Abstract
Concerns regarding the overconsumption of natural resources has provoked the recovery of biopolymers from food processing biomass. Furthermore, the current market opportunity for pectin in other areas has increased, necessitating the search for alternative pectin resources. This is also a step towards the sustainable and circular green economy. Mango peel is the byproduct of agro-processing and has been used for high value-added components such as polysaccharide biopolymers. Pectin derived from the peel is yet to be exploited to its greatest extent, particularly in terms of its separation and physiochemical properties, which limit its applicability to dietary fiber in culinary applications. The functionality of the mango peel pectin (MPP) strongly depends on the molecular size and degree of esterification which highlight the importance of isolation and characterisation of pectin from this novel resource. This article therefore provides a useful overview of mango peel as a potential biomaterial for the recovery of MPP. Different extraction techniques and the integrated recovery were also discussed. The utilisation of MPP in different industrial schemes are also detailed out from different perspectives such as the pharmaceutical and biotechnology industries. This review convincingly expresses the significance of MPP, providing a sustainable opportunity for food and pharmaceutical development.
1. Introduction
Fruits are widely used in agri-food industry in which large quantity of by-products including pomace, peel, rind and seeds are generated [1,2]. This biomass is a potential source for valuable bioactive compound recovery such as dietary fibres, carotenoids, polyphenols, oils, vitamins and many other compounds [3]. Mango is one of the most consumed tropical fruits, known for its high nutritive values and extensively cultivated in the tropical and sub-tropical regions. Several preserved products of mango are commercially needed such as can, dried mango, frozen slices, purée, juices and nectar [4,5,6]. It is estimated that around 200,000 tons of biomass are generated during these processing and peels account for as high as 25% of the volume [7]. So far, attempts have been made in trying to value-add such the biomass from mango processing using integrated refinery approaches [8,9,10,11,12,13]. Besides its high contents of carbohydrates, proteins, fats and various classes antioxidants such as polyphenols, carotenoids and vitamins [14,15,16,17], this high-volume biomass is known as a potential source of dietary fibre [10,18,19,20]. The soluble dietary fibre is a carbohydrate polymer with more than 10 monomeric units that makes it is difficult to be hydrolysed by endogenous enzymes in the human small intestine [21,22]. They include pectin, galactomannan, inulin, gum while pectin is of high commercial need for functional foods and pharmaceutical applications [23,24,25,26]. Besides, mango peel contains high cellulose content (30%) and lignin (16%) [27,28]. As a result, it was employed as a novel source for biopolymer recovery. Additionally, it comprises of 5–20% of pectin with variable contents of galacturonic acid, dependent upon the extraction methods and the cultivars [10,18,19,20]. To extract such the value-added biopolymers, the integrated isolation approach can be used [29].
The global need for pectin as biopolymer amounted to $1 billion in 2019 and is expected to rise to $1.5 billion in 2025 [26]. Commercial pectin is mainly recovered from either apple pomace or citrus peel which are of different physicochemical functionalities based up on the presence of pectin esterase of the raw materials [30]. Apple pomace pectin forms a gel of high viscosity which is suitable as a medicinal polymer, while the lighter colour of citrus pectin is preferable in the confectionery industry. Biopolymer pectin for industry requires a minimum of 65% of galacturonic acid on ash and moisture-free substances which limit other potential new resources for pectin recovery. In recent years, the recovery of non-starch polysaccharides from fruit by-products has become a promising strategy for the development of natural biopolymers [31]. Besides these, the information on mango peel pectin (MPP) as a potential biopolymer for industrial applications is not collective. In this study, the characteristics, value adding components and biorefinery process of mango peel are discussed. Featuring the most-sought after pectin biopolymer, its chemical structures and different extraction processes are highlighted along with possible applications in different industries are collectively presented. This review provides a useful baseline for substantial production of MPP as well as a guidance for the global policy of zero-waste processing and sustainable used of natural resource.
2. Mango Peel as the Novel Source for Pectin Biopolymer
2.1. Mango Variety
A wide range of mango varieties are cultivated including those of native and new bred cultivars. Therefore, the yield and the physiological attributes are diverse, depending on their gene pools and further interaction with environmental conditions [32,33]. Physical characteristics such as fruit weight, size and peel colour have been used to describe mango varieties. These physical attributes also play a crucial role in consumption and industrial processing. The commercial attributes of mango physiology are illustrated in Table 1. The CIE colour space (L*, a*, b*) has been used to determine maturity index and the ripening process of mangoes [34,35]. The information of length, width and breadth of mango fruits are used for arithmetic mean diameter (Da) and geometric mean diameter [36] data calculation. These values are regarded as physical parameters during fruit grading [37]. Likewise, the ratio of width-to-length or aspect ratio (Ra) indicates an ellipsoid shape during the process of fruit development [38]. The greater value of Ra signifies more advanced ripening stages of the fruits [39]. In addition, specific gravity as defined as fruit soluble matters of the sugar contents along with firmness alteration can be typical used to define the stage of maturity [5]. It is worth highlighting that both parameters intercorrelate with each other, and greater values of Ra and sphericity denote an advanced stages of fruit ripening [39]. Moreover, a higher fruit ripeness leads to a greater content of pectin from fruit peel [40]. Wongkaew et al. [6] reported that the physical properties of fruit physical properties (Colour, Da, Dg, Ra, sphericity, surface area and percentage yield of fruit parts) can be used to distinguish mango varieties.
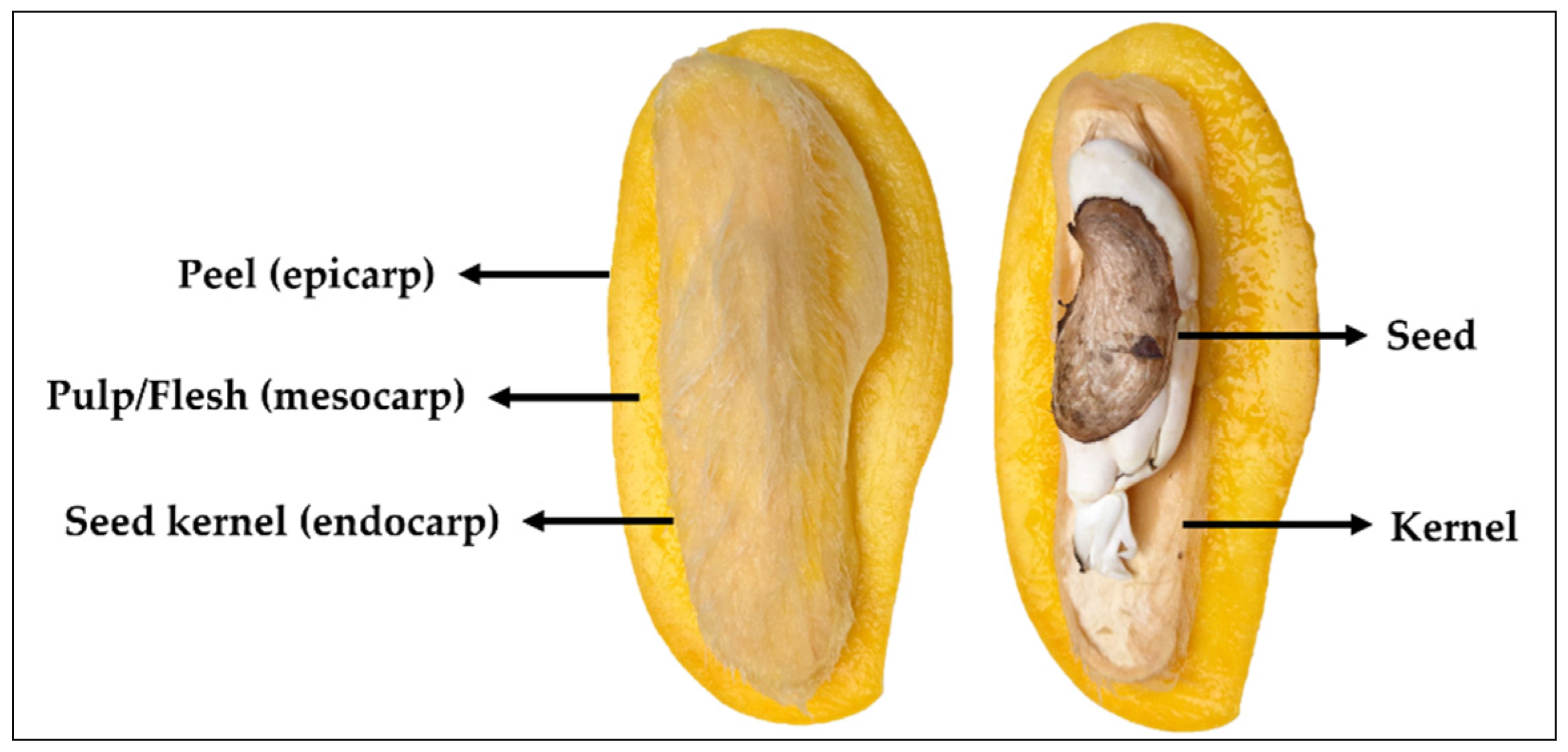
The morphology of mango fruit comprises of three parts, namely pulp (mesocarp), peel (epicarp) and seed kernel (endocarp), as illustrated in Figure 1. Mango pulp is a source of a variety of phytochemical components including those of reducing sugars, amino acids, aromatic compounds as well as functionally active ingredients, such as pectin, vitamins, anthocyanins and polyphenols [41]. In processing, pulp is the most-consumed parts of the fruit, while the peel and seed are usually discarded (accounted for 35–60% of the total fruit weight) as biomass [7]. Peel (~5–17%) and seed (~7–17%) are known as byproducts of mango processing and the amount depends on mango varieties. These are, indeed, the potential resource for natural product biorefinery.
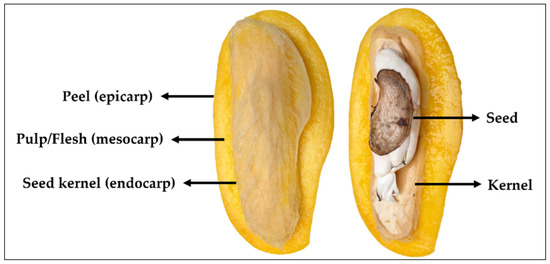
Figure 1.
The biomass composition of mango fruit.

Table 1.
Physical characteristics of different ripe mango varieties.
Table 1.
Physical characteristics of different ripe mango varieties.
| Mango Varieties | Colour | Length (mm) | Width (mm) | Breadth (mm) | Volume (mL) | Flesh Weight (%) | Peel Weight (%) | Seed Weight (%) | References | ||
|---|---|---|---|---|---|---|---|---|---|---|---|
| L* | a* | b* | |||||||||
| Mahachanok | 68.83 | 3.28 | 40.66 | 165.05 ** | 66.42 ** | 58.30 ** | 313.89 ** | 66.69 | 16.64 | 16.66 | [6] |
| Chok Anan | 69.98 | 5.55 | 43.09 | 111.77 ** | 74.80 ** | 63.1 ** | 217.49 ** | 67.32 | 14.32 | 18.29 | |
| Nam Dok Mai | 72.26 | 6.74 | 36.63 | 140.14 ** | 70.61 ** | 62.10 ** | 271.47 ** | 73.15 | 14.42 | 12.43 | |
| Kaew | 67.68 | 3.41 | 39.70 | 112.16 ** | 70.66 ** | 62.45 ** | 209.54 ** | 70.32 | 15.60 | 14.08 | |
| Rad | 47.19 | 0.26 | 17.03 | 98.47 | 51.67 | 45.89 | n/a | n/a | 5.40 | n/a | [13] |
| Ta Labnak | 33.09 | −6.01 | 9.51 | 87.26 | 81.47 | 69.17 | n/a | n/a | 6.00 | n/a | |
| Sampee | 47.43 | 3.28 | 20.36 | 93.62 | 55.69 | 47.65 | n/a | n/a | 7.60 | n/a | |
| Nyala | n/a | n/a | n/a | 83.00 | 83.00 | n/a | 150.00 | 82.10 | 10.70 | 7.20 | [42] |
| Edelfursan | n/a | n/a | n/a | 92.00 | 92.00 | n/a | 250.00 | 81.57 | 10.53 | 7.90 | |
| Kaboom | n/a | n/a | n/a | 95.00 | 95.00 | n/a | 300.00 | 82.40 | 10.60 | 7.00 | |
| Alphonso | n/a | n/a | n/a | 94.60 | 73.40 | 60.60 | 214.40 | 74.58 | 14.19 | 11.22 | [43] |
| Kesar | n/a | n/a | n/a | 95.70 | 65.70 | 58.00 | 188.80 | 71.28 | 13.06 | 15.66 | |
| Totapuri | n/a | n/a | n/a | 123.60 | 70.80 | 66.60 | 261.50 | 71.33 | 16.42 | 12.25 | |
** unpublished data by the first author; n/a = not available.
2.2. Value-Added Components from Mango Peel
As mentioned, peel and seed are the major by-products of the mango processing industry. These biomasses are usually buried in landfill or used as animal feed that; the fermentation process is generally toxic to the soil [44]. Previous reports claimed that mango peel consists of various valuable phytochemicals, including pectin, carotenoids, polyphenols and other bioactive compounds that can be used in the pharmaceutical industry [8,17,22,45]. However, these compositions are variable depending on the maturity stage, locality, variety and climatic conditions where mangoes are produced.
As presented in Table 2, mango peel contains a variety of macronutrients viz., carbohydrates, protein, lipid and crude fibre. Crude fibre is an important element to determine the significance of the by-products as sources of pectin recovery. In mango peel, the fibre content ranges between 2–20% of the total mass. While citrus peels contain as high as (>50%) dietary fibre and different varieties are currently used as raw material for citrus peel pectin including those from Valencia orange [46], Persia lime [46], lemon [47] and sweet orange [47]. Owing to its high fibre content, the peel can be used as an additive ingredient to enhance the functional properties of food and feed [48,49]. Calcium is the largest mineral constituent in the peel followed by magnesium, potassium and sodium, respectively [7]. The content of vitamin C ranges from 18–257 mg·g−1, depending again on the varieties. The vitamin E of ripened mango peel is of a greater concentration than the green peel [41].
The polyphenol content in the peel varies from 55–110 mg·g−1 dry weight and higher levels are found in the ripe fruit than they are in the unripe peel [16]. The peel is also the major source of polyphenols that are basically higher than the pulp at all growth stages [50,51]. Mangiferin (C-glucosyl xanthone), a heat-stable and pharmacologically active phytochemical, is typically found in high content of mango peel. Mangiferin illustrates various bioactivities such as antiinflammation, anti-diabetic, immunomodulatory, antitumor and antioxidant [52]. The amount of mangiferin and its derivatives is greater in the peel than in the pulp [53]. As a result of its functional properties, mangiferin is commercially used in term of therapeutic and cosmetics products [54] and food supplements [55].
Anthocyanins, water-soluble pigments, add red, blue and purple colours to the peels of mangoes. The compounds are known for their beneficial effects in the prevention of various diseases such as cancer, diabetes and neuronal and cardiovascular diseases, thereby promoting human health [56,57]. Total anthocyanin content ranges from 3600–5650 µg·g−1 in the fully ripe stage and from 2030–3260 µg·g−1 in the raw and unripe stages [16]. The major anthocyanins detected in various varieties of mangoes, namely cyanidin, pelargonidin, delphinidin, malvidin, petunidin and peonidin [58]. Regarding to their biological properties, anthocyanins are comprehensively used as a substitute for artificial colorants in foods and beverages [59,60].
Carotenoids are fat-soluble pigments that give peels and flesh their yellow, orange and red colours. Mango peel contains high concentrations of carotenoids in the form of β-carotene, a precursor for vitamin A [61]. The content of carotenoids generally increases during ripening and is the highest at the fully-ripe stage [58]. Consumption of carotenoids reduces the risk of developing certain cancers (cervical, ovarian, colorectal, prostate, breast), cardiovascular disease, bone, skin, or eye disorders, mental health, metabolic health, during pregnancy and early life and even provide cosmetic benefits [62,63]. As a result, carotenoids are also widely used in food as a colourant, antioxidant and additive [64].

Table 2.
Nutritional and phytochemical compositions of mango peel [6,13,41,49,65,66,67,68,69].
Table 2.
Nutritional and phytochemical compositions of mango peel [6,13,41,49,65,66,67,68,69].
| Compounds | Content |
|---|---|
| Macronutrients (%) | |
| Water | 31.30–76.70 |
| Carbohydrate | 10.53–30.80 |
| Protein | 2.10–8.06 |
| Total lipid | 1.40–2.48 |
| Total sugar | 25.00 |
| Total dietary fibre | 1.40–20.53 |
| Minerals (mg·100 g−1) | |
| Calcium | 150 |
| Iron | 40.6 |
| Magnesium | 100 |
| Potassium | 75 |
| Sodium | 50 |
| Copper | 10.4 |
| Vitamins | |
| Vitamin C (total ascorbic acid, mg·100 g−1) | 18–257 |
| Vitamin A (retinol activity equivalent, μg ·100 g−1) | 100 |
| Vitamin E (α-tocopherol, mg·100 g−1) | 0.25–0.59 |
| Polyphenols (mg·100 g−1) | |
| Kaempferol | 3.6 |
| Mangiferin | 169 |
| Mangiferin gallate | 321 |
| Isomangiferin | 13.4 |
| Quercetin | 6.5 |
| Rhamnetin 3-0 galactoside/glucoside | 9.4 |
| Flavonoids (catechin equivalent·100 g−1) | |
| Anthocyanins (μg) | 3600–5650 |
| Cyanidin | 22.10 |
| Pelargonidins | 22.73 |
| Delphinidins | 18.02 |
| Malvidins | 5.26 |
| Petunidins | 21.60 |
| Peonidins | 24.42 |
| Carotenoids (μg) | 3092 |
| β-carotene | 1310 |
| β-cryptoxanthin | 600 |
| Lutein and zeaxanthin | 299 |
2.3. High Value-Added Components Biorefinery
Table 3 details out research studies on the phytochemical biorefinery of mango by-product, mainly peel. The biorefinery not only value-adds the biomass but also reduces the biomass volume from the industrial processing of mango. Owing to a disposal of the loss, transportation costs and limited availability of landfills are questionable for sustainable processing. Thereby, mango peel valorisation through different techniques would undoubtedly eliminate the disposal problem.

Table 3.
Current research studies on mango peel biorefinery of various value-add products.
3. Mango Peel Pectin
3.1. Mango Peel Pectin Recovery
General pectin recovery includes a raw material pre-treatment stage, an extraction operation and a post-extraction stage [86,87]. Nevertheless, the issue on the conventional process, particularly the extraction step, is whether or not it is worth the energy and economic demands that are currently required in the practice [88]. Therefore, several sustainable and quicker alternative approaches to extract pectin from biological materials have been developed. The innovative techniques for pectin extraction include enzyme-assisted extraction, ultrasounds, subcritical fluids and microwave heating. The benefits and drawbacks of the techniques are compared as shown in Table 4.
3.1.1. Conventional Heating Extraction (CHE)
Pectin is traditionally extracted in water acidified with 0.05–2 M sulfuric, nitric, phosphoric, acetic or hydrochloric acid between 80–100 °C for 1 h with continuous stirring [89]. Conventional extraction (solid–liquid extraction) depends on a number of factors such as temperature, pH, solvent properties, solid to solvent ratio, dry solids, particle size and diffusion rate [90]. For pectin extraction, mango peel powder was initially treated with the acidified solution. Subsequently, the obtained solvent was treated with ethanol solution [91]. Through this method, an MPP yield as high as 30% can be achieved from the residue with the degree of esterification (DE) varying from approximately 60 to 90% [10,91].
3.1.2. Novel Extraction Techniques
- Microwave-Assisted Extraction (MAE)
MAE involves dielectric heating of plant molecules through the exposure of microwaves. The microwave irradiation accelerates cell rupture by a sudden temperature rise and internal pressure increase inside the cells of plant sample, which promotes the destruction of sample surface and in turns the exudation of pectin within the plant cells into the surrounding solvents and increase [92,93,94]. The conventional “on-off” microwave operation, however, may lead to the overheating of the raw material, which may ultimately result in a low quality of MPP. Consequently, a phase controller (PCMAE), which regulates the electrical power input into the magnetron thereby generating smooth and adjustable microwave power was installed additionally for a better extraction performance [10]. The applications of the MAE for pectin extraction from mango peel were reported and the obtained pectin had higher content when compared with the CHE [10,12]. The microwave provides more efficient heat than the CHE approach due to the intense formation of vapour in polar substances generated by the electromagnetic field [95].
- Enzyme-Assisted Extraction (EAE)
The enzymes are used to improve extraction process by hydrolyzing matrix of the plant cell wall. Cell wall degrading enzymes with minimum pectinolytic activity are used to hydrolyze non-pectin plant cell wall components in enzymatic extraction of pectin [96,97]. The EAE depends on reaction time, type and concentration of enzyme, temperature, pH value and particle size of plant material [98,99]. The EAE technique was applied to recover pectin from multiple bioresources such as lime [100], passion fruit [101] and apple pomace [102]. The yields of pectin were achieved with the enzymatic extraction which were greater than that obtained with the CHE method. However, the pectin extraction from mango peel using this technology has not yet been implemented.
- Ultrasound-Assisted Extraction (UAE)
Sound waves consist of mechanical vibrations, which can be applied in treatments to the solid, liquid or gas with frequencies higher than 20 kHz [99,103]. Adapted for pectin extraction, the collapse of cavitation bubbles near cell walls induced by ultrasound produces cell disruption, thus causing stronger and enhanced solvent entrance into the cells and intensification of the mass transfer [104,105]. For pectin recovery, Guandalini et al. [106] found that the UAE provided an alternative choice for pectin extraction from mango peel because through this technique an MPP yield as high as 50% can be achieved without interfering the physicochemical properties (galacturonic acid content and degree of esterification).
- Subcritical-Assisted Extraction (SWE)
Subcritical water is liquid water at elevated pressure which is able to attain temperatures higher than its normal boiling point without a change in phase. When such water is used as solvent in extraction, the process is known as subcritical water extraction (SWE) also known as pressurized hot water extraction (PHWE) and superheated water extraction (SHWE) [107]. The SWE is stated as a green route for the valorisation of mango peel in form of pectin product. Xiaa and Matharu [108] reported that the MPP extracted by the SWE with no mineral acid supplementation resulted in a great yield of 18.34%, while the DE of the pectin was more than 70%.

Table 4.
Benefits and drawbacks of the novel techniques.
Table 4.
Benefits and drawbacks of the novel techniques.
| Extraction Techniques | Benefits | Drawbacks |
|---|---|---|
| MAE |
|
|
| EAE |
|
|
| UAE |
|
|
| SWE |
|
|
3.2. MPP Functionality
Pectin is mostly extracted from various plant sources and is of great variation in term of quality. Consequently, pectin is purified and restructured in order to achieve constant and reproducible gel strength, for example HMP is improved its quality by dilution with sucrose. MPP is typical of high methoxyl content which is unable to form gel by interaction with calcium ions due to an insufficient number of carboxylic groups [8,119]. Thus, to improve its functionality for a specific purpose, de-esterification using either acidic or basic chemicals is necessary. The characteristic compositions of the extracted MPP are illustrated in Table 5.

Table 5.
Typical characteristics of mango peel pectin compared with commercial pectin.
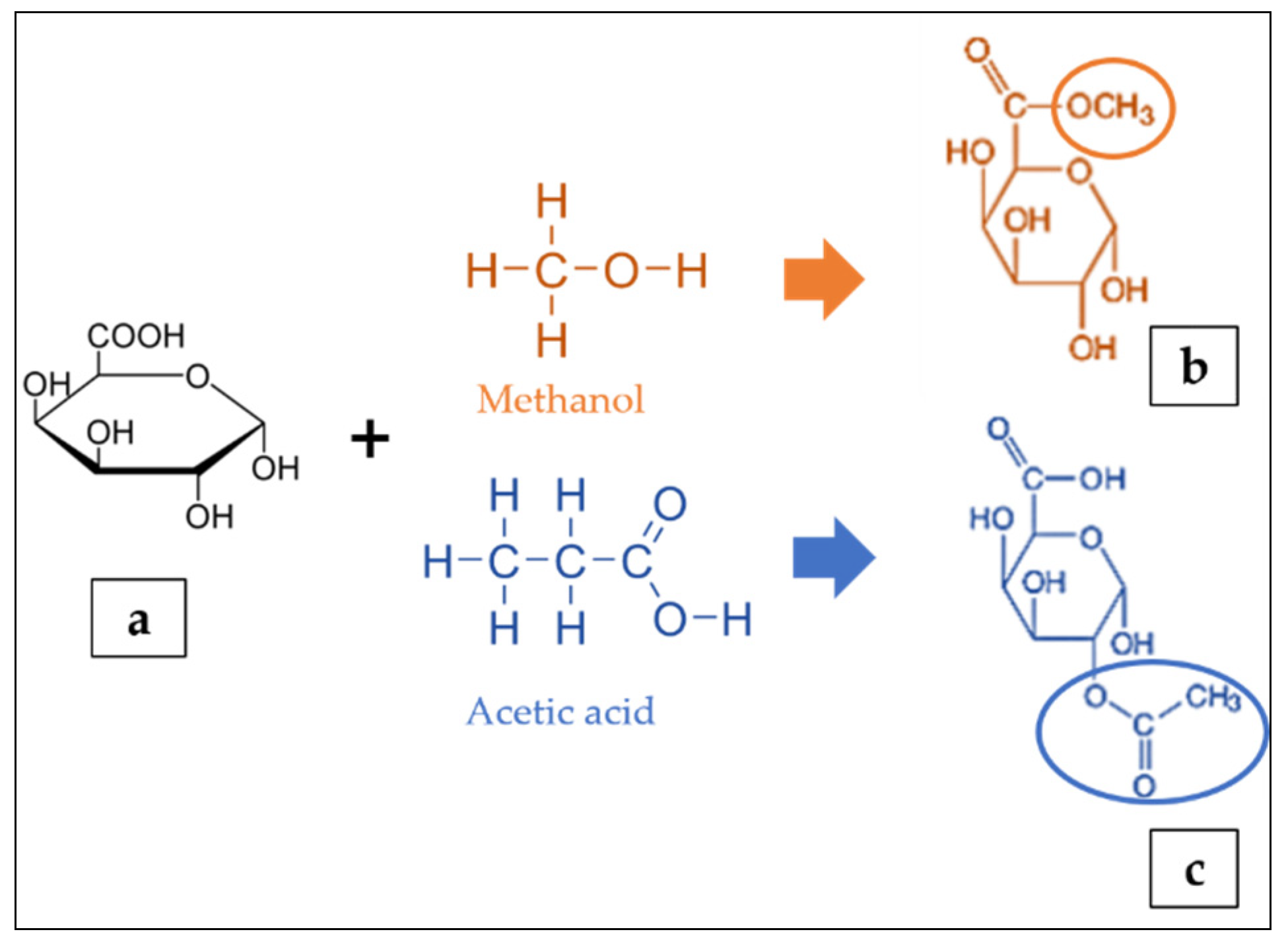
The residues of galacturonic acid (GA) (Figure 2a) are generally recognised as the backbone of the pectin structure. Its chemical structure composes of an aldehyde group at C1 and a carboxylic acid group at C6 [25]. The GA can be partially methyl-esterified at C6 with methanol and acetylated at the O2 or O3 positions with acetic acid (Figure 2b,c) [122]. The GA content can be determined by either the colorimetry [106] or high performance liquid chromatography [123]. The ratio of methyl-esterified galacturonic acid groups to the total galacturonic acid groups is defined as the degree of esterification (DE) [124,125,126]. The degree of esterification and acetylation of pectin affects the gelling properties of the pectin; a higher DE increases the capacity to form gels, whereas a higher degree of acetylation inhibits gelling [127]. The analytical quantification of DE include the titrimetric technique [106,128], gas liquid chromatography and colorimetric uronic acid analyses [129]. Furthermore, the content of GA in foods is very important because their presence can affect the chemical and sensorial characteristics of the matrix such as pH, total acidity, microbial stability, sweetness, consumer acceptability and therefore, provide precious information on the wholesome quality of the food or on the optimisation needed to impart select technical features [130]. Meanwhile, the molecular weight of pectin depends on the raw materials and the extraction techniques. Bagherian et al. [109] reported that continued heating of pectin extraction may lead to pectin networks disaggregation, thus decreasing the molecular weight.
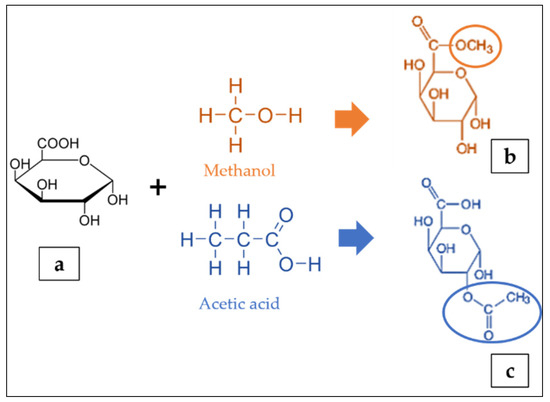
Figure 2.
Structure of galacturonic acid (a) presenting methyl esterified (b) and acetylated (c) forms adapted from [122].
In case of pectin recovered from mango peel, the GalA contents varied depending on the extraction techniques. Process optimization of extraction methods to obtain the minimal GalA level of 65% in MPP has been highlighted in many research studies [121,131,132]. Geerkens et al. [133] claimed that the preparation processes of the peel (blanching, particle size reduction) and fruit ripening stage reduced the GalA content, however the highest content obtained was 48%. Regarding the DE content, the values were in a range between 56% and 93%, which categorized it as high methoxyl pectin [134]. Both GalA and DE of pectic polysaccharides are involved in the commercial uses of pectin as gelling and thickening agents [135,136].
3.3. MPP Applications
Pectins are widely used as additive in foods and beverages such as a gelling agent, thickener, texturiser, emulsifier and stabiliser [137]. In recent years, pectin has been applied as a fat or sugar alternative in low-calorie foods [12], dietetic food [138], food packaging [139] and drug carrier [119]. Selection of pectin for a particular food depends on many factors, including the texture required, pH, processing temperature, presence of ions, proteins and the expected shelf life of the product [140]. MPP was recovered from peel of ‘Nam dok mai’ variety (Mox > 8%) and was found suitable as fat replacement in a Chinese sausage formular in its original form and colour [12]. Additionally, MPP obtained from ‘Chok anan’ variety was utilised as a substrate for pectic oligosaccharide hydrolysate with pectinase. The digested monosaccharide compositions were mainly fructose and glucose while arabinose had prominent influence on prebiotic potentials of Bifidobacterium animalis [141]. Thin films have been used as food packaging polymer and many drug delivery systems of oral, buccal, and transdermal routes. In one study, thin film was fabricated from a mixture of LMP and MPP at 1:2 ratio with 40% (w/w) glycerol. The film attained the highest elongation at break (8.80%) and lowest Young’s modulus (83.19 MPa) with an increasing hydrophobicity when the content of MPP increased [8]. For a topical drug delivery, de-esterified MPP with NaOH was proposed for thin film development [119]. In this same study, the DE decreased when a higher volume (~3.0 mL) of 1 N NaOH at 25 °C was employed in the preparation.
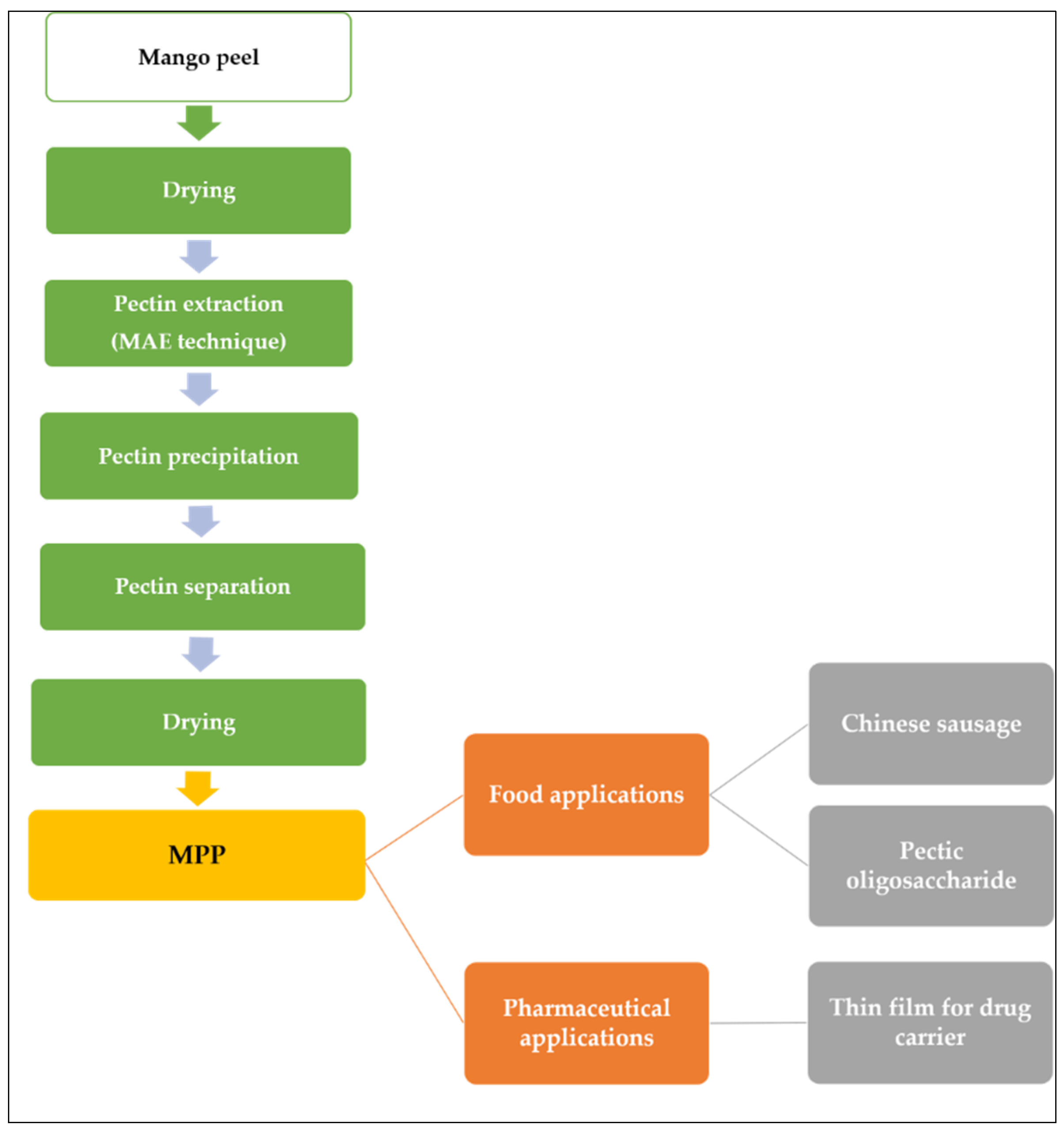
Wongkaew et al. [6] explained the industrial value chain process of MPP as illustrated in Figure 3. First, the biomass was dried and pectin extraction can be achieved with MAE techniques. The dried peel powder was suspended in diluted acidic solution (distilled H2O adjusted to pH 1.5 with 2 M HCl) and heated in a microwave oven followed by separating the residue from the solution using filtration technique. The liquid is combined with a 1:1 ethanol-water mixture to precipitate the pectin, and then it is separated by filtration. The pectin was dried at 40 °C until a consistent weight was attained. The final product can be applied to food additives or sources of prebiotic or in pharmaceutical application.
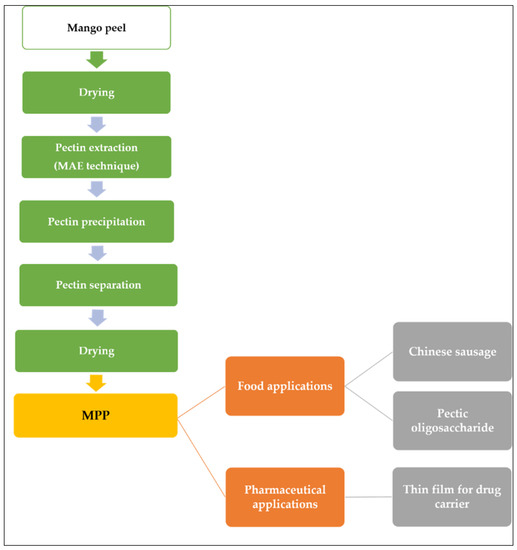
Figure 3.
MPP value chain and applications.
3.4. Future Direction of MPP Utilisation
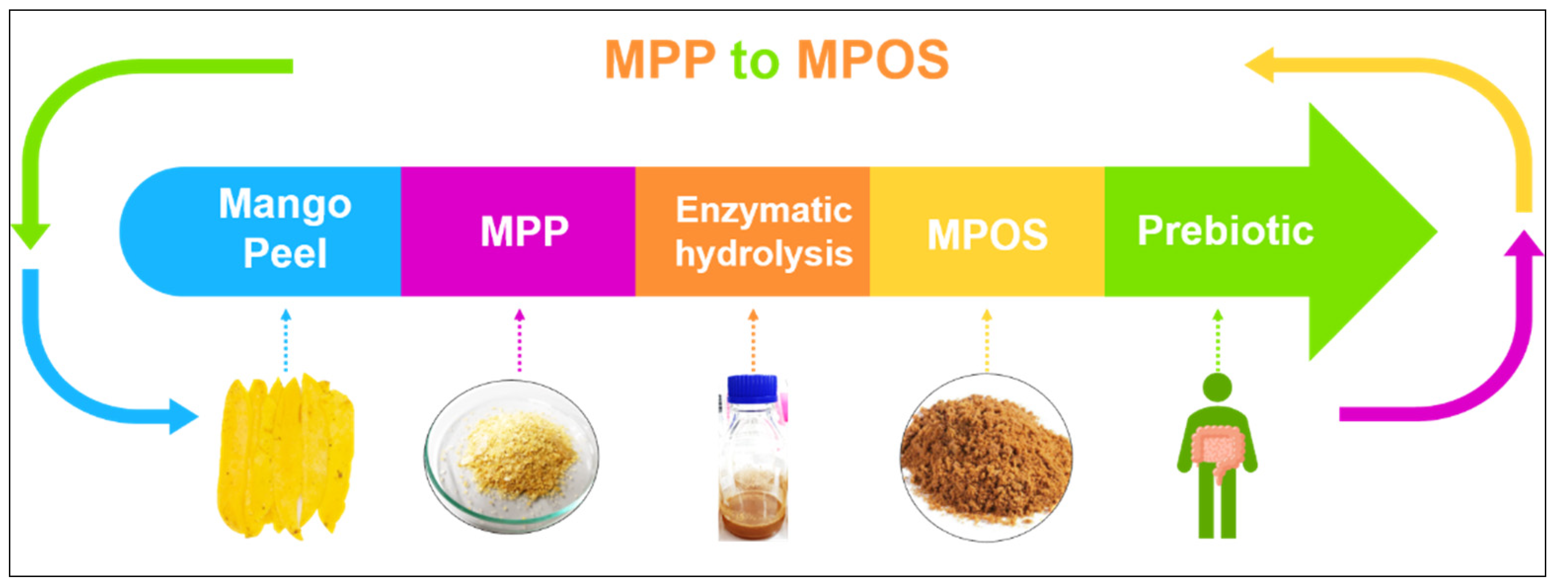
Plant polysaccharides are vital for the modulation of human gut microbiota which can impact on health generally recognised as prebiotics [142]. Among the most common prebiotic candidates, pectin oligosaccharide (POS) is receiving attention in the functional food industry [143]. MPP can possibly be hydrolysed into small molecules of pectic oligosaccharide or MPOS, as shown in Figure 4 [144]. The MPOS obtained highly stimulated the probiotic growth as well as the total short-chain fatty acids (SCFAs) production of Bifidobacterium animalis TISTR 2195 and Lactobacillus reuteri DSM 17938. It is also confirmed in our previous study that the MPOS illustrates a high potential as a prebiotic property [141]. The subsequently obtained SCFAs provide a great variety of health effects, including inhibition of pathogenic bacteria, constipation relief, reduction in blood glucose levels, improvement in mineral absorption, reduction of colonic cancer and modulation of the immune system [145].
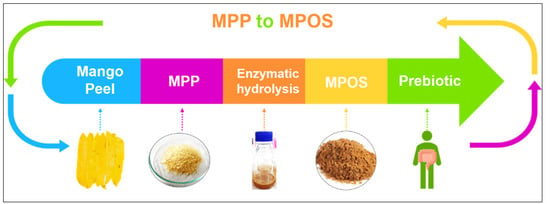
Figure 4.
Future direction of MPP utilisation to MPOS production.
4. Conclusions
Peels account for around a quarter of the entire mango fruit which is generated during the large-scale processing. Mango peel pectin can be retrieved from this biomass and its functionality depends upon the physiochemical characteristics which are largely influenced by varieties and extraction techniques. The high methoxyl content of the recovered pectin limits its use as food additive only. This biopolymer is structurally conversed by the de-esterification with alkaline treatment, resulting in its extended use as packaging or pharmaceutical-drug carrier. Future direction is heading toward the use of this potential biopolymer as functional prebiotic ingredient. This review pulls together the landscape picture of mango peel pectin biopolymer and highlights the use of the biomass as alternative biorefinery material to encourage a global sustainable development approach.
Author Contributions
Conceptualisation, S.R.S.; methodology, M.W., P.C. and S.R.S.; formal analysis, M.W. and P.C.; investigation, M.W., P.C. and S.R.S.; writing—original draft preparation, M.W. and P.C.; writing—review and editing, M.W., P.C. and S.R.S.; supervision, S.R.S., P.S., Y.P. and T.C.; project administration, M.W. and P.C.; funding acquisition, N.L., K.J., P.R., W.R. and P.J. All authors have read and agreed to the published version of the manuscript.
Funding
This research project was partially supported by Chiang Mai University.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
References
- Kumar, H.; Bhardwaj, K.; Sharma, R.; Nepovimova, E.; Kuča, K.; Dhanjal, D.S.; Verma, R.; Bhardwaj, P.; Sharma, S.; Kumar, D. Fruit and Vegetable Peels: Utilization of high value horticultural waste in novel industrial applications. Molecules 2020, 25, 2812. [Google Scholar] [CrossRef]
- Antonic, B.; Jancikova, S.; Dordevic, D.; Tremlova, B. Apple pomace as food fortification ingredient: A systematic review and meta-analysis. J. Food Sci. 2020, 85, 2977–2985. [Google Scholar] [CrossRef]
- Galanakis, C.M. Recovery of high added-value components from food wastes: Conventional, emerging technologies and commercialized applications. Trends Food Sci. Technol. 2012, 26, 68–87. [Google Scholar] [CrossRef]
- Loeillet, D. The european mango market a promising tropical fruit. Fruits 1994, 49, 332–334. [Google Scholar]
- Wongkaew, M.; Sangta, J.; Chansakaow, S.; Jantanasakulwong, K.; Rachtanapun, P.; Sommano, S.R. Volatile profiles from over-ripe purée of Thai mango varieties and their physiochemical properties during heat processing. PLoS ONE 2021, 16, e0248657. [Google Scholar] [CrossRef]
- Wongkaew, M.; Kittiwachana, S.; Phuangsaijai, N.; Tinpovong, B.; Tiyayon, C.; Pusadee, T.; Chuttong, B.; Sringarm, K.; Bhat, F.M.; Sommano, S.R. Fruit characteristics, peel nutritional compositions, and their relationships with mango peel pectin quality. Plants 2021, 10, 1148. [Google Scholar] [CrossRef]
- Larrauri, J.; Rupérez, P.; Borroto, B.; Saura-calixto, F. Mango peels as a new tropical fibre: Preparation and characterization. LWT-Food Sci. Technol. 1996, 29, 729–733. [Google Scholar] [CrossRef]
- Chaiwarit, T.; Masavang, S.; Mahe, J.; Sommano, S.; Ruksiriwanich, W.; Brachais, C.-H.; Chambin, O.; Jantrawut, P. Mango (cv. Nam Dokmai) peel as a source of pectin and its potential use as a film-forming polymer. Food Hydrocoll. 2020, 102, 105611. [Google Scholar] [CrossRef]
- Min, B.; Lim, J.; Ko, S.; Lee, K.-G.; Lee, S.H.; Lee, S. Environmentally friendly preparation of pectins from agricultural byproducts and their structural/rheological characterization. Bioresour. Technol. 2011, 102, 3855–3860. [Google Scholar] [CrossRef] [PubMed]
- Sommano, S.; Ounamornmas, P.; Nisoa, M.; Sriwattana, S.; Page, P.; Colelli, G. Characterisation and physiochemical properties of mango peel pectin extracted by conventional and phase control microwave-assisted extractions. Int. Food Res. J. 2018, 25, 2657–2665. [Google Scholar]
- Deeksha, K.; Sunita, M. Utilization of mango and its by-products by different processing methods. Asian J. Sci. Technol. 2020, 9, 8896–8910. [Google Scholar]
- Wongkaew, M.; Sommano, S.; Tangpao, T.; Rachtanapun, P.; Jantanasakulwong, K. Mango peel pectin by microwave-assisted extraction and its use as fat replacement in dried Chinese sausage. Foods 2020, 9, 450. [Google Scholar] [CrossRef]
- Sommano, S.; Ounamornmas, P.; Nisoa, M.; Sriwattana, S. Bioactive functionality of pectin from peels of seven Thai mango cultivars. Acta Hortic. 2018, 423–428. [Google Scholar] [CrossRef]
- Manthey, J.A.; Perkins-Veazie, P. Influences of harvest date and location on the levels of beta-carotene, ascorbic acid, total phenols, the in vitro antioxidant capacity, and phenolic profiles of five commercial varieties of mango (Mangifera indica L.). J. Agric. Food Chem. 2009, 57, 10825–10830. [Google Scholar] [CrossRef] [PubMed]
- Schieber, A.; Berardini, N.; Carle, R. Identification of flavonol and xanthone glycosides from mango (Mangifera indica L. Cv. “Tommy Atkins”) peels by high-performance liquid chromatography-electrospray ionization mass spectrometry. J. Agric. Food Chem. 2003, 51, 5006–5011. [Google Scholar] [CrossRef] [PubMed]
- Ajila, C.M.; Bhat, S.G.; Prasada Rao, U.J.S. Valuable components of raw and ripe peels from two Indian mango varieties. Food Chem. 2007, 102, 1006–1011. [Google Scholar] [CrossRef]
- Beerh, O.P.; Raghuramaiah, B.; Krishnamurthy, G.; Giridhar, N. Utilization of mango waste: Recovery of juice from waste pulp and peel. J. Food Sci. Technol. 1976, 13, 138–141. [Google Scholar]
- Ajila, C.M.; Prasada Rao, U.J.S. Mango peel dietary fibre: Composition and associated bound phenolics. J. Funct. Foods 2013, 5, 444–450. [Google Scholar] [CrossRef]
- Garcia-Magana Mde, L.; Garcia, H.S.; Bello-Perez, L.A.; Sayago-Ayerdi, S.G.; de Oca, M.M. Functional properties and dietary fiber characterization of mango processing by-products (Mangifera indica L., cv Ataulfo and Tommy Atkins). Plant Foods Hum. Nutr. 2013, 68, 254–258. [Google Scholar] [CrossRef] [PubMed]
- Malviya, R.; Kulkarni, G. Extraction and characterization of mango peel pectin as pharmaceutical excipient. Polym. Med. 2012, 42, 185–190. [Google Scholar]
- Villegas, D.; Handford, M.; Alcalde, J.A.; Perez-Donoso, A. Exogenous application of pectin-derived oligosaccharides to grape berries modifies anthocyanin accumulation, composition and gene expression. Plant Physiol. Biochem. 2016, 104, 125–133. [Google Scholar] [CrossRef]
- Ajila, C.M.; Jaganmohan Rao, L.; Prasada Rao, U.J.S. Characterization of bioactive compounds from raw and ripe Mangifera indica L. peel extracts. Food Chem. Toxicol. 2010, 48, 3406–3411. [Google Scholar] [CrossRef] [PubMed]
- Güzel, M.; Akpınar, Ö. Valorisation of fruit by-products: Production characterization of pectins from fruit peels. Food Bioprod. Process. 2019, 115, 126–133. [Google Scholar] [CrossRef]
- Macagnan, F.; Santos, L.; Roberto, B.; Moura, F.; Bizzani, M.; Silva, L. Biological properties of apple pomace, orange bagasse and passion fruit peel as alternative source of dietary fibre. Bioact. Carbohydr. Diet. Fibre 2015, 6, 1–6. [Google Scholar] [CrossRef]
- Mohnen, D. Pectin structure and biosynthesis. Curr. Opin. Plant Biol. 2008, 11, 266–277. [Google Scholar] [CrossRef]
- Freitas, C.M.; Coimbra, J.S.; Souza, V.G.; Sousa, R.C. Structure and applications of pectin in food, biomedical, and pharmaceutical industry: A review. Coatings 2021, 11, 922. [Google Scholar] [CrossRef]
- Reddy, L.; Obulam, V.S.; Wee, Y.-J. Production of ethanol from mango (Mangifera indica L.) peel by Saccharomyces cerevisiae CFTRI101. Afr. J. Biotechnol. 2011, 10, 4183–4189. [Google Scholar]
- Banerjee, J.; Singh, R.; Vijayaraghavan, R.; MacFarlane, D.; Patti, A.F.; Arora, A. A hydrocolloid based biorefinery approach to the valorisation of mango peel waste. Food Hydrocoll. 2018, 77, 142–151. [Google Scholar] [CrossRef]
- Yingkamhaeng, N.; Sukyai, P. The potential of mango peel utilization for cellulose extraction by hydrothermal pretreatment. In Proceedings of the 26th Annual Meeting of the Thai Society for Biotechnology and International Conference, Chiang Rai, Thailand, 26–29 November 2014; pp. 107–109. [Google Scholar]
- May, C.D. Industrial pectins: Sources, production and applications. Carbohydr. Polym. 1990, 12, 79–99. [Google Scholar] [CrossRef]
- Belkheiri, A.; Forouhar, A.; Ursu, A.V.; Dubessay, P.; Pierre, G.; Delattre, C.; Djelveh, G.; Abdelkafi, S.; Hamdami, N.; Michaud, P. Extraction, characterization, and applications of pectins from plant by-products. Appl. Sci. 2021, 11, 6596. [Google Scholar] [CrossRef]
- Méndez-Zamora, G.; García-Macías, J.A.; Santellano-Estrada, E.; Chávez-Martínez, A.; Durán-Meléndez, L.A.; Silva-Vázquez, R.; Quintero-Ramos, A. Fat reduction in the formulation of frankfurter sausages using inulin and pectin. Food Sci. Technol. 2015, 35, 25–31. [Google Scholar] [CrossRef]
- Iqbal, M.; Saeed, A.; Kalim, I.J.S.S. Characterization of adsorptive capacity and investigation of mechanism of Cu2+, Ni2+ and Zn2+ adsorption on mango peel waste from constituted metal solution and genuine electroplating effluent. Sep. Sci. Technol. 2009, 44, 3770–3791. [Google Scholar] [CrossRef]
- Jha, S.N.; Chopra, S.; Kingsly, A.R.P. Modeling of color values for nondestructive evaluation of maturity of mango. J. Food Eng. 2007, 78, 22–26. [Google Scholar] [CrossRef]
- Malevski, Y.; Brito, L.; Peleg, M.; Silberg, M. External color as maturity index of mango. J. Food Sci. 2006, 42, 1316–1318. [Google Scholar] [CrossRef]
- Burana-Osot, J.; Soonthornchareonnon, N.; Chaidedgumjorn, A.; Hosoyama, S.; Toida, T. Determination of galacturonic acid from pomelo pectin in term of galactose by HPAEC with fluorescence detection. Carbohydr. Polym. 2010, 81, 461–465. [Google Scholar] [CrossRef]
- Nunak, N.; Suesut, T. Measuring Geometric Mean Diameter of fruits and vegetables using Light Sectioning Method. Songklanakarin J. Sci. Technol. 2009, 31, 629–633. [Google Scholar]
- Nordey, T.; Léchaudel, M.; Saudreau, M.; Joas, J.; Génard, M. Model-assisted analysis of spatial and temporal variations in fruit temperature and transpiration highlighting the role of fruit development. PLoS ONE 2014, 9, e92532. [Google Scholar] [CrossRef]
- Athmaselvi, K.; Jenney, P.; Pavithra, C.; Roy, I. Physical and biochemical properties of selected tropical fruits. Int. Agrophys. 2014, 28, 383–388. [Google Scholar] [CrossRef]
- Nguyen, H.D.H.; Nguyen, H.V.H.; Savage, G.P. Properties of pectin extracted from Vietnamese mango peels. Foods 2019, 8, 629. [Google Scholar] [CrossRef]
- Lebaka, V.R.; Wee, Y.-J.; Ye, W.; Korivi, M. Nutritional Composition and bioactive compounds in three different parts of mango fruit. Int. J. Environ. Res. Public Health 2021, 18, 741. [Google Scholar] [CrossRef]
- Abdualrahm, M. Physico-chemical characteristics of different types of mango (Mangifera indica L.) fruits grown in Drafur regions and its use in jam processing. Sci. Int. 2013, 1, 144–147. [Google Scholar] [CrossRef]
- Madalageri, D.D.; Bharati, P.; Kage, U. Physicochemical properties, nutritional and antinutritional composition of pulp and peel of three mango varieties. Int. J. Educ. Sci. Res. 2017, 7, 81–94. [Google Scholar]
- Vieira, W.A.S.; Michereff, S.J.; de Morais, M.A.; Hyde, K.D.; Câmara, M.P.S. Endophytic species of Colletotrichum associated with mango in northeastern Brazil. Fungal Divers. 2014, 67, 181–202. [Google Scholar] [CrossRef]
- Larrauri, J. New approaches in the preparation of high dietary fibre powders from fruit by-products. Trends Food Sci. Technol. 1999, 10, 3–8. [Google Scholar] [CrossRef]
- Larrauri, J.; Rupérez, P.; Bravo, L.; Saura-Calixto, F. High dietary fibre powders from orange and lime peels: Associated polyphenols and antioxidant capacity. Food Res. Int. 1996, 29, 757–762. [Google Scholar] [CrossRef]
- Marín, F.R.; Soler-Rivas, C.; Benavente-García, O.; Castillo, J.; Pérez-Alvarez, J.A. By-products from different citrus processes as a source of customized functional fibres. Food Chem. 2007, 100, 736–741. [Google Scholar] [CrossRef]
- Abbasi, A.; Guo, X.; Fu, X.; Zhou, L.; Chen, Y.; Zhu, Y.; Yan, H.; Liu, R. Comparative assessment of phenolic content and in vitro antioxidant capacity in the pulp and peel of mango cultivars. Int. J. Mol. Sci. 2015, 16, 13507–13527. [Google Scholar] [CrossRef]
- Tokas, J.P.H.; Baloda, S.; Sheokand, R.N. Mango peel a potential source of bioactive compounds and phytochemicals. Austin Food Sci. 2020, 5, 1–7. [Google Scholar]
- Berardini, N.; Fezer, R.; Conrad, J.; Beifuss, U.; Carle, R.; Schieber, A. Screening of Mango (Mangifera indica L.) Cultivars for their contents of flavonol o- and xanthone c-glycosides, anthocyanins, and pectin. J. Agric. Food Chem. 2005, 53, 1563–1570. [Google Scholar] [CrossRef]
- Lakshminarayana, S.; Subhadra, N.V.; Subramanyam, H. Some aspects of developmental physiology of the mango fruit. J. Hortic. Sci. 1970, 45, 133–142. [Google Scholar] [CrossRef]
- Luo, F.; Lv, Q.; Zhao, Y.; Hu, G.; Huang, G.; Zhang, J.; Sun, C.; Li, X.; Chen, K. Quantification and purification of mangiferin from Chinese mango (Mangifera indica L.) cultivars and its protective effect on human umbilical vein endothelial cells under H2O2-induced stress. Int. J. Mol. Sci. 2012, 13, 11260–11274. [Google Scholar] [CrossRef] [PubMed]
- Ramirez, J.E.; Zambrano, R.; Sepúlveda, B.; Simirgiotis, M.J. Antioxidant properties and hyphenated HPLC-PDA-MS profiling of Chilean Pica mango fruits (Mangifera indica L. Cv. piqueño). Molecules 2013, 19, 438–458. [Google Scholar] [CrossRef] [PubMed]
- Telang, M.; Dhulap, S.; Mandhare, A.; Hirwani, R. Therapeutic and cosmetic applications of mangiferin: A patent review. Expert Opin. Ther. Patents 2013, 23, 1561–1580. [Google Scholar] [CrossRef]
- Imran, M.; Arshad, M.S.; Butt, M.S.; Kwon, J.-H.; Sultan, M.T. Mangiferin: A natural miracle bioactive compound against lifestyle related disorders. Lipids Health Dis. 2017, 16, 1–17. [Google Scholar] [CrossRef]
- Giampieri, F.; Alvarez-Suarez, J.M.; Mazzoni, L.; Forbes-Hernandez, T.Y.; Gasparrini, M.; Gonzàlez-Paramàs, A.M.; Santos-Buelga, C.; Quiles, J.L.; Bompadre, S.; Mezzetti, B.; et al. An anthocyanin-rich strawberry extract protects against oxidative stress damage and improves mitochondrial functionality in human dermal fibroblasts exposed to an oxidizing agent. Food Funct. 2014, 5, 1939–1948. [Google Scholar] [CrossRef] [PubMed]
- Rahman, M.M.; Ichiyanagi, T.; Komiyama, T.; Sato, S.; Konishi, T. Effects of anthocyanins on psychological stress-induced oxidative stress and neurotransmitter status. J. Agric. Food Chem. 2008, 56, 7545–7550. [Google Scholar] [CrossRef]
- Ranganath, K.G.; Shivashankara, K.S.; Roy, T.K.; Dinesh, M.R.; Geetha, G.A.; Pavithra, K.C.; Ravishankar, K.V. Profiling of anthocyanins and carotenoids in fruit peel of different colored mango cultivars. J. Food Sci. Technol. 2018, 55, 4566–4577. [Google Scholar] [CrossRef]
- Kayesh, E.; Shangguan, L.; Korir, N.K.; Sun, X.; Bilkish, N.; Zhang, Y.; Han, J.; Song, C.; Cheng, Z.-M.; Fang, J. Fruit skin color and the role of anthocyanin. Acta Physiol. Plant. 2013, 35, 2879–2890. [Google Scholar] [CrossRef]
- El Gharras, H. Polyphenols: Food sources, properties and applications—A review. Int. J. Food Sci. Technol. 2009, 44, 2512–2518. [Google Scholar] [CrossRef]
- Ribeiro, S.M.R.; Barbosa, L.C.A.; Queiroz, J.H.; Knödler, M.; Schieber, A. Phenolic compounds and antioxidant capacity of Brazilian mango (Mangifera indica L.) varieties. Food Chem. 2008, 110, 620–626. [Google Scholar] [CrossRef]
- Meléndez-Martínez, A.J. An overview of carotenoids, apocarotenoids, and vitamin a in agro-food, nutrition, health, and disease. Mol. Nutr. Food Res. 2019, 63, e1801045. [Google Scholar] [CrossRef] [PubMed]
- Melendez-Martinez, A.J.; Mapelli-Brahm, P.; Stinco, C.M. The colourless carotenoids phytoene and phytofluene: From dietary sources to their usefulness for the functional foods and nutricosmetics industries. J. Food Compos. Anal. 2018, 67, 91–103. [Google Scholar] [CrossRef]
- Meléndez-Martínez, A.J.; Mandić, A.I.; Bantis, F.; Böhm, V.; Borge, G.I.A.; Brnčić, M.; Bysted, A.; Cano, M.P.; Dias, M.G.; Elgersma, A. A comprehensive review on carotenoids in foods and feeds: Status quo, applications, patents, and research needs. Crit. Rev. Food Sci. Nutr. 2021, 1–51. [Google Scholar] [CrossRef] [PubMed]
- Akther, S.; Sultana, A.; Badsha, R.; Rahman, M.; Alim, A.; Amin, A.M. Physicochemical properties of mango (Amropali cultivar) powder and its reconstituted product as affected by drying methods. Int. J. Food Prop. 2020, 23, 2201–2216. [Google Scholar] [CrossRef]
- Burton-Freeman, B.M.; Sandhu, A.K.; Edirisinghe, I. Mangos and their bioactive components: Adding variety to the fruit plate for health. Food Funct. 2017, 8, 3010–3032. [Google Scholar] [CrossRef]
- Maldonado-Celis, M.E.; Yahia, E.M.; Bedoya, R.; Landázuri, P.; Loango, N.; Aguillón, J.; Restrepo, B.; Ospina, J.C.G. Chemical composition of mango (Mangifera indica L.) fruit: Nutritional and phytochemical compounds. Front. Plant Sci. 2019, 10, 1073. [Google Scholar] [CrossRef]
- Saleem Dar, M.; Oak, P.; Chidley, H.; Deshpande, A.; Giri, A.; Gupta, V. Chapter 19—Nutrient and flavor content of mango (Mangifera indica L.) cultivars: An appurtenance to the list of staple foods. In Nutritional Composition of Fruit Cultivars; Simmonds, M.S.J., Preedy, V.R., Eds.; Academic Press: San Diego, CA, USA, 2016; pp. 445–467. [Google Scholar]
- Tharanathan, R.N.; Yashoda, H.M.; Prabha, T.N. Mango (Mangifera indica L.), “The King of Fruits”—An overview. Food Rev. Int. 2006, 22, 95–123. [Google Scholar] [CrossRef]
- Arumugam, R. Fermentation of pretreated hydrolyzates of banana and mango fruit wastes for ethanol production. Asian J. Exp. Biol. Sci. 2011, 2, 246–256. [Google Scholar]
- Sadineni, V.; Kumar, Y.; Obulam, V.S. Carotenoid composition of mango (Mangifera indica L.) wine and its antioxidant activity. J. Food Biochem. 2011, 35, 1538–1547. [Google Scholar] [CrossRef]
- Kumar, D.; Ashfaque, M.; Muthukumar, M.; Singh, M.; Garg, N. Production and characterization of carboxymethyl cellulase from Paenibacillus polymyxa using mango peel as substrate. J. Environ. Biol. 2012, 33, 81–84. [Google Scholar]
- Kumar, Y.S.; Kumar, P.V.; Reddy, O.V.S. Pectinase production from mango peel using Aspergillus foetidusand its application in processing of mango juice. Food Biotechnol. 2012, 26, 107–123. [Google Scholar] [CrossRef]
- Saravanan, P.; Muthuvelayudham, R.; Thangavelu, V. Application of Statistical Design for the Production of Cellulase by Trichoderma reesei Using Mango Peel. Enzym. Res. 2012, 2012, 157643. [Google Scholar] [CrossRef] [PubMed]
- Jawad, A.H.; Alkarkhi, A.; Ogugbue, C.; Easa, A.; Norulaini, N. Production of the lactic acid from mango peel waste—Factorial experiment. J. King Saud Univ. Sci. 2013, 25, 39–45. [Google Scholar] [CrossRef]
- Rashad, M.M.; Moharib, S.A.; Jwanny, E.W. Yeast conversion of mango waste or methanol to single cell protein and other metabolites. Biol. Wastes 1990, 32, 277–284. [Google Scholar] [CrossRef]
- Kumar, C.S.C.; Mythily, R.; Chandraju, S. Utilization of mango peels (Mangifera indica) for the extraction of sugars. Der Pharma Chem. 2012, 4, 2422–2426. [Google Scholar]
- Ajila, C.; Aalami, M.; Leelavathi, K.; Rao, U.P. Mango peel powder: A potential source of antioxidant and dietary fiber in macaroni preparations. Innov. Food Sci. Emerg. Technol. 2010, 11, 219–224. [Google Scholar] [CrossRef]
- Palmeira, S.; Gois, L.M.; Souza, L.D. Extraction of phenolic compounds from mango peels. Lat. Am. Appl. Res. 2012, 42, 77–81. [Google Scholar]
- Tunchaiyaphum, S.; Eshtiaghi, M.N.; Yoswathana, N. Extraction of bioactive compounds from mango peels using green technology. Int. J. Chem. Eng. Appl. 2013, 4, 194–198. [Google Scholar] [CrossRef]
- Aziz, N.A.A.; Wong, L.M.; Bhat, R.; Cheng, L.H. Evaluation of processed green and ripe mango peel and pulp flours (Mangifera indica var. Chokanan) in terms of chemical composition, antioxidant compounds and functional properties. J. Sci. Food Agric. 2011, 92, 557–563. [Google Scholar] [CrossRef]
- Ruiz, C.; Ramírez, C.; Piñeres, C.; Angulo, M.; Hedreira, J. Obtaining and characterization of mango peel powder and its use as a source of fiber and a functional ingredient in natural yogurt. In Proceedings of the 11th International Congress on Engineering and Food (ICEF11), Athens, Greece, 22–26 May 2011. [Google Scholar]
- Abdeldaiem, M.H.; Ali, G.M.H. Use of irradiated mango (Mangifera Indica) peels powder as potential source of dietary fiber and antioxidant in beef burger. J. Appl. Sci. Res. 2012, 8, 3677–3687. [Google Scholar]
- Iqbal, M.; Saeed, A.; Zafar, S.I. FTIR spectrophotometry, kinetics and adsorption isotherms modeling, ion exchange, and EDX analysis for understanding the mechanism of Cd2+ and Pb2+ removal by mango peel waste. J. Hazard. Mater. 2009, 164, 161–171. [Google Scholar] [CrossRef] [PubMed]
- Malviya, R.; Srivastava, P.; Bansal, M.; Sharma, P. Mango peel pectin as superdisintegrating agent. J. Sci. Ind. Res. 2010, 69, 688–690. [Google Scholar]
- Gentilini, R.; Bozzini, S.; Munarin, F.; Petrini, P.; Visai, L.; Tanzi, M.C. Pectins from Aloe Vera: Extraction and production of gels for regenerative medicine. J. Appl. Polym. Sci. 2014, 131, 1–9. [Google Scholar] [CrossRef]
- Peng, K.; Zhang, Y.; Wang, S.; Liao, X.; Hu, X. Effect of microwave drying pretreatment on extraction of pectin from apple pomace. Int. Food Res. J. 2008, 24, 222–226. [Google Scholar]
- Casas-Orozco, D.; Villa, A.L.; Bustamante, F.; Gonzalez-Rodriguez, L.-M. Process development and simulation of pectin extraction from orange peels. Food Bioprod. Process. 2015, 96, 86–98. [Google Scholar] [CrossRef]
- Georgiev, Y.; Ognyanov, M.; Yanakieva, I.; Kussovski, V.; Kratchanova, M. Isolation, characterization and modification of citrus pectins. J. Biosci. Biotechnol. 2013, 2012, 223–233. [Google Scholar]
- Marić, M.; Grassino, A.N.; Zhu, Z.; Barba, F.J.; Brnčić, M.; Brnčić, S.R. An overview of the traditional and innovative approaches for pectin extraction from plant food wastes and by-products: Ultrasound-, microwaves-, and enzyme-assisted extraction. Trends Food Sci. Technol. 2018, 76, 28–37. [Google Scholar] [CrossRef]
- Oliveira, A.D.N.; Paula, D.D.A.; de Oliveira, E.B.; Saraiva, S.H.; Stringheta, P.C.; Ramos, A.M. Optimization of pectin extraction from Ubá mango peel through surface response methodology. Int. J. Biol. Macromol. 2018, 113, 395–402. [Google Scholar] [CrossRef]
- Maran, J.P.; Sivakumar, V.; Thirugnanasambandham, K.; Sridhar, R. Optimization of microwave assisted extraction of pectin from orange peel. Carbohydr. Polym. 2013, 97, 703–709. [Google Scholar] [CrossRef] [PubMed]
- Maran, J.P.; Prakash, K.A. Process variables influence on microwave assisted extraction of pectin from waste Carcia papaya L. peel. Int. J. Biol. Macromol. 2015, 73, 202–206. [Google Scholar] [CrossRef] [PubMed]
- Pandit, S.G.; Vijayanand, P.; Kulkarni, S. Pectic principles of mango peel from mango processing waste as influenced by microwave energy. LWT 2015, 64, 1010–1014. [Google Scholar] [CrossRef]
- Kratchanova, M.; Pavlova, E.; Panchev, I. The effect of microwave heating of fresh orange peels on the fruit tissue and quality of extracted pectin. Carbohydr. Polym. 2004, 56, 181–185. [Google Scholar] [CrossRef]
- Fissore, E.N.; Ponce, N.M.; Wider, E.A.; Stortz, C.A.; Gerschenson, L.N.; Rojas, A.M. Commercial cell wall hydrolytic enzymes for producing pectin-enriched products from butternut (Cucurbita moschata, Duchesne ex Poiret). J. Food Eng. 2009, 93, 293–301. [Google Scholar] [CrossRef]
- Puri, M.; Sharma, D.; Barrow, C.J. Enzyme-assisted extraction of bioactives from plants. Trends Biotechnol. 2012, 30, 37–44. [Google Scholar] [CrossRef] [PubMed]
- Poojary, M.M.; Orlien, V.; Passamonti, P.; Olsen, K. Enzyme-assisted extraction enhancing the umami taste amino acids recovery from several cultivated mushrooms. Food Chem. 2017, 234, 236–244. [Google Scholar] [CrossRef]
- Roselló-Soto, E.; Parniakov, O.; Deng, Q.; Patras, A.; Koubaa, M.; Grimi, N.; Boussetta, N.; Tiwari, B.K.; Vorobiev, E.; Lebovka, N. Application of non-conventional extraction methods: Toward a sustainable and green production of valuable compounds from mushrooms. Food Eng. Rev. 2016, 8, 214–234. [Google Scholar] [CrossRef]
- Dominiak, M.; Søndergaard, K.M.; Wichmann, J.; Vidal-Melgosa, S.; Willats, W.G.; Meyer, A.S.; Mikkelsen, J.D. Application of enzymes for efficient extraction, modification, and development of functional properties of lime pectin. Food Hydrocoll. 2014, 40, 273–282. [Google Scholar] [CrossRef]
- Liew, S.; Chin, N.; Yusof, Y.; Sowndhararajan, K. Comparison of acidic and enzymatic pectin extraction from passion fruit peels and its gel properties. J. Food Process. Eng. 2016, 39, 501–511. [Google Scholar] [CrossRef]
- Dranca, F.; Oroian, M. Optimization of pectin enzymatic extraction from malus domestica ‘fălticeni’ apple pomace with Celluclast 1.5L. Molecules 2019, 24, 2158. [Google Scholar] [CrossRef]
- Zinoviadou, K.G.; Galanakis, C.; Brnčić, M.; Grimi, N.; Boussetta, N.; Mota, M.; Saraiva, J.A.; Patras, A.; Tiwari, B.K.; Barba, F.J. Fruit juice sonication: Implications on food safety and physicochemical and nutritional properties. Food Res. Int. 2015, 77, 743–752. [Google Scholar] [CrossRef]
- Barba, F.J.; Brianceau, S.; Turk, M.; Boussetta, N.; Vorobiev, E. Effect of alternative physical treatments (ultrasounds, pulsed electric fields, and high-voltage electrical discharges) on selective recovery of bio-compounds from fermented grape pomace. Food Bioprocess Technol. 2015, 8, 1139–1148. [Google Scholar] [CrossRef]
- Zhu, Z.; Wu, Q.; Di, X.; Li, S.; Barba, F.J.; Koubaa, M.; Roohinejad, S.; Xiong, X.; He, J. Multistage recovery process of seaweed pigments: Investigation of ultrasound assisted extraction and ultra-filtration performances. Food Bioprod. Process. 2017, 104, 40–47. [Google Scholar] [CrossRef]
- Guandalini, B.B.V.; Rodrigues, N.P.; Marczak, L.D.F. Sequential extraction of phenolics and pectin from mango peel assisted by ultrasound. Food Res. Int. 2019, 119, 455–461. [Google Scholar] [CrossRef]
- Zakaria, S.M.; Kamal, S.M.M. Subcritical water extraction of bioactive compounds from plants and algae: Applications in pharmaceutical and food ingredients. Food Eng. Rev. 2016, 8, 23–34. [Google Scholar] [CrossRef]
- Xia, H.; Matharu, A.S. Unavoidable food supply chain waste: Acid-free pectin extraction from mango peel via subcritical water. Faraday Discuss. 2017, 202, 31–42. [Google Scholar] [CrossRef]
- Bagherian, H.; Ashtiani, F.Z.; Fouladitajar, A.; Mohtashamy, M. Comparisons between conventional, microwave- and ultrasound-assisted methods for extraction of pectin from grapefruit. Chem. Eng. Process. Process. Intensif. 2011, 50, 1237–1243. [Google Scholar] [CrossRef]
- Fishman, M.L.; Chau, H.K.; Hoagland, P.; Ayyad, K. Characterization of pectin, flash-extracted from orange albedo by microwave heating, under pressure. Carbohydr. Res. 1999, 323, 126–138. [Google Scholar] [CrossRef]
- Adetunji, L.R.; Adekunle, A.; Orsat, V.; Raghavan, V. Advances in the pectin production process using novel extraction techniques: A review. Food Hydrocoll. 2017, 62, 239–250. [Google Scholar] [CrossRef]
- Ptichkina, N.; Markina, O.; Rumyantseva, G. Pectin extraction from pumpkin with the aid of microbial enzymes. Food Hydrocoll. 2008, 22, 192–195. [Google Scholar] [CrossRef]
- Sowbhagya, H.B.; Chitra, V.N. Enzyme-assisted extraction of flavorings and colorants from plant materials. Crit. Rev. Food Sci. Nutr. 2010, 50, 146–161. [Google Scholar] [CrossRef]
- Wikiera, A.; Mika, M.; Grabacka, M. Multicatalytic enzyme preparations as effective alternative to acid in pectin extraction. Food Hydrocoll. 2015, 44, 156–161. [Google Scholar] [CrossRef]
- Azmir, J.; Zaidul, I.S.M.; Rahman, M.M.; Sharif, K.M.; Mohamed, A.; Sahena, F.; Jahurul, M.H.A.; Ghafoor, K.; Norulaini, N.A.N.; Omar, A.K.M. Techniques for extraction of bioactive compounds from plant materials: A review. J. Food Eng. 2013, 117, 426–436. [Google Scholar] [CrossRef]
- Wang, L.; Weller, C.L. Recent advances in extraction of nutraceuticals from plants. Trends Food Sci. Technol. 2006, 17, 300–312. [Google Scholar] [CrossRef]
- Ueno, H.; Tanaka, M.; Hosino, M.; Sasaki, M.; Goto, M. Extraction of valuable compounds from the flavedo of Citrus junos using subcritical water. Sep. Purif. Technol. 2008, 62, 513–516. [Google Scholar] [CrossRef]
- Khajavi, S.H.; Kimura, Y.; Oomori, T.; Matsuno, R.; Adachi, S. Degradation kinetics of monosaccharides in subcritical water. J. Food Eng. 2005, 68, 309–313. [Google Scholar] [CrossRef]
- Chaiwarit, T.; Rachtanapun, P.; Kantrong, N.; Jantrawut, P. Preparation of clindamycin hydrochloride loaded de-esterified low-methoxyl mango peel pectin film used as a topical drug delivery system. Polymers 2020, 12, 1006. [Google Scholar] [CrossRef] [PubMed]
- Thibault, J.F.; Ralet, M.-C. Physico-Chemical Properties of Pectins in the Cell Walls and After Extraction; Springer: Berlin/Heidelberg, Germany, 2003; pp. 91–105. [Google Scholar]
- Wang, M.; Huang, B.; Fan, C.; Zhao, K.; Hu, H.; Xu, X.; Pan, S.; Liu, F. Characterization and functional properties of mango peel pectin extracted by ultrasound assisted citric acid. Int. J. Biol. Macromol. 2016, 91, 794–803. [Google Scholar] [CrossRef]
- Melton, L.D.; Smith, B.G. Determining the degree of methylation and acetylation of pectin. Curr. Protoc. Food Anal. Chem. 2001, 1, E3.4.1–E3.4.6. [Google Scholar] [CrossRef]
- Moreira, M.M.; Guido, L.F.; Cruz, J.M.; Barros, A.A. Determination of galacturonic acid content in pectin from fruit juices by liquid chromatographydiode array detection-electrospray ionization tandem mass spectrometry. Open Chem. 2010, 8, 1236–1243. [Google Scholar] [CrossRef]
- Gnanasambandam, R.; Proctor, A. Preparation of soy hull pectin. Food Chem. 1999, 65, 461–467. [Google Scholar] [CrossRef]
- Salminen, H.; Weiss, J. Effect of pectin type on association and ph stability of whey protein—Pectin complexes. Food Biophys. 2013, 9, 29–38. [Google Scholar] [CrossRef]
- Yavuz-Düzgün, M.; Zeeb, B.; Dreher, J.; Özçelik, B.; Weiss, J. The impact of esterification degree and source of pectins on complex coacervation as a tool to mask the bitterness of potato protein isolates. Food Biophys. 2020, 15, 376–385. [Google Scholar] [CrossRef]
- Brouns, F.; Theuwissen, E.; Adam, A.; Bell, M.G.; Berger, A.P.A.; Mensink, R.P. Cholesterol-lowering properties of different pectin types in mildly hyper-cholesterolemic men and women. Eur. J. Clin. Nutr. 2012, 66, 591–599. [Google Scholar] [CrossRef]
- Pinheiro, E.s.R.; Silva, I.M.; Gonzaga, L.V.; Amante, E.R.; Teófilo, R.F.; Ferreira, M.M.; Amboni, R.D. Optimization of extraction of high-ester pectin from passion fruit peel (Passiflora edulis flavicarpa) with citric acid by using response surface methodology. Bioresour. Technol. 2008, 99, 5561–5566. [Google Scholar] [CrossRef] [PubMed]
- Maness, N.O.; Ryan, J.D.; Mort, A.J. Determination of the degree of methyl esterification of pectins in small samples by selective reduction of esterified galacturonic acid to galactose. Anal. Biochem. 1990, 185, 346–352. [Google Scholar] [CrossRef]
- Chinnici, F.; Spinabelli, U.; Riponi, C.; Amati, A. Optimization of the determination of organic acids and sugars in fruit juices by ion-exclusion liquid chromatography. J. Food Compos. Anal. 2005, 18, 121–130. [Google Scholar] [CrossRef]
- Kermani, Z.J.; Shpigelman, A.; Pham, T.T.H.; Van Loey, A.; Hendrickx, M.E. Functional properties of citric acid extracted mango peel pectin as related to its chemical structure. Food Hydrocoll. 2015, 44, 424–434. [Google Scholar] [CrossRef]
- Nagel, A.; Neidhart, S.; Anders, T.; Elstner, P.; Korhummel, S.; Sulzer, T.; Wulfkühler, S.; Winkler, C.; Qadri, S.; Rentschler, C. Improved processes for the conversion of mango peel into storable starting material for the recovery of functional co-products. Ind. Crop. Prod. 2014, 61, 92–105. [Google Scholar] [CrossRef]
- Geerkens, C.H.; Nagel, A.; Just, K.M.; Miller-Rostek, P.; Kammerer, D.R.; Schweiggert, R.M.; Carle, R. Mango pectin quality as influenced by cultivar, ripeness, peel particle size, blanching, drying, and irradiation. Food Hydrocoll. 2015, 51, 241–251. [Google Scholar] [CrossRef]
- Sharma, B.R.; Dhuldhoya, N.; Merchant, S.U.; Merchant, U. An overview on pectins. Times Food Proc. J. 2006, 23, 44–51. [Google Scholar]
- Koubala, B.; Kansci, G.; Mbome, L.; Crépeau, M.-J.; Thibault, J.-F.; Ralet, M.-C. Effect of extraction conditions on some physicochemical characteristics of pectins from “Améliorée” and “Mango” mango peels. Food Hydrocoll. 2008, 22, 1345–1351. [Google Scholar] [CrossRef]
- Willats, W.G.; Knox, P.; Mikkelsen, J.D. Pectin: New insights into an old polymer are starting to gel. Trends Food Sci. Technol. 2006, 17, 97–104. [Google Scholar] [CrossRef]
- Thakur, B.R.; Singh, R.K.; Handa, A.K.; Rao, M.A. Chemistry and uses of pectin—A review. Crit. Rev. Food Sci. Nutr. 1997, 37, 47–73. [Google Scholar] [CrossRef]
- Ciurzyńska, A.; Szerszeń, J.; Lenart, A. Pectin—A functional component of diet. Int. J. Res. Stud. Sci. Eng. Technol. 2016, 3, 20–27. [Google Scholar]
- Zhang, Y.; Rempel, C.; McLaren, D. Chapter 12-Edible coating and film materials: Carbohydrates. In Innovations in Food Packaging; Elsevier: Amsterdam, The Netherlands, 2013; pp. 305–323. [Google Scholar]
- Speiser, R.; Copley, M.J.; Nutting, G.C. Effect of molecular association and charge distribution on the gelation of pectin. J. Phys. Chem. 1947, 51, 117–133. [Google Scholar] [CrossRef]
- Wongkaew, M.; Tinpovong, B.; Sringarm, K.; Leksawasdi, N.; Jantanasakulwong, K.; Rachtanapun, P.; Hanmoungjai, P.; Sommano, S. Crude pectic oligosaccharide recovery from Thai Chok Anan mango peel using pectinolytic enzyme hydrolysis. Foods 2021, 10, 627. [Google Scholar] [CrossRef]
- Gibson, G.R.; Roberfroid, M.B. Dietary modulation of the human colonic microbiota-introducing the concept of prebiotics. J. Nutr. 1995, 125, 1401–1412. [Google Scholar] [CrossRef]
- Zhang, S.; Hu, H.; Wang, L.; Liu, F.; Pan, S. Preparation and prebiotic potential of pectin oligosaccharides obtained from citrus peel pectin. Food Chem. 2018, 244, 232–237. [Google Scholar] [CrossRef] [PubMed]
- Chung, W.S.F.; Meijerink, M.; Zeuner, B.; Holck, J.; Louis, P.; Meyer, A.S.; Wells, J.M.; Flint, H.J.; Duncan, S.H. Prebiotic potential of pectin and pectic oligosaccharides to promote anti-inflammatory commensal bacteria in the human colon. FEMS Microbiol. Ecol. 2017, 93, fix127. [Google Scholar] [CrossRef]
- Gullón, B.; Gómez, B.; Martínez-Sabajanes, M.; Yáñez, R.; Parajó, J.C.; Alonso, J.L. Pectic oligosaccharides: Manufacture and functional properties. Trends Food Sci. Technol. 2013, 30, 153–161. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).