Functionalized MoS2 Nanoflowers with Excellent Near-Infrared Photothermal Activities for Scavenging of Antibiotic Resistant Bacteria
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
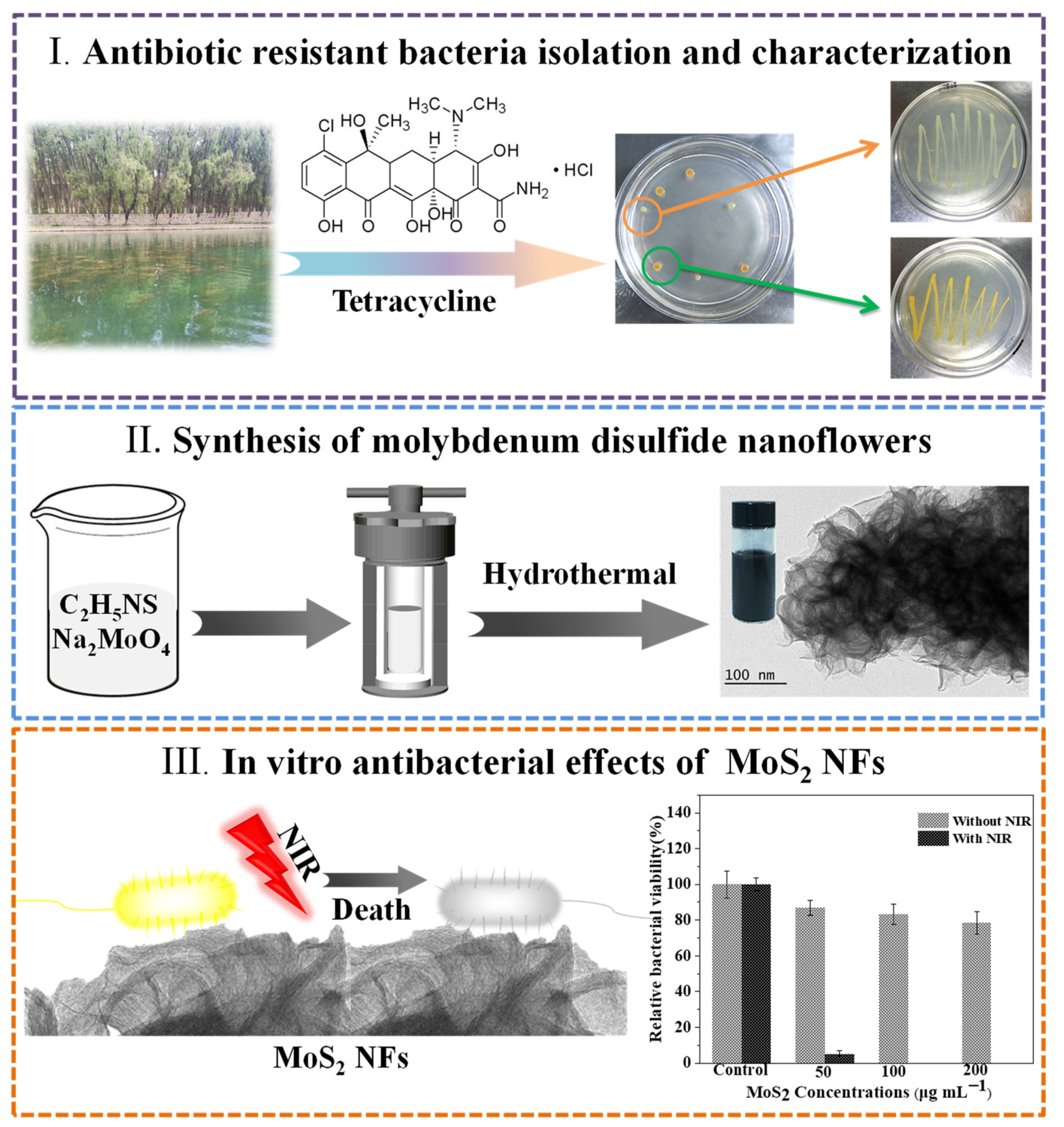
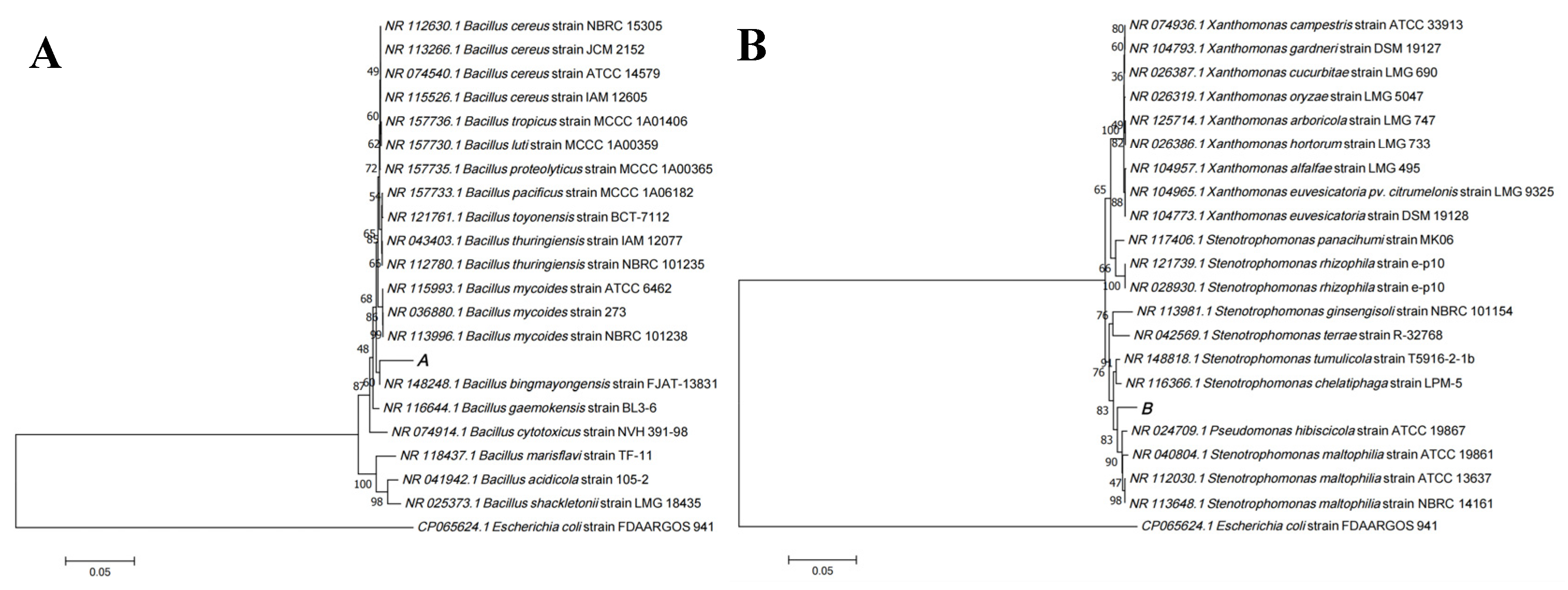
2.2. Antibiotic Resistant Bacteria Isolation and Characterization
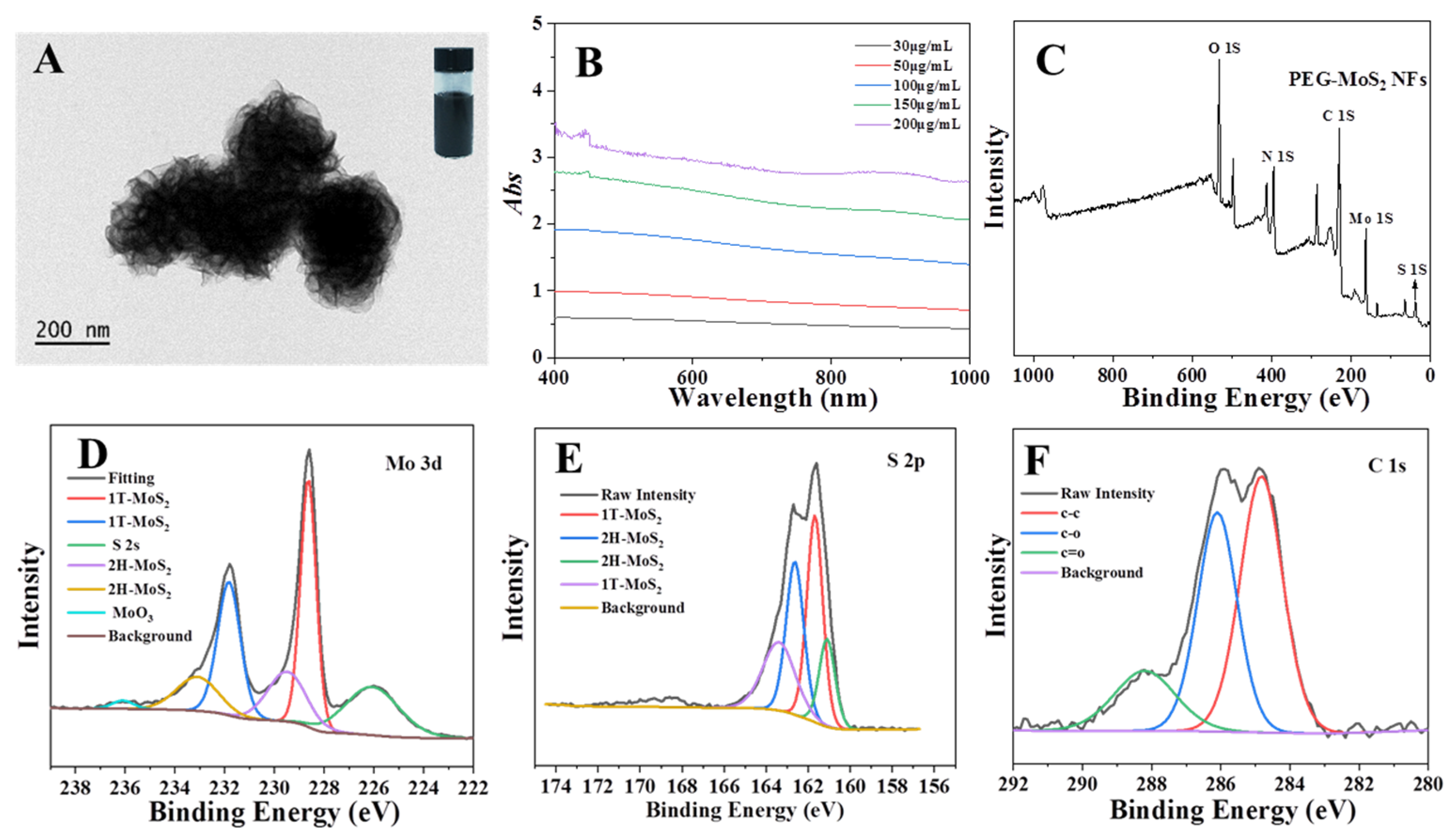
2.3. Synthesis of Molybdenum Disulfide Nanoflowers
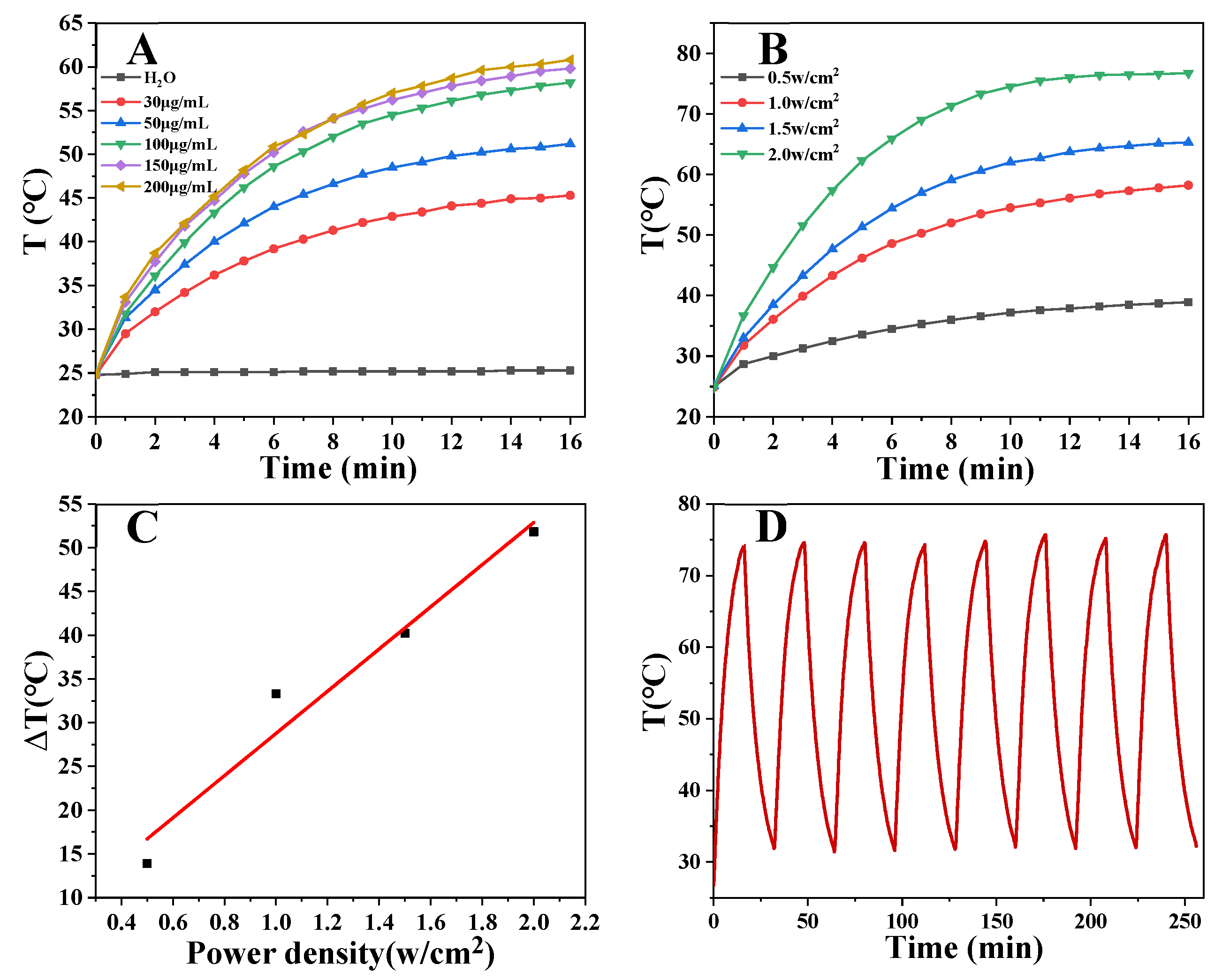
2.4. Photothermal Effect of PEG-MoS2 NFs
2.4.1. Effect of Concentration on Photothermal Effect of PEG-MoS2 NFs
2.4.2. Effect of Power on Photothermal Effect of PEG-MoS2 NFs
2.4.3. Photothermal Stability Test
2.4.4. Photothermal Conversion Efficiency
2.5. In Vitro Antibacterial Effects of PEG-MoS2 NFs
2.5.1. Preparation of Bacterial Suspensions
2.5.2. In Vitro Antibacterial Effect of NIR Photothermal Therapy
2.6. Ellman’s Assay
3. Results and Discussion
3.1. Identification of Antibiotic Resistant Bacteria
3.2. Synthesis and Characterization of Molybdenum Disulfide Nanoflowers
3.3. In Vitro Antibacterial Effects of PEG-MoS2 NFs
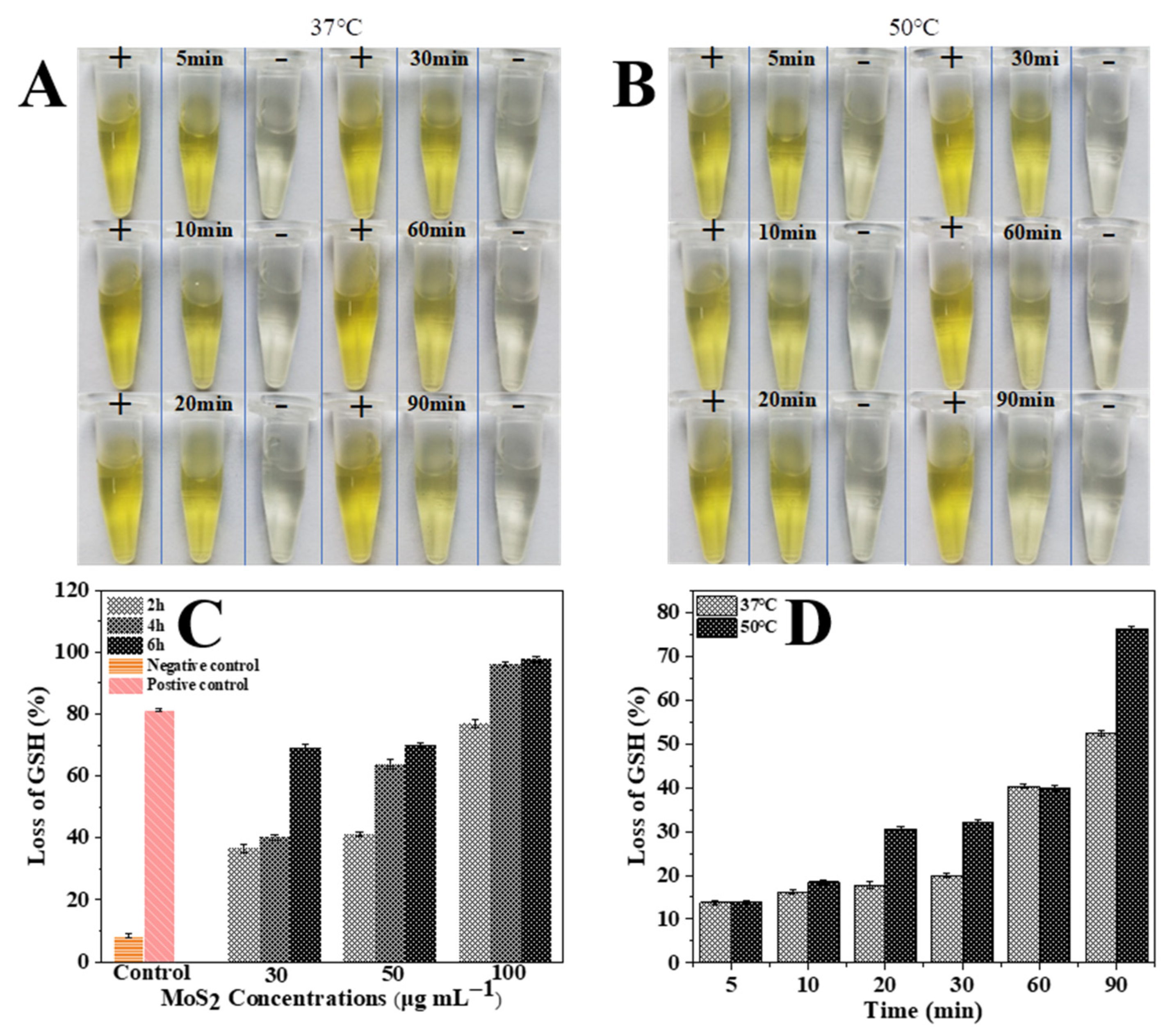
3.4. Oxidation and Quantification of GSH
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Han, Q.; Lau, J.W.; Do, T.C.; Zhang, Z.; Xing, B. Near-infrared light brightens bacterial disinfection: Recent progress and perspectives. ACS Appl. Bio Mater. 2020, 4, 3937–3961. [Google Scholar] [CrossRef]
- Finnegan, M.; Linley, E.; Denyer, S.P.; Mcdonnell, G.; Simons, C.; Maillard, J.Y. Mode of action of hydrogen peroxide and other oxidizing agents: Differences between liquid and gas forms. J. Antimicrob. Chemother. 2010, 65, 2108–2115. [Google Scholar] [CrossRef] [PubMed]
- Theuretzbacher, U.; Gottwalt, S.; Beyer, P.; Butler, M.; Czaplewski, L.; Lienhardt, C.; Moja, L.; Paul, M.; Paulin, S.; Rex, J.H.; et al. Analysis of the clinical antibacterial and antituberculosis pipeline. Lancet 2018, 19, E40–E50. [Google Scholar] [CrossRef]
- Busscher, H.J.; Van, D.; Subbiahdoss, G.; Jutte, P.C.; Van Den Dungen, J.J.A.M.; Zaat, S.A.J.; Schultz, M.J.; Grainger, D.W. Biomaterial-associated infection: Locating the finish line in the race for the surface. Sci. Transl. Med. 2012, 4, 153rv10. [Google Scholar] [CrossRef]
- Chen, J.; Sun, R.; Pan, C.; Sun, Y.; Li, Q. Antibiotics and food safety in aquaculture. J. Agric. Food Chem. 2020, 68, 11908–11919. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Zhang, Y.; Zhu, D.; Li, H. Assessment of bioavailability of biochar-sorbed tetracycline to escherichia coli for activation of antibiotic resistance genes. Environ. Sci. Technol. 2020, 54, 12920–12928. [Google Scholar] [CrossRef]
- Finley, R.L.; Collignon, P.; Larsson, D.G.J.; Mcewen, S.A.; Li, X.Z.; Gaze, W.H.; Reid-Smith, R.; Timinouni, M.; Graham, D.W.; Topp, E. The scourge of antibiotic resistance: The important role of the environment. Clin. Infect. Dis. 2013, 57, 704–710. [Google Scholar] [CrossRef] [PubMed]
- Knapp, C.W.; Dolfing, J.; Ehlert, P.A.I.; Graham, D.W. Evidence of increasing antibiotic resistance gene abundances in archived soils since 1940. Environ. Sci. Technol. 2010, 44, 580–587. [Google Scholar] [CrossRef]
- Fasciani, C.; Silvero, M.J.; Anghel, M.A.; Argueello, G.A.; Becerra, M.C.; Scaiano, J.C. Aspartame-stabilized gold–silver bimetallic biocompatible nanostructures with plasmonic photothermal properties, antibacterial activity, and long-term stability. J. Am. Chem. Soc. 2014, 136, 17394–17397. [Google Scholar] [CrossRef]
- Chen, J.; Peng, H.; Wang, X.; Shao, F.; Yuan, Z.; Han, H. Graphene oxide exhibits broad-spectrum antimicrobial activity against bacterial phytopathogens and fungal conidia by intertwining and membrane perturbation. Nanoscale 2014, 6, 1879–1889. [Google Scholar] [CrossRef]
- Mangadlao, J.D.; Santos, C.M.; Felipe, M.J.L.; de Leon, A.C.C.; Rodrigues, D.F.; Advincula, R.C. On the antibacterial mechanism of graphene oxide (GO) langmuir-blodgett films. Chem. Commun. 2015, 51, 2886–2889. [Google Scholar] [CrossRef]
- Sun, H.; Gao, N.; Dong, K.; Ren, J.; Qu, X. Graphene quantum dots-band-aids used for wound disinfection. ACS Nano 2014, 8, 6202–6210. [Google Scholar] [CrossRef] [PubMed]
- Yin, W.; Yu, J.; Lv, F.; Yan, L.; Zheng, L.R.; Gu, Z.; Zhao, Y. Functionalized nano-MoS2 with peroxidase catalytic and near-Infrared photothermal activities for safe and synergetic wound antibacterial applications. ACS Nano 2016, 10, 11000–11011. [Google Scholar] [CrossRef] [PubMed]
- Tang, Y.; Qiu, Z.Y.; Xu, Z.B.; Gao, L.Z. Antibacterial mechanism and applications of nanozymes. Prog. Biochem. Biophys. 2018, 45, 118–128. [Google Scholar] [CrossRef]
- Huh, A.J.; Kwon, Y.J. “Nanoantibiotics”: A new paradigm for treating infectious diseases using nanomaterials in the antibiotics resistant era. J. Control Release 2011, 156, 128–145. [Google Scholar] [CrossRef] [PubMed]
- Cao, F.; Ju, E.; Zhang, Y.; Wang, Z.; Liu, C.; Li, W.; Huang, Y.; Dong, K.; Ren, J.; Qu, X. An efficient and benign antimicrobial depot based on silver-infused MoS2. ACS Nano 2017, 11, 4651–4659. [Google Scholar] [CrossRef]
- Wahab, M.A.; Hasan, C.M.; Alothman, Z.A.; Hossain, M.S.A. In-situ incorporation of highly dispersed silver nanoparticles in nanoporous carbon nitride for the enhancement of antibacterial activities. J. Hazard Mater. 2021, 408, 124919. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.S.D.; Rajendran, N.K.; Houreld, N.N.; Abrahamse, H. Recent advances on silver nanoparticle and biopolymer based biomaterials for wound healing applications. Int. J. Biol. Macromol. 2018, 115, 165–175. [Google Scholar] [CrossRef]
- Dicastillo, C.; Patio, C.; Galotto, M.J.; Vásquez-Martínez, Y.; Escrig, J. Novel hollow titanium dioxide nanospheres with antimicrobial activity against resistant bacteria. Beilstein J. Nanotechnol. 2019, 10, 1716–1725. [Google Scholar] [CrossRef]
- Zhang, H. Ultrathin two-dimensional nanomaterials. ACS Nano 2015, 9, 9451–9469. [Google Scholar] [CrossRef]
- Yin, W.; Yan, L.; Yu, J.; Tian, G.; Zhou, L.; Zheng, X.; Zhang, X.; Yong, Y.; Li, J.; Gu, Z.; et al. High-throughput synthesis of single-layer MoS2 nanosheets as a near-infrared photothermal-triggered drug delivery for effective cancer therapy. ACS Nano 2014, 8, 6922–6933. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; Yin, W.; Zheng, X.; Tian, G.; Zheng, X.; Bao, T.; Dong, X.; Wang, Z.; Gu, Z.; Ma, X.; et al. Smart MoS2/Fe3O4 nanotheranostic for magnetically targeted photothermal therapy guided by magnetic resonance/photoacoustic imaging. Theranostics 2015, 5, 931–945. [Google Scholar] [CrossRef]
- Sang, Y.; Li, W.; Liu, H.; Zhang, L.; Wang, H.; Liu, Z.; Ren, J.; Qu, X. Construction of nanozyme-hydrogel for enhanced capture and elimination of bacteria. Adv. Funct. Mater. 2019, 29, 1900518. [Google Scholar] [CrossRef]
- Feng, L.; Zhang, L.; Zhang, S.; Chen, X.; Li, P.; Gao, Y.; Xie, S.; Zhang, A.; Wang, H. Plasma-assisted controllable doping of nitrogen into MoS2 nanosheets as efficient nanozymes with enhanced peroxidase-like catalysis activity. ACS Appl. Mater. Interfaces 2020, 12, 17547–17556. [Google Scholar] [CrossRef]
- Lin, T.; Zhong, L.; Guo, L.; Fu, F.; Chen, G. Seeing diabetes: Visual detection of glucose based on the intrinsic peroxidase-like activity of MoS2 nanosheets. Nanoscale 2014, 6, 11856–11862. [Google Scholar] [CrossRef] [PubMed]
- Dey, S.; Matte, H.S.S.R.; Shirodkar, S.N.; Waghmare, U.V.; Rao, C.N.R. Charge-transfer interaction between few-layer MoS2 and tetrathiafulvalene. Chem. Asian J. 2013, 8, 1780–1784. [Google Scholar] [CrossRef] [PubMed]
- Chou, S.S.; Kaehr, B.; Kim, J.; Foley, B.M.; De, M.; Hopkins, P.E.; Huang, J.; Brinker, C.J.; Dravid, V.P. Chemically exfoliated MoS2 as near-infrared photothermal agents. Angew. Chem. 2013, 52, 4160–4164. [Google Scholar] [CrossRef] [PubMed]
- Feng, W.; Chen, L.; Qin, M.; Zhou, X.; Zhang, Q.; Miao, Y.; Qiu, K.; Zhang, Y.; He, C. Flower-like PEGylated MoS2 nanoflakes for near-infrared photothermal cancer therapy. Sci. Rep. 2015, 5, 17422. [Google Scholar] [CrossRef]
- Yang, X.; Li, J.; Liang, T.; Ma, C.; Zhang, Y.; Chen, H.; Hanagata, N.; Su, H.; Xu, M. Antibacterial activity of two-dimensional MoS2 sheets. Nanoscale 2014, 6, 10126–10133. [Google Scholar] [CrossRef]
- Remanan, S.; Samantaray, P.K.; Bose, S.; Das, N.C. Phase transited lysozyme particles and MoS2 nanosheets modified elastomer-like antibacterial and antifouling microfiltration membrane derived from poly(ethylene-co-methyl acrylate)/poly(vinylidene fluoride) (EMA/PVDF) blend for water purification application. Microporous Mesoporous Mater. 2021, 316, 110945. [Google Scholar] [CrossRef]
- Zhang, X.; Zhao, Z.; Yang, P.; Liu, W.; Fan, J.; Zhang, B.; Yin, S. MoS2@C nanosphere as near infrared/pH dual response platform for chemical photothermal combination treatment. Colloid Surf. B 2020, 192, 111054. [Google Scholar] [CrossRef]
- Liu, S.; Zeng, T.H.; Hofmann, M.; Burcombe, E.; Wei, J.; Jiang, R.; Kong, J.; Chen, Y. Antibacterial activity of graphite, graphite oxide, graphene oxide, and reduced graphene oxide: Membrane and oxidative stress. ACS Nano 2011, 5, 6971–6980. [Google Scholar] [CrossRef]
- Han, S.; Kimb, C.; Kwon, D. Thermal degradation of poly(ethyleneglyco1). Polym. Degrad. Stabil. 1995, 47, 203–208. [Google Scholar] [CrossRef]
- Zhang, M.; Wang, K.; Zeng, S.; Xu, Y.; Nie, W.; Chen, P.; Zhou, Y. Visible light-induced antibacterial effect of MoS2: Effect of the synthesis methods. Chem. Eng. J. 2021, 411, 128517. [Google Scholar] [CrossRef]
- Pan, J.; Yang, Y.; Fang, W.; Liu, W.; Le, K.; Xu, D.; Li, X. Fluorescent phthalocyanine-graphene conjugate with enhanced NIR absorbance for imaging and multi-modality therapy. ACS Appl. Energy Mater. 2018, 1, 2785–2795. [Google Scholar] [CrossRef]
- Wang, B.; Wang, J.H.; Liu, Q.; Huang, H.; Chen, M.; Li, K.; Li, C.; Yu, X.F.; Chu, P.K. Rose-bengal-conjugated gold nanorods for in vivo photodynamic and photothermal oral cancer therapies. Biomaterials 2014, 35, 1954–1966. [Google Scholar] [CrossRef]
- Zhang, Q.; Huang, W.; Yang, C.; Wang, F.; Song, C.; Gao, Y.; Qiu, Y.; Yan, M.; Yang, B.; Guo, C. Theranostic nanoagent of Mo2C for multi-modal imaging-guided cancer synergistic phototherapy. Biomater. Sci. 2019, 7, 2729–2739. [Google Scholar] [CrossRef]
- Tian, Q.; Jiang, F.; Zou, R.; Liu, Q.; Chen, Z.; Zhu, M.; Yang, S.; Wang, J.; Wang, J.; Hu, J. Hydrophilic Cu9S5 nanocrystals: A photothermal agent with a 25.7% heat conversion efficiency for photothermal ablation of cancer cells in vivo. ACS Nano 2011, 5, 9761–9771. [Google Scholar] [CrossRef]
- Hessel, C.M.; Pattani, V.P.; Rasch, M.; Panthani, M.G.; Koo, B.; Tunnell, J.W.; Korgel, B.A. Copper selenide nanocrystals for photothermal therapy. Nano Lett. 2011, 11, 2560–2566. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Zhu, D.; Hu, Y.; Swihart, M.T.; Wei, W. Controlled synthesis of Cu2-xSe nanoparticles as near-Infrared photothermal agents and irradiation wavelength dependence of their photothermal conversion efficiency. Langmuir 2018, 34, 13905–13909. [Google Scholar] [CrossRef]
- Han, J.; Feng, Y.; Liu, Z.; Chen, Q.; Shen, Y.; Feng, F.; Liu, L.; Zhong, M.; Zhai, Y.; Bockstaller, M.; et al. Degradable GO-Nanocomposite hydrogels with synergistic photothermal and antibacterial response. Polymer 2021, 230, 124018. [Google Scholar] [CrossRef]
- Smirnova, G.V.; Oktyabrsky, O.N. Glutathione in Bacteria. Biochemistry 2005, 70, 1199–1211. [Google Scholar] [CrossRef] [PubMed]


| Photothermal Nanomaterials | Wavelength | Photothermal Conversion Efficiency | Ref. |
|---|---|---|---|
| MoS2 nanosheets | 808 nm | 24.37% | [21] |
| Silicon phthalocyanine -graphene oxide | 808 nm | 20.20% | [35] |
| Rose-bengal-conjugated gold nanorods | 810 nm | 21% | [36] |
| Mo2C nanospheres | 1064 nm | 24.95% | [37] |
| Cu9S5 nanocrystals | 980 nm | 25.7% | [38] |
| Ligand-stabilized copper selenide nanocrystals | 800 nm | 22% | [39] |
| copper selenide nanoparticles | 980 nm | 18.9% | [40] |
| PEG-MoS2 NFs | 808 nm | 30.6% | This work |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, L.; Wu, W.; Fang, Y.; Liu, H.; Chen, F.; Zhang, M.; Qin, Y. Functionalized MoS2 Nanoflowers with Excellent Near-Infrared Photothermal Activities for Scavenging of Antibiotic Resistant Bacteria. Nanomaterials 2021, 11, 2829. https://doi.org/10.3390/nano11112829
Liu L, Wu W, Fang Y, Liu H, Chen F, Zhang M, Qin Y. Functionalized MoS2 Nanoflowers with Excellent Near-Infrared Photothermal Activities for Scavenging of Antibiotic Resistant Bacteria. Nanomaterials. 2021; 11(11):2829. https://doi.org/10.3390/nano11112829
Chicago/Turabian StyleLiu, Lulu, Wanfeng Wu, Yan Fang, Haoqiang Liu, Fei Chen, Minwei Zhang, and Yanan Qin. 2021. "Functionalized MoS2 Nanoflowers with Excellent Near-Infrared Photothermal Activities for Scavenging of Antibiotic Resistant Bacteria" Nanomaterials 11, no. 11: 2829. https://doi.org/10.3390/nano11112829
APA StyleLiu, L., Wu, W., Fang, Y., Liu, H., Chen, F., Zhang, M., & Qin, Y. (2021). Functionalized MoS2 Nanoflowers with Excellent Near-Infrared Photothermal Activities for Scavenging of Antibiotic Resistant Bacteria. Nanomaterials, 11(11), 2829. https://doi.org/10.3390/nano11112829







