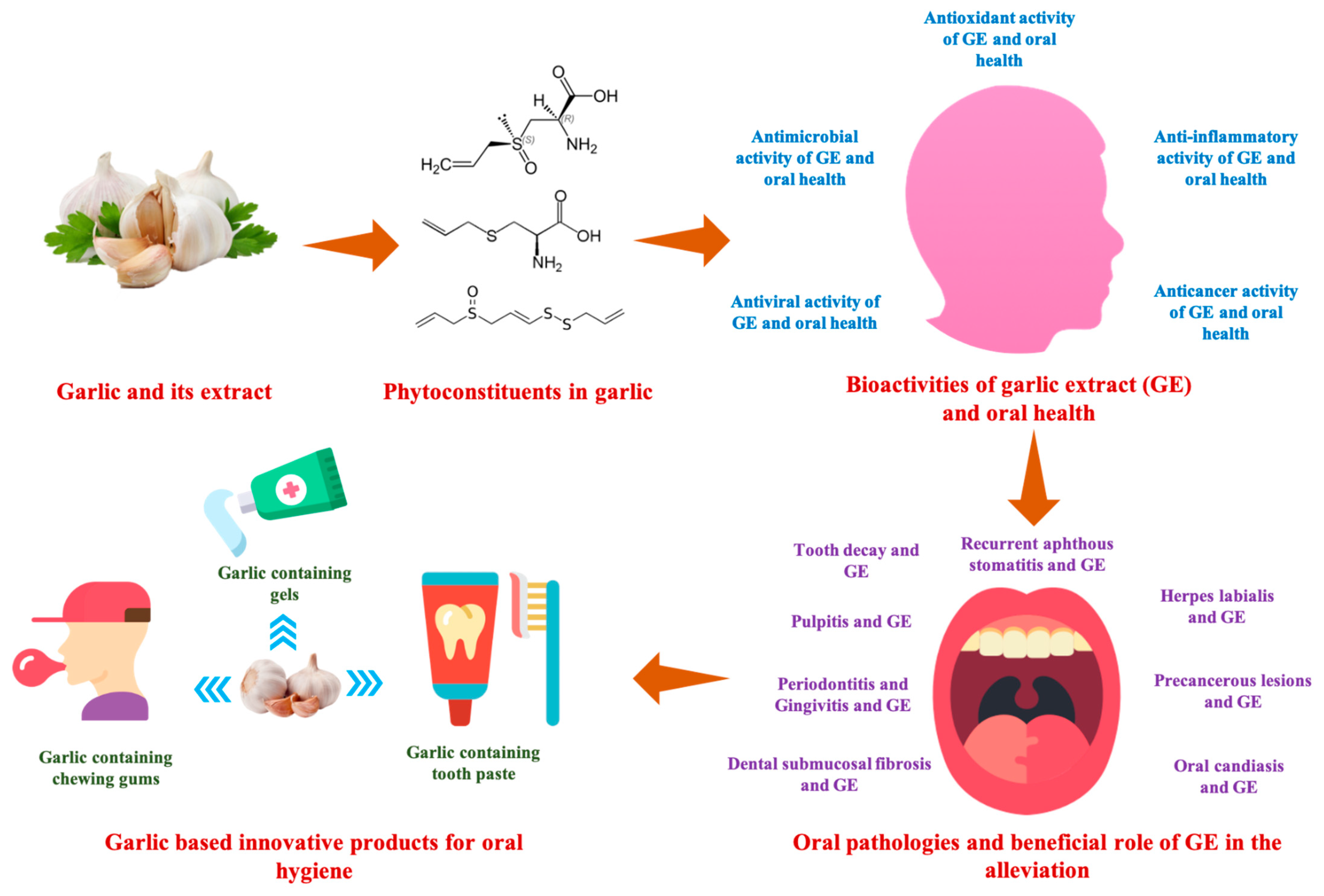
Garlic (Allium sativum L.) Bioactives and Its Role in Alleviating Oral Pathologies
Abstract
1. Introduction
2. Methodology
2.1. Selection Criteria
2.2. Phytochemicals from Garlic with Relation to Oral Health
2.3. Garlic Extract Preparation
2.4. Comparison of the Components of Fresh GE and BGE
3. Bioactivities of GE in Alleviating the Oral Pathologies
3.1. Antioxidant Activity of GE
3.2. Anti-Microbial Activity of GE
3.2.1. Antibacterial Activity of GE
3.2.2. Antifungal Activity of GE
3.3. Antiviral Activity of GE
3.4. Anti-Inflammatory Activity of GE
Mechanism of Modulation of Immunomodulatory Factors like TNF-α, IL by GE in Oral Diseases
3.5. Anti-Cancer Activity of GE
4. Oral Pathologies and Beneficial Role of GE in the Alleviation
4.1. Tooth Decay
4.2. Pulpitis and GE
4.3. Periodontitis and Gingivitis and GE
4.4. Recurrent Aphthous Stomatitis and GE
4.5. Herpes Labialis and GE
4.6. Precancerous Lesions and GE
4.7. Dental Submucosal Fibrosis and GE
4.8. Oral Candiasis and GE
4.9. Dental Plaque and Anti-Biofilm Potential
4.10. Oral Microflora and Antibiosis
5. Garlic Based Innovative Products for Oral Hygiene
5.1. Chewing Gum
5.2. Breath-Freshening Agent/Toothpaste
5.3. Garlic Gel
6. Safety of Garlic
7. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Kumar, M.; Devi, H.; Prakash, S.; Rathore, S.; Thakur, M.; Puri, S.; Pundir, A.; Bangar, S.P.; Changan, S.; Ilakiya, T.; et al. Ethnomedicinal plants used in the health care system: Survey of the mid hills of solan district, Himachal Pradesh, India. Plants 2021, 10, 1842. [Google Scholar] [CrossRef]
- Prakash, P.; Kumar, M.; Kumari, N.; Prakash, S.; Rathour, S.; Thakur, M.; Jamwal, R.; Janjua, S.; Ali, M.; Pundir, A.; et al. Therapeutic uses of wild plants by rural inhabitants of Maraog region in district Shimla, Himachal pradesh, India. Horticulturae 2021, 7, 343. [Google Scholar] [CrossRef]
- Prakash, P.; Kumar, M.; Pundir, A.; Puri, S.; Prakash, S.; Kumari, N.; Thakur, M.; Rathour, S.; Jamwal, R.; Janjua, S.; et al. Documentation of Commonly Used Ethnoveterinary Medicines from Wild Plants of the High Mountains in Shimla District, Himachal Pradesh, India. Horticulturae 2021, 7, 351. [Google Scholar] [CrossRef]
- Srivastava, S.C.; Sharma, U.C.; Singh, B.K.; Yadava, H.S. A profile of garlic production in India: Facts, trends and opportunities. Int. J. Agric. Environ. Biotechnol. 2012, 5, 477–482. [Google Scholar]
- Green, O.C.; Polydoris, N.G. Garlic, Cancer and Heart Disease: Review and Recommendations; GN Communications (Pub.) Limited: Chicago, IL, USA, 1993; Volume 3, pp. 21–41. [Google Scholar]
- Lawson, L.D. Garlic: A review of its medicinal effects and indicated active compounds. In Phytomedicines of Europe. Chemistry and Biological Activity; ACS Symposium Series 691; American Chemical Society: Washington, DC, USA, 1998; Volume 3, pp. 176–209. [Google Scholar]
- Woodward, P.W. Garlic and Friends: The History, Growth and Use of Edible Alliums; Hyland House: Melbourne, Australia, 1996; Volume 2, pp. 248–276. [Google Scholar]
- Zhang, Y.; Liu, X.; Ruan, J.; Zhuang, X.; Zhang, X.; Li, Z. Phytochemicals of garlic: Promising candidates for cancer therapy. Biomed. Pharmacother. 2020, 123, 109730. [Google Scholar] [CrossRef] [PubMed]
- Ceccanti, C.; Rocchetti, G.; Lucini, L.; Giuberti, G.; Landi, M.; Biagiotti, S.; Guidi, L. Comparative phytochemical profile of the elephant garlic (Allium ampeloprasum var. holmense) and the common garlic (Allium sativum) from the Val di Chiana area (Tuscany, Italy) before and after in vitro gastrointestinal digestion. Food Chem. 2021, 338, 128011. [Google Scholar] [CrossRef]
- Harini, K.; Babu, S.; Ajila, V.; Hegde, S. Garlic: It’s role in oral and systemic health. J. Health Allied Sci. NU 2013, 3, 17–22. [Google Scholar]
- Fenwick, G.R.; Hanley, A.B.; Whitaker, J.R. The genus Allium—Part 1. Crit. Rev. Food Sci. Nutr. 1985, 22, 199–271. [Google Scholar] [CrossRef]
- Block, E.; Ahmad, S.; Jain, M.K.; Crecely, R.W.; Apitz-Castro, R.; Cruz, M.R. The chemistry of alkyl thiosulfate esters. (E, Z)-Ajoene: A potent antithrombotic agent from garlic. J. Am. Chem. Soc. 1984, 106, 8295–8296. [Google Scholar] [CrossRef]
- Gazzani, G.; Daglia, M.; Papetti, A. Food components with anticaries activity. Curr. Opin. Biotechnol. 2012, 23, 153–159. [Google Scholar] [CrossRef]
- Kumar, M.; Prakash, S.; Kumari, N.; Pundir, A.; Punia, S.; Saurabh, V.; Choudhary, P.; Changan, S.; Dhumal, S.; Pradhan, P.C.; et al. Beneficial role of antioxidant secondary metabolites from medicinal plants in maintaining oral health. Antioxidants 2021, 10, 1061. [Google Scholar] [CrossRef]
- Hoglund, K.B.; Barnett, B.K.; Watson, S.A.; Melgarejo, M.B.; Kang, Y. Activity of bioactive garlic compounds on the oral microbiome: A literature review. Gen. Dent. 2020, 68, 27–33. [Google Scholar]
- Singh, R.P.; Prakash, S.; Bhatia, R.; Negi, M.; Singh, J.; Bishnoi, M.; Kondepudi, K.K. Generation of structurally diverse pectin oligosaccharides having prebiotic attributes. Food Hydrocoll. 2020, 108, 105988. [Google Scholar] [CrossRef]
- Kumar, M.; Tomar, M.; Punia, S.; Dhakane, J.; Dhumal, S.; Changan, S.; Senapathy, M.; Berwal, M.K.; Vellaikumar, S.; Sayed, A.A.; et al. Plant-based proteins and their multifaceted industrial applications. LWT 2021, 154, 112620. [Google Scholar] [CrossRef]
- Prakash, S.; Kumar, M.; Kumari, N.; Thakur, M.; Rathour, S.; Pundir, A.; Sharma, A.K.; Bangar, S.P.; Dhumal, S.; Singh, S.; et al. Plant-Based Antioxidant Extracts and Compounds in the Management of Oral Cancer. Antioxidants 2021, 10, 1358. [Google Scholar] [CrossRef]
- Mitra, S.; Anand, U.; Sanyal, R.; Jha, N.K.; Behl, T.; Mundhra, A.; Ghosh, A.; Radha Kumar, M.; Proćków, J.; Dey, A. Neoechinulins: Molecular, cellular, and functional attributes as promising therapeutics against cancer and other human diseases. Biomed. Pharmacother. 2022, 145, 112378. [Google Scholar] [CrossRef]
- Kumar, M.; Tomar, M.; Bhuyan, D.J.; Punia, S.; Grasso, S.; Sá, A.G.A.; Carciofi, B.A.M.; Arrutia, F.; Changan, S.; Singh, S.; et al. Tomato (Solanum lycopersicum L.) seed: A review on bioactives and biomedical activities. Biomed. Pharmacother. 2021, 142, 112018. [Google Scholar] [CrossRef]
- Mann, J.; Bernstein, Y.; Findler, M. Periodontal disease and its prevention, by traditional and new avenues. Exp. Ther. Med. 2020, 19, 1504–1506. [Google Scholar] [CrossRef]
- Tsai, C.W.; Chen, H.W.; Sheen, L.Y.; Lii, C.K. Garlic: Health benefits and actions. BioMedicine 2012, 2, 17–29. [Google Scholar] [CrossRef]
- Ahmad, T.A.; El-Sayed, B.A.; El-Sayed, L.H. Development of immunization trials against Eimeria spp. Trials Vaccinol. 2016, 5, 38–47. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. Syst. Rev. 2021, 10, 89. [Google Scholar] [CrossRef] [PubMed]
- El-Saber Batiha, G.; Magdy Beshbishy, A.; GWasef, L.; Elewa, Y.H.A.; AAl-Sagan, A.; Abd El-Hack, M.E.; Taha, A.E.; Abd-Elhakim, Y.M.; Prasad Devkota, H. Chemical Constituents and Pharmacological Activities of Garlic (Allium sativum L.): A Review. Nutrients 2020, 12, 872. [Google Scholar] [CrossRef] [PubMed]
- Neeraj, S.; Sushila, K.; Neeraj, D.; Milind, P.; Minakshi, P. Garlic: A Pungent wonder from nature. Int. Res. J. Phamacy 2014, 5, 523–529. [Google Scholar]
- Shang, A.; Cao, S.Y.; Xu, X.Y.; Gan, R.Y.; Tang, G.Y.; Corke, H.; Mavumengwana, V.; Li, H.B. Bioactive Compounds and Biological Functions of Garlic (Allium sativum L.). Foods 2019, 8, 246. [Google Scholar] [CrossRef]
- Yun, H.M.; Ban, J.O.; Park, K.R.; Lee, C.K.; Jeong, H.S.; Han, S.B.; Hong, J.T. Potential therapeutic effects of functionally active compounds isolated from garlic. Pharmacol. Ther. 2014, 142, 183–195. [Google Scholar] [CrossRef]
- Santhosha, S.; Jamuna, P.; Prabhavathi, S. Bioactive components of garlic and their physiological role in health maintenance: A review. Food Biosci. 2013, 3, 59–74. [Google Scholar] [CrossRef]
- Diretto, G.; Rubio-Moraga, A.; Argandoña, J.; Castillo, P.; Gomez-Gomez, L.; Ahrazem, O. Tissuespecific accumulation of sulfur compounds and saponins in different parts of garlic cloves from purple and white ecotypes. Molecules 2017, 22, 1359. [Google Scholar] [CrossRef]
- Butt, M.S.; Sultan, M.T.; Butt, M.S.; Iqbal, J. Garlic: Nature’s protection against physiological threats. Crit. Rev. Food Sci. Nutr. 2009, 49, 538–551. [Google Scholar] [CrossRef]
- Shooriabi, M. Effects of Allium sativum (Garlic) and Its Derivatives on Oral Diseases: A Narrative Review. J. Res. Dent. Maxillofac. Sci. 2021, 6, 36–44. [Google Scholar]
- Vlachojannis, C.; Chrubasik-Hausmann, S.; Hellwig, E.; Vach, K.; Al-Ahmad, A. Activity of preparations from Spilanthes oleracea, propolis, Nigella sativa, and black garlic on different microorganisms involved in oral diseases and on total human salivary bacteria: A pilot study. Phytother. Res. 2018, 32, 1992–2001. [Google Scholar] [CrossRef]
- Durairaj, S.; Srinivasan, S.; Lakshmanaperumalsamy, P. In vitro antibacterial activity and stability of garlic extract at different pH and temperature. Electron. J. Biol. 2009, 5, 5–10. [Google Scholar]
- Elosta, A.; Slevin, M.; Rahman, K.; Ahmed, N. Aged garlic has more potent antiglycation and antioxidant properties compared to fresh garlic extract in vitro. Sci. Rep. 2017, 7, 39613. [Google Scholar] [CrossRef]
- Nasri, H.; Nematbakhsh, M.; Rafieian-Kopaei, M. Ethanolic extract of garlic for attenuation of gentamicin-induced nephrotoxicity in Wistar rats. Iran. J. Kidney Dis. 2013, 7, 376–382. [Google Scholar] [PubMed]
- Zaini, A.S.; Putra, N.R.; Idham, Z.; Norodin, N.M.; Rasidek, N.M.; Yunus, M.C. Mini Review: Extraction of Allicin from Allium sativum using Subcritical Water Extraction. IOP Conf. Ser. Mater. Sci. Eng. 2020, 932, 012023. [Google Scholar] [CrossRef]
- Moreno-Ortega, A.; Pereira-Caro, G.; Ordonez, J.L.; Moreno-Rojas, R.; Ortíz-Somovilla, V.; Moreno-Rojas, J.M. Bioaccessibility of bioactive compounds of fresh garlic and black garlic through in vitro gastrointestinal digestion. Foods 2020, 9, 1582. [Google Scholar] [CrossRef] [PubMed]
- Simpson, D.S.; Oliver, P.L. ROS generation in microglia: Understanding oxidative stress and inflammation in neurodegenerative disease. Antioxidants 2020, 9, 743. [Google Scholar] [CrossRef] [PubMed]
- Lourenço, S.C.; Moldão-Martins, M.; Alves, V.D. Antioxidants of Natural Plant Origins: From Sources to Food Industry Applications. Molecules 2019, 24, 4132. [Google Scholar] [CrossRef]
- Locatelli, D.A.; Nazareno, M.A.; Fusari, C.M.; Camargo, A.B. Cooked garlic and antioxidant activity: Correlation with organosulfur compound composition. Food Chem. 2017, 220, 219–224. [Google Scholar] [CrossRef]
- Kanzaki, H.; Wada, S.; Narimiya, T.; Yamaguchi, Y.; Katsumata, Y.; Itohiya, K.; Nakamura, Y. Pathways that Regulate ROS Scavenging Enzymes, and Their Role in Defense Against Tissue Destruction in Periodontitis. Front. Physiol. 2017, 8, 351. [Google Scholar] [CrossRef]
- Ahmadi-Motamayel, F.; Goodarzi, M.T.; Jamshidi, Z.; Kebriaei, R. Evaluation of Salivary and Serum Antioxidant and Oxidative Stress Statuses in Patients with Chronic Periodontitis: A Case-Control Study. Front. Physiol. 2017, 8, 189. [Google Scholar] [CrossRef]
- Pradeep, A.; Rao, N.S.; Bajaj, P.; Agarwal, E. 8-Isoprostane: A lipid peroxidation product in gingival crevicular fluid in healthy, gingivitis and chronic periodontitis subjects. Arch. Oral Biol. 2013, 58, 500–504. [Google Scholar] [CrossRef]
- Shahidi, F.; Ambigaipalan, P. Phenolics and polyphenolics in foods, beverages and spices: Antioxidant activity and health effects—A review. J. Funct. Foods 2015, 18, 820–897. [Google Scholar]
- Feng, Y.; Xu, B.; Yagoub, A.E.A.; Ma, H.; Sun, Y.; Xu, X.; Zhou, C. Role of drying techniques on physical, rehydration, flavor, bioactive compounds and antioxidant characteristics of garlic. Food Chem. 2021, 343, 128404. [Google Scholar] [CrossRef]
- Tvarijonaviciute, A.; Aznar-Cayuela, C.; Rubio, C.P.; Ceron, J.J.; Lopez-Jornet, P. Evaluation of salivary oxidate stress biomarkers, nitric oxide and C-reactive protein in patients with oral lichen planus and burning mouth syndrome. J. Oral Pathol. Med. 2017, 46, 387–392. [Google Scholar] [CrossRef] [PubMed]
- Shiva, A.; Arab, S. Evaluation of Uric Acid, Total Antioxidant and Lipid Peroxidation Parameters in Serum and Saliva of Patients with Oral Lichen Planus. Glob. J. Health Sci. 2016, 8, 225. [Google Scholar] [CrossRef][Green Version]
- Shetty, S.; Thomas, B.; Shetty, V.; Bhandary, R.; Shetty, R.M. An in-vitro evaluation of the efficacy of garlic extract as an antimicrobial agent on periodontal pathogens: A microbiological study. AYU 2013, 34, 445–451. [Google Scholar] [CrossRef]
- Ravi, B.S.; Nirupad, S.; Chippagiri, P.; Pandurangappa, R. Antibacterial effects of natural herbal extracts on Streptococcus mutans: Can they be potential additives in dentifrices. Int. J. Dent. 2017, 2017, 1–5. [Google Scholar] [CrossRef]
- Prabu, G.R.; Gnanamani, A.; Sadulla, S. Guaijaverina plant flavonoid as potential antiplaque agent against Streptococcus mutans. J. Appl. Microbiol. 2006, 101, 487–495. [Google Scholar] [CrossRef] [PubMed]
- Shetty, S.; Shetty, R.M.; Rahman, B.; Vannala, V.; Desai, V.; Shetty, S.R. Efficacy of Psidium guajava and Allium sativum extracts as antimicrobial agents against periodontal pathogens. J. Pharm. Bioallied Sci. 2020, 12, 589–S594. [Google Scholar] [CrossRef]
- Groppo, F.; Ramacciato, J.; Simões, R.; Flório, F.; Sartoratto, A. Antimicrobial activity of garlic, tea tree oil, and chlorhexidine against oral microorganisms. Int. Dent. J. 2002, 52, 433–437. [Google Scholar] [CrossRef] [PubMed]
- Almehmady, A.; Ali, S. Transdermal Film Loaded with Garlic Oil-Acyclovir Nanoemulsion to Overcome Barriers for Its Use in Alleviating Cold Sore Conditions. Pharmaceutics 2021, 13, 669. [Google Scholar] [CrossRef] [PubMed]
- Goncagul, G. Antimicrobial Effect of Garlic (Allium sativum). Recent Pat. Anti-Infect. Drug Discov. 2010, 5, 91–93. [Google Scholar] [CrossRef] [PubMed]
- Borhan-Mojabi, K.; Sharifi, M.; Karagah, T. Efficacy of different concentrations of garlic extract in reduction of oral salivary microorganisms. Arch. Iran. Med. 2012, 15, 99–101. [Google Scholar] [PubMed]
- Fujisawa, H.; Watanabe, K.; Suma, K.; Origuchi, K.; Matsufuji, H.; Seki, T.; Ariga, T. Antibacterial potential of garlic-derived allicin and its cancellation by sulfhydryl compounds. Biosci. Biotechnol. Biochem. 2009, 73, 1948–1955. [Google Scholar] [CrossRef] [PubMed]
- Zini, A.; Mann, J.; Mazor, S.; Vered, Y. The Efficacy of Aged Garlic Extract on Gingivitis—A Randomized Clinical Trial. J. Clin. Dent. 2018, 29, 52–56. [Google Scholar]
- Bakri, I.M.; Douglas, C.W.I. Inhibitory effect of garlic extract on oral bacteria. Arch. Oral Biol. 2005, 50, 645–651. [Google Scholar] [CrossRef]
- Hutomo, S.; Putri, D.U.; Welviyanda, B.C.; Susilowati, H. Inhibition Effect of Garlic (Allium sativum) Extract on Streptococcus sanguinis Biofilm Formation Involving Bacterial Motility Mechanism. Malays. J. Med. Health Sci. 2021, 17, 169–174. [Google Scholar]
- Bin, C.; Al-Dhabi, N.A.; Esmail, G.A.; Arokiyaraj, S.; Arasu, M.V. Potential effect of Allium sativum bulb for the treatment of biofilm forming clinical pathogens recovered from periodontal and dental caries. Saudi J. Biol. Sci. 2020, 27, 1428–1434. [Google Scholar] [CrossRef]
- Muniz, I.D.A.F.; Campos, D.E.S.; Shinkai, R.S.A.; Trindade, T.G.D.; Cosme-Trindade, D.C. Case report of oral mucosa garlic burn during COVID-19 pandemic outbreak and role of teledentistry to manage oral health in an older adult woman. Spec. Care Dent. 2021, 41, 639–643. [Google Scholar] [CrossRef]
- Samaranayake, L.P.; Cheung, L.K.; Samaranayake, Y.H. Candidiasis and other fungal diseases of the mouth. Dermatol. Ther. 2002, 15, 251–269. [Google Scholar] [CrossRef]
- Papu, S.; Jaivir, S.; Sweta, S.; Singh, B.R. Medicinal values of garlic (Allium sativum L.) in human life: An overview. Greener J. Agric. Sci. 2014, 4, 265–280. [Google Scholar]
- Abdelhameed, B.; Abdullah, E. Clinical and Microbiological Evaluation of the Effect of Heat Killed Lactobacillus acidophilus and Garlic Extract on Candida albicans in a Group of Elderly Denture Wearers. Egypt. Dent. J. 2021, 67, 1475–1486. [Google Scholar] [CrossRef]
- Thomas, A.; Thakur, S.; Habib, R. Comparison of antimicrobial efficacy of green tea, garlic with lime and sodium fluoride mouth rinse against Streptococcus mutans, Lactobacilli species, and Candida albicans in children: A randomized dou-ble-blined controlled clinical trial. Int. J. Clin. Pediatr. Dent. 2017, 10, 234–239. [Google Scholar]
- Sabitha, P.; Adhikari, P.M.; Shenoy, S.; Kamath, A.; John, R.; Prabhu, M.V.; Padmaja, U. Efficacy of garlic paste in oral candidiasis. Trop. Dr. 2005, 35, 99–100. [Google Scholar] [CrossRef] [PubMed]
- Bakhshi, M.; Taheri, J.B.; Basir, S.S.; Tanik, A.; Pahlevan, R. Comparison of Therapeutic effect of aqueous extract of garlic and nystatin mouthwash in denture stomatistis. Gerodontology 2012, 29, 680–684. [Google Scholar] [CrossRef]
- Fattahi, H.F.; Alirezaei, S.; Goodarzi, H.; Khalesi, M.A. Investigation of antifungal effect of aqueous extract of garlic on Candida albicans (Invitro). J. Res. Dent. Sci. 2019, 16, 20–26. [Google Scholar] [CrossRef]
- Sreedhar, A.; Haritha, T.V.; Keenari, S.; Walvekar, A.; Uthappa, K.B.; Hari, A.; Emmanuel, A.A. Comparative evaluation of the efficacy of garlic and propolis extracts against Candida albicans with amphotericin-B as control- an invitro study. Int. J. Sci. Res. 2019, 8, 53–56. [Google Scholar]
- Mendoza-Juache, A.; Aranda-Romo, S.; Bermeo-Escalona, J.R.; Gómez-Hernández, A.; Pozos-Guillén, A.; Sánchez-Vargas, L.O. The essential oil of Allium sativum as an alternative agent against Candida isolated from dental prostheses. Rev. Iberoam. Micol. 2017, 34, 158–164. [Google Scholar] [CrossRef]
- Mirabadi, M.; Azadeghan Qomi, H.; Didehdar, M. In vitro activities of garlic essential oil against Candida species. Tabari Biomed. Stud. Res. J. 2019, 1, 12–17. [Google Scholar]
- Santosh, A.B.R.; Muddana, K. Viral infections of oral cavity. J. Fam. Med. Prim. Care 2020, 9, 36–42. [Google Scholar] [CrossRef]
- Asai, D.; Nakashima, H. Pathogenic Viruses Commonly Present in the Oral Cavity and Relevant Antiviral Compounds Derived from Natural Products. Medicines 2018, 5, 120. [Google Scholar] [CrossRef]
- Santacroce, L.; Di Cosola, M.D.; Bottalico, L.; Topi, S.; Charitos, I.A.; Ballini, A.; Dipalma, G. Focus on HPV Infection and the Molecular Mechanisms of Oral Carcinogenesis. Viruses 2021, 13, 559. [Google Scholar] [CrossRef] [PubMed]
- La Rosa, G.R.M.; Libra, M.; De Pasquale, R.; Ferlito, S.; Pedulla, E. Association of viral infections with oral cavity lesions: Role of SARS-CoV-2 infection. Front. Med. 2021, 7, 1059. [Google Scholar] [CrossRef] [PubMed]
- Sinha, A.K.; Farooqui, S.A.; Sharma, A.; Mishra, A.; Verma, V. Reactivity of allyl methyl sulphide, the in-vitro metabolite of garlic, with some amino acids and with phospholipid involved in viral infections. J. Biomol. Struct. Dyn. 2020, 1–7. [Google Scholar] [CrossRef]
- Tsai, Y.; Cole, L.L.; Davis, L.E.; Lockwood, S.J.; Simmons, V.; Wild, G.C. Antiviral properties of garlic: In vitro effects on influenza B, herpes simplex and coxsackie viruses. Planta Med. 1985, 6, 460–461. [Google Scholar] [CrossRef] [PubMed]
- Weber, N.D.; Andersen, D.O.; North, J.A.; Murray, B.K.; Lawson, L.D.; Hughes, B.G. In vitro virucidal effects of Allium sativum (garlic) extract and compounds. Planta Med. 1992, 58, 417–423. [Google Scholar] [CrossRef]
- Romeilah, R.M.; Fayed, S.A.; Mahmoud, G.I. Chemical compositions, antiviral and antioxidant activities of seven essential oils. J. Appl. Sci. Res. 2010, 6, 50–62. [Google Scholar]
- Razavi, S.M.; Azizolahi, B.; Rahimi, H. An investigation on antiviral effect of garlic extract on herpes simplex virus via cell culture. J. Dent. Sch. Shahid Beheshti Univ. Med. Sci. 2006, 24, 86093. [Google Scholar]
- Ban, J.O.; Oh, J.H.; Kim, T.M.; Kim, D.J.; Jeong, H.S.; Han, S.B.; Hong, J.T. Anti-inflammatory and arthritic effects of thiacremonone, a novel sulfurcompound isolated from garlic via inhibition of NF-κB. Arthritis Res. Ther. 2009, 11, 1–13. [Google Scholar] [CrossRef]
- Ohtani, M.; Nishimura, T. The preventive and therapeutic application of garlic and other plant ingredients in the treatment of periodontal diseases. Exp. Ther. Med. 2020, 19, 1507–1510. [Google Scholar] [CrossRef]
- Kim, H.K. Garlic supplementation ameliorates UV-induced photoaging in hairless mice by regulating antioxidative activity and MMPs expression. Molecules 2016, 21, 70. [Google Scholar] [CrossRef] [PubMed]
- Iciek, M.; Kwiecień, I.; Włodek, L. Biological properties of garlic and garlic-derived organosulfur compounds. Environ. Mol. Mutagen. 2009, 50, 247–265. [Google Scholar] [CrossRef]
- Zini, A.; Mann, J.; Mazor, S.; Vered, Y. Beneficial Effect of Aged Garlic Extract on Periodontitis: A Randomized Controlled Double-Blind Clinical Study. J. Clin. Biochem. Nutr. 2020, 67, 297–301. [Google Scholar] [CrossRef] [PubMed]
- Arreola, R.; Quintero-Fabian, S.; Lopez-Roa, R.; Flores-Gutierrez, E.; Reyes-Grajeda, J.; Carrera-Quintanar, L.; Ortuño-Sahagun, D. Immunomodulation and Anti-Inflammatory Effects of Garlic Compounds: Discovery Service for Endeavour College of Natural Health Library. J. Immunol. Res. 2015, 13, 1–13. [Google Scholar]
- Hodge, G.; Hodge, S.; Han, P. Allium sativum (garlic) suppresses leukocyte inflammatory cytokine production in vitro: Potential therapeutic use in the treatment of inflammatory bowel disease. Cytometry 2002, 48, 209–215. [Google Scholar] [CrossRef]
- Salman, H.; Bergman, M.; Bessler, H.; Punsky, I.; Djaldetti, M. Effect of a garlic derivative (alliin) on peripheral blood cell immune responses. Int. J. Immunopharmacol. 1999, 21, 589–597. [Google Scholar] [CrossRef]
- Li, Z.; Le, W.; Cui, Z. A novel therapeutic anticancer property of raw garlic extract via injection but not ingestion. Cell Death Discov. 2018, 4, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Tang, F.Y.; Chiang, E.P.I.; Chung, J.G.; Lee, H.Z.; Hsu, C.Y. S-allylcysteine modulates the expression of E-cadherin and inhibits the malignant progression of human oral cancer. J. Nutr. Biochem. 2009, 20, 1013–1020. [Google Scholar] [CrossRef]
- Schultz, C.R.; Gruhlke, M.C.; Slusarenko, A.J.; Bachmann, A.S. Allicin, a Potent New Ornithine Decarboxylase Inhibitor in Neuroblastoma Cells. J. Nat. Prod. 2020, 83, 2518–2527. [Google Scholar] [CrossRef]
- Meng, C.L.; Shyu, K.W. Inhibition of experimental carcinogenesis by painting with garlic extract. Nutr. Cancer 1990, 14, 207–217. [Google Scholar] [CrossRef]
- Pai, M.H.; Kuo, Y.H.; Chiang, E.P.I.; Tang, F.Y. S-Allylcysteine inhibits tumour progression and the epithelial–mesenchymal transition in a mouse xenograft model of oral cancer. Br. J. Nutr. 2012, 108, 28–38. [Google Scholar] [CrossRef]
- Muramatsu, T.; Shima, K.; Ohta, K.; Kizaki, H.; Ro, Y.; Kohno, Y.; Shimono, M. Inhibition of osteopontin expression and function in oral cancer cell lines by antisense oligonucleotides. Cancer Lett. 2005, 217, 87–95. [Google Scholar] [CrossRef] [PubMed]
- Bessho, T.; Roy, R.; Yamamoto, K.; Kasai, H.; Nishimura, S.; Tano, K.; Mitra, S. Repair of 8-hydroxyguanine in DNA by mammalian N-methylpurine-DNA glycosylase. Proc. Natl. Acad. Sci. USA 1993, 90, 8901–8904. [Google Scholar] [CrossRef]
- Banerjee, S.; Mukherjee, P.K.; Maulik, S.K. Garlic as an antioxidant: The good, the bad and the ugly. Phytother. Res. 2003, 17, 97–106. [Google Scholar] [CrossRef] [PubMed]
- Groppo, F.C.; Ramacciato, J.C.; Motta, R.H.L.; Ferraresi, P.M.; Sartoratto, A. Antimicrobial activity of garlic against oral streptococci. Int. J. Dent. Hyg. 2007, 5, 109–115. [Google Scholar] [CrossRef]
- Abdelkader, H.S.; Alayafi, A.A.; Ahmed, H.E.; Bin Osail, R.A. The Antibacterial Activity of Nanosilver Coupled Edible Plant Extracts Against Streptococcus mutans, the Cause of Dental Caries. J. Pharm. Res. Int. 2021, 33, 167–186. [Google Scholar] [CrossRef]
- Tamai, I.A.; Pakbin, B.; Fasaei, B.N. Genetic diversity and antifungal susceptibility of Candida albicans isolates from Iranian HIV-infected patients with oral candidiasis. BMC Res. Notes 2021, 14, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Guo, N.L.; Lu, D.P.; Woods, G.L.; Reed, E.; Zhou, G.Z.; Zhang, L.B.; Waldman, R.H. Demonstration of the anti-viral activity of garlic extract against human cytomegalovirus in vitro. Chin. Med. J. 1993, 106, 93–96. [Google Scholar]
- Ohtani, M.; Nishimura, T. Sulfur containing amino acids in aged garlic extract inhibit inflammation in human gingival ep-ithelial cells by suppressing intercellular adhesion molecule 1 expression and IL 6 secretion. Biomed. Rep. 2020, 12, 99–108. [Google Scholar] [CrossRef]
- Alamir, A.H.; Patil, S. Allicin Could Potentially Alleviate Oral Cancer Pain by Inhibiting “Pain Me-diators” TNF-α, IL-8, and Endothelin. Curr. Issues Mol. Biol. 2021, 43, 187–196. [Google Scholar] [CrossRef]
- Kshirsagar, M.M.; Dodamani, A.S.; Karibasappa, G.N.; Vishwakarma, P.K.; Vathar, J.B.; Sonawane, K.R.; Khobragade, V.R. Antibacterial activity of garlic extract on cariogenic bacteria: An in vitro study. Ayu 2018, 39, 165–172. [Google Scholar] [CrossRef]
- Prabhakaran, P.; Mariswamy, A.B. A scanning electron microscope evaluation of efficacy of sodium hypochlorite and Allium sativum in smear layer removal in root canals with the use of modified evacuation system: An ex vivo study. J. Conserv. Dent. 2018, 21, 401–407. [Google Scholar] [CrossRef] [PubMed]
- Mahfouz, S.; Wahba, O.M. Comparative Evaluation of Pulpal Response to Tri-antibiotic Paste and Allium Sativum with Formacresol as Pulpotomy Medication in Primary Teeth: An in vivo Study. Egypt. Dent. J. 2019, 65, 3131–3142. [Google Scholar] [CrossRef][Green Version]
- Lobene, R.R.; Mankodi, S.M.; Ciancio, S.G.; Lamm, R.A.; Charles, C.H.; Ross, N.M. Correlations among gingival indices: A methodology study. J. Periodontol. 1989, 60, 159–162. [Google Scholar] [CrossRef] [PubMed]
- Pistorius, A.; Brita, W.; Eva-Maria, S.; Matthias, K. Efficacy of Sub-gingival Irrigation Using Herbal Extracts on Gingival Inflammation. J. Periodontol. 2003, 74, 616–622. [Google Scholar] [CrossRef]
- Rezaei, S.; Rezaei, K.; Mahboubi, M.; Jarahzadeh, M.H.; Momeni, E.; Bagherinasab, M.; Memarzadeh, M.R. Comparison the efficacy of herbal mouthwash with chlorhexidine on gingival index of intubated patients in Intensive Care Unit. J. Indian Soc. Periodontol. 2016, 20, 404–408. [Google Scholar] [CrossRef]
- Chiang, C.P.; Yu-Fong Chang, J.; Wang, Y.P.; Wu, Y.H.; Wu, Y.C.; Sun, A. Recurrent aphthous stomatitis—Etiology, serum autoantibodies, anemia, hematinic deficiencies, and management. J. Formos. Med. Assoc. 2019, 118, 1279–1289. [Google Scholar] [CrossRef]
- Nair, P.K.; Dyasanoor, S. Clinical efficacy of allicin–A novel alternative therapeutic agent in the management of minor recurrent aphthous stomatitis. J. Adv. Clin. Res. Insights 2015, 2, 231–236. [Google Scholar] [CrossRef]
- Peter, A.E.; Sandeep, B.V.; Rao, B.G.; Kalpana, V.L. Calming the Storm: Natural Immunosuppressants as Adjuvants to Target the Cytokine Storm in COVID-19. Front. Pharmacol. 2021, 11, 2305. [Google Scholar] [CrossRef]
- Rouf, R.; Uddin, S.J.; Sarker, D.K.; Islam, M.T.; Ali, E.S.; Shilpi, J.A.; Sarker, S.D. Anti-viral potential of garlic (Allium sativum) and it’s organosulfur compounds: A systematic update of pre-clinical and clinical data. Trends Food Sci. Technol. 2020, 104, 219–234. [Google Scholar] [CrossRef]
- Meshri, S.M.; Zaki, A.M.; Raslan, H.S.; Shams El-Din, M.A. Chemopreventive effect of topical ap-plication of s-allylcysteine in the management of oral dysplastic potentially malignant disorders. Alex. Dent. J. 2017, 42, 33–39. [Google Scholar] [CrossRef]
- Jiang, X.; Zhang, Y.; Li, F.; Zhu, Y.; Chen, Y.; Yang, S.; Sun, G. Allicin as a possible adjunctive therapeutic drug for stage II oral submucous fibrosis: A preliminary clinical trial in a Chinese cohort. Int. J. Oral Maxillofac. Surg. 2015, 44, 1540–1546. [Google Scholar] [CrossRef]
- Jiang, X.W.; Zhang, Y.; Song, G.D.; Li, F.F.; Peng, H.Y.; Yang, S.K.; Sun, G.L. Clinical evaluation of allicin oral adhesive tablets in the treatment of recurrent aphthous ulceration. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2012, 113, 500–504. [Google Scholar] [CrossRef]
- Prabhakar, A.R.; Ahuja, V.; Basappa, N. Effect of Curry Leaves, Garlic and Tea Tree Oil on Streptococcus mutans and Lactobacilli in Children: A Clinical and Microbiological Study. Braz. Res. Pediatr. Dent. Integr. Clin. 2009, 9, 259–263. [Google Scholar]
- Bachrach, G.; Jamil, A.; Naor, R.; Tal, G.; Ludmer, Z.; Steinberg, D. Garlic Allicin as a Potential Agent for Controlling Oral Pathogens. J. Med. Food 2011, 14, 1338–1343. [Google Scholar] [CrossRef]
- Birring, O.J.; Viloria, I.L.; Nunez, P. Anti-microbial efficacy of Allium sativum extract against Enterococcus faecalis biofilm and its penetration into the root dentin: An in vitro study. Indian J. Dent. Res. 2015, 26, 477–482. [Google Scholar] [CrossRef]
- Octavia, A.; Budiardjo, S.B.; Indiarti, I.S.; Fauziah, E.; Suharsini, M.; Sutadi, H.; Rizal, M.F. Garlic extract efficacy against the viability of Enterococcus faecalis (In vitro). Int. J. Appl. Pharm. 2019, 194–197. [Google Scholar] [CrossRef]
- Chen, P.; Yao, H.; Yuan, Q.; Li, P.; Wang, X.; Su, W.; Wang, Y.; Li, P. Discovery of the possible mechanisms in kouyanqing granule for treatment of oral ulcers based on network pharmacology. BMC Complement Med. Ther. 2020, 20, 258–270. [Google Scholar] [CrossRef] [PubMed]
- Xie, X.L.; Liu, B.J.; Yuan, S.H. Clinical effect of garlic plaster on recurrent oral ulcer. J. Cent. South Univ. 2004, 29, 330–331. [Google Scholar]
- Jain, N.; Annigeri, R.G.; Pipalia, P.R. Efficacy of garlic in conjunction with pentoxifylline in the management of oral submucous fibrosis-a preliminary study. Int. J. Pharm. Sci. Res. 2016, 7, 5017. [Google Scholar]
- Marsh, P.D. Dental Plaque as a Microbial Biofilm. Caries Res. 2004, 38, 204–211. [Google Scholar] [CrossRef]
- Rakshanaa, T.V.R.; Geetha, R.V. Evaluation of Antimicrobial Action of Honey on Cariogenic Bacteria—An in Vitro Study. J. Pharm. Sci. Res. 2017, 9, 705–715. [Google Scholar]
- Houshmand, B.; Mahjour, F.; Dianat, O. Antibacterial effect of different concentrations of garlic (Al-lium sativum) extract on dental plaque bacteria. Indian J. Dent. Res. 2013, 24, 71. [Google Scholar] [PubMed]
- Khan, L.; Paulino, E.G.M.; Lim, D.; Nadela, F.; Yadav, R.; Birring, O.J.S. Anti-microbial efficacy of Allium sativum against Streptococcus mutans biofilm formation on orthodontic mini-implants. J. Orthod. Res. 2014, 2, 129. [Google Scholar] [CrossRef]
- Girish, V.M.; Liang, H.; Aguilan, J.T.; Nosanchuk, J.D.; Friedman, J.M.; Nacharaju, P. Anti-biofilm activity of garlic extract loaded nanoparticles. Nanomed. Nanotechnol. Biol. Med. 2019, 20, 102009. [Google Scholar] [CrossRef]
- Beshr, A.K.; Abdelrahim, R.A. Antibacterial efficacy of Allium sativum (garlic) and chitosan incorporated into two root canal sealers against Enterococcus faecalis: Comparative study. Tanta Dent. J. 2019, 16, 94. [Google Scholar] [CrossRef]
- Isani, A.; Masih, U.; Joshi, K. Ex vivo comparative evaluation of efficacy of disinfecting ability of garlic oil, neem oil, clove oil and tulsi oil with autoclaving on endodontic K files, tested against aerobic bacteria. Univ. J. Dent. Sci. 2020, 6, 27–31. [Google Scholar] [CrossRef]
- Karic, V.; Jaiswal, A.; Abrahamse, H.; Thakur, A.; Ganeshpurkar, A. Effectiveness of Allium sativum on Bacterial Oral Infection. Nat. Oral Care Dent. Ther. 2020, 345–369. [Google Scholar] [CrossRef]
- Zied, S.T.A.; Eissa, S.A. Comparative Study on Antibacterial Activities of Two Natural Plants versus Three Different Intracanal Medications; Endodontic Department, Faculty of Oral and Dental Medicine, Cairo University: Giza, Egypt, 2011; Volume 12, pp. 1–2. [Google Scholar]
- Staphane, J. In Vitro Evaluation of the Efficacy of an Aqueous Extract of Allium Sativum as an Antibacterial Agent on Three Major Periodontal Pathogens. J. Oral Dent. Health Res. 2021, 3, 121. [Google Scholar]
- Ataee, R.A.; Araqizade, H.; Yoosefi, R.; Tavana, A.M.; Ataee, M.H. Effect of Allium sativum Extract on Erythromycin and Methicillin Resistant Bacteria Isolated from Hospital Operating Room. J. Med. Bacteriol. 2016, 5, 7–14. [Google Scholar]
- Arbach, M.; Santana, T.M.; Moxham, H.; Tinson, R.; Anwar, A.; Groom, M.; Hamilton, C.J. Antimicrobial garlic-derived diallyl polysulfanes: Interactions with biological thiols in Bacillus subtilis. Biochim. Biophys. Acta (BBA) Gen. Subj. 2019, 1863, 1050–1058. [Google Scholar] [CrossRef]
- Kimura, S.; Tung, Y.C.; Pan, M.H.; Su, N.W.; Lai, Y.J.; Cheng, K.C. Black garlic: A critical review of its production, bioactivity, and application. J. Food Drug Anal. 2017, 25, 62–70. [Google Scholar] [CrossRef]
- Dewhirst, F.E.; Tamer, M.A.; Ericson, R.E.; Lau, C.N.; Levanos, V.A.; Boches, S.K.; Galvin, J.L.; Paster, B.J. The Diversity of Periodontal Spirochetes by 16S RRNA Analysis. Oral Microbiol. Immunol. 2000, 15, 196–202. [Google Scholar] [CrossRef] [PubMed]
- Porciani, P.F.; Grandini, S. The effect of zinc acetate and magnolia bark extract added to chewing gum on volatile sulfur-containing compounds in the oral cavity. J. Clin. Dent. 2012, 23, 76–79. [Google Scholar]
- Cun, L. Toothpaste with Black Garlic Extract, Issued 2020. CN Patent CN111888315A, 6 November 2020. [Google Scholar]
- Li, X. Garlic Antitoxic Bactericidal Toothpaste, Issued 2003. CN Patent CN1555777A, 30 December 2003. [Google Scholar]
- Sagar, H.; Jha, K.K.; Sharma, S. Formulation and Evaluation of Garlic Gel for Tongue Ulcer. Crjournals 2020, 1, 1–24. [Google Scholar]
- Sagar, H.; Jha, K.K.; Sharma, S.; Kumar, A. Therapeutic Study of Garlic Gel Formulation for Tongue Ulcer Healing. J. Adv. Pharmacogn. 2020, 1, 9–29. [Google Scholar]
- Hegziy, A.; Elshaib, S. Pharmaceutical Composition Containing Garlic Extract, Issued 2009. WO Patent WO2009092387A2, 1 October 2009. [Google Scholar]
- Kuichang, Z.; Zhang, Z. Black Garlic Chewing Gum, Issued 2012. CN Patent CN102763759A, 7 November 2012. [Google Scholar]
- Pompeius, M. Improve the Composition of Oral Health, Issued 2016. CN Patent CN103384526B, 13 October 2016. [Google Scholar]
- Steven, E. National Center for Biotechnology Information. Adherent Oral Pharmabiotic Delivery Strip, Issued 2021. U.S. Patent US2020155447-A1, 13 July 2021. [Google Scholar]
- Steiner, M.; Khan, A.H.; Holbert, D.; Lin, R.I. A double-blind crossover study in moderately hypercholesterolemic men that compared the effect of aged garlic extract and placebo administration on blood lipids. Am. J. Clin. Nutr. 1996, 64, 866–870. [Google Scholar] [CrossRef]
- Elheeny, A.A.H. Allium sativum extract as an irrigant in pulpectomy of primary molars: A 12-month short-term evaluation. Clin. Exp. Dent. Res. 2019, 5, 420–426. [Google Scholar] [CrossRef]




| Variety | Type of Extract | Bioactive Compounds Identified | References |
|---|---|---|---|
| Raw garlic bulb | AGE & EGE | Sulphur containing compounds (2.3%) (Thiosulphinates such as allicin, allylmethyl-, methylallyl- and trans-1-propenyl-thiosulfinate); (OrganoSulphur volatiles such as DADS, DAS, DATS, sulfur dioxide, E/Z-ajoene, SAC, and S-allyl-cysteine sulfoxide (alliin)); (Vinyldithiins such as 2-vinyl-4H-1,3 dithiin) | [27,31] |
| Raw garlic bulb | AGE & EGE | Phenols (1.5%), (β-resorcylic acid, pyrogallol, gallic acid, rutin, protocatechuic acid and quercetin) | [27] |
| Raw garlic bulb | AGE & EGE | Saponins (diosgenin, gitogenin and β-cholorogenin) | [30] |
| Raw garlic bulb | AGE & EGE | Carbohydrate (starch, sucrose, glucose, fructose) | [27] |
| Raw garlic bulb | AGE & EGE | Fatty acids (palmitic acid, oleic acid, linoleic acid, linolenic acid) | [27] |
| Plant Source | Type of Extract | Bioactive Compounds Identified | Type of Study | Major Findings and Mechanism of Action | References |
|---|---|---|---|---|---|
| Antioxidant Activity | |||||
| Garlic | Lyophilized garlic powder | Allicin, ajoene, DAS, DADS, DATS, 2-VD | In vitro study on antioxidant activity of garlic and the mechanisms involved in this activity | Garlic samples depicted TPC (2.43–11.21), DPPH (0.05–0.58 mg GAE/100 g DW), ABTS (0.02–164.80 mg GAE/100 g DW) and FRAP (12.30–164.80 mM TEAC/10 mg DW). Allicin, ajoenes and 2-VD showed higher antioxidant activity compared to DAS, DADS and DATS. | [41] |
| Garlic | Lyophilized garlic powder | Allicin | In vitro study on antioxidant activities of garlic | TPC (1.48–3.48 mg GAE/g DW), DPPH (IC50: 6.25–33.28 mg/mL), ABTS (IC50: 11.46–46.53 mg/mL), Allicin content (127.33–165.43 mg/100 g DW) | [46] |
| Antimicrobial Activity | |||||
| Garlic | AGE EGE | Allicin and DAS | In vitro study of the efficacy of GE as antibacterial agents against periodontal pathogens including Porphyromonas gingivalis and Aggregatibacter actinomycetemcomitans | AGE with different concentrations—25, 50, and 75 μL showed 17 mm, 21 mm, and 26 mm zone of inhibition, respectively. The AGE showed higher bacteriostatic activity than EGE against the P. gingivalis with MIC determined at 16.7 μL/mL. | [52] |
| Garlic | Hydro alcoholic GE | Allicin and other thiosulfonates | Clinical study on efficiency of different concentrations (40 and 70%) of GE in alleviating of oral salivary bacteria in a culture medium of Trypticase Soy Agar (TSA) (Saliva samples: 40 Patients) | The 40% concentration at 30 s revealed a 78.5% reduction, and at 60 s, there was an 83.3% reduction in CFU. The 70% concentrations showed 86.6% reduction at 30 s and 90.9% reduction in CFU at 60 s. | [56] |
| Garlic | GE in ethanol, hexane, acetone, water diethyl ether | Organosulfur compounds alkaloids saponins flavonoids tannins steroids | Human clinical study on potential effect of GE for the treatment of biofilm-forming clinical pathogens Lactobacillus acidophilus, Streptococcus sanguis, S. salivarius, S. mutans and Staphylococcus aureus recovered from periodontal and dental caries | GE showed high antibacterial activity against Staphylococcus aureus and Streptococcus mutans. EGE were particularly more effective against the pathogens, probably due to the more phytochemical content in the EGE | [61] |
| Garlic | AGE | Allicin allicin thiosulfonates ajoene | In vitro study on evaluation of the antimicrobial activity of two garlic clones (1: purple and 2: white) crude extracts against nine oral Streptococci strains was carried out | The white garlic clone was more effective than the purple one. MIC ranged from 0.5–33.0 mg mL for purple clone and from 7.0 to 63.0 mg mL for the white clone. MBC ranged from 1.0 to 129.0 mg mL and from 9.0 to 129.0 mg mL regarding purple and white clones, respectively | [98] |
| Garlic | DMSO extracts of garlic bulbs with 1:1 Nano silver 25 nm | Allyl 2-propenethiosulfinate | In vitro study to evaluate the antibacterial property of nanosilver coupled edible plant extracts against Streptococcus mutans | The synergism of silver nanoparticles and GE on S. mutans isolates produced bigger-sized inhibition zones than GE alone and the MIC ranged between 52.5 ± 7.73 to 103.6 ± 5.91 mg/mL. | [99] |
| Antifungal Activity | |||||
| Garlic Green tea | AGE | Allicin | Randomized double-blinded controlled clinical trial aimed to evaluate and compare the antimicrobial efficacy of green tea, garlic with lime and 0.05% NAF mouth rinses against Candida albicans (45 Patients, Time period: Once daily for 2 weeks) | Colony count of C. albicans NAF Mean baseline, 7.7 × 104 CFU/mL Mean postrinse, 4.5 × 104 CFU/mL Garlic with lime Mean base line, 7.1 × 104 CFU/mL Mean postrinse, 4.3 × 104 CFU/mL Green tea Mean base line, 6.4 × 104 CFU/mL Mean Post-rinse, 2.3 × 104 CFU/ml | [68] |
| Garlic | Paste | Human clinical study (randomized trial) aimed to evaluate the clinical efficacy of topical garlic paste in comparison with clotrimazole solution in 56 patients for 14 days with oral candidiasis | Percentage of patients with clinical response: The percentage of patients cured was 50%, improved was 36.7% whereas, 13.3% showed no change in clotrimazole group. For garlic group, the percentage of patients cured was 61.5%, improved was 38.5%, every patient showed positive improvements by garlic paste. | [69] | |
| Garlic | AGE | Allicin | Randomized clinical trial aimed to compare the antifungal effect of nystatin (N) mouth wash with AGE on denture stomatitis (4 Weeks, 40 Patients) | Mean width of erythema At the start of treatment,
At the start of treatment,
| [70] |
| Garlic | AGE | Allicin | In vitro study aimed to evaluate the efficacy of garlic and propolis extracts against Candida albicans and compare it with Amphotericin-B as control at 3 different concentrations. | Anticandidal actions at different concentration at 24 and 48 h duration (i) Amphotericin-B (Control) 24 h 48 h 10% 0.002000 0.000667 (ii) Propolis 24 h 48 h 10% 0.056000 0.098667 20% 0.082333 0.133333 30% 0.003333 0.131000 (iii) Garlic 24 h 48 h 10% 0.000667 0.004000 20% 0.000000 0.000000 30% 0.000000 0.000000 | [72] |
| Garlic | Essential’s oil |
| In vitro study aimed to evaluate the effect of garlic essential oil against Candida species | The MIC was lowest for C. albicans (0.4 µg/mL) followed by C. tropicalis (0.5 µg/mL) and C.glabrata (0.6 µg/mL). Similarly, MFC was lowest for C. albicans (0.7 µg/mL) and similar for C. tropicalis (0.8 µg/mL) and C. glabrata (0.8 µg/mL). C. albicans has more susceptibility to garlic essential oil. | [74] |
| Garlic | Powder |
| Clinical study aimed to assess the effect of heat killed Lactobacillus acidophilus as probiotic and GE as a prebiotic on salivary Candida albicans | Candida albicans counts: Control: 42 GE + LB: 3.33–1.8 C. albicans bioform forming ability Control: 5.0 GE + LB: 0.31–0.26 | [100] |
| Antiviral Activity | |||||
| Garlic | NS | allicin | In vitro rabbit skin cells and plaque count assay | Dose of 0.015 mg/mL and higher significantly reduce the HSV-1 population | [81] |
| Garlic | Fresh GE (juice), polar fraction and garlic associated compound | In GE: Thiosulfinates (Allicin) ailyl and aliyi methyl thiosulfinates and trans-1-propenyl allyl thiosulfinate In aqueous (polar) fraction: y-giutamyl-S-trans-1-propenyl-cysteine, y-glutamyl-SAC and y-gluta- myi-S-methylcysteine | In vitro plaque assay techniques on HeLa and Vero cells Cytotoxicity assay | The compounds were toxic against HSV-1 & HSV-2. Highest viricidal activity was in ajoene followed by allicin, allyl methyl thiosulfinate and methyl allyl thiosulfinate | [82] |
| Garlic | Oil using hydro-distillation | 3,3’-thiobis-1-Propene, Disulfide, 3 Methyl-trans-propenyl-disulfide, cis-Propenyl methyl disulphide, Propanedioic acid, Dimethyl trisulfide, Limonene, 8 Di-2-propenyl disulphide, Methyl-2-propenyl trisulfide, 3-vinyl-[4H]-1,2-dithiin, 2,4,5,6-Tetramethylpyrimidine, 2-vinyl-[4H]-1,3-dithiin, Di-2-propenyl trisulfide, Isobutyl isothiocyanate, 3,3’-thiobis-1-propene, 2,3-Dicarboxythiophene, Diallyl tetrasulphide, Diallyl pentasulfide, Diallyl hexasulfide, Methyl allyl, Mentasulfide, Methyl allyl hexasulfide | In vitro study Vero cells in cytopathicity assay antiviral activity against HSV-1 under | Increased the longevity of cells treated with GEO (Garlic essential oil) | [83] |
| Garlic | AGE and alcoholic extract | - | In vitro Hela cell culture | Very effective against HSV-1 Required higher dose for cytotoxic effect compared to antiviral effect | [84] |
| Garlic | NS | NS | In-vitro cells plaque reduction and early antigen assay | Antiviral activity against CMV was in dose dependent manner and author recommended as prophylactic use | [101] |
| Anti-Inflammatory Activity | |||||
| Garlic | AGE tablets (300 mg AGE powder) | SAC, S1PC and SAMC | Randomized controlled double-blind clinical study aimed to assess the long-term efficacy of AGE for the treatment of periodontitis (201 participants, 18-month study period) | Prevent or improve periodontal disease. SAC and SAMC inhibit LPS-induced inflammation in human gingival epithelial cells by suppressing intercellular adhesion molecule-1 expression and IL-6 secretion. | [90] |
| Garlic | AGE tablets | - | Randomized controlled clinical study to assess the effectivity of AGE extract in reducing gingivitis and gingival bleeding (151 participants, 4-month study period) | Decrease in Gingival bleeding index and Gingival index score with treatment of AGE is observed compared to placebo group (p < 0.001). | [91] |
| Garlic | Aged garlic extract (AGE > 10 months in ethanol) | SAC, S1PC and SAMC | In vitro study aimed to examine the role of SAC, S1PC and SAMC in AGE induced anti-inflammatory effects | Alleviation of gingivitis by reducing the inflammation markers IL-1β, IL-2, IL-6, IFN-γ | [102] |
| Anticancer activity | |||||
| Garlic | SAC standard purchased from LKT laboratories (USA) | SAC | In vivo study on mouse xenograft model | SAC showed anti-oral cancer activity by suppressing the osteopontin and N-methylpurine DNA glycosylase and by inhibiting the APK/ERK and phosphatidylinositol-3-kinase/Akt signalling pathways | [99] |
| Garlic | Allicin standard extracts | Allicin | In vitro primary oral tumors from oral squamous cell carcinoma patients were collected | Allicin alleviates the pain in oral squamous cell carcinoma by attenuating the TNF-α, pain mediators, endothelin and IL-8 | [103] |
| Plant Source | Type of Extract | Bioactive Compounds Identified | Disease Studied | Major Findings and Mechanism of Action | References |
|---|---|---|---|---|---|
| 2.5% garlic, Karnataka, India | 100 mL AGE | Allicin and thiosulfinates | Tooth decay | Growth of cariogenic bacteria, Streptococcus mutans were inhibited at MIC range of 4–32 mg/mL | [117] |
| Chinese garlic cloves | 100 g diethyl ether | Allicin and DAS | Periodontal | Porphyromonas gingivalis, an anaerobic, gram-negative pathogen linked to chronic periodontitis, has the lowest allicin sensitivity (2, 400 μg/mL). | [83,118] |
| Garlic, Manila | 70% aqueous | Allicin | Pulpitis | GE 70% was able to disrupt and prevent the production of Enterococcus faecalis biofilm in root canals. | [119,120] |
| Garlic, Changsha, China | Garlic plaster including 0.1% garlicin | Garlicin | Recurrent oral ulcers | Overall effective rate was 100%, while the complete effective rate was 83.3%. | [121,122] |
| Garlic, Chenzhou, China | Allicin adhesive tablets contained 5 mg allicin magnesium stearate, sodium carboxymethylcellulose, and flavoring additives | Allicin | Recurrent aphthous ulceration | Allicin adhesive tablets reduced ulcer size (72.5%) and pain (75.7%) dramatically. | [116] |
| Fresh garlic, Provo, USA | Aqueous alcohol | Diallyl thio-sulfinate (allicin), allyl methyl thiosulfinate, methyl allyl thiosulfinate, ajoene, alliin, deoxyalliin, DADS, and DATS | Herpes simplex virus type 1,2, Parainfluenza virus type 3, vaccinia virus, vesicular stomatitis virus type 2 | Disruption of the viral envelope and cell membrane to prevent virus entry | [54,79] |
| Garlic, Davangere, Karnataka, India | Garlic pearl and 0.25% garlic oil | Dimethyl trisulfide | Oral submucous fibrosis | 95.68% reduction in burning sensation and a 5.37 mm increase in mouth opening | [123] |
| Garlic, Mangalore, Karnataka, India | Garlic paste with one drop of 2% lignocaine jelly and Clotrimazole solution 1% w/v (2–4 drops), | Ajoenes, allyl sulfides | Oral candiasis | More than 50% reductions in the colony count, 61.5% complete eradication of lesions | [72] |
| Type of Product | Product | Patents | Plant Part Used | Intended Use | Reference |
|---|---|---|---|---|---|
| Toothpaste | Toothpaste with BGE | CN111888315A | Black garlic blub | To realise the broad-spectrum sterilization effect targeting various oral pathogens. | [139] |
| Garlic antitoxic bactericidal toothpaste | CN1555777A | Garlicin and Garlic oil | To disinfect respiratory tract and oral cavity and treating foul breath | [140] | |
| Garlic gel | Garlic gel-Pharmaceutical composition containing a GE | WO2009092387A2 | AGE | Anti-Acute and Chronic inflammation and ToothPain Relief | [143] |
| Chewing Gum | Black garlic chewing gum | CN102763759A | Black garlic blub | To promote elimination of human radicals, enhance human immunity, stabilize blood pressure, lower blood lipid and blood sugar, lower cholesterol and reduce weight, and fine health caring effect | [144] |
| Mouth fresheners | oral cavity nursing agent | CN103384526B | Garlic clove | To improve the composition of oral health and prevent dental caries, or calculus dentalis, treating oral malodour and halitosis | [145] |
| Pharmabiotic strips | Adherent oral pharmabiotic delivery strip | US 20200155447 A1 | AGE | An oral pharmabiotic system is disclosed for improving oral, dental, and systemic health by repopulating and reshaping the flora within a patient’s oral environment in a manner that overcomes the deficiencies of prior oral probiotic products. | [146] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sasi, M.; Kumar, S.; Kumar, M.; Thapa, S.; Prajapati, U.; Tak, Y.; Changan, S.; Saurabh, V.; Kumari, S.; Kumar, A.; et al. Garlic (Allium sativum L.) Bioactives and Its Role in Alleviating Oral Pathologies. Antioxidants 2021, 10, 1847. https://doi.org/10.3390/antiox10111847
Sasi M, Kumar S, Kumar M, Thapa S, Prajapati U, Tak Y, Changan S, Saurabh V, Kumari S, Kumar A, et al. Garlic (Allium sativum L.) Bioactives and Its Role in Alleviating Oral Pathologies. Antioxidants. 2021; 10(11):1847. https://doi.org/10.3390/antiox10111847
Chicago/Turabian StyleSasi, Minnu, Sandeep Kumar, Manoj Kumar, Sandhya Thapa, Uma Prajapati, Yamini Tak, Sushil Changan, Vivek Saurabh, Shweta Kumari, Ashok Kumar, and et al. 2021. "Garlic (Allium sativum L.) Bioactives and Its Role in Alleviating Oral Pathologies" Antioxidants 10, no. 11: 1847. https://doi.org/10.3390/antiox10111847
APA StyleSasi, M., Kumar, S., Kumar, M., Thapa, S., Prajapati, U., Tak, Y., Changan, S., Saurabh, V., Kumari, S., Kumar, A., Hasan, M., Chandran, D., Radha, Bangar, S. P., Dhumal, S., Senapathy, M., Thiyagarajan, A., Alhariri, A., Dey, A., ... Mekhemar, M. (2021). Garlic (Allium sativum L.) Bioactives and Its Role in Alleviating Oral Pathologies. Antioxidants, 10(11), 1847. https://doi.org/10.3390/antiox10111847












