Spore Formers as Beneficial Microbes for Humans and Animals
Abstract
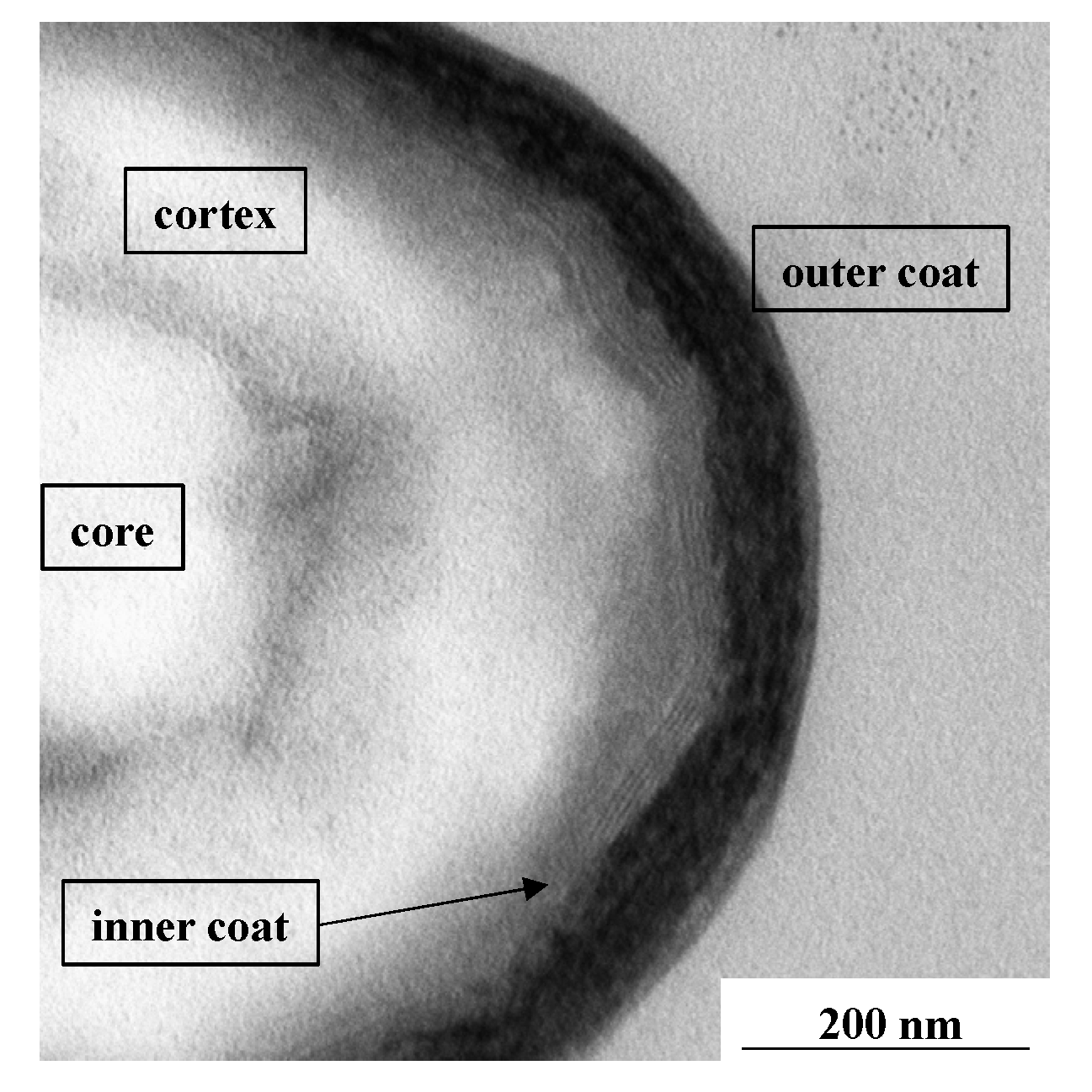
:1. Introduction
2. Bacillus Spores and Vegetative Cells Interact with Epithelial and Immune Cells
2.1. Interaction of Bacillus Spores with Epithelial and Immune Cells
2.2. Interaction of Bacillus Vegetative Cells with Epithelial and Immune Cells
3. Beneficial Effects of Bacillus Probiotics
3.1. Safety of Bacillus Probiotics
3.2. Bacillus Probiotics for Animal Use
3.3. Bacillus Probiotics for Human Use
4. Future Development
4.1. Functional Spores as Probiotics
4.2. Bacillus Probiotics and the Nervous System
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Fritze, D. Taxonomy and systematics of the aerobic endospore forming bacteria: Bacillus and related genera. In Bacterial Spore Formers; Ricca, E., Henriques, A.O., Cutting, S.M., Eds.; Horizon Biosience: Norfolk, UK, 2004; pp. 17–34. [Google Scholar]
- Yutin, N.; Galperin, M.Y. A genomic update on clostridial phylogeny: Gram-negative spore formers and other misplaced clostridia. Environ. Microbiol. 2013, 15, 2631–2641. [Google Scholar] [CrossRef] [PubMed]
- Browne, H.P.; Foster, S.C.; Anonye, B.O.; Kumar, N.; Neville, B.A.; Stares, M.D.; Goulding, D.; Lawley, T.D. Culturing of ‘unculturable’ human microbiota reveals novel taxa and extensive sporulation. Nature 2016, 533, 543–546. [Google Scholar] [CrossRef] [Green Version]
- Galperin, M.Y.; Mekhedov, S.L.; Puigbo, P.; Smirnov, S.; Wolf, Y.I.; Rigden, D.J. Genomic determinants of sporulation in Bacilli and Clostridia: Towards the minimal set of sporulation-specific genes. Environ. Microbiol. 2012, 14, 2870–2890. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Egan, M.; Dempsey, E.; Ryan, A.C.; Ross, P.R.; Stanton, C. The Sporobiota of the human gut. Gut Microbes 2021, 13, e1863134. [Google Scholar] [CrossRef]
- McKenney, P.T.; Driks, A.; Eichenberger, P. The Bacillus subtilis endospore: Assembly and functions of the multilayered coat. Nat. Rev. Microbiol. 2013, 11, 33–44. [Google Scholar] [CrossRef]
- Christie, G.; Setlow, P. Bacillus spore germination: Knowns, unknowns and what we need to learn. Cell. Signal. 2020, 74, 109729. [Google Scholar] [CrossRef]
- Henriques, A.O.; Moran, C.P., Jr. Structure, assembly, and function of the spore surface layers. Ann. Rev. Microbiol. 2007, 61, 555–588. [Google Scholar] [CrossRef] [PubMed]
- Eckburg, P.B.; Bik, E.M.; Bernstein, C.N.; Purdom, E.; Dethlefsen, M.; Sargent, L.; Gill, S.R.; Nelson, K.E.; Relman, D.A. Diversity of the human intestinal microbial flora. Science 2005, 308, 1635–1638. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mahowald, M.A.; Rey, F.E.; Seedorf, H.; Turnbaugh, P.J.; Fulton, R.S. Characterizing a model human gut microbiota composed of members of its two dominant bacterial phyla. Proc. Natl. Acad. Sci. USA 2009, 106, 5859–5864. [Google Scholar] [CrossRef] [Green Version]
- Nicholson, W.L. Roles of Bacillus endospores in the environment. Cell. Mol. Life Sci. 2002, 59, 410–416. [Google Scholar] [CrossRef]
- Nicholson, W.L. Ubiquity, longevity, and ecological roles of Bacillus spores. In Bacterial Spore Formers; Ricca, E., Henriques, A.O., Cutting, S.M., Eds.; Horizon Biosience: Norfolk, UK, 2004; pp. 1–15. [Google Scholar]
- Fakhry, S.; Sorrentini, I.; Ricca, E.; De Felice, M.; Baccigalupi, L. Characterization of spore forming Bacilli isolated from the human gastrointestinal tract. J. Appl. Microbiol. 2008, 105, 2178–2186. [Google Scholar] [CrossRef]
- Hong, H.A.; To, E.; Fakhry, S.; Baccigalupi, L.; Ricca, E.; Cutting, S.M. Defining the natural habitat of Bacillus spore-formers. Res. Microbiol. 2009, 160, 375–379. [Google Scholar] [CrossRef] [PubMed]
- Cutting, S.M.; Hong, H.A.; Baccigalupi, L.; Ricca, E. Oral Vaccine Delivery by Recombinant Spore Probiotics. Int. Rev. Immunol. 2009, 28, 487–505. [Google Scholar] [CrossRef] [PubMed]
- Spinosa, M.R.; Braccini, T.; Ricca, E.; De Felice, M.; Morelli, L.; Pozzi, G.; Oggioni, M.R. On the fate of ingested Bacillus spores. Res. Microbiol. 2000, 151, 361–368. [Google Scholar] [CrossRef]
- Casula, G.; Cutting, S.M. Bacillus probiotics: Spore germination in the gastrointestinal tract. Appl. Environ. Microbiol. 2002, 68, 2344–2352. [Google Scholar] [CrossRef] [Green Version]
- Hoa, T.-T.; Duc, L.H.; Isticato, R.; Baccigalupi, L.; Ricca, E.; Van, P.H.; Cutting, S.M. Fate and dissemination of Bacillus subtilis spores in a murine model. Appl. Environ. Microbiol. 2001, 67, 3819–3823. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tam, N.K.; Uyen, N.Q.; Hong, H.A.; Duc, L.H.; Hoa, T.T.; Serra, C.R.; Henriques, A.O.; Cutting, S.M. The intestinal life cycle of Bacillus subtilis and close relatives. J. Bacteriol. 2006, 188, 2692–2700. [Google Scholar] [CrossRef] [Green Version]
- Margulis, L.; Jorgensen, J.Z.; Dolan, S.; Kolchinsky, R.; Rainey, F.A.; Lo, S.C. The Arthromitus stage of Bacillus cereus: Intestinal symbionts of animals. Proc. Natl. Acad. Sci. USA 1998, 95, 1236–1241. [Google Scholar] [CrossRef] [Green Version]
- Feinberg, L.; Jorgensen, J.; Haselton, A.; Pitt, A.; Rudner, R.; Margulis, L. Arthromitus (Bacillus cereus) symbionts in the cockroach Blaberus giganteus: Dietary influences on bacterial development and population density. Symbiosis 1999, 2, 109–123. [Google Scholar]
- Jadamus, A.; Vahjen, W.; Simon, O. Growth behaviour of a spore forming probiotic strain in the gastrointestinal tract of broiler chicken and piglets. Arch. Anim. Nutr. 2001, 54, 1–17. [Google Scholar] [CrossRef]
- Jadamus, A.; Vahjen, W.; Schafer, K.; Simon, O. Influence of the probiotic strain Bacillus cereus var. toyoi on the development of enterobacterial growth and on selected parameters of bacterial metabolism in digested samples of piglets. J. Anim. Physiol. Anim. Nutr. 2002, 86, 42–54. [Google Scholar] [CrossRef]
- Sumi, H.; Hamada, H.; Tsushima, H.; Mihara, H.; Muraki, H. A novel fibrinolytic enzyme (nattokinase) in the vegetable cheese Natto; a typical and popular soybean food in the Japanese diet. Experientia 1987, 43, 1110–1111. [Google Scholar] [CrossRef]
- Kim, J.Y.; Gum, S.N.; Paik, J.K.; Lim, H.H.; Kim, K.C.; Ogasawara, K.; Inoue, K.; Park, S.; Jang, Y.; Lee, J.H. Effects of nattokinase on blood pressure: A randomized, controlled trial. Hypertens. Res. 2008, 31, 1583–1588. [Google Scholar] [CrossRef] [Green Version]
- Chang, H.C. Healthy and safe Korean traditional fermented foods: Kimki and chongkukjang. J. Ethnic Foods 2018, 5, 161–166. [Google Scholar] [CrossRef]
- Binda, S.; Hill, C.; Johansen, E.; Obis, D.; Pot, B.; Sanders, M.E.; Tremblay, A.; Ouwehand, A.C. Criteria to qualify microorganisms as “Probiotic” in foods and dietary supplements. Front. Microbiol. 2020, 11, 1662. [Google Scholar] [CrossRef]
- Fenster, K.; Freeburg, B.; Hollard, C.; Wong, C.; Laursen, R.R.; Ouwehand, A.C. The Production and Delivery of Probiotics: A Review of a Practical Approach. Microorganisms 2019, 7, 83. [Google Scholar] [CrossRef] [Green Version]
- Duc, L.H.; Hong, A.H.; Nguyen, Q.U.; Cutting, S.M. Intracellular fate and immunogenicity of B. subtilis spores. Vaccine 2004, 22, 1873–1885. [Google Scholar] [CrossRef] [PubMed]
- Ceragioli, M.; Cangiano, G.; Esin, S.; Ghelardi, E.; Ricca, E.; Senesi, S. Phagocytosis, germination and killing of Bacillus subtilis spores presenting heterologous antigens in human macrophages. Microbiology 2009, 155, 338–346. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Duc, L.H.; Hong, H.A.; Fairweather, N.; Ricca, E.; Cutting, S.M. Bacterial spores as vaccine vehicles. Infect. Immun. 2003, 71, 2810–2818. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, J.-M.; La Ragione, R.; Nunez, A.; Cutting, S.M. Immunostimulatory activity of Bacillus spores. FEMS Immunol. Med. Microbiol. 2008, 53, 195–203. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Severson, K.M.; Mallozzi, M.; Driks, A.; Knight, K.L. B cell development in GALT: Role of bacterial superantigen-like molecules. J. Immunol. 2010, 184, 6782–6789. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Petruk, G.; Donadio, G.; Lanzilli, M.; Isticato, R.; Monti, D.M. Alternative use of Bacillus subtilis spores: Protection against environmental oxidative stress in human normal keratinocytes. Sci. Rep. 2018, 8, 1745. [Google Scholar] [CrossRef]
- Mazzoli, A.; Donadio, G.; Lanzilli, M.; Saggese, A.; Guarino, A.M.; Rivetti, M.; Crescenzo, R.; Ricca, E.; Ferrandino, I.; Iossa, S.; et al. Bacillus megaterium SF185 spores exert protective effects against oxidative stress in vivo and in vitro. Sci. Rep. 2019, 9, 12082. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rhee, K.-J.; Sethupathi, P.; Driks, A.; Lanning, D.K.; Knight, K.L. Role of commensal bacteria in development of gut-associated lymphoid tissue and preimmune antibody repertoire. J. Immunol. 2004, 172, 1118–1124. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fujita, M.; Musch, M.W.; Nakagawa, Y.; Hu, S.; Alverdy, J.; Kohgo, Y.; Schneewind, O.; Jabri, B.; Chang, E.B. The Bacillus subtilis quorum-sensing molecule CSF contribute to intestinal homoestasis via OCTN2, a host cell membrane transporter. Cell Host Microbe 2007, 1, 299–308. [Google Scholar] [CrossRef] [Green Version]
- Okamoto, K.; Fujita, M.; Nata, T.; Ueno, N.; Inaba, Y.; Ishikawa, C.; Ito, T.; Moriichi, K.; Tanabe, H.; Mizukami, Y.; et al. Competence and sporulation factor derived from Bacillus subtilis improves epithelial cell injury in intestinal inflammation via immunomodulation and cytoprotection. Int. J. Colorectal Dis. 2012, 27, 1039–1046. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Di Luccia, B.; D’Apuzzo, E.; Varriale, F.; Baccigalupi, L.; Ricca, E.; Pollice, A. Bacillus megaterium SF185 induces stress pathways and affects the cell cycle distribution of human intestinal epithelial cells. Benef. Microbes 2016, 7, 609–620. [Google Scholar] [CrossRef] [Green Version]
- D’Arienzo, R.; Maurano, F.; Mazzarella, G.; Luongo, D.; Stefanile, R.; Ricca, E.; Rossi, M. Bacillus subtilis spores reduce susceptibility to Citrobacter rodentium-mediated enteropathy in a mouse model. Res. Microbiol. 2006, 157, 891–897. [Google Scholar] [CrossRef] [PubMed]
- Schauer, D.B.; Falkow, S. The eae gene of Citrobacter freundii biotype 4280 is necessary for colonization in transmissible murine colonic hyperplasia. Infect. Immun. 1993, 61, 4654–4661. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khaneja, R.; Perez-Fons, L.; Fakhry, S.; Baccigalupi, L.; Steiger, S.; To, E.; Sandmann, G.; Dong, T.C.; Ricca, E.; Fraser, P.D.; et al. Carotenoids Found in Bacillus. J. Appl. Microbiol. 2010, 108, 1889–1902. [Google Scholar] [PubMed]
- Crescenzo, R.; Mazzoli, A.; Cancelliere, R.; Bucci, A.; Naclerio, G.; Baccigalupi, L.; Cutting, S.M.; Ricca, E.; Iossa, S. Beneficial effects of carotenoid-producing cells of Bacillus indicus HU16 in a rat model of diet-induced metabolic syndrome. Benef. Microbes 2017, 8, 823–831. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Song, M.; Hong, H.A.; Huang, J.-M.; Colenutt, C.; Khang, D.D.; Nguyen, T.V.A.; Park, S.-M.; Shim, B.-S.; Song, H.H.; Cheon, I.S.; et al. Killed Bacillus subtilis spores as a mucosal adjuvant for an H5N1 vaccine. Vaccine 2012, 30, 3266–3277. [Google Scholar] [CrossRef]
- Paparo, L.; Tripodi, L.; Bruno, C.; Pisapia, L.; Damiano, C.; Pastore, L.; Berni Canani, R. Protective action of Bacillus clausii probiotic strains in an in vitro model of Rotavirus infection. Sci. Rep. 2020, 10, 12636. [Google Scholar] [CrossRef]
- Mingmongkolchai, S.; Panbangred, W. Bacillus probiotics: An alternative to antibiotics for livestock production. J. Appl. Microbiol. 2018, 124, 1334–1346. [Google Scholar] [CrossRef] [PubMed]
- La Ragione, R.M.; Casula, G.; Cutting, S.M.; Woodward, M.J. Bacillus subtilis spores competitively exclude Escherichia coli O78:K80 in poultry. Vet. Microbiol. 2001, 79, 113–142. [Google Scholar] [CrossRef]
- La Ragione, R.M.; Woodward, M.J. Competitive exclusion by Bacillus subtilis spores of Salmonella enterica serotype Enteritidis and Clostridium perfringens in young chickens. Vet. Microbiol. 2003, 94, 245–256. [Google Scholar] [CrossRef]
- Šabatková, J.; Kumprecht, I.; Zoba, P.; Suchý, P.; Čermák, B. The Probiotic BioPlus 2B as an Alternative to Antibiotics in Diets for Broiler Chickens. Acta Vet. Brno 2008, 77, 569–574. [Google Scholar]
- Alexopoulos, C.; Georgoulakis, I.E.; Tzivara, A.; Kyriakis, C.S.; Govaris, A.; Kyriakis, S.C. Field evaluation of the effect of a probiotic containing Bacillus licheniformis and Bacillus subtilis spores on the health status, performance, and carcass quality of grower and finisher pigs. J. Vet. Med. A Physiol. Pathol. Clin. Med. 2004, 51, 306–312. [Google Scholar] [CrossRef] [PubMed]
- Scharek-Tedin, L.; Pieper, R.; Vahjen, W.; Tedin, K.; Neumann, K.; Zentek, J. Bacillus cereus var. Toyoi modulates the immune reaction and reduces the occurrence of diarrhea in piglets challenged with Salmonella Typhimurium DT104. J. Anim. Sci. 2013, 91, 5696–5704. [Google Scholar] [CrossRef]
- Williams, L.D.; Burdock, G.A.; Jimenez, G.; Castillo, M. Literature review on the safety of Toyocerin, a non-toxigenic and nonpathogenic Bacillus cereus var. toyoi preparation. Toxicol. Pharmacol. 2009, 55, 236–246. [Google Scholar]
- Mohapatra, S.; Chakraborty, T.; Kumar, V.; Deboeck, G.; Mohanta, K.N. Aquaculture and stress management: A review of probiotic intervention. J. Anim. Physiol. Anim. Nutr. 2013, 97, 405–430. [Google Scholar] [CrossRef] [PubMed]
- Liu, K.F.; Chiu, C.S.; Shiu, Y.L.; Cheng, W.; Liu, C.H. Effects of the probiotic, Bacillus subtilis E20, on the survival, development, stress tolerance, and immune status of white shrimp, Litopenaeus vannamei larvae. Fish Shellfish Immunol. 2010, 28, 837–844. [Google Scholar] [CrossRef]
- Liu, C.; Chiu, C.; Wang, S.; Cheng, W. Dietary administration of the probiotic, Bacillus subtilis E20, enhances the growth, innate immune responses, and disease resistance of the grouper, Epinephelus coioides. Fish Shellfish Immunol. 2012, 33, 699–706. [Google Scholar] [CrossRef] [PubMed]
- Das, A.; Nakhro, K.; Chowdhury, S.; Kamilya, D. Effect of potential probiotic Bacillus amyloliquefaciens FPTB16 on systemic and cutaneous mucosal immune responses and desease resistance of catla (Catla catla). Fish Shellfish Immunol. 2013, 35, 1547–1553. [Google Scholar] [CrossRef]
- Piewngam, P.; Zheng, Y.; Nguyen, T.H.; Dickey, S.W.; Joo, H.S.; Villaruz, A.E.; Glose, K.A.; Fisher, E.L.; Hunt, R.L.; Li, B.; et al. Pathogen elimination by probiotic Bacillus via signaling interference. Nature 2018, 562, 532–537. [Google Scholar] [CrossRef]
- Stevens, Y.; Pinheiro, I.; Salden, B.; Duysburgh, C.; Bolca, S.; Degroote, J.; Majdeddin, M.; Van Noten, N.; Gleize, B.; Caris-Veyrat, C.; et al. Effect of a carotenoid-producing Bacillus strain on intestinal barrier integrity and systemic delivery of cartenoids: A randomised trial in animals and humans. J. Funct. Foods 2021, 80, 104445. [Google Scholar] [CrossRef]
- Sirec, T.; Strazzulli, A.; Isticato, R.; De Felice, M.; Moracci, M.; Ricca, E. Adsorption of beta-galactosidase of Alicyclobacillus acidocaldarius on wild type and mutant spores of Bacillus subtilis. Microb. Cell Factories 2012, 11, 100. [Google Scholar] [CrossRef] [Green Version]
- Sirec, T.; Cangiano, G.; Baccigalupi, L.; Ricca, E.; Isticato, R. Human intestinal isolates of Bacillus subtilis: Characterization of the spore surface and use as display systems. FEMS Microbiol. Lett. 2014, 358, 194–201. [Google Scholar] [CrossRef] [Green Version]
- Lanzilli, M.; Donadio, G.; Fusco, F.A.; Sarcinelli, C.; Limauro, D.; Ricca, E.; Isticato, R. Display of the peroxiredoxin Bcp1 of Sulfolobus solfataricus on probiotic spores of Bacillus megaterium. New Biotechnol. 2018, 46, 38–44. [Google Scholar] [CrossRef]
- Ricca, E.; Baccigalupi, L.; Isticato, R. Spore-adsorption: Mechanism and applications of a non-recombinant display system. Biotechnol. Adv. 2021, 47, 107693. [Google Scholar] [CrossRef]
- Cogliati, S.; Clementi, V.; Francisco, M.; Crespo, C.; Arfanaraz, F.; Grau, R. Bacillus subtilis delays neurodegeneration and behavioral impairment in the Alzheimer’s disease model Caenorhabditis elegans. J. Alzheimer’s Dis. 2020, 73, 1035–1052. [Google Scholar] [CrossRef] [PubMed]
- Goya, M.A.; Xue, F.; Sampedro-Torres-Quevedo, C.; Arnaouteli, S.; Riquelme-Dominquez, L.; Romanowski, A.; Brydon, J.; Ball, K.L.; Stanley-Wall, N.R.; Doitsidou, M. Probotic Bacillus subtilis protects against alpha-synucleinaggregation in C. elegans. Cell Rep. 2020, 30, 367–380. [Google Scholar] [CrossRef] [PubMed]

| Bacillus amyloliquefaciens Bacillus atrophaeus | Bacillus megaterium |
|---|---|
| Bacillus circulans | Bacillus mojavensis |
| Bacillus clausii | Bacillus paralicheniformis Bacillus pumilus |
| Bacillus coagulans | Bacillus smithii |
| Bacillus flexus | Bacillus subtilis |
| Bacillus fusiformis | Bacillus vallismortis |
| Bacillus lentus | Bacillus velenzensis |
| Bacillus licheniformis |
| Antibiotic | Microbiological Breakpoint (mL/L) |
|---|---|
| Vancomycin | 4 |
| Gentamycin | 4 |
| Kanamycin | 8 |
| Streptomycin | 8 |
| Erythromycin | 4 |
| Clindamycin | 4 |
| Quinupristin + Dalfopristin | 4 |
| Product | Manufacturer | Species (Spores/Dose) * | Animal |
|---|---|---|---|
| AlCare | Alpharma Inc. (Australia) | B. licheniformis NCTC 13123 (109–1010) | Swine |
| BioGrow | Provita Eurotech Ltd. (UK) | B. licheniformis1 (1.6 × 109) and B. subtilis 1 (1.6 × 109) | Poultry, calves and swine |
| BioPlus 2B | Christian Hansen Hoersholm (Denmark) | B. licheniformis DSM 5749(1.6 × 109) and B. subtilis DSM 5750 (1.6 × 109) | Piglets, chickens, turkeys |
| Esporafeed Plus | Norel, S.A. (Spain) | B. cereus CECT 953 (109) | Swine |
| Lactopure | Pharmed Medicare (India) | B. coagulans1 | Poultry, calves and swine |
| Neoferm BS 10 | Sanofi Sante Nutrition Animale (France) | B. clausii CNCM MA23/3V and CNCM MA66/4M | Poultry, calves and swine |
| Toyocerin | Asahi Vet S.A. (Japan) | B. cereus var. toyoi NCIMB-40112/CNCM-1012 (>1010) | Calves, poultry, rabbits and swine. |
| Product | Manufacturer | Species (Spores/Dose) * |
|---|---|---|
| Biostart | Microbial Solutions (South Africa); Advanced Microbial Systems (USA) | B. megaterium1, B. licheniformis1, P. polymyxa1 and B. subtilis 1 |
| BioZyme-Aqua | Sino-Aqua Corp. (Taiwan) | B. subtilis Wu-S and Wu-T (108) |
| Liqualife | Cargill (USA) | Bacillus ssp. 1 |
| Promarine | Sino-Aqua Corp. (Taiwan) | B. subtilis1 |
| Sanocare Sanolife | INVE Technologies | Bacillus ssp. 1 |
| Sanoguard | Belgium | Bacillus ssp. 1 |
| Product | Manufacturer | Species (Spores/Dose) * |
|---|---|---|
| Bactisubtil | Marion Merrell (France); Casella-Med (Germany) | B. cereus ATCC 14893 (109) |
| Bibactyl | UPHACE (Vietnam) | B. subtilis var. natto (107–108)9) |
| Bidisubtilis | Bidiphar (Vietnam) | B. cereus1 (106)9) |
| Bio-Acimin | Viet-Duc Pharm. (Vietnam) | B. cereus1 and other bacteria |
| Bio-Kult | Probiotics international Ltd. (UK) | B. subtilis1 and other bacteria |
| Biobaby | Ildong Pharma (Korea) | B. subtilis1 (3 × 106), C. butyricum 1 (107), B. coagulans 1 (5 × 107) |
| Biosubtyl | Biophar Company (Vietnam) | B. cereus1 (106–107) |
| Biosubtyl DL | IVAC (Vietnam) | B. subtilis1 (107–108) and other bacteria |
| Biosubtyl I and II | Biophar Company (Vietnam) | B. pumilus1 (106–107) |
| Biosporin | Biofarm (Ukraine)/Garars (Russia) | B. subtilis 2335 and B. licheniformis 2336 (ratio is 3:1) |
| Biovicerin | Geyer Medicamentos S.A. (Brazil) | B. cereus GM (106) |
| Bispan | Binex Co. (Korea) | B. polyfermenticus SCD (1.7 × 107) |
| Domuvar | BioProgress SpA (Italy) | B. clausii1 (109) |
| Enterogermina | Sanofi Winthrop SpA (Italy) | B. clausii1 (106) |
| Flora-Balance | Flora-Balance (USA) | B. laterosporus BOD (>106) |
| Flora3 | USA | S. boulardii1 and B. coagulans 1 |
| GanedenBC30 | USA | B. coagulans GBI-30 |
| Ildong Biovita | Ildong Pharma (Korea) | B. subtilis1 (3 × 106), C.butyricum 1 (107), L. sporogenes 1 (5 × 107) |
| Just Thrive | USA | B. indicus HU36, B. coagulans 1, B. clausii 1, B. subtilis HU58 |
| Lacbon, Lacris | Uni- Sankyo (Japan) | B. coagulans1 |
| Lactipan Plus | Istituto Biochimico Italiano SpA (Italy) | B. subtilis1 (2 × 109) |
| Lactospore | Sabinsa Corp. (USA) | B. coagulans1 (109) |
| Latero-Flora | GHC (USA) | B. laterosporus BOD (>106) |
| LifeinU | LHC Lesaffre Human Care Ltd. (France) | B. subtilis CU1 |
| Medilac-Vita | Hanmi Pharmaceutical Co. (China) | B. subtilis RO179 (108) and other bacteria |
| MegaSporeBiotic | UK | B. indicus1, B. subtilis1, B. coagulans1 |
| Nature’s First Food | Nature’s First Law (California) | B. laterosporus1, B. polymyxa1, B. subtilis1, B. pumilus1 |
| Neolactoflorene | Newpharma S.r.l. (Italy) | B. coagulans1 and other bacteria |
| NutriCommit | USA | B. subtilis1 and B. coagulans 1 |
| Pastylbio | Pasteur Institute (Vietnam) | B. subtilis1 (108) |
| Primal Defense | Garden of Life (USA) | B. subtilis1 (108) and other bacteria |
| Subtyl | Mekophar (Vietnam) | B. cereus vietnami (106–107) |
| SunnyGreen Cleansing | USA | B. coagulans1 |
| Sustenex | Ganeden Biotech Inc. (USA) | B. coagulans Ganeden BC30 |
| THORNE | USA | B. coagulans1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Saggese, A.; Baccigalupi, L.; Ricca, E. Spore Formers as Beneficial Microbes for Humans and Animals. Appl. Microbiol. 2021, 1, 498-509. https://doi.org/10.3390/applmicrobiol1030032
Saggese A, Baccigalupi L, Ricca E. Spore Formers as Beneficial Microbes for Humans and Animals. Applied Microbiology. 2021; 1(3):498-509. https://doi.org/10.3390/applmicrobiol1030032
Chicago/Turabian StyleSaggese, Anella, Loredana Baccigalupi, and Ezio Ricca. 2021. "Spore Formers as Beneficial Microbes for Humans and Animals" Applied Microbiology 1, no. 3: 498-509. https://doi.org/10.3390/applmicrobiol1030032
APA StyleSaggese, A., Baccigalupi, L., & Ricca, E. (2021). Spore Formers as Beneficial Microbes for Humans and Animals. Applied Microbiology, 1(3), 498-509. https://doi.org/10.3390/applmicrobiol1030032








