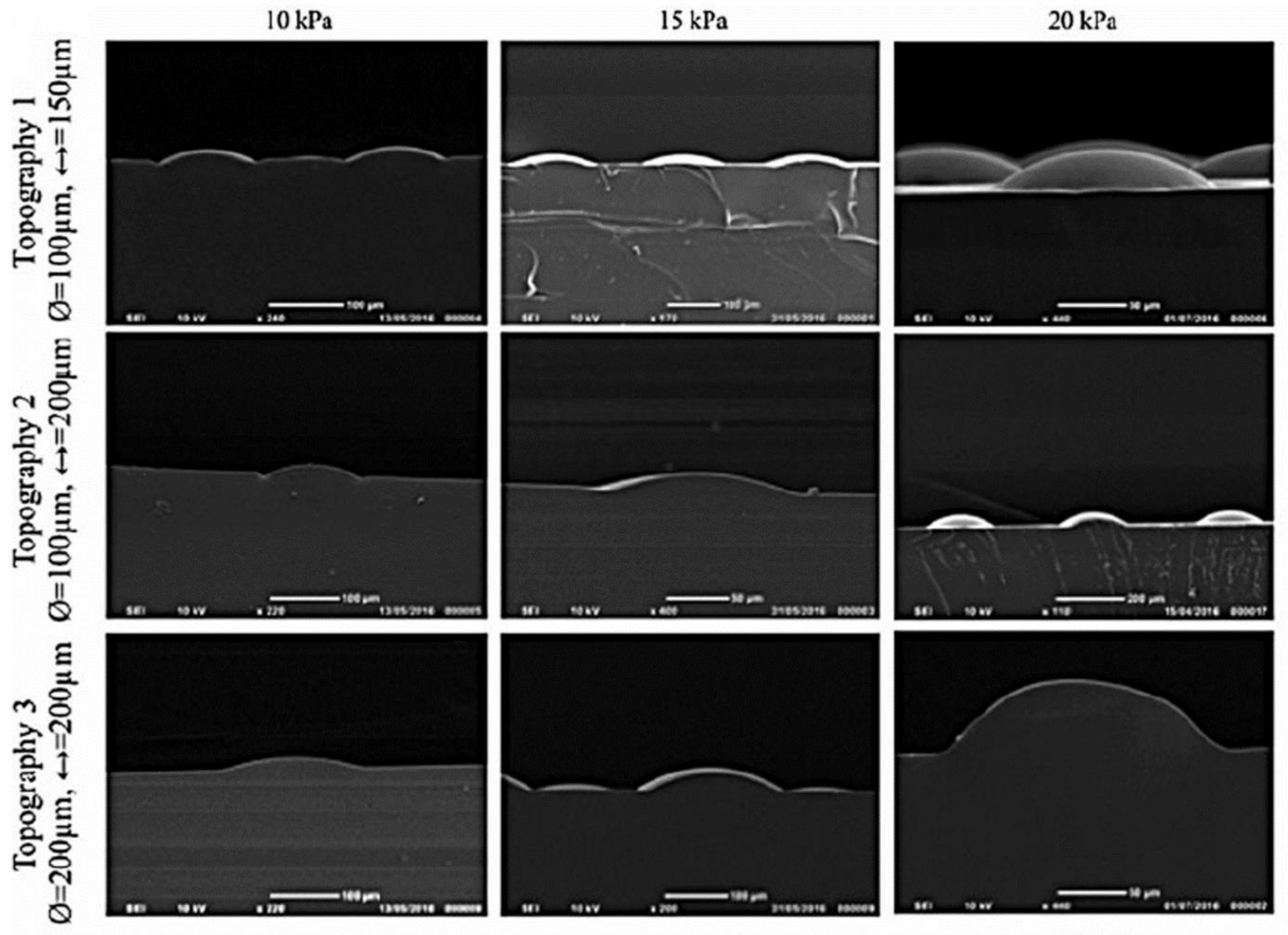
| PHEMA (mold, negative patterns) & PDMS (film; positive patterns) coated by Col type I *Static model* | 1. SEM 2. NHKs for 2 days 3. IHC & DAPI | S1: the best pattern => recreated SCs distribution in the basal layer. Clusters of β1 integrin bright cells on the tips of topographies, particularly S1. Altering wavelength spacing & amplitude => changing pattern in the integrin-bright cells expression. No Ks differentiation on the tips [112]. | |
| 1. SEM 2. NHKs for 4 days 3. IHC, DAPI, Live/Dead & AFM (cell stiffness) | Expression & accumulation on the tips: β1 integrin bright cells. ↑ F-actin, Desmoglein 3 & ↓ MAL. ↑ Cell stiffness on the base. Rho-kinase activity => maintain adheren junctions. Rho kinase activity => differential stiffness of the cells. Forces exerted by cells on the slopes of the topographies => regulating SC patterning [113]. | ||
| Laser | PLGA (membrane) coated by COL I & Polyimide (template) *Dynamic model* | 1. SEM 2. NHKs for 2 days 3. IHC & DAPI | Formation of stratified basal sheets (β1 integrin-positive) & suprabasal. Differentiating cells (involucrin-positive). Clustering β1 integrin bright cells in the holes.YAP localization to the cell nucleus. At the edge of largest holes (topography 3) => DEJ formation by integrin bright cells with nuclear YAP [114]. |
| Electrospinning | PHBV (scaffold) & PEGDA ( template by Stereolithography) | 1. SEM 2 & 3. HKs; MTT (1, 3, & 7 days) & Live/dead (1, 3, & 7 days) | ↑ Colonies formation retained within the microfeatures. Migration within the niche-like structures. Showing the typical K morphology [14]. |
| PCL (scaffold) & RS-F2-GPGR-04 (template by microtereolithography) | 1. SEM & H&E 2 & 3. HFs & HKs co-culture; A/L interface; 1, 3, 6 (Resazurin), 10, & 12 days (in vitro skin model); IHC, DAPI & Lightsheet Microscopy | ↑ Cell metabolic activity. HFs & HKs accumulation at the bottom of the microfeatures. COL IV & integrin β1expression at the bottom of the microfeatures. Pattern B: the best-promoting DEJ [115]. | |
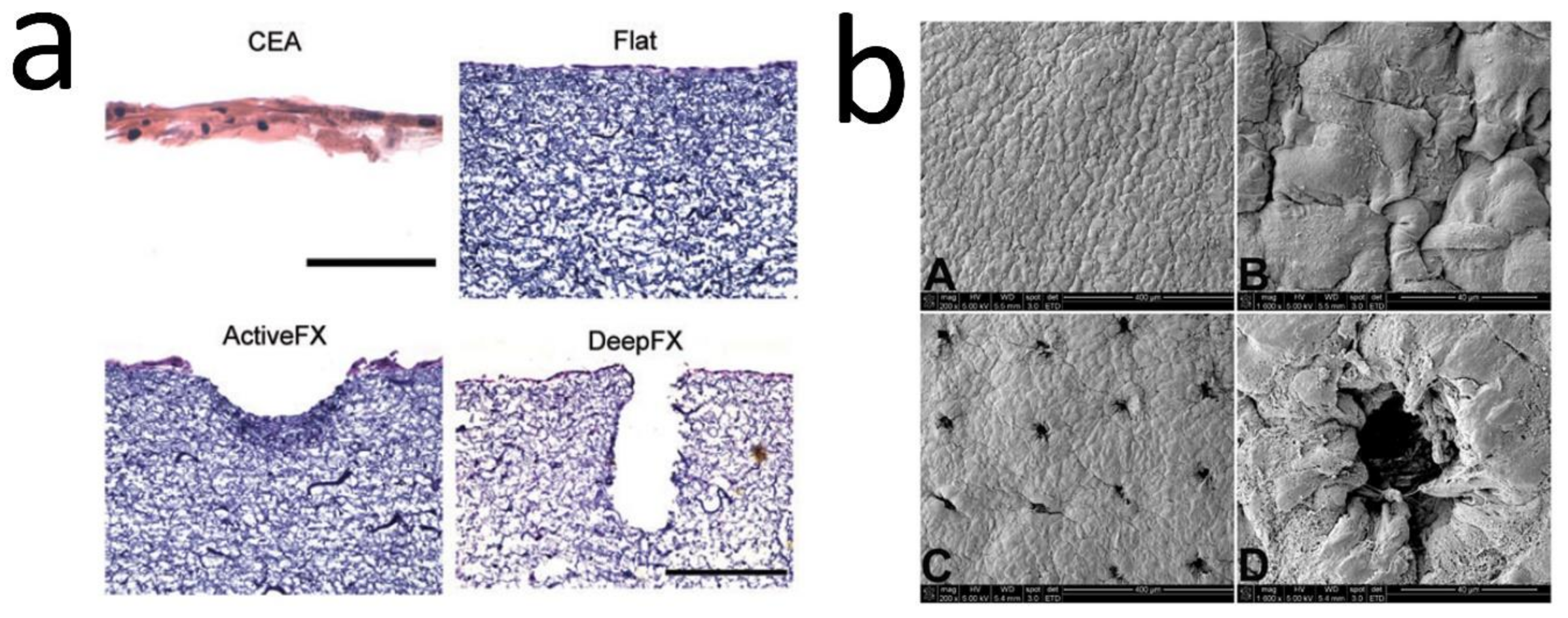
| Laser structuring of electrospun mats | CI | 1. H&E 2. HFs (5 days, on this day, the ridges generated) & HKs (20 days). 4. IM (grafting: 2 weeks & monitoring post-grafting: 4 weeks); Contraction evaluation; IHC & DAPI | ↑ Epidermal barrier function (started at 2 weeks). ActiveFX & DeepFX: Dermal papilla-like generation. ↑ BM proteins levels (COL IV & rat anti-integrin alpha 6 (ITGA6)). ↑ Epidermal thickness & proliferative Ks. DeepFX grafts: the best-promoting DEJ, epidermal viability, & barrier function [33]. |
| 1. SEM & H&E 2. HFs (5 days, ridges generation) & HKs (A-L; 3 & 11 days) 3. MTT, DAPI, IHC, & Quantitative gene expression 4. IM (grafting: 2 weeks & monitoring post-grafting: 4 weeks); Contraction evaluation; TEWL; PCR; IHC. | Organization of Ks in ridged samples. Formation discrete projections into the dermis. ↑ Ln expression. Expression of epidermal SC marker genes [15]. | ||
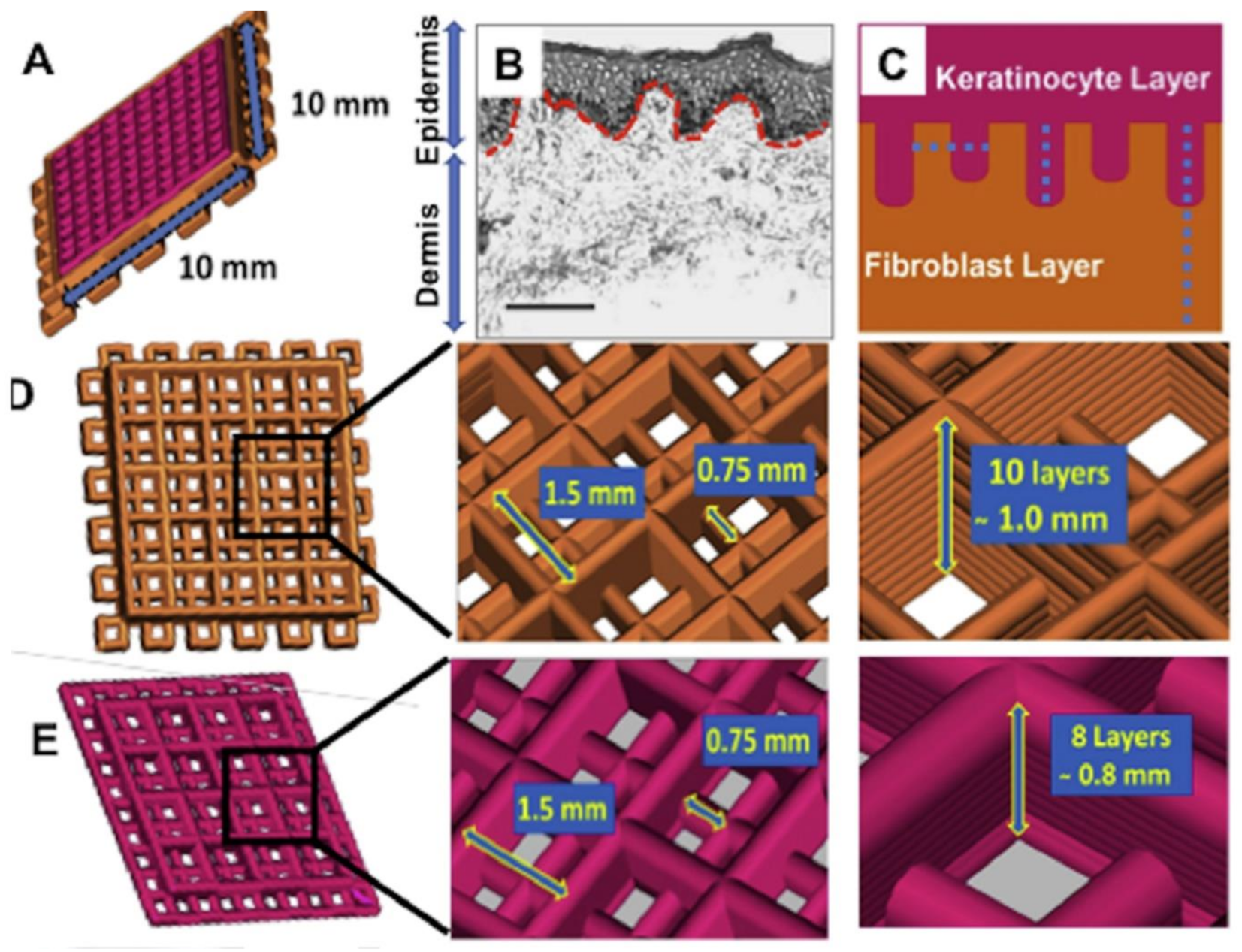
| 3D bioprinting | Silk fibroin & Gelatin | 1. Light microscopy 2. HFs (3 days) & HKs (next 3 days); dual-layered 3D bioprinted constructs: A-L (21 days) 3. Live/dead, gene expression: RT-PCR (COL1A1, fibronectin (FN1) & Ln 1), total COL estimation (hydroxyproline) & IHC, genomic, & proteomic analysis | BM proteins expression => ↑ Mechanical strength. ↑ migration of cultured Ks. ↑ stretching of actin cytoskeleton & cell polarization (close the pores). ↑ integrins & focal adhesion => developing anchorage within pericellular niche. An akin FN distribution (similar to the native skin). Expression of ECM producing genes & differentiation proteins [116]. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aleemardani, M.; Trikić, M.Z.; Green, N.H.; Claeyssens, F. The Importance of Mimicking Dermal-Epidermal Junction for Skin Tissue Engineering: A Review. Bioengineering 2021, 8, 148. https://doi.org/10.3390/bioengineering8110148
Aleemardani M, Trikić MZ, Green NH, Claeyssens F. The Importance of Mimicking Dermal-Epidermal Junction for Skin Tissue Engineering: A Review. Bioengineering. 2021; 8(11):148. https://doi.org/10.3390/bioengineering8110148
Chicago/Turabian StyleAleemardani, Mina, Michael Zivojin Trikić, Nicola Helen Green, and Frederik Claeyssens. 2021. "The Importance of Mimicking Dermal-Epidermal Junction for Skin Tissue Engineering: A Review" Bioengineering 8, no. 11: 148. https://doi.org/10.3390/bioengineering8110148
APA StyleAleemardani, M., Trikić, M. Z., Green, N. H., & Claeyssens, F. (2021). The Importance of Mimicking Dermal-Epidermal Junction for Skin Tissue Engineering: A Review. Bioengineering, 8(11), 148. https://doi.org/10.3390/bioengineering8110148