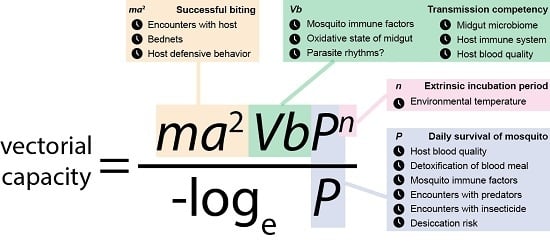
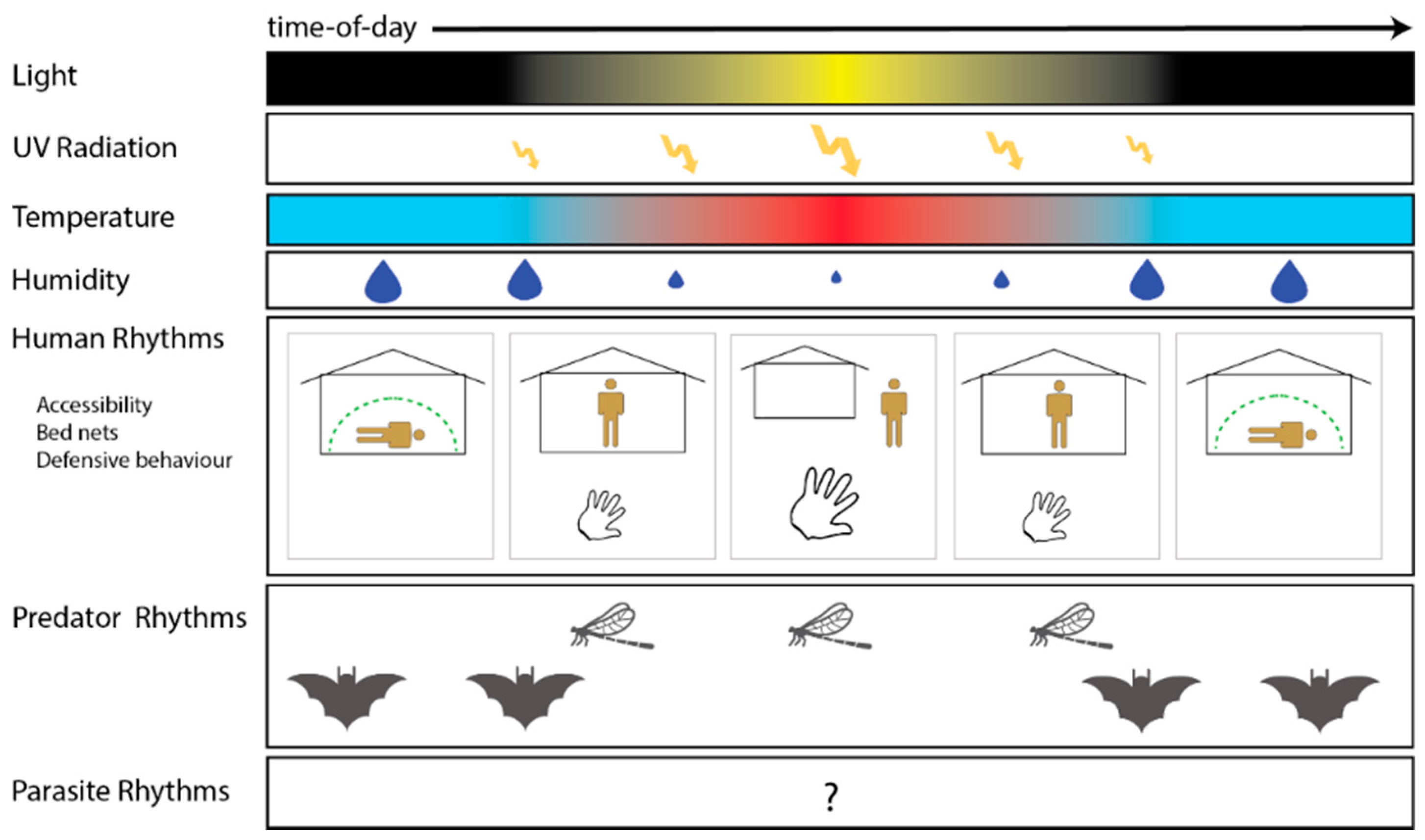
Daily Rhythms in Mosquitoes and Their Consequences for Malaria Transmission
Abstract
:1. Introduction
2. The Chronobiology of Mosquitoes
2.1. Anopheles Mosquitoes Live in a Rhythmic World
2.2. Behavioral Rhythms

2.3. Physiological Rhythms in Sensory Processes
2.4. Rhythms in Insecticide Metabolism
2.5. Rhythmic Detoxification of Reactive Oxygen Species
2.6. Rhythms in Osmoregulation
2.7. Rhythms in Immune Factors
2.8. Further Complexity
3. Interactions between Mosquito, Host and Parasite Rhythms
3.1. Rhythms in Host Blood Composition
3.2. Rhythms in Parasite Infectivity to Mosquitoes
3.3. Rhythms in Infectivity to Hosts
4. The Impact of Rhythms for Interventions
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Dunlap, J.C.; Loros, J.J.; Decoursey, P.J. Chronobiology: Biological Timekeeping; Sinauer Associates: Sunderland, MA, USA, 2004. [Google Scholar]
- Dodd, A.N.; Salathia, N.; Hall, A.; Kevei, E.; Toth, R.; Nagy, F.; Hibberd, J.M.; Millar, A.J.; Webb, A.A. Plant circadian clocks increase photosynthesis, growth, survival, and competitive advantage. Science 2005, 309, 630–633. [Google Scholar] [CrossRef] [PubMed]
- Ouyang, Y.; Andersson, C.R.; Kondo, T.; Golden, S.S.; Johnson, C.H. Resonating circadian clocks enhance fitness in cyanobacteria. Proc. Natl. Acad. Sci. USA 1998, 95, 8660–8664. [Google Scholar] [CrossRef] [PubMed]
- Chiesa, J.J.; Duhart, J.M.; Casiraghi, L.P.; Paladino, N.; Bussi, I.L.; Golombek, D.A. Effects of circadian disruption on physiology and pathology: From bench to clinic (and back). In Mechanisms of Circadian Systems in Animals and Their Clinical Relevance; Aguilar-Roblero, R., Díaz-Muñoz, M., Fanjul-Moles, M.L., Eds.; Springer: Gewerbestrasse, Switzerland, 2015; pp. 289–320. [Google Scholar]
- Cloudsley-Thompson, J.L. Adaptive functions of circadian rhythms. Cold Spring Harb. Symp. Quant. Biol. 1960, 25, 345–355. [Google Scholar] [CrossRef] [PubMed]
- Garnham, P.C.; Powers, K.G. Periodicity of infectivity of plasmodial gametocytes: The “Hawking phenomenon”. Int. J. Parasitol. 1974, 4, 103–106. [Google Scholar] [CrossRef]
- Gautret, P.; Gantier, J.C.; Baccam, D.; Miltgen, F.; Saulai, M.; Chabaud, A.G.; Landau, I. The gametocytes of Plasmodium vinckei petteri, their morphological stages, periodicity and infectivity. Int. J. Parasitol. 1996, 26, 1095–1101. [Google Scholar] [CrossRef]
- Gautret, P.; Miltgen, F.; Gantier, J.C.; Chabaud, A.G.; Landau, I. Enhanced gametocyte formation by Plasmodium chabaudi in immature erythrocytes: Pattern of production, sequestration, and infectivity to mosquitoes. J. Parasitol. 1996, 82, 900–906. [Google Scholar] [CrossRef] [PubMed]
- Shah, K.S. The periodic development of sexual forms of Plasmodium cathemerium in the peripheral circulation of canaries. Am. J. Epidemiol. 1934, 19, 392–403. [Google Scholar]
- Gautret, P.; Chabaud, A.G.; Landau, I. Plasmodium vinckei vinckei and P. yoelii nigeriensis: Pattern of gametocyte production and development. Parassitologia 1995, 37, 17–24. [Google Scholar] [PubMed]
- Gautret, P.; Motard, A. Periodic infectivity of Plasmodium gametocytes to the vector. A review. Parasite 1999, 6, 103–111. [Google Scholar] [CrossRef] [PubMed]
- Hawking, F.; Worms, M.J.; Gammage, K. 24- and 48-hour cycles of malaria parasites in the blood: Their purpose, production and control. Trans. R. Soc. Trop. Med. Hyg. 1968, 62, 731–765. [Google Scholar] [CrossRef]
- Young, M.W.; Kay, S.A. Time zones: A comparative genetics of circadian clocks. Nat. Rev. Genet. 2001, 2, 702–715. [Google Scholar] [CrossRef] [PubMed]
- O’Neill, J.S.; Reddy, A.B. Circadian clocks in human red blood cells. Nature 2011, 469, 498–503. [Google Scholar] [CrossRef] [PubMed]
- O’Neill, J.S.; van Ooijen, G.; Dixon, L.E.; Troein, C.; Corellou, F.; Bouget, F.; Reddy, A.B.; Millar, A.J. Circadian rhythms persist without transcription in a eukaryote. Nature 2011, 469, 554–558. [Google Scholar] [CrossRef] [PubMed]
- Zhu, H.; Yuan, Q.; Froy, O.; Casselman, A.; Reppert, S.M. The two CRYs of the butterfly. Curr. Biol. 2005, 15, R953–R954. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Q.; Metterville, D.; Briscoe, A.D.; Reppert, S.M. Insect cryptochromes: Gene duplication and loss define diverse ways to construct insect circadian clocks. Mol. Biol. Evol. 2007, 24, 948–955. [Google Scholar] [CrossRef] [PubMed]
- Rund, S.S.C.; Hou, T.Y.; Ward, S.M.; Collins, F.H.; Duffield, G.E. Genome-wide profiling of diel and circadian gene expression in the malaria vector Anopheles gambiae. Proc. Natl. Acad. Sci. USA 2011, 108, E421–E430. [Google Scholar] [CrossRef] [PubMed]
- Gentile, C.; Rivas, G.B.S.; Meireles-Filho, A.C.A.; Lima, J.B.P.; Peixoto, A.A. Circadian expression of clock genes in two mosquito disease vectors: cry2 is different. J. Biol. Rhythm. 2009, 24, 444–451. [Google Scholar] [CrossRef] [PubMed]
- Leming, M.T.; Rund, S.S.C.; Behura, S.K.; Duffield, G.E.; O’Tousa, J.E. A database of circadian and diel rhythmic gene expression in the yellow fever mosquito Aedes aegypti. BMC Genom. 2014. [Google Scholar] [CrossRef] [PubMed]
- Rund, S.S.C.; Bonar, N.A.; Champion, M.C.; Ghazi, J.P.; Houk, C.H.; Leming, M.T.; Syed, Z.; Duffield, G.E. Daily rhythms in antennal protein and olfactory sensitivity in the malaria mosquito Anopheles gambiae. Sci. Rep. 2013. [Google Scholar] [CrossRef] [PubMed]
- Tuchinda, P.; Kitaoka, M.; Ogata, T.; Kurihara, T. On the diurnal rhythmus of biting behavior of Aëdes aegypti in relation to the age and to the hemorrhagic fever in Bangkok, 1964. Jpn. J. Trop. Med. 1969, 10, 1–6. [Google Scholar] [CrossRef]
- Bioclock, Database of Circadian Gene Expression. Developed By the Duffield Laboratory. Available online: http://www3.nd.edu/~bioclock/ (accessed on 2 October 2015).
- Balmert, N.J.; Rund, S.S.C.; Ghazi, J.P.; Zhou, P.; Duffield, G.E. Time-of-day specific changes in metabolic detoxification and insecticide resistance in the malaria mosquito Anopheles gambiae. J. Insect Physiol. 2014, 64, 30–39. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Liu, Y.; Teng, H.; Sauman, I.; Sehnal, F.; Lee, H. Circadian control of permethrin-resistance in the mosquito Aedes aegypti. J. Insect Physiol. 2010, 56, 1219–1223. [Google Scholar] [CrossRef] [PubMed]
- Bainbridge, C.A.; Margham, P.; Thomas, M. Diurnal fluctuations in susceptibility to insecticides in several strains of the yellow fever mosquito (Aedes aegypti L.). Pestic. Sci. 1982, 13, 92–96. [Google Scholar] [CrossRef]
- Roberts, D.; Smolensky, M.; Hsi, B.; Scanlon, J. Circadian pattern in susceptibility of Aedes aegypti (L.) larvae to Dursban. In Chronobiology; Scheving, L.E., Halberg, F., Pauly, J.E., Eds.; Igaku Schoin: Tokyo, Japan, 1974; pp. 612–616. [Google Scholar]
- Moon, Y.M.; Metoxen, A.J.; Leming, M.T.; Whaley, M.A.; O’Tousa, J.E. Rhodopsin management during the light-dark cycle of Anopheles gambiae mosquitoes. J. Insect Physiol. 2014, 70, 88–93. [Google Scholar] [CrossRef] [PubMed]
- Jenkins, A.M.; Muskavitch, M.A. Crepuscular behavioral variation and profiling of opsin genes in Anopheles gambiae and Anopheles stephensi (Diptera: Culicidae). J. Med. Entomol. 2015, 52, 296–307. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.; Leming, M.T.; Metoxen, A.J.; Whaley, M.A.; O’Tousa, J.E. Light-mediated control of rhodopsin movement in mosquito photoreceptors. J. Neurosci. 2012, 32, 13661–13667. [Google Scholar] [CrossRef] [PubMed]
- Kawada, H.; Takemura, S.Y.; Arikawa, K.; Takagi, M. Comparative study on nocturnal behavior of Aedes aegypti and Aedes albopictus. J. Med. Entomol. 2005, 42, 312–318. [Google Scholar] [CrossRef]
- Cooke, M.K.; Kahindi, S.C.; Oriango, R.M.; Owaga, C.; Ayoma, E.; Mabuka, D.; Nyangau, D.; Abel, L.; Atieno, E.; Awuor, S. “A bite before bed”: Exposure to malaria vectors outside the times of net use in the highlands of western Kenya. Malar. J. 2015, 14, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Geissbühler, Y.; Chaki, P.; Emidi, B.; Govella, N.J.; Shirima, R.; Mayagaya, V.; Mtasiwa, D.; Mshinda, H.; Fillinger, U.; Lindsay, S.W.; et al. Interdependence of domestic malaria prevention measures and mosquito-human interactions in urban Dar es Salaam, Tanzania. Malar. J. 2007. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bockarie, M.J.; Alexander, N.; Bockarie, F.; Ibam, E.; Barnish, G.; Alpers, M. The late biting habit of parous Anopheles mosquitoes and pre-bedtime exposure of humans to infective female mosquitoes. Trans. R. Soc. Trop. Med. Hyg. 1996, 90, 23–25. [Google Scholar] [CrossRef]
- Moiroux, N.; Damien, G.B.; Egrot, M.; Djenontin, A.; Chandre, F.; Corbel, V.; Killeen, G.F.; Pennetier, C. Human exposure to early morning Anopheles funestus biting behavior and personal protection provided by long-lasting insecticidal nets. PLoS ONE 2014, 9, e104967. [Google Scholar]
- Moiroux, N.; Gomez, M.B.; Pennetier, C.; Elanga, E.; Djènontin, A.; Chandre, F.; Djègbé, I.; Guis, H.; Corbel, V. Changes in Anopheles funestus biting behavior following universal coverage of long-lasting insecticidal nets in Benin. J. Infect. Dis. 2012, 206, 1622–1629. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Harris, A.F.; Matias-Arnéz, A.; Hill, N. Biting time of Anopheles darlingi in the Bolivian Amazon and implications for control of malaria. Trans. R. Soc. Trop. Med. Hyg. 2006, 100, 45–47. [Google Scholar] [CrossRef] [PubMed]
- Yohannes, M.; Boelee, E. Early biting rhythm in the afro-tropical vector of malaria, Anopheles arabiensis, and challenges for its control in Ethiopia. Med. Vet. Entomol. 2012, 26, 103–105. [Google Scholar] [CrossRef] [PubMed]
- Klowden, M.J.; Briegel, H. Mosquito gonotrophic cycle and multiple feeding potential: Contrasts between Anopheles and Aedes. (Diptera: Culicidae). J. Med. Entomol. 1994, 31, 618–622. [Google Scholar] [CrossRef] [PubMed]
- Matowo, N.S.; Moore, J.; Mapua, S.; Madumla, E.P.; Moshi, I.R.; Kaindoa, E.W.; Mwangungulu, S.P.; Kavishe, D.R.; Sumaye, R.D.; Lwetoijera, D.W. Using a new odour-baited device to explore options for luring and killing outdoor-biting malaria vectors: A report on design and field evaluation of the Mosquito Landing Box. Parasit. Vectors 2013. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ndiath, M.O.; Mazenot, C.; Sokhna, C.; Trape, J.F. How the malaria vector Anopheles gambiae adapts to the use of insecticide-treated nets by African populations. PLoS ONE 2014, 9, e97700. [Google Scholar] [CrossRef] [PubMed]
- Charlwood, J.D.; Graves, P.M. The effect of permethrin-impregnated bednets on a population of Anopheles farauti in coastal Papua New Guinea. Med. Vet. Entomol. 1987, 1, 319–327. [Google Scholar] [CrossRef] [PubMed]
- Fornadel, C.M.; Norris, L.C.; Glass, G.E.; Norris, D.E. Analysis of Anopheles arabiensis blood feeding behavior in southern Zambia during the two years after introduction of insecticide-treated bed nets. Am. J. Trop. Med. Hyg. 2010, 83, 848–853. [Google Scholar] [CrossRef] [PubMed]
- Fritz, M.L.; Walker, E.D.; Yunker, A.J.; Dworkin, I. Daily blood feeding rhythms of laboratory-reared North American Culex pipiens. J. Circadian Rhythm. 2014. [Google Scholar] [CrossRef] [PubMed]
- Veronesi, R.; Gentile, G.; Carrieri, M.; Maccagnani, B.; Stermieri, L.; Bellini, R. Seasonal pattern of daily activity of Aedes caspius, Aedes detritus, Culex modestus, and Culex pipiens in the Po Delta of northern Italy and significance for vector-borne disease risk assessment. J. Vector Ecol. 2012, 37, 49–61. [Google Scholar] [CrossRef] [PubMed]
- Rund, S.S.C.; Gentile, J.E.; Duffield, G.E. Extensive circadian and light regulation of the transcriptome in the malaria mosquito Anopheles gambiae. BMC Genom. 2013. [Google Scholar] [CrossRef] [PubMed]
- Mathias, D.; Jacky, L.; Bradshaw, W.E.; Holzapfel, C.M. Geographic and developmental variation in expression of the circadian rhythm gene, timeless, in the pitcher-plant mosquito, Wyeomyia smithii. J. Insect Physiol. 2005, 51, 661–667. [Google Scholar] [CrossRef] [PubMed]
- Chahad-Ehlers, S.; Gentile, C.; Lima, J.B.P.; Peixoto, A.A.; Bruno, R.V. Analysis of cycle gene expression in Aedes aegypti brains by in situ hybridization. PLoS ONE 2013, 8, e52559. [Google Scholar]
- Gentile, C.; Meireles-Filho, A.C.; Britto, C.; Lima, J.B.; Valle, D.; Peixoto, A.A. Cloning and daily expression of the timeless gene in Aedes aegypti (Diptera: Culicidae). Insect Biochem. Mol. Biol. 2006, 36, 878–884. [Google Scholar] [CrossRef] [PubMed]
- González-Alvarez, R.; Villanueva-Segura, O.K.; Ponce-García, G.; de la Luz Martínez-Fierro, M.; Delgado-Enciso, I.; Flores-Suárez, A.E.; Garza-Guajardo, R.; de Jesús Zamudio, M.; Barrera-Saldaña, H.A.; Barboza-Quintana, O.; et al. Molecular cloning, sequence analysis, and gene expression of the circadian clock gene Period. in Culex quinquefasciatus Say (Diptera: Culicidae). Southwest. Entomol. 2015, 40, 71–80. [Google Scholar]
- Rodriguez-Sanchez, I.P.; Villanueva-Segura, O.K.; Gonzalez-Alvarez, R.; Flores-Suarez, A.E.; Garza-Rodriguez, M.L.; Delgado-Enciso, I.; Martinez-de-Villarreal, L.E.; Castillo, R.C.; Favela-Lara, S.; Garza-Guajardo, R.; et al. Molecular cloning and characterization of the circadian clock Timeless gene in Culex quinquefasciatus Say (Diptera: Culicidae). Southwest. Entomol. 2015, 40, 53–70. [Google Scholar] [CrossRef]
- Summa, K.; Urbanski, J.M.; Zhao, X.; Poelchau, M.; Armbruster, P. Cloning and sequence analysis of the circadian clock genes period and timeless in Aedes albopictus (Diptera: Culicidae). J. Med. Entomol. 2012, 49, 777–782. [Google Scholar] [CrossRef] [PubMed]
- Tormey, D.; Colbourne, J.K.; Mockaitis, K.; Choi, J.; Lopez, J.; Burkhart, J.; Bradshaw, W.; Holzapfel, C. Evolutionary divergence of core and post-translational circadian clock genes in the pitcher-plant mosquito, Wyeomyia smithii. BMC Genom. 2015. [Google Scholar] [CrossRef] [PubMed]
- Ptitsyn, A.; Reyes Solis, G.; Saavedra Rodriguez, K.; Betz, J.; Suchman, E.; Carlson, J. Rhythms and synchronization patterns in gene expression in the Aedes aegypti mosquito. BMC Genom. 2011. [Google Scholar] [CrossRef] [PubMed]
- Sumba, L.A.; Okoth, K.; Deng, A.L.; Githure, J.; Knols, B.G.J.; Beier, J.C.; Hassanali, A. Daily oviposition patterns of the African malaria mosquito Anopheles gambiae Giles (Diptera: Culicidae) on different types of aqueous substrates. J. Circadian Rhythm. 2004. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fritz, M.L.; Huang, J.; Walker, E.D.; Bayoh, M.N.; Vulule, J.; Miller, J.R. Ovipositional periodicity of caged Anopheles gambiae individuals. J. Circadian Rhythm. 2008. [Google Scholar] [CrossRef] [PubMed]
- Chahad-Ehlers, S.; Lozovei, A.L.; Marques, M.D. Reproductive and post-embryonic daily rhythm patterns of the malaria vector Anopheles (Kerteszia.) cruzii: Aspects of the life cycle. Chronobiol. Int. 2007, 24, 289–304. [Google Scholar] [CrossRef] [PubMed]
- McCrae, A.W.R. Oviposition by African malaria vector mosquitoes. I. Temporal activity patterns of caged, wild-caught, freshwater Anopheles gambiae Giles sensu lato. Ann. Trop. Med. Parasitol. 1983, 77, 615–625. [Google Scholar] [PubMed]
- Chadee, D.D. The diel oviposition periodicity of Aedes aegypti (L.) (Diptera: Culicidae) in Trinidad, West Indies: Effects of forced egg retention. Bull. Entomol. Res. 2010, 100, 599–603. [Google Scholar] [CrossRef] [PubMed]
- Chadee, D.D. Effects of forced egg-retention on the oviposition patterns of female Aedes aegypti (Diptera: Culicidae). Bull. Entomol. Res. 1997, 87, 649–651. [Google Scholar] [CrossRef]
- Jones, M.D.R.; Cubbin, C.M.; Marsh, D. The circadian rhythm of flight activity of the mosquito Anopheles gambiae: The light-response rhythm. J. Exp. Biol. 1972, 57, 337–346. [Google Scholar]
- Jones, M.D.R.; Gubbins, S.J.; Cubbin, C.M. Circadian flight activity in four sibling species of Anopheles gambiae complex (Diptera, Culicidae.). Bull. Entomol. Res. 1974, 64, 241–246. [Google Scholar] [CrossRef]
- Jones, M.D.R.; Hill, M.; Hope, A.M. The circadian flight activity of the mosquito Anopheles gambiae: Phase setting by the light regime. J. Exp. Biol. 1967, 47, 503–511. [Google Scholar] [PubMed]
- Jones, M.D.R.; Gubbins, S.J. Changes in circadian flight activity of the mosquito Anopheles gambiae in relation to insemination, feeding and oviposition. Physiol. Entomol. 1978, 3, 213–220. [Google Scholar] [CrossRef]
- Jones, M.D.R.; Reiter, P. Entrainment of pupation and adult activity rhythms during development in the mosquito Anopheles gambiae. Nature 1975, 254, 242–244. [Google Scholar] [CrossRef] [PubMed]
- Rowland, M. Changes in the circadian flight activity of the mosquito Anopheles stephensi associated with insemination, blood-feeding, oviposition and nocturnal light-intensity. Physiol. Entomol. 1989, 14, 77–84. [Google Scholar] [CrossRef]
- Rund, S.S.C.; Lee, S.J.; Bush, B.R.; Duffield, G.E. Strain- and sex-specific differences in daily flight activity and the circadian clock of Anopheles gambiae mosquitoes. J. Insect Physiol. 2012, 58, 1609–1619. [Google Scholar] [CrossRef] [PubMed]
- Jones, M.D.R.; Cubbin, C.M.; Marsh, D. Light-on effects and the question of bimodality in the circadian flight activity of the mosquito Anopheles gambiae. J. Exp. Biol. 1972, 57, 347–357. [Google Scholar]
- Rowland, M. Flight activity of insecticide resistant and susceptible Anopheles stephensi mosquitos in actograph chambers lined with malathion, γ HCH or dieldrin. Med. Vet. Entomol. 1990, 4, 397–404. [Google Scholar] [CrossRef] [PubMed]
- Taylor, B. Biological clocks in mosquitoes. Available online: http://antbase.org/ants/africa/personal/crhtml/covercr.htm (accessed on 2 October 2015).
- Kawada, H.; Takagi, M. Photoelectric sensing device for recording mosquito host-seeking behavior in the laboratory. J. Med. Entomol. 2004, 41, 873–881. [Google Scholar] [CrossRef] [PubMed]
- Shinkawa, Y.; Takeda, S.; Tomioka, K.; Matsumoto, A.; Oda, T.; Chiba, Y. Variability in circadian activity patterns within the Culex pipiens complex (Diptera: Culcidae). J. Med. Entomol. 1994, 31, 49–56. [Google Scholar] [CrossRef] [PubMed]
- Jones, M.D.R. The programming of circadian flight-activity in relation to mating and the gonotrophic cycle in the mosquito, Aedes aegypti. Physiol. Entomol. 1981, 6, 307–313. [Google Scholar] [CrossRef]
- Taylor, B.; Jones, M.D.R. The circadian rhythm of flight activity in the mosquito Aedes aegypti (L.): The phase-setting effects of light-on and light-off. J. Exp. Biol. 1969, 51, 59–70. [Google Scholar] [PubMed]
- Peterson, E.L. The temporal pattern of mosquito flight activity. Behaviour 1980, 72, 1–25. [Google Scholar] [CrossRef]
- Peterson, E.L. Phase-resetting a mosquito circadian oscillator. J. Comp. Physiol. 1980, 138, 201–211. [Google Scholar] [CrossRef]
- Chiba, Y.; Shinkawa, Y.; Yoshii, M.; Matsumoto, A.; Tomioka, K.; Takahashi, S. A comparative study on insemination dependency of circadian activity pattern in mosquitoes. Physiol. Entomol. 1992, 17, 213–218. [Google Scholar] [CrossRef]
- Yee, W.L.; Foster, W.A. Diel sugar-feeding and host-seeking rhythms in mosquitoes (Diptera: Culicidae) under laboratory conditions. J. Med. Entomol. 1992, 29, 784–791. [Google Scholar] [CrossRef] [PubMed]
- Charlwood, J.D.; Jones, M.D.R. Mating behaviour in the mosquito, Anopheles gambiae s.l. I. Close range and contact behaviour. Physiol. Entomol. 1979, 4, 111–120. [Google Scholar] [CrossRef]
- Nijhout, H.F. Control of antennal hair erection in male mosquitoes. Biol. Bull. 1977, 153, 591–603. [Google Scholar] [CrossRef]
- Sawadogo, S.P.; Costantini, C.; Pennetier, C.; Diabaté, A.; Gibson, G.; Dabiré, K. Differences in timing of mating swarms in sympatric populations of Anopheles coluzzii and Anopheles gambiae ss (formerly An gambiae M and S molecular forms) in Burkina Faso, West Africa. Parasit. Vectors 2013. [Google Scholar] [CrossRef] [PubMed]
- Jones, M.D.R.; Gubbins, S.J. Modification of female circadian flight-activity by a male accessory-gland pheromone in the mosquito, Culex pipiens quinquefasciatus. Physiol. Entomol. 1979, 4, 345–351. [Google Scholar] [CrossRef]
- Benelli, G. The best time to have sex: Mating behaviour and effect of daylight time on male sexual competitiveness in the Asian tiger mosquito, Aedes albopictus (Diptera: Culicidae). Parasitol. Res. 2015, 114, 887–894. [Google Scholar] [CrossRef] [PubMed]
- Reiter, P.; Jones, M.D.R. Eclosion timing mechanism in the mosquito Anopheles gambiae. J. Entomol. Ser. A 1976, 50, 161–168. [Google Scholar] [CrossRef]
- Buffington, J. A circadian pattern in the respiration of larvae of the mosquito Culex pipiens. Mosquito News 1968, 28, 95–98. [Google Scholar]
- Yap, H.H.; Cutkomp, L.K. Activity and rhythm of ATPases in larvae of the mosquito, Aedes aegypti L. Life Sci. 1970, 9, 1419–1425. [Google Scholar] [CrossRef]
- Yap, H.H.; Cutkomp, L.K.; Halberg, F. Circadian rhythms in rate of oxygen consumption by larvae of the mosquito, Aedes aegypti (L). Chronobiologia 1974, 1, 54–61. [Google Scholar] [PubMed]
- Gary, R.E., Jr.; Foster, W.A. Diel timing and frequency of sugar feeding in the mosquito Anopheles gambiae, depending on sex, gonotrophic state and resource availability. Med. Vet. Entomol. 2006, 20, 308–316. [Google Scholar] [CrossRef] [PubMed]
- Gillett, J.D.; Haddow, A.J.; Corbet, P.S. The sugar-feeding-cycle in a cage-population of mosquitoes. Entomol. Exp. Appl. 1962, 5, 223–232. [Google Scholar] [CrossRef]
- Holliday-Hanson, M.L.; Yuval, B.; Washino, R.K. Energetics and sugar-feeding of field-collected Anopheline females. J. Vector Ecol. 1997, 22, 83–89. [Google Scholar] [PubMed]
- Gray, E.M.; Bradley, T.J. Metabolic rate in female Culex tarsalis (Diptera: Culicidae): Age, size, activity, and feeding effects. J. Med. Entomol. 2003, 40, 903–911. [Google Scholar] [CrossRef] [PubMed]
- Berlin, O.; Dwarakanath, S.; Pandian, R. Relation between diel activity and respiration in Armigeres subalbatus (Coquillett)(Diptera: Culicidae). J. Med. Entomol. 1975, 12, 479–480. [Google Scholar] [CrossRef] [PubMed]
- Schlein, Y.; Gratz, N.G. Determination of the age of some anopheline mosquitos by daily growth layers of skeletal apodemes. Bull. World Health Organ. 1973, 49, 371–375. [Google Scholar] [PubMed]
- Schlein, Y.; Gratz, N.G. Age determination of some flies and mosquitoes by daily growth layers of skeletal apodemes. Bull. World Health Organ. 1972, 47, 71–76. [Google Scholar] [PubMed]
- Yuval, B.; Bouskila, A. Temporal dynamics of mating and predation in mosquito swarms. Oecologia 1993, 95, 65–69. [Google Scholar] [CrossRef]
- Murdock, C.C.; Moller-Jacobs, L.L.; Thomas, M.B. Complex environmental drivers of immunity and resistance in malaria mosquitoes. Proc. Biol. Sci. 2013. [Google Scholar] [CrossRef] [PubMed]
- Meuti, M.E.; Stone, M.; Ikeno, T.; Denlinger, D.L. Functional circadian clock genes are essential for the overwintering diapause of the Northern house mosquito, Culex pipiens. J. Exp. Biol. 2015, 218, 412–422. [Google Scholar] [CrossRef] [PubMed]
- Meuti, M.E.; Denlinger, D.L. Evolutionary links between circadian clocks and photoperiodic diapause in insects. Integr. Comp. Biol. 2013, 53, 131–143. [Google Scholar] [CrossRef] [PubMed]
- Keating, J.A.; Bhattacharya, D.; Rund, S.S.C.; Hoover, S.; Dasgupta, R.; Lee, S.J.; Duffield, G.E.; Striker, R. Mosquito protein kinase G phosphorylates flavivirus NS5 and alters flight behavior in Aedes aegypti and Anopheles gambiae. Vector Borne Zoonotic Dis. 2013, 13, 590–600. [Google Scholar] [CrossRef] [PubMed]
- Lima-Camara, T.N.; Bruno, R.V.; Luz, P.M.; Castro, M.G.; Lourenço-de-Oliveira, R.; Sorgine, M.H.; Peixoto, A.A. Dengue infection increases the locomotor activity of Aedes aegypti females. PLoS ONE 2011, 6, e17690. [Google Scholar] [CrossRef] [PubMed]
- Berry, W.J.; Rowley, W.A.; Clarke, J.L.; Swack, N.S.; Hausler, W.J. Spontaneous flight activity of Aedes trivittatus (Diptera: Culicidae) infected with trivittatus virus (Bunyaviridae: California serogroup). J. Med. Entomol. 1987, 24, 286–289. [Google Scholar] [CrossRef] [PubMed]
- Blanford, J.I.; Blanford, S.; Crane, R.G.; Mann, M.E.; Paaijmans, K.P.; Schreiber, K.V.; Thomas, M.B. Implications of temperature variation for malaria parasite development across Africa. Sci. Rep. 2013. [Google Scholar] [CrossRef] [PubMed]
- Paaijmans, K.P.; Blanford, S.; Bell, A.S.; Blanford, J.I.; Read, A.F.; Thomas, M.B. Influence of climate on malaria transmission depends on daily temperature variation. Proc. Natl. Acad. Sci. USA 2010, 107, 15135–15139. [Google Scholar] [CrossRef] [PubMed]
- Lambrechts, L.; Paaijmans, K.P.; Fansiri, T.; Carrington, L.B.; Kramer, L.D.; Thomas, M.B.; Scott, T.W. Impact of daily temperature fluctuations on dengue virus transmission by Aedes aegypti. Proc. Natl. Acad. Sci. USA 2011, 108, 7460–7465. [Google Scholar] [CrossRef] [PubMed]
- Jones, M.D.R. Delayed effect of light on the mosquito “clock”. Nature 1973, 245, 384–385. [Google Scholar] [CrossRef] [PubMed]
- Hoffman, S.; Subramanian, G.M. Light deprivation affects larval development and arrestin gene expression in Anopheles stephensi. J. Med. Entomol. 2005, 42, 801–804. [Google Scholar] [CrossRef]
- Hori, M.; Shibuya, K.; Sato, M.; Saito, Y. Lethal effects of short-wavelength visible light on insects. Sci. Rep. 2014. [Google Scholar] [CrossRef] [PubMed]
- Honnen, A.; Johnston, P.R.; Monaghan, M.T. Sex-specific gene expression in the mosquito Culex pipiens f. molestus in response to artificial light at night. BMC Genom. 2016. [Google Scholar] [CrossRef] [PubMed]
- Sharma, V.K. Adaptive significance of circadian clocks. Chronobiol. Int. 2003, 20, 901–919. [Google Scholar] [CrossRef] [PubMed]
- Neafsey, D.E.; Waterhouse, R.M.; Abai, M.R.; Aganezov, S.S.; Alekseyev, M.A.; Allen, J.E.; Amon, J.; Arca, B.; Arensburger, P.; Artemov, G.; et al. Mosquito genomics. Highly evolvable malaria vectors: The genomes of 16 Anopheles mosquitoes. Science 2015. [Google Scholar] [CrossRef] [PubMed]
- Garrett-Jones, C.; Shidrawi, G.R. Malaria vectorial capacity of a population of Anopheles gambiae: An exercise in epidemiological entomology. Bull. World Health Organ. 1969, 40, 531–545. [Google Scholar] [PubMed]
- Garrett-Jones, C.; Ferreira Neto, J.A. The Prognosis for Interruption of Malaria Transmission Through Assessment of the Mosquito’s Vectorial Capacity; World Health Organization: Geneva, Switzerland, 1964. [Google Scholar]
- Detinova, T.S. Age grouping methods in Diptera of medical importance with special reference to some Vectors of Malaria. In World Health Organization monograph series; no. 47; World Health Organization: Geneva, Switzerland, 1962. [Google Scholar]
- Ranson, H.; Lissenden, N. Insecticide resistance in African Anopheles mosquitoes: A worsening situation that needs urgent action to maintain malaria control. Trends Parasitol. 2016, 32, 187–196. [Google Scholar] [CrossRef] [PubMed]
- Tirados, I.; Costantini, C.; Gibson, G.; Torr, S.J. Blood-feeding behaviour of the malarial mosquito Anopheles arabiensis: Implications for vector control. Med. Vet. Entomol. 2006, 20, 425–437. [Google Scholar] [CrossRef] [PubMed]
- Dekker, T.; Takken, W.; Braks, M.A. Innate preference for host-odor blends modulates degree of anthropophagy of Anopheles gambiae sensu lato (Diptera: Culicidae). J. Med. Entomol. 2001, 38, 868–871. [Google Scholar] [CrossRef] [PubMed]
- Mathenge, E.M.; Gimnig, J.E.; Kolczak, M.; Ombok, M.; Irungu, L.W.; Hawley, W.A. Effect of permethrin-impregnated nets on exiting behavior, blood feeding success, and time of feeding of malaria mosquitoes (Diptera: Culicidae) in western Kenya. J. Med. Entomol. 2001, 38, 531–536. [Google Scholar] [CrossRef] [PubMed]
- Stone, C.; Chitnis, N.; Gross, K. Environmental inuences on mosquito foraging and integrated vector management can delay the evolution of behavioral resistance. Evol. Appl. 2015, 9, 502–517. [Google Scholar] [CrossRef] [PubMed]
- Knols, B.G.; Meuerink, J. Odors influence mosquito behavior. Sci. Med. 1997, 4, 56–63. [Google Scholar]
- Bailey, S.L.; Heitkemper, M.M. Circadian rhythmicity of cortisol and body temperature: Morningness-eveningness effects. Chronobiol. Int. 2001, 18, 249–261. [Google Scholar] [CrossRef] [PubMed]
- Spengler, C.M.; Czeisler, C.A.; Shea, S.A. An endogenous circadian rhythm of respiratory control in humans. J. Physiol. 2000, 526, 683–694. [Google Scholar] [CrossRef] [PubMed]
- Fleissner, G.; Fleissner, G. Efferent Control of Visual Sensitivity in Arthropod Eyes: With Emphasis on Circadian Rhythms; G. Fischer Verlag: New York, NY, USA, 1987. [Google Scholar]
- Oxborough, R.M.; N’Guessan, R.; Jones, R.; Kitau, J.; Ngufor, C.; Malone, D.; Mosha, F.W.; Rowland, M.W. The activity of the pyrrole insecticide chlorfenapyr in mosquito bioassay: Towards a more rational testing and screening of non-neurotoxic insecticides for malaria vector control. Malar. J. 2015. [Google Scholar] [CrossRef] [PubMed]
- Shipp, E.; Otton, J. Orcadian rhythms of sensitivity to insecticides in Musca Domestica. (Diptera, Muscidae). Entomol. Exp. Appl. 1976, 19, 163–171. [Google Scholar] [CrossRef]
- West, P.A.; Protopopoff, N.; Wright, A.; Kivaju, Z.; Tigererwa, R.; Mosha, F.W.; Kisinza, W.; Rowland, M.; Kleinschmidt, I. Indoor residual spraying in combination with insecticide-treated nets compared to insecticide-treated nets alone for protection against malaria: A cluster randomised trial in Tanzania. PLoS Med. 2014, 11, e1001630. [Google Scholar] [CrossRef] [PubMed]
- Corbel, V.; Akogbeto, M.; Damien, G.B.; Djenontin, A.; Chandre, F.; Rogier, C.; Moiroux, N.; Chabi, J.; Banganna, B.; Padonou, G.G. Combination of malaria vector control interventions in pyrethroid resistance area in Benin: A cluster randomised controlled trial. Lancet Infect. Dis. 2012, 12, 617–626. [Google Scholar] [CrossRef]
- Pinder, M.; Jawara, M.; Jarju, L.B.; Salami, K.; Jeffries, D.; Adiamoh, M.; Bojang, K.; Correa, S.; Kandeh, B.; Kaur, H. Efficacy of indoor residual spraying with dichlorodiphenyltrichloroethane against malaria in Gambian communities with high usage of long-lasting insecticidal mosquito nets: A cluster-randomised controlled trial. Lancet 2015, 385, 1436–1446. [Google Scholar] [CrossRef]
- Krishnan, N.; Davis, A.J.; Giebultowicz, J.M. Circadian regulation of response to oxidative stress in Drosophila melanogaster. Biochem. Biophys. Res. Commun. 2008, 374, 299–303. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Baldwin, I.T. New insights into plant responses to the attack from insect herbivores. Annu. Rev. Genet. 2010, 44, 1–24. [Google Scholar] [CrossRef] [PubMed]
- Graça-Souza, A.V.; Maya-Monteiro, C.; Paiva-Silva, G.O.; Braz, G.R.; Paes, M.C.; Sorgine, M.H.; Oliveira, M.F.; Oliveira, P.L. Adaptations against heme toxicity in blood-feeding arthropods. Insect Biochem. Mol. Biol. 2006, 36, 322–335. [Google Scholar] [CrossRef] [PubMed]
- Magalhaes, T.; Brackney, D.E.; Beier, J.C.; Foy, B.D. Silencing an Anopheles gambiae catalase and sulfhydryl oxidase increases mosquito mortality after a blood meal. Arch. Insect Biochem. Physiol. 2008, 68, 134–143. [Google Scholar] [CrossRef] [PubMed]
- DeJong, R.J.; Miller, L.M.; Molina-Cruz, A.; Gupta, L.; Kumar, S.; Barillas-Mury, C. Reactive oxygen species detoxification by catalase is a major determinant of fecundity in the mosquito Anopheles gambiae. Proc. Natl. Acad. Sci. USA 2007, 104, 2121–2126. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, J.H.M.; Gonçalves, R.L.; Lara, F.A.; Dias, F.A.; Gandara, A.C.P.; Menna-Barreto, R.F.; Edwards, M.C.; Laurindo, F.R.; Silva-Neto, M.A.; Sorgine, M.H. Blood meal-derived heme decreases ROS levels in the midgut of Aedes aegypti and allows proliferation of intestinal microbiota. PLoS Pathog. 2011, 7, e1001320. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Christophides, G.K.; Cantera, R.; Charles, B.; Han, Y.S.; Meister, S.; Dimopoulos, G.; Kafatos, F.C.; Barillas-Mury, C. The role of reactive oxygen species on Plasmodium melanotic encapsulation in Anopheles gambiae. Proc. Natl. Acad. Sci. USA 2003, 100, 14139–14144. [Google Scholar] [CrossRef] [PubMed]
- Molina-Cruz, A.; DeJong, R.J.; Charles, B.; Gupta, L.; Kumar, S.; Jaramillo-Gutierrez, G.; Barillas-Mury, C. Reactive oxygen species modulate Anopheles gambiae immunity against bacteria and Plasmodium. J. Biol. Chem. 2008, 283, 3217–3223. [Google Scholar] [CrossRef] [PubMed]
- Beyenbach, K.W.; Petzel, D.H. Diuresis in mosquitoes: Role of a natriuretic factor. Physiology 1987, 2, 171–175. [Google Scholar]
- Roitberg, B.D.; Mondor, E.B.; Tyerman, J.G. Pouncing spider, flying mosquito: Blood acquisition increases predation risk in mosquitoes. Behav. Ecol. 2003, 14, 736–740. [Google Scholar] [CrossRef]
- Overend, G.; Cabrero, P.; Halberg, K.A.; Ranford-Cartwright, L.C.; Woods, D.J.; Davies, S.A.; Dow, J.A. A comprehensive transcriptomic view of renal function in the malaria vector, Anopheles gambiae. Insect. Biochem. Mol. Biol. 2015, 67, 47–58. [Google Scholar] [CrossRef] [PubMed]
- Blumberg, B.J.; Trop, S.; Das, S.; Dimopoulos, G. Bacteria-and IMD pathway-independent immune defenses against Plasmodium falciparum in Anopheles gambiae. PLoS ONE 2013, 8, e72130. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.E.; Edery, I. Circadian regulation in the ability of Drosophila to combat pathogenic infections. Curr. Biol. 2008, 18, 195–199. [Google Scholar] [CrossRef] [PubMed]
- Thaiss, C.A.; Zeevi, D.; Levy, M.; Zilberman-Schapira, G.; Suez, J.; Tengeler, A.C.; Abramson, L.; Katz, M.N.; Korem, T.; Zmora, N. Transkingdom control of microbiota diurnal oscillations promotes metabolic homeostasis. Cell 2014, 159, 514–529. [Google Scholar] [CrossRef] [PubMed]
- Dong, Y.; Manfredini, F.; Dimopoulos, G. Implication of the mosquito midgut microbiota in the defense against malaria parasites. PLoS Pathog. 2009, 5, e1000423. [Google Scholar] [CrossRef] [PubMed]
- Gill, S.; Le, H.D.; Melkani, G.C.; Panda, S. Time-restricted feeding attenuates age-related cardiac decline in Drosophila. Science 2015, 347, 1265–1269. [Google Scholar] [CrossRef] [PubMed]
- Xu, K.; DiAngelo, J.R.; Hughes, M.E.; Hogenesch, J.B.; Sehgal, A. The circadian clock interacts with metabolic physiology to influence reproductive fitness. Cell. Metab. 2011, 13, 639–654. [Google Scholar] [CrossRef] [PubMed]
- Minors, D.S.; Waterhouse, J.M. Circadian Rhythms and the Human; John Wright: Boston, MA, USA, 1981. [Google Scholar]
- Feigin, R.D.; Klainer, A.S.; Beisel, W.R. Circadian periodicity of blood amino-acids in adult men. Nature 1967, 215, 512–514. [Google Scholar] [CrossRef] [PubMed]
- Malherbe, C.; de Gasparo, M.; de Hertogh, R.; Hoem, J. Circadian variations of blood sugar and plasma insulin levels in man. Diabetologia 1969, 5, 397–404. [Google Scholar] [CrossRef] [PubMed]
- Van Cauter, E.; Blackman, J.D.; Roland, D.; Spire, J.P.; Refetoff, S.; Polonsky, K.S. Modulation of glucose regulation and insulin secretion by circadian rhythmicity and sleep. J. Clin. Investig. 1991, 88, 934–942. [Google Scholar] [CrossRef] [PubMed]
- Curtis, A.M.; Bellet, M.M.; Sassone-Corsi, P.; O’Neill, L.A. Circadian clock proteins and immunity. Immunity 2014, 40, 178–186. [Google Scholar] [CrossRef] [PubMed]
- Scheiermann, C.; Kunisaki, Y.; Frenette, P.S. Circadian control of the immune system. Nat. Rev. Immunol. 2013, 13, 190–198. [Google Scholar] [CrossRef] [PubMed]
- Briegel, H. Fecundity, metabolism, and body size in Anopheles (Diptera, Culicidae), vectors of malaria. J. Med. Entomol. 1990, 27, 839–850. [Google Scholar] [CrossRef] [PubMed]
- Surachetpong, W.; Pakpour, N.; Cheung, K.W.; Luckhart, S. Reactive oxygen species-dependent cell signaling regulates the mosquito immune response to Plasmodium falciparum. Antioxid. Redox Signal. 2011, 14, 943–955. [Google Scholar] [CrossRef] [PubMed]
- Kang, M.A.; Mott, T.M.; Tapley, E.C.; Lewis, E.E.; Luckhart, S. Insulin regulates aging and oxidative stress in Anopheles stephensi. J. Exp. Biol. 2008, 211, 741–748. [Google Scholar] [CrossRef] [PubMed]
- Pakpour, N.; Corby-Harris, V.; Green, G.P.; Smithers, H.M.; Cheung, K.W.; Riehle, M.A.; Luckhart, S. Ingested human insulin inhibits the mosquito NF-κB-dependent immune response to Plasmodium falciparum. Infect. Immun. 2012, 80, 2141–2149. [Google Scholar] [CrossRef] [PubMed]
- Hawking, F. The clock of the malaria parasite. Sci. Am. 1970, 222, 123–131. [Google Scholar] [CrossRef] [PubMed]
- Taliaferro, W.H.; Taliaferro, L.G. Morphology, periodicity and course of infection of Plasmodium brasilianum in Panamanian monkeys. Am. J. Epidemiol. 1934, 20, 1–49. [Google Scholar]
- Cambie, G.; Landau, I.; Chabaud, A.G. Timing niches of 3 species of Plasmodium coexisting in a rodent in Central Africa. C. R. Acad. Sci. III 1989, 310, 183–188. (In French) [Google Scholar]
- Chimanuka, B.; Francois, G.; Timperman, G.; Vanden Driessche, T.; Plaizier-Vercammen, J. Chronobiology of Plasmodium chabaudi chabaudi: Analysis of hourly recorded total and differential parasitaemia during a schizogonic cycle. Parasite 1997, 4, 319–323. [Google Scholar] [CrossRef] [PubMed]
- Gautret, P.; Deharo, E.; Chabaud, A.G.; Ginsburg, H.; Landau, I. Plasmodium vinckei vinckei, P.v. lentum and P. yoelii yoelii—Chronobiology of the asexual cycle in the blood. Parasite 1994, 1, 235–239. [Google Scholar] [CrossRef] [PubMed]
- Shungu, D.M.; Arnold, J.D. Induction of growth and division synchrony in Plasmodium vinckei chabaudi by photoperiodic rhythm. J. Parasitol. 1972, 58, 142–147. [Google Scholar] [CrossRef] [PubMed]
- Garnham, P.C.C. Malaria Parasites and Other Haemosporidia; Blackwell Scientific: Oxford, UK, 1966. [Google Scholar]
- Hawking, F.; Gammage, K. The timing of the asexual cycles of Plasmodium lophurae and of P. cathemerium. J. Parasitol. 1970, 56, 17–26. [Google Scholar] [CrossRef] [PubMed]
- Garcia, C.R.; Markus, R.P.; Madeira, L. Tertian and quartan fevers: Temporal regulation in malarial infection. J. Biol. Rhythm. 2001, 16, 436–443. [Google Scholar] [CrossRef]
- Mideo, N.; Reece, S.E.; Smith, A.L.; Metcalf, C.J. The Cinderella syndrome: Why do malaria-infected cells burst at midnight? Trends Parasitol. 2013, 29, 10–16. [Google Scholar] [CrossRef] [PubMed]
- Arnold, J.D.; Lalli, F.; Martin, D.C. Augmentation of growth and division synchrony and of vascular sequestration of Plasmodium berghei by the photoperiodic rhythm. J. Parasitol. 1969, 55, 597–608. [Google Scholar] [CrossRef] [PubMed]
- Boyd, G.H. Induced variations in the asexual cycle of Plasmodium cathemerium. Am. J. Epidemiol. 1929, 9, 181–187. [Google Scholar]
- Boyd, G.H. Experimental modification of the reproductive activity of Plasmodium cathemerium. J. Exp. Zool. 1929, 54, 111–126. [Google Scholar] [CrossRef]
- Gautret, P.; Deharo, E.; Tahar, R.; Chabaud, A.G.; Landau, I. The adjustment of the schizogonic cycle of Plasmodium chabaudi chabaudi in the blood to the circadian rhythm of the host. Parasite 1995, 2, 69–74. [Google Scholar] [CrossRef] [PubMed]
- Taliaferro, W.H.; Taliaferro, L.G. Alteration in the time of sporulation of Plasmodium brasilianum in monkeys by reversal of light and dark. Am. J. Epidemiol. 1934, 20, 50–59. [Google Scholar]
- O’Donnell, A.J.; Mideo, N.; Reece, S.E. Disrupting rhythms in Plasmodium chabaudi: Costs accrue quickly and independently of how infections are initiated. Malar. J. 2013. [Google Scholar] [CrossRef] [PubMed]
- O’Donnell, A.; Schneider, P.; McWatters, H.; Reece, S. Fitness costs of disrupting circadian rhythms in malaria parasites. Proc. Biol. Sci. 2011, 278, 2429–2436. [Google Scholar] [CrossRef] [PubMed]
- Taliaferro, L.G. Return to normal of the asexual cycle in bird malaria after retardation by low temperatures in vitro. J. Prev. Med. 1928, 2, 525–541. [Google Scholar]
- Hotta, C.T.; Gazarini, M.L.; Beraldo, F.H.; Varotti, F.P.; Lopes, C.; Markus, R.P.; Pozzan, T.; Garcia, C.R. Calcium-dependent modulation by melatonin of the circadian rhythm in malarial parasites. Nat. Cell Biol. 2000, 2, 466–468. [Google Scholar] [PubMed]
- Shea, S.A.; Hilton, M.F.; Orlova, C.; Ayers, R.T.; Mantzoros, C.S. Independent circadian and sleep/wake regulation of adipokines and glucose in humans. J. Clin. Endocrinol. Metab. 2005, 90, 2537–2544. [Google Scholar] [CrossRef] [PubMed]
- Reddy, A.B.; Karp, N.A.; Maywood, E.S.; Sage, E.A.; Deery, M.; O’Neill, J.S.; Wong, G.K.Y.; Chesham, J.; Odell, M.; Lilley, K.S.; et al. Circadian orchestration of the hepatic proteome. Curr. Biol. 2006, 16, 1107–1115. [Google Scholar] [CrossRef] [PubMed]
- Engelbrecht, D.; Coetzer, T.L. Sunlight inhibits growth and induces markers of programmed cell death in Plasmodium falciparum in vitro. Malar. J. 2015, 14, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Magesa, S.M.; Mdira, Y.K.; Akida, J.A.; Bygbjerg, I.C.; Jakobsen, P.H. Observations on the periodicity of Plasmodium falciparum gametocytes in natural human infections. Acta Trop. 2000, 76, 239–246. [Google Scholar] [CrossRef]
- Bray, R.S.; McCrae, A.W.R.; Smalley, M.E. Lack of a circadian rhythm in the ability of the gametocytes of Plasmodium falciparum to infect Anopheles gambiae. Int. J. Parasitol. 1976, 6, 399–401. [Google Scholar] [CrossRef]
- Githeko, A.K.; Brandling-Bennett, A.D.; Beier, M.; Mbogo, C.M.; Atieli, F.K.; Owaga, M.L.; Juma, F.; Collins, F.H. Confirmation that Plasmodium falciparum has aperiodic infectivity to Anopheles gambiae. Med. Vet. Entomol. 1993, 7, 373–376. [Google Scholar] [CrossRef] [PubMed]
- Naotunne, T.S.; Karunaweera, N.D.; del Giudice, G.; Kularatne, M.U.; Grau, G.E.; Carter, R.; Mendis, K.N. Cytokines kill malaria parasites during infection crisis: Extracellular complementary factors are essential. J. Exp. Med. 1991, 173, 523–529. [Google Scholar] [CrossRef] [PubMed]
- Ramiro, R.S.; Alpedrinha, J.; Carter, L.; Gardner, A.; Reece, S.E. Sex and death: The effects of innate immune factors on the sexual reproduction of malaria parasites. PLoS Pathog. 2011, 7, e1001309. [Google Scholar] [CrossRef] [PubMed]
- Gravenor, M.B.; Kwiatkowski, D. An analysis of the temperature effects of fever on the intra-host population dynamics of Plasmodium falciparum. Parasitology 1998, 117, 97–105. [Google Scholar] [CrossRef] [PubMed]
- Hawking, F.; Worms, M.J.; Gammage, K. Host temperature and control of 24-hour and 48-hour cycles in malaria parasites. Lancet 1968, 291, 506–509. [Google Scholar] [CrossRef]
- Kwiatkowski, D. Febrile temperatures can synchronize the growth of Plasmodium falciparum in vitro. J. Exo. Med. 1989, 169, 357–361. [Google Scholar] [CrossRef]
- Pavithra, S.R.; Banumathy, G.; Joy, O.; Singh, V.; Tatu, U. Recurrent fever promotes Plasmodium falciparum development in human erythrocytes. J. Biol. Chem. 2004, 279, 46692–46699. [Google Scholar] [CrossRef] [PubMed]
- Macdonald, G. The Epidemiology and Control of Malaria; Oxford University Press: London, UK, 1957; pp. 10–11. [Google Scholar]
- Mori, T.; Binder, B.; Johnson, C.H. Circadian gating of cell division in cyanobacteria growing with average doubling times of less than 24 hours. Proc. Natl. Acad. Sci. USA 1996, 93, 10183–10188. [Google Scholar] [CrossRef] [PubMed]
- Dong, G.; Yang, Q.; Wang, Q.; Kim, Y.; Wood, T.L.; Osteryoung, K.W.; van Oudenaarden, A.; Golden, S.S. Elevated ATPase activity of KaiC applies a circadian checkpoint on cell division in Synechococcus elongatus. Cell 2010, 140, 529–539. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Deuchert, E.; Wunsch, C. Evaluating nationwide health interventions: Malawi’s insecticide-treated-net distribution programme. J. R. Stat. Soc. Ser. A 2014, 177, 523–552. [Google Scholar] [CrossRef]
- Martinez-Bakker, M.; Helm, B. The influence of biological rhythms on host-parasite interactions. Trends Ecol. Evol. 2015, 30, 314–326. [Google Scholar] [CrossRef] [PubMed]

| Mosquito Rhythms in: | Anophelines | Other Mosquito Species |
|---|---|---|
| Insecticide response | [24] | [25] *, [26,27] |
| Vision | [28,29] | [30,31] |
| Olfaction | [21] | |
| Biting behavior (including bed net use and biting time) | [21] *, [32,33,34,35,36,37,38,39,40,41,42,43] | [22,39,40,44,45] |
| Molecular clock genes | [18,46] * | [19,20] *, [47,48,49,50,51,52,53] |
| Genome-wide transcriptomics | [18,23,46] * | [20] *, [54] |
| Oviposition | [55,56,57,58] | [59,60] |
| Locomotor flight activity | [61,62,63,64,65,66,67] *, [68,69,70,71] | [72,73,74,75,76] *, [31,70,71,77,78] |
| Mating | [67] *, [79,80,81] | [73,82] *, [77,83] |
| Larval/pupal rhythms | [65,84] * | [85] *, [26,27,86,87] |
| Sugar feeding | [78,88] | [89] *, [78] |
| Metabolism | [90] | [85] *, [86,87,91,92] |
| Cuticle development | [93] | [94] |
| Predation risk | [95] | |
| Immunity | [96] | |
| Related Work with Time-of-Day Aspects: | ||
| Diapause induction | [97,98] | |
| Behavioral changes during infection | [99] | [99,100,101] |
| Environmental temperature rhythms | [102,103] | [104] |
| The role and effect of light and the light:dark cycle | [46,63,66,68,105,106] | [74,76,107,108] |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rund, S.S.C.; O’Donnell, A.J.; Gentile, J.E.; Reece, S.E. Daily Rhythms in Mosquitoes and Their Consequences for Malaria Transmission. Insects 2016, 7, 14. https://doi.org/10.3390/insects7020014
Rund SSC, O’Donnell AJ, Gentile JE, Reece SE. Daily Rhythms in Mosquitoes and Their Consequences for Malaria Transmission. Insects. 2016; 7(2):14. https://doi.org/10.3390/insects7020014
Chicago/Turabian StyleRund, Samuel S. C., Aidan J. O’Donnell, James E. Gentile, and Sarah E. Reece. 2016. "Daily Rhythms in Mosquitoes and Their Consequences for Malaria Transmission" Insects 7, no. 2: 14. https://doi.org/10.3390/insects7020014
APA StyleRund, S. S. C., O’Donnell, A. J., Gentile, J. E., & Reece, S. E. (2016). Daily Rhythms in Mosquitoes and Their Consequences for Malaria Transmission. Insects, 7(2), 14. https://doi.org/10.3390/insects7020014