The Medial Prefrontal Cortex and Fear Memory: Dynamics, Connectivity, and Engrams
Abstract
:1. Introduction
| Although prefrontal areas have been widely studied and implicated in various brain functions and disorders, there is a surprising lack of commonly accepted nomenclature and delineation of its subdivisions. Rodent stereotaxic atlases, on which experimentalists rely the most, are regularly updated as no consensus is found [15]. In the absence of clear landmarks to define the mPFC, a lot is left to individual appreciation which can lead to apparent incoherencies between studies and overall misinterpretation. Until a unified nomenclature is accepted in the field, it is necessary that authors report precise stereotaxic coordinates and explicitly define the brain region(s) they study. | |
For simplicity here, we will use the following
nomenclature for the 3 major subdivisions of the mPFC:
| |
| Although the human PFC evolved to be relatively bigger and more complex than the rodent PFC, notably with more clearly defined subregions, homologies in embryological development, layer organization, cell-type distribution and connectivity patterns advocate for potentially shared functions. For an anatomical definition and a comparison between human and rodent PFC, see Carlén, 2017 [16]. For a very detailed description of the cytoarchitecture of the mouse PFC, see Van de Werd et al., 2010 [17], and for a comparison between mouse reference atlases, see Le Merre et al., 2021 [15]. | |
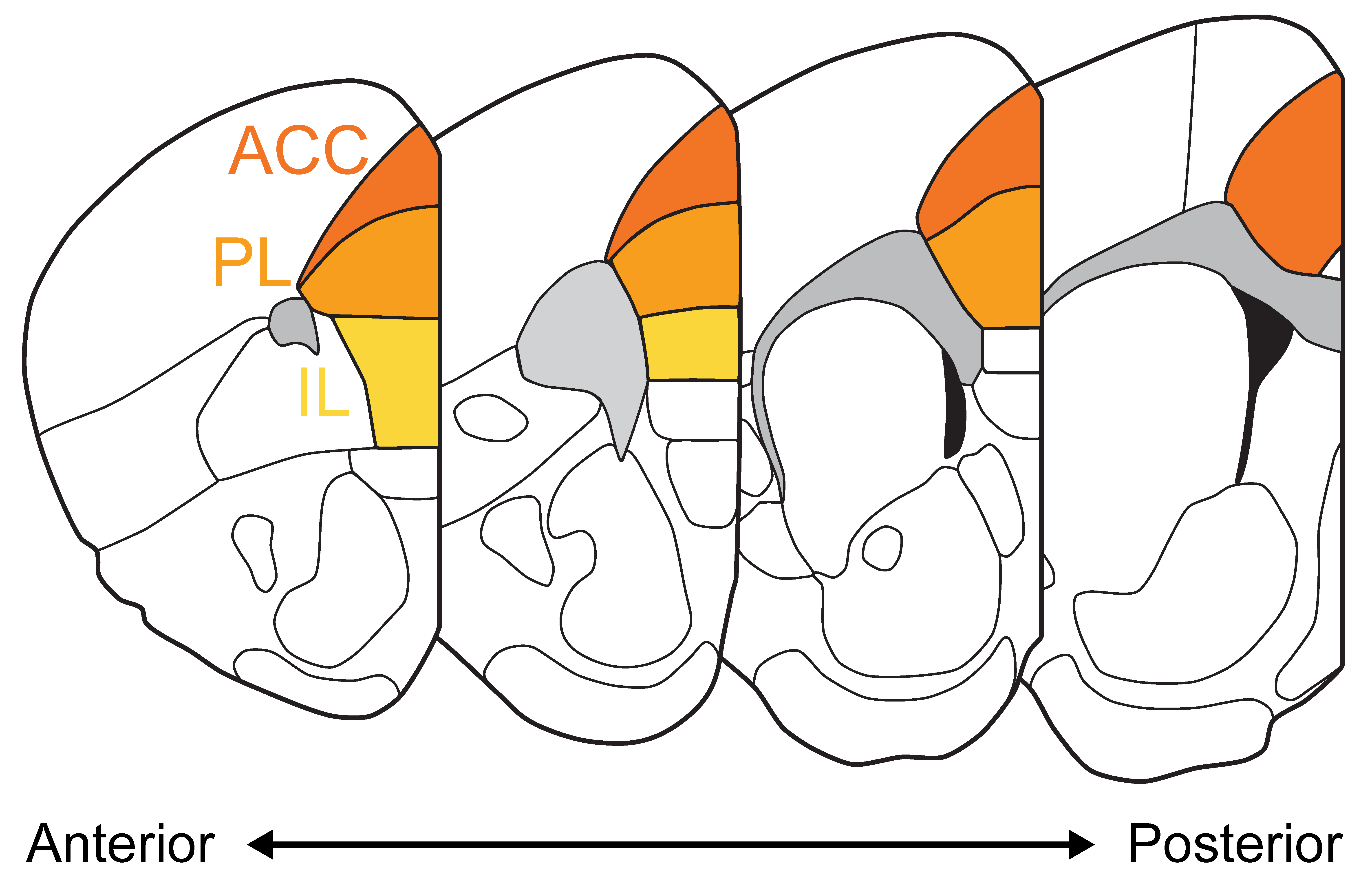 | Box figure. Coronal sections of the mouse brain along the antero-posterior axis with the 3 major subdivions of the mPFC highlighted (Anterior cingulate cortex ACC, Prelimbic cortex PL, Infralimbic cortex IL) based on the Allen Brain Atlas. |
2. The mPFC in Remote Memory Recall
- Neuronal activity can be visualized using several techniques with their unique advantages and disadvantages depending on the research question being asked.
- Electrophysiology enables direct monitoring of the electrical activity of a single neuron ex vivo or in vivo, with a high temporal and spatial resolution, and can be used to identify specific neuronal subtypes based on their distinct firing patterns [19,20]. At a lower spatial resolution, it is also possible to record local field potentials or global electrical oscillation patterns at the scale of an entire brain region [21,22].
- Calcium imaging is based on a fluorescent reporter, the activity of which correlates with intracellular calcium concentration, considered as a proxy for neuronal activation [23]. It has a lower temporal resolution than electrophysiology, but allows for monitoring of many neurons at once, with a potentially high spatial resolution depending on the type of imaging technique it is paired with it [24]. In both cases, technological advances are increasing the power of those techniques to monitor live brain activity in freely behaving animals with minimum tissue damage [25,26].
- Immediate early genes (IEGs), such as cFos, Arc, Npas4, Zif 268, etc., are transcribed upon neuronal activation. Visualizing the corresponding mRNA or protein expressed after behavior enables identification of recently active neurons. Taking advantage of the specific dynamics of each of those IEGs, they can also be combined to disentangle neurons that take part in two successive tasks [27,28,29,30], but they cannot provide permanent labelling themselves.
- To this end, conditional reporter expression using IEG promoters have then been developed, allowing for the long-lasting tagging of neurons that were active at a given point in time. Two main strategies have been used: (1) the Tettag system uses the Doxycyclin-dependant transcription factor tTA, expressed under the cFos promoter, to restrict the expression of a chosen protein under a Tet promoter to only tag neurons active during the desired tagging time-window [31]; (2) the TRAP system uses a Tamoxifen-dependent Cre recombinase Cre-ERT2 under a cFos or Arc promoter to restrict recombination of a chosen gene to activated neurons at the time of Tamoxifen injection [32,33]. In both cases, those systems can be used within transgenic mouse lines or through stereotaxic delivery of viral constructs. They allow restriction in time and, if desired, in space, of the initial tagging, and subsequent manipulation of engram neurons by either chemogenetic [34,35,36] or optogenetic tools [37,38].
- Furthermore, GRASP techniques (GFP Reconstitution Across Synaptic Partners) coupled with the Tettag system enable visualization of direct synaptic contact between engram and/or non-engram cells that are located in different brain areas, and specifically manipulate those pair types [41].
- Lastly, using cell-type specific promoters or viral vectors with restricted tropism or antero- and retrograde transport, the scope of action of these techniques can be even more refined. These methods can also be coupled to enhance their potential. For instance, calcium imaging and Tettag tagging were used together to monitor replay of engram cells during sleep [42].
3. The mPFC in Early Memory Phases
3.1. At Memory Encoding
3.2. At Recent Memory Recall
4. The mPFC Engram
5. Post-Learning Molecular Modifications in the mPFC
6. The mPFC Functional Connectivity
6.1. mPFC Functional Inputs
6.2. mPFC Functional Outputs
7. Models of Memory Formation
7.1. The Standard Model of Memory Formation and Its Limitations
7.2. The Indexing Theory (IT)
7.3. The Multiple Trace Theory (MTT)
8. Memories Are Dynamic: Discussion and Outlook
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Scoville, W.B.; Milner, B. Loss of Recent Memory After Bilateral Hippocampal Lesions. J. Neurol. Neurosurg. Psychiatry 1957, 20, 11–21. [Google Scholar] [CrossRef] [Green Version]
- Josselyn, S.A.; Kohler, S.; Frankland, P.W. Finding the engram. Nat. Rev. Neurosci. 2015, 16, 521–534. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Ramirez, S.; Pang, P.T.; Puryear, C.B.; Govindarajan, A.; Deisseroth, K.; Tonegawa, S. Optogenetic stimulation of a hippocampal engram activates fear memory recall. Nat. Cell Biol. 2012, 484, 381–385. [Google Scholar] [CrossRef] [Green Version]
- Ramirez, S.; Liu, X.; Lin, P.-A.; Suh, J.; Pignatelli, M.; Redondo, R.L.; Ryan, T.J.; Tonegawa, S. Creating a False Memory in the Hippocampus. Science 2013, 341, 387–391. [Google Scholar] [CrossRef]
- Tonegawa, S.; Liu, X.; Ramirez, S.; Redondo, R. Memory Engram Cells Have Come of Age. Neuron 2015, 87, 918–931. [Google Scholar] [CrossRef] [Green Version]
- Bontempi, B.; Laurent-Demir, C.; Destrade, C.; Jaffard, R. Time-dependent reorganization of brain circuitry underlying long-term memory storage. Nat. Cell Biol. 1999, 400, 671–675. [Google Scholar] [CrossRef] [PubMed]
- Frankland, P.W.; Bontempi, B.; Talton, L.E.; Kaczmarek, L.; Silva, A.J. The Involvement of the Anterior Cingulate Cortex in Remote Contextual Fear Memory. Science 2004, 304, 881–883. [Google Scholar] [CrossRef] [Green Version]
- Frankland, P.W.; Bontempi, B. The organization of recent and remote memories. Nat. Rev. Neurosci. 2005, 6, 119–130. [Google Scholar] [CrossRef]
- Bero, A.W.; Meng, J.; Cho, S.; Shen, A.; Canter, R.G.; Ericsson, M.; Tsai, L.-H. Early remodeling of the neocortex upon episodic memory encoding. Proc. Natl. Acad. Sci. USA 2014, 111, 11852–11857. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Einarsson, E.Ö.; Nader, K. Involvement of the anterior cingulate cortex in formation, consolidation, and reconsolidation of recent and remote contextual fear memory. Learn. Mem. 2012, 19, 449–452. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rajasethupathy, P.; Sankaran, S.; Marshel, J.; Kim, C.; Ferenczi, E.A.; Lee, S.Y.; Berndt, A.; Ramakrishnan, C.; Jaffe, A.; Lo, M.; et al. Projections from neocortex mediate top-down control of memory retrieval. Nat. Cell Biol. 2015, 526, 653–659. [Google Scholar] [CrossRef] [Green Version]
- Rizzo, V.; Touzani, K.; Raveendra, B.L.; Swarnkar, S.; Lora, J.; Kadakkuzha, B.M.; Liu, X.-A.; Zhang, C.; Betel, D.; Stackman, R.W.; et al. Encoding of Contextual Fear Memory Requires De Novo Proteins in the Prelimbic Cortex. Biol. Psychiatry Cogn. Neurosci. Neuroimaging 2017, 2, 158–169. [Google Scholar] [CrossRef] [Green Version]
- Kitamura, T.; Ogawa, S.K.; Roy, D.S.; Okuyama, T.; Morrissey, M.D.; Smith, L.M.; Redondo, R.L.; Tonegawa, S. Engrams and circuits crucial for systems consolidation of a memory. Science 2017, 356, 73–78. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Matos, M.R.; Visser, E.; Kramvis, I.; Van Der Loo, R.J.; Gebuis, T.; Zalm, R.; Rao-Ruiz, P.; Mansvelder, H.D.; Smit, A.B.; Oever, M.C.V.D. Memory strength gates the involvement of a CREB-dependent cortical fear engram in remote memory. Nat. Commun. 2019, 10, 2315. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Le Merre, P.; Ährlund-Richter, S.; Carlén, M. The mouse prefrontal cortex: Unity in diversity. Neuron 2021, 109, 1925–1944. [Google Scholar] [CrossRef]
- Carlén, M. What constitutes the prefrontal cortex? Science 2017, 358, 478–482. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Van De Werd, H.J.J.M.; Rajkowska, G.; Evers, P.; Uylings, H.B.M. Cytoarchitectonic and chemoarchitectonic characterization of the prefrontal cortical areas in the mouse. Brain Struct. Funct. 2010, 214, 339–353. [Google Scholar] [CrossRef] [Green Version]
- Maviel, T.; Durkin, T.P.; Menzaghi, F.; Bontempi, B. Sites of Neocortical Reorganization Critical for Remote Spatial Memory. Science 2004, 305, 96–99. [Google Scholar] [CrossRef] [Green Version]
- Lynch, G.; Schubert, P. The Use of In Vitro Brain Slices for Multidisciplinary Studies of Synaptic Function. Annu. Rev. Neurosci. 1980, 3, 1–22. [Google Scholar] [CrossRef]
- Tao, C.; Zhang, G.; Xiong, Y.; Zhou, Y. Functional dissection of synaptic circuits: In vivo patch-clamp recording in Neuroscience. Front. Neural Circuits 2015, 9, 23. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Buzsáki, G.; Draguhn, A. Neuronal Oscillations in Cortical Networks. Science 2004, 304, 1926–1929. [Google Scholar] [CrossRef] [Green Version]
- Whittington, M.A.; Traub, R.D.; Adams, N.E. A future for neuronal oscillation research. Brain Neurosci. Adv. 2018, 2, 2398212818794827. [Google Scholar] [CrossRef] [Green Version]
- Nakai, J.; Ohkura, M.; Imoto, K. A high signal-to-noise Ca2+ probe composed of a single green fluorescent protein. Nat. Biotechnol. 2001, 19, 137–141. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.; Yuste, W.Y.R. In vivo imaging of neural activity. Nat. Methods 2017, 14, 349–359. [Google Scholar] [CrossRef]
- Hamel, E.J.; Grewe, B.; Parker, J.G.; Schnitzer, M.J. Cellular Level Brain Imaging in Behaving Mammals: An Engineering Approach. Neuron 2015, 86, 140–159. [Google Scholar] [CrossRef] [Green Version]
- Hong, G.; Lieber, C.M. Novel electrode technologies for neural recordings. Nat. Rev. Neurosci. 2019, 20, 330–345. [Google Scholar] [CrossRef]
- Khalaf, O.; Resch, S.; Dixsaut, L.; Gorden, V.; Glauser, L.; Gräff, J. Reactivation of recall-induced neurons contributes to remote fear memory attenuation. Science 2018, 360, 1239–1242. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guzowski, J.F.; McNaughton, B.L.; Barnes, C.A.; Worley, P.F. Environment-specific expression of the immediate-early gene Arc in hippocampal neuronal ensembles. Nat. Neurosci. 1999, 2, 1120–1124. [Google Scholar] [CrossRef]
- Khalaf, O.; Gräff, J. Reactivation of Recall-Induced Neurons in the Infralimbic Cortex and the Basolateral Amygdala After Remote Fear Memory Attenuation. Front. Mol. Neurosci. 2019, 12, 70. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nonaka, A.; Toyoda, T.; Miura, Y.; Hitora-Imamura, N.; Naka, M.; Eguchi, M.; Yamaguchi, S.; Ikegaya, Y.; Matsuki, N.; Nomura, H. Synaptic Plasticity Associated with a Memory Engram in the Basolateral Amygdala. J. Neurosci. 2014, 34, 9305–9309. [Google Scholar] [CrossRef] [Green Version]
- Reijmers, L.G.; Perkins, B.L.; Matsuo, N.; Mayford, M. Localization of a Stable Neural Correlate of Associative Memory. Science 2007, 317, 1230–1233. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- DeNardo, L.A.; Liu, C.D.; Allen, W.E.; Adams, E.L.; Friedmann, D.; Fu, L.; Guenthner, C.J.; Tessier-Lavigne, M.; Luo, L. Temporal evolution of cortical ensembles promoting remote memory retrieval. Nat. Neurosci. 2019, 22, 460–469. [Google Scholar] [CrossRef]
- Guenthner, C.J.; Miyamichi, K.; Yang, H.; Heller, H.C.; Luo, L. Permanent Genetic Access to Transiently Active Neurons via TRAP: Targeted Recombination in Active Populations. Neuron 2013, 78, 773–784. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alexander, G.; Rogan, S.C.; Abbas, A.; Armbruster, B.N.; Pei, Y.; Allen, J.A.; Nonneman, R.J.; Hartmann, J.; Moy, S.S.; Nicolelis, M.A.; et al. Remote Control of Neuronal Activity in Transgenic Mice Expressing Evolved G Protein-Coupled Receptors. Neuron 2009, 63, 27–39. [Google Scholar] [CrossRef] [Green Version]
- Roth, B.L. DREADDs for Neuroscientists. Neuron 2016, 89, 683–694. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cruz, F.C.; Koya, E.; Guez-Barber, D.H.; Bossert, J.M.; Lupica, C.; Shaham, Y.; Hope, B.T. New technologies for examining the role of neuronal ensembles in drug addiction and fear. Nat. Rev. Neurosci. 2013, 14, 743–754. [Google Scholar] [CrossRef] [Green Version]
- Boyden, E.S.; Zhang, F.; Bamberg, E.; Nagel, G.; Deisseroth, K. Millisecond-timescale, genetically targeted optical control of neural activity. Nat. Neurosci. 2005, 8, 1263–1268. [Google Scholar] [CrossRef] [PubMed]
- Deisseroth, K. Optogenetics: 10 years of microbial opsins in Neuroscience. Nat. Neurosci. 2015, 18, 1213–1225. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sun, X.; Bernstein, M.J.; Meng, M.; Rao, S.; Sorensen, A.; Yao, L.; Zhang, X.; Anikeeva, P.O.; Lin, Y. Functionally Distinct Neuronal Ensembles within the Memory Engram. Cell 2020, 181, 410–423.e17. [Google Scholar] [CrossRef]
- Sorensen, A.; Cooper, Y.A.; Baratta, M.V.; Weng, F.-J.; Zhang, Y.; Ramamoorthi, K.; Fropf, R.; Laverriere, E.K.; Xue, J.; Young, A.; et al. A robust activity marking system for exploring active neuronal ensembles. eLife 2016, 5, e13918. [Google Scholar] [CrossRef] [Green Version]
- Choi, J.-H.; Sim, S.-E.; Kim, J.-I.; Choi, D.I.; Oh, J.; Ye, S.; Lee, J.; Kim, T.; Ko, H.-G.; Lim, C.-S.; et al. Interregional synaptic maps among engram cells underlie memory formation. Science 2018, 360, 430–435. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ghandour, K.; Ohkawa, N.; Fung, C.C.A.; Asai, H.; Saitoh, Y.; Takekawa, T.; Okubo-Suzuki, R.; Soya, S.; Nishizono, H.; Matsuo, M.; et al. Orchestrated ensemble activities constitute a hippocampal memory engram. Nat. Commun. 2019, 10, 2637. [Google Scholar] [CrossRef] [Green Version]
- Silva, B.A.; Burns, A.M.; Gräff, J. A cFos activation map of remote fear memory attenuation. Psychopharmacology 2018, 236, 369–381. [Google Scholar] [CrossRef] [Green Version]
- Wheeler, A.L.; Teixeira, C.; Wang, A.H.; Xiong, X.; Kovacevic, N.; Lerch, J.P.; McIntosh, A.; Parkinson, J.; Frankland, P.W. Identification of a Functional Connectome for Long-Term Fear Memory in Mice. PLoS Comput. Biol. 2013, 9, e1002853. [Google Scholar] [CrossRef] [PubMed]
- Aceti, M.; Vetere, G.; Novembre, G.; Restivo, L.; Ammassari-Teule, M. Progression of activity and structural changes in the anterior cingulate cortex during remote memory formation. Neurobiol. Learn. Mem. 2015, 123, 67–71. [Google Scholar] [CrossRef]
- Restivo, L.; Vetere, G.; Bontempi, B.; Ammassari-Teule, M. The Formation of Recent and Remote Memory Is Associated with Time-Dependent Formation of Dendritic Spines in the Hippocampus and Anterior Cingulate Cortex. J. Neurosci. 2009, 29, 8206–8214. [Google Scholar] [CrossRef] [PubMed]
- Corcoran, K.A.; Frick, B.J.; Radulovic, J.; Kay, L.M. Analysis of coherent activity between retrosplenial cortex, hippocampus, thalamus, and anterior cingulate cortex during retrieval of recent and remote context fear memory. Neurobiol. Learn. Mem. 2016, 127, 93–101. [Google Scholar] [CrossRef] [Green Version]
- Makino, Y.; Polygalov, D.; Bolaños, F.; Benucci, A.; McHugh, T.J. Physiological Signature of Memory Age in the Prefrontal-Hippocampal Circuit. Cell Rep. 2019, 29, 3835–3846.e5. [Google Scholar] [CrossRef] [Green Version]
- Goshen, I.; Brodsky, M.; Prakash, R.; Wallace, J.; Gradinaru, V.; Ramakrishnan, C.; Deisseroth, K. Dynamics of Retrieval Strategies for Remote Memories. Cell 2011, 147, 678–689. [Google Scholar] [CrossRef] [Green Version]
- Beeman, C.L.; Bauer, P.S.; Pierson, J.L.; Quinn, J.J. Hippocampus and medial prefrontal cortex contributions to trace and contextual fear memory expression over time. Learn. Mem. 2013, 20, 336–343. [Google Scholar] [CrossRef] [Green Version]
- Varela, C.; Weiss, S.; Meyer, R.; Halassa, M.; Biedenkapp, J.; Wilson, M.A.; Goosens, K.A.; Bendor, D. Tracking the Time-Dependent Role of the Hippocampus in Memory Recall Using DREADDs. PLoS ONE 2016, 11, e0154374. [Google Scholar] [CrossRef]
- Doyère, V.; Burette, F.; Negro, C.R.-D.; Laroche, S. Long-term potentiation of hippocampal afferents and efferents to prefrontal cortex: Implications for associative learning. Neuropsychologia 1993, 31, 1031–1053. [Google Scholar] [CrossRef]
- Tang, J.; Ko, S.; Ding, H.-K.; Qiu, C.-S.; Calejesan, A.A.; Zhuo, M. Pavlovian Fear Memory Induced by Activation in the Anterior Cingulate Cortex. Mol. Pain 2005, 1, 6. [Google Scholar] [CrossRef] [Green Version]
- Cho, J.-H.; Rendall, S.D.; Gray, J.M. Brain-wide maps of Fos expression during fear learning and recall. Learn. Mem. 2017, 24, 169–181. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu, W.; Morishita, W.; Buckmaster, P.S.; Pang, Z.; Malenka, R.C.; Südhof, T.C. Distinct Neuronal Coding Schemes in Memory Revealed by Selective Erasure of Fast Synchronous Synaptic Transmission. Neuron 2012, 73, 990–1001. [Google Scholar] [CrossRef] [Green Version]
- Vetere, G.; Restivo, L.; Cole, C.J.; Ross, P.; Ammassari-Teule, M.; Josselyn, S.A.; Frankland, P.W. Spine growth in the anterior cingulate cortex is necessary for the consolidation of contextual fear memory. Proc. Natl. Acad. Sci. USA 2011, 108, 8456–8460. [Google Scholar] [CrossRef] [Green Version]
- Zelikowsky, M.; Bissiere, S.; Hast, T.A.; Bennett, R.Z.; Abdipranoto, A.; Vissel, B.; Fanselow, M.S. Prefrontal microcircuit underlies contextual learning after hippocampal loss. Proc. Natl. Acad. Sci. USA 2013, 110, 9938–9943. [Google Scholar] [CrossRef] [Green Version]
- Moscarello, J.M.; LeDoux, J.E. Active Avoidance Learning Requires Prefrontal Suppression of Amygdala-Mediated Defensive Reactions. J. Neurosci. 2013, 33, 3815–3823. [Google Scholar] [CrossRef]
- Yizhar, O.; Fenno, L.E.; Prigge, M.; Schneider, F.; Davidson, T.; O’Shea, D.J.; Sohal, V.; Goshen, I.; Finkelstein, J.; Paz, J.T.; et al. Neocortical excitation/inhibition balance in information processing and social dysfunction. Nat. Cell Biol. 2011, 477, 171–178. [Google Scholar] [CrossRef] [PubMed]
- Do-Monte, F.H.; Quiñones-Laracuente, K.; Quirk, G.J. A temporal shift in the circuits mediating retrieval of fear memory. Nat. Cell Biol. 2015, 519, 460–463. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sierra-Mercado, D.; Padilla-Coreano, N.; Quirk, G.J. Dissociable Roles of Prelimbic and Infralimbic Cortices, Ventral Hippocampus, and Basolateral Amygdala in the Expression and Extinction of Conditioned Fear. Neuropsychopharmacology 2010, 36, 529–538. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dejean, C.; Courtin, J.; Karalis, N.; Chaudun, F.; Wurtz, H.; Bienvenu, T.; Herry, C. Prefrontal neuronal assemblies temporally control fear behaviour. Nat. Cell Biol. 2016, 535, 420–424. [Google Scholar] [CrossRef] [PubMed]
- Lehmann, H.; Lacanilao, S.; Sutherland, R.J. Complete or partial hippocampal damage produces equivalent retrograde amnesia for remote contextual fear memories. Eur. J. Neurosci. 2007, 25, 1278–1286. [Google Scholar] [CrossRef] [PubMed]
- Zelikowsky, M.; Hersman, S.; Chawla, M.K.; Barnes, C.A.; Fanselow, M.S. Neuronal Ensembles in Amygdala, Hippocampus, and Prefrontal Cortex Track Differential Components of Contextual Fear. J. Neurosci. 2014, 34, 8462–8466. [Google Scholar] [CrossRef] [Green Version]
- Pereira, L.M.; De Castro, C.M.; Guerra, L.T.L.; Queiroz, T.M.; Marques, J.T.; Pereira, G.S. Hippocampus and Prefrontal Cortex Modulation of Contextual Fear Memory Is Dissociated by Inhibiting De Novo Transcription During Late Consolidation. Mol. Neurobiol. 2019, 56, 5507–5519. [Google Scholar] [CrossRef]
- Lesburguères, E.; Gobbo, O.L.; Alaux-Cantin, S.; Hambucken, A.; Trifilieff, P.; Bontempi, B. Early Tagging of Cortical Networks Is Required for the Formation of Enduring Associative Memory. Science 2011, 331, 924–928. [Google Scholar] [CrossRef] [Green Version]
- Chen, M.B.; Jiang, X.; Quake, S.R.; Südhof, T.C. Persistent transcriptional programmes are associated with remote memory. Nat. Cell Biol. 2020, 587, 437–442. [Google Scholar] [CrossRef]
- Kol, A.; Adamsky, A.; Groysman, M.; Kreisel, T.; London, M.; Goshen, I. Astrocytes contribute to remote memory formation by modulating hippocampal–cortical communication during learning. Nat. Neurosci. 2020, 23, 1229–1239. [Google Scholar] [CrossRef] [PubMed]
- Ye, X.; Kapeller-Libermann, D.; Travaglia, A.; Inda, M.C.; Alberini, C.M. Direct dorsal hippocampal–prelimbic cortex connections strengthen fear memories. Nat. Neurosci. 2016, 20, 52–61. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Herry, C.; Ciocchi, S.; Senn, V.; Demmou, L.; Müller, C.; Lüthi, A. Switching on and off fear by distinct neuronal circuits. Nat. Cell Biol. 2008, 454, 600–606. [Google Scholar] [CrossRef]
- Bayer, H.; Bertoglio, L.J. Infralimbic cortex controls fear memory generalization and susceptibility to extinction during consolidation. Sci. Rep. 2020, 10, 15827. [Google Scholar] [CrossRef] [PubMed]
- Do-Monte, F.H.; Nieves, G.M.; Quiñones-Laracuente, K.; Ramos-Medina, L.; Quirk, G.J. Revisiting the Role of Infralimbic Cortex in Fear Extinction with Optogenetics. J. Neurosci. 2015, 35, 3607–3615. [Google Scholar] [CrossRef] [PubMed]
- Giustino, T.; Emaren, S. The Role of the Medial Prefrontal Cortex in the Conditioning and Extinction of Fear. Front. Behav. Neurosci. 2015, 9, 298. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Karalis, N.; Dejean, C.; Chaudun, F.; Khoder, S.; Rozeske, R.R.; Wurtz, H.; Bagur, S.; Benchenane, K.; Sirota, N.K.A.; Courtin, J.; et al. 4-Hz oscillations synchronize prefrontal–amygdala circuits during fear behavior. Nat. Neurosci. 2016, 19, 605–612. [Google Scholar] [CrossRef] [Green Version]
- Knapska, E.; Macias, M.; Mikosz, M.; Nowak, A.; Owczarek, D.; Wawrzyniak, M.; Pieprzyk, M.; Cymerman, I.A.; Werka, T.; Sheng, M.; et al. Functional anatomy of neural circuits regulating fear and extinction. Proc. Natl. Acad. Sci. USA 2012, 109, 17093–17098. [Google Scholar] [CrossRef] [Green Version]
- Bloodgood, D.W.; Sugam, J.A.; Holmes, A.; Kash, T.L. Fear extinction requires infralimbic cortex projections to the basolateral amygdala. Transl. Psychiatry 2018, 8, 60. [Google Scholar] [CrossRef] [Green Version]
- Ye, L.; Allen, W.E.; Thompson, K.R.; Tian, Q.; Hsueh, B.; Ramakrishnan, C.; Wang, A.-C.; Jennings, J.H.; Adhikari, A.; Halpern, C.H.; et al. Wiring and Molecular Features of Prefrontal Ensembles Representing Distinct Experiences. Cell 2016, 165, 1776–1788. [Google Scholar] [CrossRef] [Green Version]
- Taylor, J.A.; Hasegawa, M.; Benoit, C.M.; Freire, J.A.; Theodore, M.; Ganea, D.A.; Innocenti, S.M.; Lu, T.; Gründemann, J. Single cell plasticity and population coding stability in auditory thalamus upon associative learning. Nat. Commun. 2021, 12, 2438. [Google Scholar] [CrossRef]
- Silva, B.A.; Astori, S.; Burns, A.M.; Heiser, H.; Heuvel, L.V.D.; Santoni, G.; Martinez-Reza, M.F.; Sandi, C.; Gräff, J. A thalamo-amygdalar circuit underlying the extinction of remote fear memories. Nat. Neurosci. 2021, 24, 964–974. [Google Scholar] [CrossRef]
- Vetere, G.; Xia, F.; Ramsaran, A.I.; Tran, L.M.; Josselyn, S.A.; Frankland, P.W. An inhibitory hippocampal–thalamic pathway modulates remote memory retrieval. Nat. Neurosci. 2021, 24, 685–693. [Google Scholar] [CrossRef]
- Squire, L.R. Memory and the hippocampus: A synthesis from findings with rats, monkeys, and humans. Psychol. Rev. 1992, 99, 195–231. [Google Scholar] [CrossRef] [PubMed]
- Squire, L.R.; Cohen, N.J.; Nadel, L. The Medial Temporal Region and Memory Consolidation: A New Hypothesis. In Memory Consolidation; Psychology Press: Hove, UK, 1984; ISBN 978-1-315-80262-6. [Google Scholar]
- Teyler, T.J.; DiScenna, P. The hippocampal memory indexing theory. Behav. Neurosci. 1986, 100, 147–154. [Google Scholar] [CrossRef]
- Goode, T.D.; Tanaka, K.Z.; Sahay, A.; McHugh, T.J. An Integrated Index: Engrams, Place Cells, and Hippocampal Memory. Neuron 2020, 107, 805–820. [Google Scholar] [CrossRef]
- Teyler, T.J.; Rudy, J.W. The hippocampal indexing theory and episodic memory: Updating the index. Hippocampus 2007, 17, 1158–1169. [Google Scholar] [CrossRef]
- Nadel, L.; Moscovitch, M. Memory consolidation, retrograde amnesia and the hippocampal complex. Curr. Opin. Neurobiol. 1997, 7, 217–227. [Google Scholar] [CrossRef]
- Moscovitch, M.; Nadel, L.; Winocur, G.; Gilboa, A.; Rosenbaum, R.S. The cognitive Neuroscience of remote episodic, semantic and spatial memory. Curr. Opin. Neurobiol. 2006, 16, 179–190. [Google Scholar] [CrossRef]
- Tse, D.; Takeuchi, T.; Kakeyama, M.; Kajii, Y.; Okuno, H.; Tohyama, C.; Bito, H.; Morris, R.G.M. Schema-Dependent Gene Activation and Memory Encoding in Neocortex. Science 2011, 333, 891–895. [Google Scholar] [CrossRef] [Green Version]
- Redondo, R.L.; Kim, J.; Arons, A.L.; Ramirez, S.; Liu, X.; Tonegawa, S. Bidirectional switch of the valence associated with a hippocampal contextual memory engram. Nat. Cell Biol. 2014, 513, 426–430. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ryan, T.J.; Roy, D.S.; Pignatelli, M.; Arons, A.; Tonegawa, S. Engram cells retain memory under retrograde amnesia. Science 2015, 348, 1007–1013. [Google Scholar] [CrossRef] [Green Version]
- Rashid, A.J.; Yan, C.; Mercaldo, V.; Hsiang, H.-L. (Liz); Park, S.; Cole, C.J.; De Cristofaro, A.; Yu, J.; Ramakrishnan, C.; Lee, S.Y.; et al. Competition between engrams influences fear memory formation and recall. Science 2016, 353, 383–387. [Google Scholar] [CrossRef] [Green Version]
- Todd, T.P.; Mehlman, M.L.; Keene, C.S.; Deangeli, N.E.; Bucci, D.J. Retrosplenial cortex is required for the retrieval of remote memory for auditory cues. Learn. Mem. 2016, 23, 278–288. [Google Scholar] [CrossRef] [Green Version]
- Cowansage, K.K.; Shuman, T.; Dillingham, B.; Chang, A.; Golshani, P.; Mayford, M. Direct Reactivation of a Coherent Neocortical Memory of Context. Neuron 2014, 84, 432–441. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Milczarek, M.M.; Vann, S.D.; Sengpiel, F. Spatial Memory Engram in the Mouse Retrosplenial Cortex. Curr. Biol. 2018, 28, 1975–1980.e6. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Haubrich, J.; Cassini, L.F.; Diehl, F.; Santana, F.; de Oliveira, L.F.; Alvares, L.D.O.; Quillfeldt, J.A. Novel learning accelerates systems consolidation of a contextual fear memory. Hippocampus 2016, 26, 924–932. [Google Scholar] [CrossRef]
- Pedraza, L.K.; Sierra, R.O.; Crestani, A.P.; Quillfeldt, J.A.; Alvares, L.D.O. Sequential learning during contextual fear conditioning guides the rate of systems consolidation: Implications for consolidation of multiple memory traces. Hippocampus 2017, 27, 518–528. [Google Scholar] [CrossRef] [PubMed]
- Wartman, B.C.; Holahan, M.R. The use of sequential hippocampal-dependent and -non-dependent tasks to study the activation profile of the anterior cingulate cortex during recent and remote memory tests. Neurobiol. Learn. Mem. 2013, 106, 334–342. [Google Scholar] [CrossRef] [PubMed]
- Grella, S.L.; Fortin, A.H.; McKissick, O.; Leblanc, H.; Ramirez, S. Odor modulates the temporal dynamics of fear memory consolidation. Learn. Mem. 2020, 27, 150–163. [Google Scholar] [CrossRef]
- Cai, D.; Aharoni, D.; Shuman, T.; Shobe, J.; Biane, J.; Song, W.; Wei, B.; Veshkini, M.; La-Vu, M.; Lou, J.; et al. A shared neural ensemble links distinct contextual memories encoded close in time. Nat. Cell Biol. 2016, 534, 115–118. [Google Scholar] [CrossRef] [Green Version]
- Wang, C.; Yue, H.; Hu, Z.; Shen, Y.; Ma, J.; Li, J.; Wang, X.-D.; Wang, L.; Sun, B.; Shi, P.; et al. Microglia mediate forgetting via complement-dependent synaptic elimination. Science 2020, 367, 688–694. [Google Scholar] [CrossRef]
- Pan, S.; Mayoral, S.R.; Choi, H.S.; Chan, J.R.; Kheirbek, M.A. Preservation of a remote fear memory requires new myelin formation. Nat. Neurosci. 2020, 23, 487–499. [Google Scholar] [CrossRef] [Green Version]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dixsaut, L.; Gräff, J. The Medial Prefrontal Cortex and Fear Memory: Dynamics, Connectivity, and Engrams. Int. J. Mol. Sci. 2021, 22, 12113. https://doi.org/10.3390/ijms222212113
Dixsaut L, Gräff J. The Medial Prefrontal Cortex and Fear Memory: Dynamics, Connectivity, and Engrams. International Journal of Molecular Sciences. 2021; 22(22):12113. https://doi.org/10.3390/ijms222212113
Chicago/Turabian StyleDixsaut, Lucie, and Johannes Gräff. 2021. "The Medial Prefrontal Cortex and Fear Memory: Dynamics, Connectivity, and Engrams" International Journal of Molecular Sciences 22, no. 22: 12113. https://doi.org/10.3390/ijms222212113
APA StyleDixsaut, L., & Gräff, J. (2021). The Medial Prefrontal Cortex and Fear Memory: Dynamics, Connectivity, and Engrams. International Journal of Molecular Sciences, 22(22), 12113. https://doi.org/10.3390/ijms222212113




