Exercise Cuts Both Ways with ROS in Remodifying Innate and Adaptive Responses: Rewiring the Redox Mechanism of the Immune System during Exercise
Abstract
:1. Introduction
2. The Roles of Acute- and Chronic-Exercise-Induced ROS in Immune Function
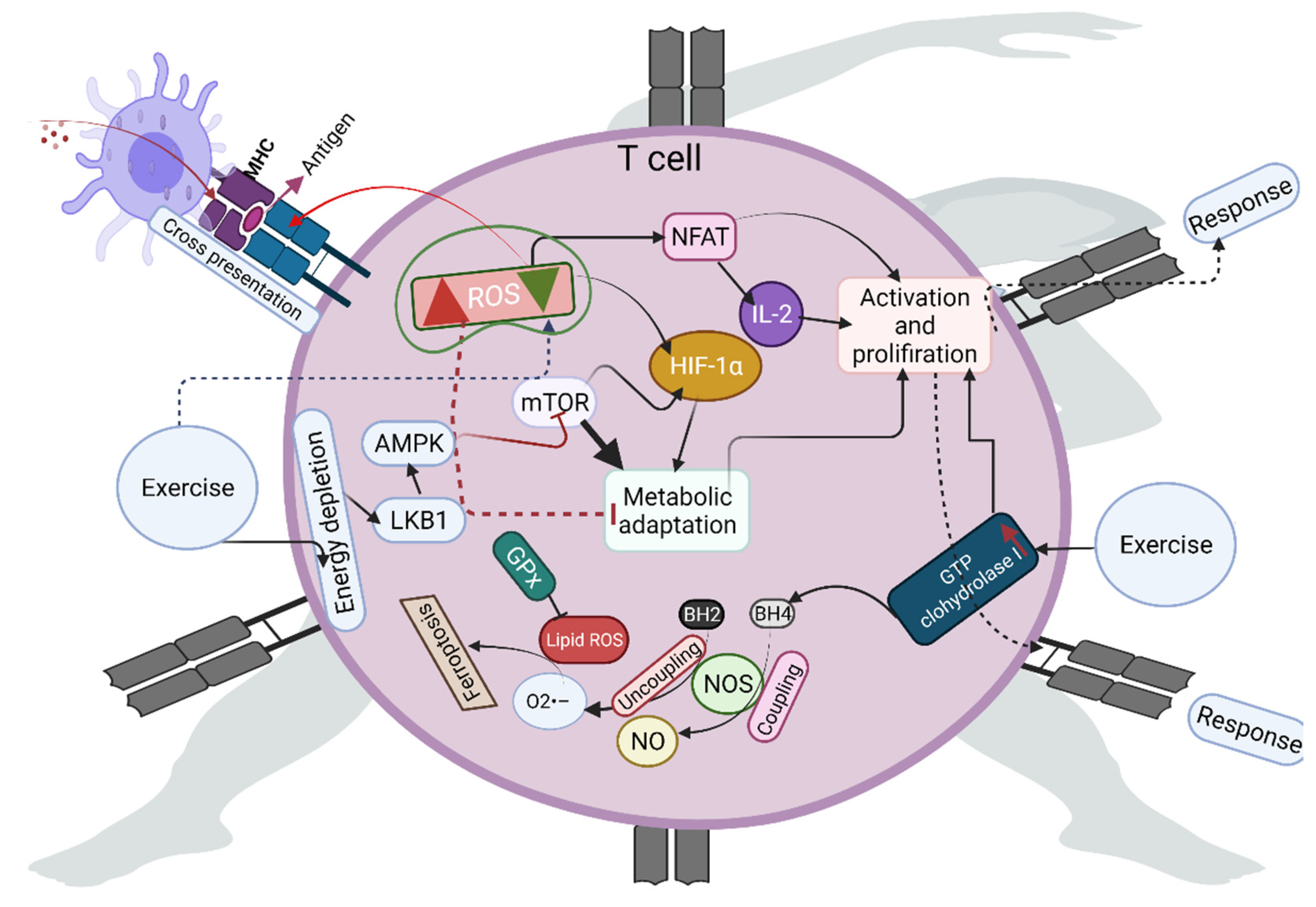
3. Role of Exercise-Induced ROS in T-Cell Activation
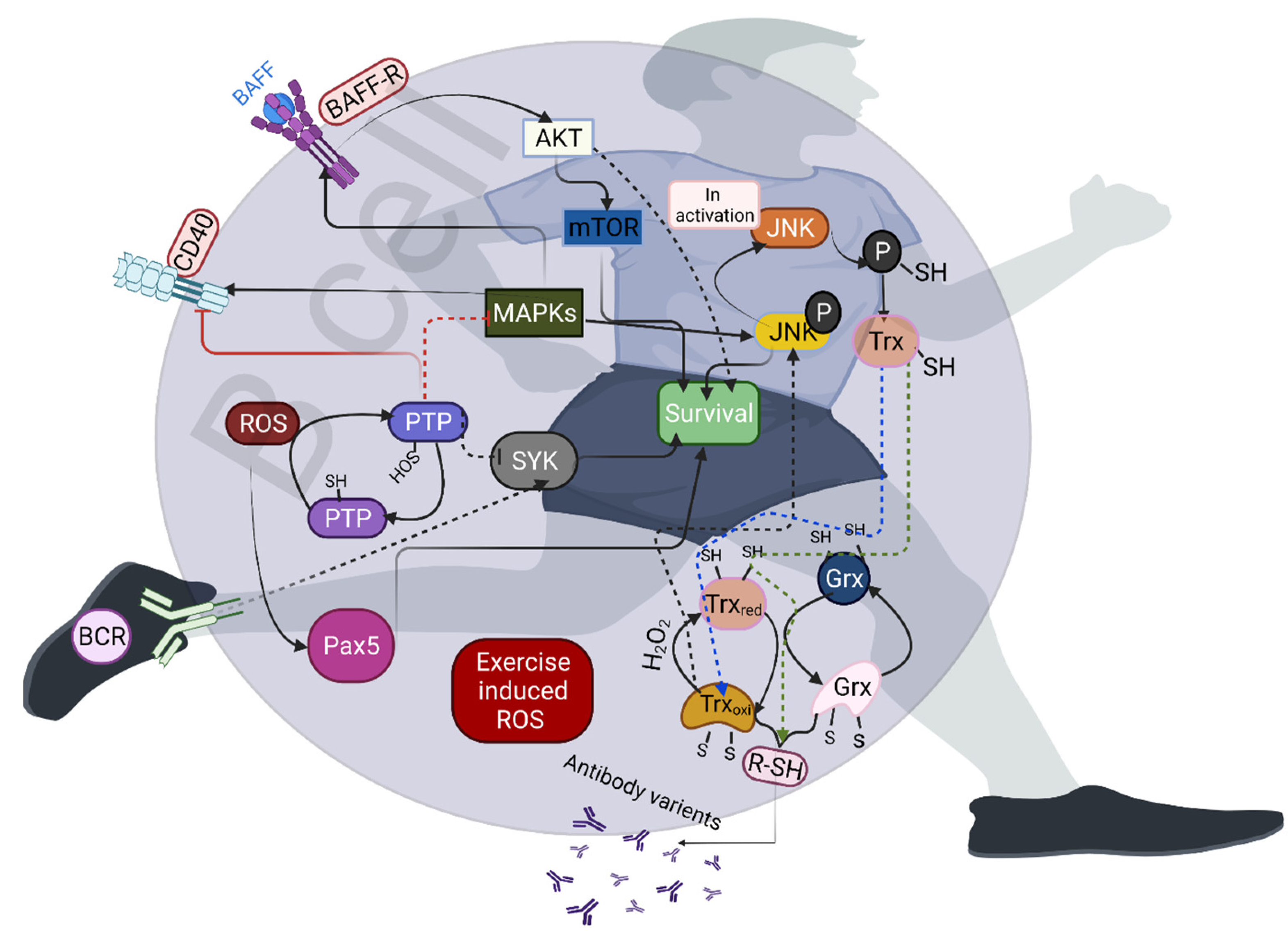
4. Effects of Exercise-Induced Redox Homeostasis on B-Cell Activation
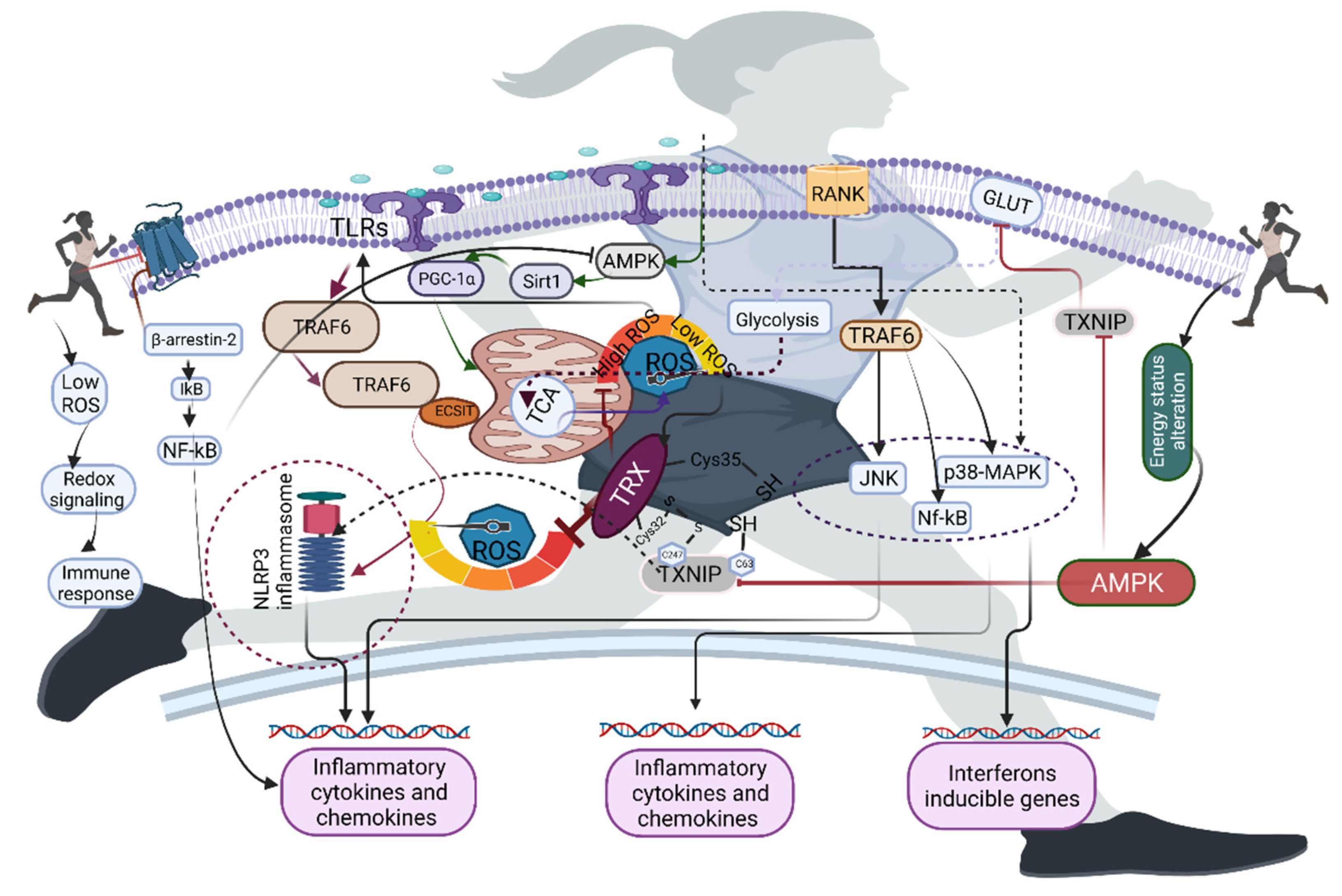
5. Redox Homeostasis and Exercise in Macrophages
6. Role of Exercise-Induced ROS in the Activation of Immune Receptors
7. Exercise in Activating Neutrophil Extracellular Traps (NETs)
8. Therapeutic Opportunities and Future Directions for Exercise-Induced ROS
9. ROS-Mediated Clinical Evidence of Immunity Changes in Athletes
10. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Muri, J.; Kopf, M. Redox regulation of immunometabolism. Nat. Rev. Immunol. 2021, 21, 363–381. [Google Scholar] [CrossRef] [PubMed]
- Mak, T.W.; Grusdat, M.; Duncan, G.S.; Dostert, C.; Nonnenmacher, Y.; Cox, M.; Binsfeld, C.; Hao, Z.; Brüstle, A.; Itsumi, M.; et al. Glutathione Primes T Cell Metabolism for Inflammation. Immunity 2017, 46, 1089–1090. [Google Scholar] [CrossRef] [PubMed]
- Previte, D.M.; O’Connor, E.C.; Novak, E.A.; Martins, C.P.; Mollen, K.P.; Piganelli, J.D. Reactive oxygen species are required for driving efficient and sustained aerobic glycolysis during CD4+ T cell activation. PLoS ONE 2017, 12, e0175549. [Google Scholar] [CrossRef] [PubMed]
- Meissner, F.; Seger, R.A.; Moshous, D.; Fischer, A.; Reichenbach, J.; Zychlinsky, A. Inflammasome activation in NADPH oxidase defective mononuclear phagocytes from patients with chronic granulomatous disease. Blood 2010, 116, 1570–1573. [Google Scholar] [CrossRef] [Green Version]
- Nieman, D.C.; Dumke, C.I.; Henson, D.A.; McAnulty, S.R.; McAnulty, L.S.; Lind, R.H.; Morrow, J.D. Immune and oxidative changes during and following the Western States Endurance Run. Int. J. Sports Med. 2003, 24, 541–547. [Google Scholar]
- Takahashi, A.; Hanson, M.G.; Norell, H.R.; Havelka, A.M.; Kono, K.; Malmberg, K.J.; Kiessling, R.V. Preferential cell death of CD8+ effector memory (CCR7-CD45RA-) T cells by hydrogen peroxide-induced oxidative stress. J. Immunol. 2005, 174, 6080–6087. [Google Scholar] [CrossRef] [Green Version]
- Bartlett, D.B.; Shepherd, S.O.; Wilson, O.J.; Adlan, A.M.; Wagenmakers, A.J.M.; Shaw, C.S.; Lord, J.M. Neutrophil and Monocyte Bactericidal Responses to 10 Weeks of Low-Volume High-Intensity Interval or Moderate-Intensity Continuous Training in Sedentary Adults. Oxid. Med. Cell. Longev. 2017, 2017, 8148742. [Google Scholar] [CrossRef]
- Cunningham, K.S.; Gotlieb, A.I. The role of shear stress in the pathogenesis of atherosclerosis. Lab Invest. 2005, 85, 9–23. [Google Scholar] [CrossRef] [Green Version]
- Kolasa-Trela, R.; Konieczynska, M.; Bazanek, M.; Undas, A. Specific changes in circulating cytokines and growth factors induced by exercise stress testing in asymptomatic aortic valve stenosis. PLoS ONE 2017, 2, e0173787. [Google Scholar] [CrossRef] [Green Version]
- Tanaka, T.; Narazaki, M.; Kishimoto, T. IL-6 in inflammation, immunity, and disease. Cold Spring Harb. Perspect. Biol. 2014, 6, a016295. [Google Scholar] [CrossRef]
- Hennigar, S.R.; McClung, J.P.; Pasiakos, S.M. Nutritional interventions and the IL-6 response to exercise. FASEB J. 2017, 31, 3719–3728. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Levada-Pires, A.C.; Lambertucci, R.H.; Mohamad, M.; Hirabara, S.M.; Curi, R.; Pithon-Curi, T.C. Exercise training raises expression of the cytosolic components of NADPH oxidase in rat neutrophils. Eur. J. Appl. Physiol. 2007, 100, 153–160. [Google Scholar] [CrossRef] [PubMed]
- Belambri, S.A.; Rolas, L.; Raad, H.; Hurtado-Nedelec, M.; Dang, P.M.; El-Benna, J. NADPH oxidase activation in neutrophils: Role of the phosphorylation of its subunits. Eur. J. Clin. Invest. 2018, 48, e12951. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wright, V.P.; Reiser, P.J.; Clanton, T.L. Redox Modulation of Global Phosphatase Activity and Protein Phosphorylation in Intact Skeletal Muscle. J. Physiol. 2009, 587, 5767–5781. [Google Scholar] [CrossRef]
- Juarez, J.C.; Manuia, M.; Burnett, M.E.; Betancourt, O.; Boivin, B.; Shaw, D.E.; Tonks, N.K.; Mazar, A.P.; Doñate, F. Superoxide dismutase 1 (SOD1) is essential for H2O2-mediated oxidation and inactivation of phosphatases in growth factor signaling. Proc. Natl. Acad. Sci. USA 2008, 105, 7147–7152. [Google Scholar] [CrossRef] [Green Version]
- Townsend, J.R.; Hoffman, J.R.; Fragala, M.S.; Jajtner, A.R.; Gonzalez, A.M.; Wells, A.J.; Mangine, G.T.; Fukuda, D.H.; Stout, J.R. TNF-α and TNFR1 responses to recovery therapies following acute resistance exercise. Front. Physiol. 2015, 18, 48. [Google Scholar] [CrossRef] [Green Version]
- Antunes, B.M.; Campos, E.Z.; Dos Santos, R.V.T.; Rosa-Neto, J.C.; Franchini, E.; Bishop, N.C.; Lira, F.S. Anti-inflammatory response to acute exercise is related with intensity and physical fitness. J. Cell. Biochem. 2019, 120, 5333–5342. [Google Scholar] [CrossRef] [Green Version]
- Gleeson, M.; McDonald, W.A.; Cripps, A.W.; Pyne, D.B.; Clancy, R.L.; Fricker, P.A. The effect on immunity of long-term intensive training in elite swimmers. Clin. Exp. Immunol. 1995, 102, 210–216. [Google Scholar] [CrossRef]
- Kjølhede, T.; Dalgas, U.; Gade, A.B.; Bjerre, M.; Stenager, E.; Petersen, T.; Vissing, K. Acute and chronic cytokine responses to resistance exercise and training in people with multiple sclerosis. Scand. J. Med. Sci. Sports. 2016, 26, 824–834. [Google Scholar] [CrossRef]
- Lancaster, G.I.; Khan, Q.; Drysdale, P.T.; Wallace, F.; Jeukendrup, A.E.; Drayson, M.T.; Gleeson, M. Effect of prolonged strenuous exercise and carbohydrate ingestion on type 1 and type 2 T lymphocyte distribution and intracellular cytokine production in humans. J. Appl. Physiol. 2005, 98, 565–571. [Google Scholar] [CrossRef]
- Kwon, J.; Shatynski, K.E.; Chen, H.; Morand, S.; de Deken, X.; Miot, F.; Leto, T.L.; Williams, M.S. The nonphagocytic NADPH oxidase Duox1 mediates a positive feedback loop during T cell receptor signaling. Sci. Signal. 2010, 3, ra59. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sena, L.A.; Li, S.; Jairaman, A.; Prakriya, M.; Ezponda, T.; Hildeman, D.A.; Wang, C.R.; Schumacker, P.T.; Licht, J.D.; Perlman, H.; et al. Mitochondria are required for antigen-specific T cell activation through reactive oxygen species signaling. Immunity 2013, 38, 225–236. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Devadas, S.; Zaritskaya, L.; Rhee, S.G.; Oberley, L.; Williams, M.S. Discrete generation of superoxide and hydrogen peroxide by T cell receptor stimulation: Selective regulation of mitogen-activated protein kinase activation and fas ligand expression. J. Exp. Med. 2002, 195, 59–70. [Google Scholar] [CrossRef] [Green Version]
- Hwang, J.R.; Byeon, Y.; Kim, D.; Park, S.G. Recent insights of T cell receptor-mediated signaling pathways for T cell activation and development. Exp. Mol. Med. 2020, 52, 750–761. [Google Scholar] [CrossRef] [PubMed]
- Case, A.J.; McGill, J.L.; Tygrett, L.T.; Shirasawa, T.; Spitz, D.R.; Waldschmidt, T.J.; Legge, K.L.; Domann, F.E. Elevated mitochondrial superoxide disrupts normal T cell development, impairing adaptive immune responses to an influenza challenge. Free Radic. Biol. Med. 2011, 50, 448–458. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Powers, S.K.; Deminice, R.; Ozdemir, M.; Yoshihara, T.; Bomkamp, M.P.; Hyatt, H. Exercise-induced oxidative stress: Friend or foe? J. Sport. Health Sci. 2020, 9, 415–425. [Google Scholar] [CrossRef]
- Kuo, I.Y.; Ehrlich, B.E. Signaling in muscle contraction. Cold Spring Harb. Perspect. Biol. 2015, 7, a006023. [Google Scholar] [CrossRef]
- Murphy, M.P.; Siegel, R.M. Mitochondrial ROS fire up T cell activation. Immunity. 2013, 38, 201–202. [Google Scholar] [CrossRef] [Green Version]
- Liu, H.W.; Chang, S.J. Moderate Exercise Suppresses NF-κB Signaling and Activates the SIRT1-AMPK-PGC1α Axis to Attenuate Muscle Loss in Diabetic db/db Mice. Front. Physiol. 2018, 9, 636. [Google Scholar] [CrossRef]
- Rundqvist, H.; Veliça, P.; Barbieri, L.; Gameiro, P.A.; Bargiela, D.; Gojkovic, M.; Mijwel, S.; Reitzner, S.M.; Wulliman, D.; Ahlstedt, E.; et al. Cytotoxic T-cells mediate exercise-induced reductions in tumor growth. Elife 2020, 23, e59996. [Google Scholar] [CrossRef]
- Chi, H. Regulation and function of mTOR signalling in T cell fate decisions. Nat. Rev. Immunol. 2012, 12, 325–338. [Google Scholar] [CrossRef] [PubMed]
- Chen, T.; Moore, T.M.; Ebbert, M.T.; McVey, N.L.; Madsen, S.R.; Hallowell, D.M.; Harris, A.M.; Char, R.E.; Mackay, R.P.; Hancock, C.R.; et al. Liver kinase B1 inhibits the expression of inflammation-related genes postcontraction in skeletal muscle. J. Appl. Physiol. 2016, 120, 876–888. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dumauthioz, N.; Tschumi, B.; Wenes, M.; Marti, B.; Wang, H.; Franco, F.; Li, W.; Lopez-Mejia, I.C.; Fajas, L.; Ho, P.C.; et al. Enforced PGC-1α expression promotes CD8 T cell fitness, memory formation and antitumor immunity. Cell. Mol. Immunol. 2021, 18, 1761–1771. [Google Scholar] [CrossRef] [Green Version]
- Lepez, A.; Pirnay, T.; Denanglaire, S.; Perez-Morga, D.; Vermeersch, M.; Leo, O.; Andris, F. Long-term T cell fitness and proliferation is driven by AMPK-dependent regulation of reactive oxygen species. Sci. Rep. 2020, 10, 21673. [Google Scholar] [CrossRef]
- Yarosz, E.L.; Chang, C.H. The Role of Reactive Oxygen Species in Regulating T Cell-mediated Immunity and Disease. Immune Netw. 2018, 18, e14. [Google Scholar] [CrossRef]
- Rall, L.C.; Roubenoff, R.; Cannon, J.G.; Abad, L.W.; Dinarello, C.A.; Meydani, S.N. Effects of progressive resistance training on immune response in aging and chronic inflammation. Med. Sci. Sports Exerc. 1996, 28, 1356–1365. [Google Scholar] [CrossRef]
- Pedersen, B.K.; Hoffman-Goetz, L. Exercise and the immune system: Regulation, integration and adaptation. Physiol. Rev. 2000, 80, 1055–1081. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pedersen, B.K.; Febbraio, M.A. Muscle as an endocrine organ: Focus on muscle-derived interleukin-6. Physiol. Rev. 2008, 88, 1379–1406. [Google Scholar] [CrossRef] [Green Version]
- Sandstrom, P.A.; Tebbey, P.W.; Van Cleave, S.; Buttke, T.M. Lipid hydroperoxides induce apoptosis in T cells displaying a HIV-associated glutathione peroxidase deficiency. J. Biol. Chem. 1994, 269, 798–801. [Google Scholar] [CrossRef]
- Ando, T.; Mimura, K.; Johansson, C.C.; Hanson, M.G.; Mougiakakos, D.; Larsson, C.; Martins da Palma, T.; Sakurai, D.; Norell, H.; Li, M.; et al. Transduction with the antioxidant enzyme catalase protects human T cells against oxidative stress. J. Immunol. 2008, 181, 8382–8390. [Google Scholar] [CrossRef] [Green Version]
- Matsushita, M.; Freigang, S.; Schneider, C.; Conrad, M.; Bornkamm, G.W.; Kopf, M. T cell lipid peroxidation induces ferroptosis and prevents immunity to infection. J. Exp. Med. 2015, 212, 555–568. [Google Scholar] [CrossRef] [Green Version]
- Guerrero, F.; Thioub, S.; Goanvec, C.; Theunissen, S.; Feray, A.; Balestra, C.; Mansourati, J. Effect of tetrahydrobiopterin and exercise training on endothelium-dependent vasorelaxation in SHR. J. Physiol. Biochem. 2013, 69, 277–287. [Google Scholar] [CrossRef]
- Couto, G.K.; Paula, S.M.; Gomes-Santos, I.L.; Negrão, C.E.; Rossoni, L.V. Exercise training induces eNOS coupling and restores relaxation in coronary arteries of heart failure rats. Am. J. Physiol. Heart Circ. Physiol. 2018, 314, H878–H887. [Google Scholar] [CrossRef] [PubMed]
- Garcia, A.; Fraga, G.A.; Vieira, R.C., Jr.; Silva, C.M.; Trombeta, J.C.; Navalta, J.W.; Prestes, J.; Voltarelli, F.A. Effects of combined exercise training on immunological, physical and biochemical parameters in individuals with HIV/AIDS. J. Sports Sci. 2014, 32, 785–792. [Google Scholar] [CrossRef]
- Wheeler, M.L.; Defranco, A.L. Prolonged production of reactive oxygen species in response to B cell receptor stimulation promotes B cell activation and proliferation. J. Immunol. 2012, 189, 4405–4416. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Merluzzi, S.; Moretti, M.; Altamura, S.; Zwollo, P.; Sigvardsson, M.; Vitale, G.; Pucillo, C. CD40 stimulation induces Pax5/BSAP and EBF activation through a APE/Ref-1-dependent redox mechanism. J. Biol. Chem. 2004, 279, 1777–1786. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Polikowsky, H.G.; Wogsland, C.E.; Diggins, K.E.; Huse, K.; Irish, J.M. Cutting Edge: Redox Signaling Hypersensitivity Distinguishes Human Germinal Center B Cells. J. Immunol. 2015, 195, 1364–1367. [Google Scholar] [CrossRef] [Green Version]
- de Moura, L.P.; Souza Pauli, L.S.; Cintra, D.E.; de Souza, C.T.; da Silva, A.S.R.; Marinho, R.; de Melo, M.A.R.; Ropelle, E.R.; Pauli, J.R. Acute exercise decreases PTP-1B protein level and improves insulin signaling in the liver of old rats. Immun. Ageing 2013, 10, 8. [Google Scholar] [CrossRef] [Green Version]
- Aronson, D.; Violan, M.A.; Dufresne, S.D.; Zangen, D.; Fielding, R.A.; Goodyear, L.J. Exercise stimulates the mitogen-activated protein kinase pathway in human skeletal muscle. J. Clin. Invest. 1997, 99, 1251–1257. [Google Scholar] [CrossRef] [Green Version]
- Bouviere, J.; Fortunato, R.S.; Dupuy, C.; Werneck-de-Castro, J.P.; Carvalho, D.P.; Louzada, R.A. Exercise-Stimulated ROS Sensitive Signaling Pathways in Skeletal Muscle. Antioxidants 2021, 10, 537. [Google Scholar] [CrossRef]
- Craxton, A.; Shu, G.; Graves, J.D.; Saklatvala, J.; Krebs, E.G.; Clark, E.A. p38 MAPK is required for CD40-induced gene expression and proliferation in B lymphocytes. J. Immunol. 1998, 161, 3225–3236. [Google Scholar] [PubMed]
- Akimoto, T.; Pohnert, S.C.; Li, P.; Zhang, M.; Gumbs, C.; Rosenberg, P.B.; Williams, R.S.; Yan, Z. Exercise Stimulates Pgc-1α Transcription in Skeletal Muscle through Activation of the P38 MAPK Pathway. J. Biol. Chem. 2005, 280, 19587–19593. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kefaloyianni, E.; Gaitanaki, C.; Beis, I. ERK1/2 and p38-MAPK signalling pathways, through MSK1, are involved in NF-kappaB transactivation during oxidative stress in skeletal myoblasts. Cell Signal. 2006, 18, 2238–2251. [Google Scholar] [CrossRef] [PubMed]
- Wei, Y.; An, Z.; Zou, Z.; Sumpter, R.; Su, M.; Zang, X.; Sinha, S.; Gaestel, M.; Levine, B. The stress-responsive kinases MAPKAPK2/MAPKAPK3 activate starvation-induced autophagy through Beclin 1 phosphorylation. Elife 2015, 18, e05289. [Google Scholar] [CrossRef]
- Markuns, J.F.; Wojtaszewski, J.F.; Goodyear, L.J. Insulin and exercise decrease glycogen synthase kinase-3 activity by different mechanisms in rat skeletal muscle. J. Biol. Chem. 1999, 274, 24896–24900. [Google Scholar] [CrossRef] [Green Version]
- Kang, E.B.; Cho, J.Y. Effect of treadmill exercise on PI3K/AKT/mTOR, autophagy, and Tau hyperphosphorylation in the cerebral cortex of NSE/htau23 transgenic mice. J. Exerc. Nutr. Biochem. 2015, 19, 199–209. [Google Scholar] [CrossRef] [Green Version]
- Zhou, Y.; Wu, H.; Zhao, M.; Chang, C.; Lu, Q. The Bach Family of Transcription Factors: A Comprehensive Review. Clin. Rev. Allergy Immunol. 2016, 50, 345–356. [Google Scholar] [CrossRef]
- Trewin, A.J.; Berry, B.J.; Wojtovich, A.P. Exercise and Mitochondrial Dynamics: Keeping in Shape with ROS and AMPK. Antioxidants 2018, 7, 7. [Google Scholar] [CrossRef] [Green Version]
- Bertolotti, M.; Yim, S.H.; Garcia-Manteiga, J.M.; Masciarelli, S.; Kim, Y.J.; Kang, M.H.; Iuchi, Y.; Fujii, J.; Vené, R.; Rubartelli, A.; et al. B-to-plasma-cell terminal differentiation entails oxidative stress and profound reshaping of the antioxidant responses. Antioxid. Redox Signal. 2010, 13, 1133–1144. [Google Scholar] [CrossRef]
- Ra, S.G.; Kawamoto, E.; Koshinaka, K.; Iwabe, M.; Tomiga, Y.; Iizawa, H.; Honda, H.; Higaki, Y.; Kawanaka, K. Acute bout of exercise downregulates thioredoxin-interacting protein expression in rat contracting skeletal muscles. Physiol. Rep. 2020, 8, e14388. [Google Scholar] [CrossRef]
- Junn, E.; Han, S.H.; Im, J.Y.; Yang, Y.; Cho, E.W.; Um, H.D.; Lee, K.W.; Han, P.L.; Rhee, S.G.; Choi, I. Vitamin D3 up-regulated protein 1 mediates oxidative stress via suppressing the thioredoxin function. J. Immunol. 2000, 164, 6287–6295. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hildebrandt, W.; Kinscherf, R.; Hauer, K.; Holm, E.; Dröge, W. Plasma cystine concentration and redox state in aging and physical exercise. Mech. Ageing Dev. 2002, 123, 1269–1281. [Google Scholar] [CrossRef]
- Liu, H.; May, K. Disulfide bond structures of IgG molecules: Structural variations, chemical modifications and possible impacts to stability and biological function. MAbs 2012, 4, 17–23. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Crotzer, V.L.; Matute, J.D.; Arias, A.A.; Zhao, H.; Quilliam, L.A.; Dinauer, M.C.; Blum, J.S. Cutting edge: NADPH oxidase modulates MHC class II antigen presentation by B cells. J. Immunol. 2012, 189, 3800–3804. [Google Scholar] [CrossRef]
- Moir, H.; Hughes, M.G.; Potter, S.; Sims, C.; Butcher, L.R.; Davies, N.A.; Verheggen, K.; Jones, K.P.; Thomas, A.W.; Webb, R. Exercise-induced immunosuppression: Roles of reactive oxygen species and 5’-AMP-activated protein kinase dephosphorylation within immune cells. J. Appl. Physiol. 2010, 108, 1284–1292. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Adams, V.; Linke, A.; Kränkel, N.; Erbs, S.; Gielen, S.; Möbius-Winkler, S.; Gummert, J.F.; Mohr, F.W.; Schuler, G.; Hambrecht, R. Impact of regular physical activity on the NAD(P)H oxidase and angiotensin receptor system in patients with coronary artery disease. Circulation 2005, 111, 555–562. [Google Scholar] [CrossRef] [Green Version]
- Campbell, J.P.; Turner, J.E. Debunking the Myth of Exercise-Induced Immune Suppression: Redefining the Impact of Exercise on Immunological Health across the Lifespan. Front. Immunol. 2018, 9, 648. [Google Scholar] [CrossRef] [PubMed]
- Sitlinger, A.; Brander, D.M.; Bartlett, D.B. Impact of exercise on the immune system and outcomes in hematologic malignancies. Blood Adv. 2020, 4, 1801–1811. [Google Scholar] [CrossRef]
- Wang, J.S.; Chiu, Y.T. Systemic hypoxia enhances exercise-mediated bactericidal and subsequent apoptotic responses in human neutrophils. J. Appl. Physiol. 2009, 107, 1213–1222. [Google Scholar] [CrossRef] [Green Version]
- Thirupathi, A.; de Souza, C.T. Multi-regulatory network of ROS: The interconnection of ROS, PGC-1 alpha, and AMPK-SIRT1 during exercise. J. Physiol. Biochem. 2017, 73, 487–494. [Google Scholar] [CrossRef]
- Chen, Y.C.; Chou, W.Y.; Fu, T.C.; Wang, J.S. Effects of normoxic and hypoxic exercise training on the bactericidal capacity and subsequent apoptosis of neutrophils in sedentary men. Eur. J. Appl. Physiol. 2018, 118, 1985–1995. [Google Scholar] [CrossRef]
- Ferrer, M.D.; Capó, X.; Martorell, M.; Busquets-Cortés, C.; Bouzas, C.; Carreres, S.; Mateos, D.; Sureda, A.; Tur, J.A.; Pons, A. Regular Practice of Moderate Physical Activity by Older Adults Ameliorates Their Anti-Inflammatory Status. Nutrients 2018, 10, 1780. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yoshizaki, T.; Schenk, S.; Imamura, T.; Babendure, J.L.; Sonoda, N.; Bae, E.J.; Oh, D.Y.; Lu, M.; Milne, J.C.; Westphal, C.; et al. SIRT1 inhibits inflammatory pathways in macrophages and modulates insulin sensitivity. Am. J. Physiol. Endocrinol. Metab. 2010, 298, E419–E428. [Google Scholar] [CrossRef] [Green Version]
- Chiang, C.F.; Chao, T.T.; Su, Y.F.; Hsu, C.C.; Chien, C.Y.; Chiu, K.C.; Shiah, S.G.; Lee, C.H.; Liu, S.Y.; Shieh, Y.S. Metformin-treated cancer cells modulate macrophage polarization through AMPK-NF-κB signaling. Oncotarget 2017, 8, 20706–20718. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, S.; Liu, R.; Yu, Q.; Dong, L.; Bi, Y.; Liu, G. Metabolic reprogramming of macrophages during infections and cancer. Cancer Lett. 2019, 452, 14–22. [Google Scholar] [CrossRef]
- Llimona, F.; de Lima, T.M.; Moretti, A.I.; Theobaldo, M.; Jukemura, J.; Velasco, I.T.; Machado, M.C.; Souza, H.P. PGC-1α expression is increased in leukocytes in experimental acute pancreatitis. Inflammation 2014, 37, 1231–1239. [Google Scholar] [CrossRef] [PubMed]
- Kizaki, T.; Takemasa, T.; Sakurai, T.; Izawa, T.; Hanawa, T.; Kamiya, S.; Haga, S.; Imaizumi, K.; Ohno, H. Adaptation of macrophages to exercise training improves innate immunity. Biochem. Biophys. Res. Commun. 2008, 372, 152–156. [Google Scholar] [CrossRef]
- Martín-Cordero, L.; Gálvez, I.; Hinchado, M.D.; Ortega, E. β2 Adrenergic Regulation of the Phagocytic and Microbicide Capacity of Macrophages from Obese and Lean Mice: Effects of Exercise. Nutrients 2019, 11, 2721. [Google Scholar] [CrossRef] [Green Version]
- Martín-Cordero, L.; Gálvez, I.; Hinchado, M.D.; Ortega, E. Influence of Obesity and Exercise on β2-Adrenergic-Mediated Anti-Inflammatory Effects in Peritoneal Murine Macrophages. Biomedicines 2020, 8, 556. [Google Scholar] [CrossRef]
- Calbet, J.A.L.; Martín-Rodríguez, S.; Martin-Rincon, M.; Morales-Alamo, D. An integrative approach to the regulation of mitochondrial respiration during exercise: Focus on high-intensity exercise. Redox Biol. 2020, 35, 101478. [Google Scholar] [CrossRef]
- Meiser, J.; Krämer, L.; Sapcariu, S.C.; Battello, N.; Ghelfi, J.; D’Herouel, A.F.; Skupin, A.; Hiller, K. Pro-inflammatory Macrophages Sustain Pyruvate Oxidation through Pyruvate Dehydrogenase for the Synthesis of Itaconate and to Enable Cytokine Expression. J. Biol. Chem. 2016, 291, 3932–3946. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, H.; Chen, T.; Ren, J.; Xia, Y.; Onuma, A.; Wang, Y.; He, J.; Wu, J.; Wang, H.; Hamad, A.; et al. Pre-operative exercise therapy triggers anti-inflammatory trained immunity of Kupffer cells through metabolic reprogramming. Nat. Metab. 2021, 3, 843–858. [Google Scholar] [CrossRef] [PubMed]
- Mills, E.L.; Ryan, D.G.; Prag, H.A.; Dikovskaya, D.; Menon, D.; Zaslona, Z.; Jedrychowski, M.P.; Costa, A.S.; Higgins, M.; Hams, E.; et al. Itaconate is an anti-inflammatory metabolite that activates Nrf2 via alkylation of KEAP1. Nature 2018, 556, 113–117. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, E.H.; Suzuki, T.; Funayama, R.; Nagashima, T.; Hayashi, M.; Sekine, H.; Tanaka, N.; Moriguchi, T.; Motohashi, H.; Nakayama, K.; et al. Nrf2 suppresses macrophage inflammatory response by blocking proinflammatory cytokine transcription. Nat. Commun. 2016, 7, 11624. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tan, Z.; Xie, N.; Cui, H.; Moellering, D.R.; Abraham, E.; Thannickal, V.J.; Liu, G. Pyruvate dehydrogenase kinase 1 participates in macrophage polarization via regulating glucose metabolism. J. Immunol. 2015, 194, 6082–6089. [Google Scholar] [CrossRef] [PubMed]
- Sailani, M.R.; Halling, J.F.; Møller, H.D.; Lee, H.; Plomgaard, P.; Pilegaard, H.; Snyder, M.P.; Regenberg, B. Lifelong physical activity is associated with promoter hypomethylation of genes involved in metabolism, myogenesis, contractile properties and oxidative stress resistance in aged human skeletal muscle. Sci. Rep. 2019, 9, 3272. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zbinden-Foncea, H.; Raymackers, J.M.; Deldicque, L.; Renard, P.; Francaux, M. TLR2 and TLR4 activate p38 MAPK and JNK during endurance exercise in skeletal muscle. Med. Sci. Sports Exerc. 2012, 44, 1463–1472. [Google Scholar] [CrossRef]
- Franchi, L.; Warner, N.; Viani, K.; Nuñez, G. Function of Nod-like receptors in microbial recognition and host defense. Immunol. Rev. 2009, 227, 106–128. [Google Scholar] [CrossRef] [Green Version]
- Khakroo Abkenar, I.; Rahmani-Nia, F.; Lombardi, G. The Effects of Acute and Chronic Aerobic Activity on the Signaling Pathway of the Inflammasome NLRP3 Complex in Young Men. Medicina 2019, 55, 105. [Google Scholar] [CrossRef] [Green Version]
- van de Veerdonk, F.L.; Smeekens, S.P.; Joosten, L.A.; Kullberg, B.J.; Dinarello, C.A.; van der Meer, J.W.; Netea, M.G. Reactive oxygen species–independent activation of the IL-1β inflammasome in cells from patients with chronic granulomatous disease. Proc. Natl. Acad. Sci. USA 2010, 107, 3030–3033. [Google Scholar] [CrossRef] [Green Version]
- Kamiński, M.M.; Röth, D.; Krammer, P.H.; Gülow, K. Mitochondria as oxidative signaling organelles in T-cell activation: Physiological role and pathological implications. Arch. Immunol. Ther. Exp. 2013, 61, 367–384. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tavassolifar, M.J.; Vodjgani, M.; Salehi, Z.; Izad, M. The Influence of Reactive Oxygen Species in the Immune System and Pathogenesis of Multiple Sclerosis. Autoimmune Dis. 2020, 2020, 5793817. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Lu, Y.; Saredy, J.; Wang, X.; Drummer, C., IV; Shao, Y.; Saaoud, F.; Xu, K.; Liu, M.; Yang, W.Y.; et al. ROS systems are a new integrated network for sensing homeostasis and alarming stresses in organelle metabolic processes. Redox Biol. 2020, 37, 101696. [Google Scholar] [CrossRef] [PubMed]
- Magherini, F.; Fiaschi, T.; Marzocchini, R.; Mannelli, M.; Gamberi, T.; Modesti, P.A.; Modesti, A. Oxidative stress in exercise training: The involvement of inflammation and peripheral signals. Free Radic. Res. 2019, 53, 1155–1165. [Google Scholar] [CrossRef]
- Lee, N.K.; Choi, Y.G.; Baik, J.Y.; Han, S.Y.; Jeong, D.W.; Bae, Y.S.; Kim, N.; Lee, S.Y. A crucial role for reactive oxygen species in RANKL-induced osteoclast differentiation. Blood 2005, 106, 852–859. [Google Scholar] [CrossRef] [Green Version]
- Dougall, W.C.; Glaccum, M.; Charrier, K.; Rohrbach, K.; Brasel, K.; De Smedt, T.; Daro, E.; Smith, J.; Tometsko, M.E.; Maliszewski, C.R.; et al. RANK is essential for osteoclast and lymph node development. Genes Dev. 1999, 13, 2412–2424. [Google Scholar] [CrossRef]
- Li, J.; Sarosi, I.; Yan, X.Q.; Morony, S.; Capparelli, C.; Tan, H.L.; McCabe, S.; Elliott, R.; Scully, S.; Van, G.; et al. RANK is the intrinsic hematopoietic cell surface receptor that controls osteoclastogenesis and regulation of bone mass and calcium metabolism. Proc. Natl. Acad. Sci. USA 2000, 97, 1566–1571. [Google Scholar] [CrossRef] [Green Version]
- West, A.P.; Brodsky, I.E.; Rahner, C.; Woo, D.K.; Erdjument-Bromage, H.; Tempst, P.; Walsh, M.C.; Choi, Y.; Shadel, G.S.; Ghosh, S. TLR signalling augments macrophage bactericidal activity through mitochondrial ROS. Nature 2011, 472, 476–480. [Google Scholar] [CrossRef] [Green Version]
- Paiva, C.N.; Bozza, M.T. Are reactive oxygen species always detrimental to pathogens? Antioxid. Redox Signal. 2014, 20, 1000–1037. [Google Scholar] [CrossRef] [Green Version]
- Tatsiy, O.; McDonald, P.P. Physiological Stimuli Induce PAD4-Dependent, ROS-Independent NETosis, with Early and Late Events Controlled by Discrete Signaling Pathways. Front. Immunol. 2018, 9, 2036. [Google Scholar] [CrossRef] [Green Version]
- Beiter, T.; Fragasso, A.; Hudemann, J.; Schild, M.; Steinacker, J.; Mooren, F.C.; Niess, A.M. Neutrophils release extracellular DNA traps in response to exercise. J. Appl. Physiol. (1985) 2014, 117, 325–333. [Google Scholar] [CrossRef] [Green Version]
- Orysiak, J.; Tripathi, J.K.; Brodaczewska, K.; Sharma, A.; Witek, K.; Sitkowski, D.; Malczewska-Lenczowska, J. The impact of physical training on neutrophil extracellular traps in young male athletes—A pilot study. Biol. Sport. 2021, 38, 459–464. [Google Scholar] [CrossRef]
- Awasthi, D.; Nagarkoti, S.; Sadaf, S.; Chandra, T.; Kumar, S.; Dikshit, M. Glycolysis dependent lactate formation in neutrophils: A metabolic link between NOX-dependent and independent NETosis. Biochim. Biophys. Acta Mol. Basis Dis. 2019, 1865, 165542. [Google Scholar] [CrossRef]
- Hamam, H.J.; Palaniyar, N. Post-Translational Modifications in NETosis and NETs-Mediated Diseases. Biomolecules 2019, 9, 369. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhao, M.; Chen, H.; Ding, Q.; Xu, X.; Yu, B.; Huang, Z. Nuclear Factor Erythroid 2-related Factor 2 Deficiency Exacerbates Lupus Nephritis in B6/lpr mice by Regulating Th17 Cell Function. Sci. Rep. 2016, 6, 38619. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Suzuki, T.; Murakami, S.; Biswal, S.S.; Sakaguchi, S.; Harigae, H.; Yamamoto, M.; Motohashi, H. Systemic Activation of NRF2 Alleviates Lethal Autoimmune Inflammation in Scurfy Mice. Mol. Cell. Biol. 2017, 37, e00063-17. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, P.; Li, C.G.; Qi, Z.; Cui, D.; Ding, S. Acute exercise stress promotes Ref1/Nrf2 signalling and increases mitochondrial antioxidant activity in skeletal muscle. Exp. Physiol. 2016, 101, 410–420. [Google Scholar] [CrossRef]
- Done, A.J.; Traustadóttir, T. Nrf2 mediates redox adaptations to exercise. Redox Biol. 2016, 10, 191–199. [Google Scholar] [CrossRef] [Green Version]
- Nath, N.; Khan, M.; Rattan, R.; Mangalam, A.; Makkar, R.S.; de Meester, C.; Bertrand, L.; Singh, I.; Chen, Y.; Viollet, B.; et al. Loss of AMPK exacerbates experimental autoimmune encephalomyelitis disease severity. Biochem. Biophys. Res. Commun. 2009, 386, 16–20. [Google Scholar] [CrossRef] [Green Version]
- Nieman, D.C.; Johanssen, L.M.; Lee, J.W.; Arabatzis, K. Infectious episodes in runners before and after the Los Angeles Marathon. J. Sports Med. Phys. Fit. 1990, 30, 316–328. [Google Scholar] [PubMed]
- Peters, E.M.; Bateman, E.D. Ultramarathon running and upper respiratory tract infections. An epidemiological survey. S. Afr. Med. J. 1983, 64, 582–584. [Google Scholar]
- Ferrandi, P.J.; Fico, B.G.; Whitehurst, M.; Zourdos, M.C.; Bao, F.; Dodge, K.M.; Rodriguez, A.L.; Pena, G.; Huang, C.J. Acute high-intensity interval exercise induces comparable levels of circulating cell-free DNA and Interleukin-6 in obese and normal-weight individuals. Life Sci. 2018, 202, 161–166. [Google Scholar] [CrossRef] [PubMed]
- Khomich, O.A.; Kochetkov, S.N.; Bartosch, B.; Ivanov, A.V. Redox Biology of Respiratory Viral Infections. Viruses 2018, 10, 392. [Google Scholar] [CrossRef] [Green Version]
- Adams, G.R.; Zaldivar, F.P.; Nance, D.M.; Kodesh, E.; Radom-Aizik, S.; Cooper, D.M. Exercise and leukocyte interchange among central circulation, lung, spleen, and muscle. Brain. Behav. Immun. 2011, 25, 658–666. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bigley, A.B.; Rezvani, K.; Chew, C.; Sekine, T.; Pistillo, M.; Crucian, B.; Bollard, C.M.; Simpson, R.J. Acute exercise preferentially redeploys NK-cells with a highly-differentiated phenotype and augments cytotoxicity against lymphoma and multiple myeloma target cells. Brain Behav. Immun. 2014, 39, 160–171. [Google Scholar] [CrossRef]
- Viana, J.L.; Kosmadakis, G.C.; Watson, E.L.; Bevington, A.; Feehally, J.; Bishop, N.C.; Smith, A.C. Evidence for anti-inflammatory effects of exercise in CKD. J. Am. Soc. Nephrol. 2014, 25, 2121–2130. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nieman, D.C.; Pedersen, B.K. Exercise and immune function. Recent developments. Sports Med. 1999, 27, 73–80. [Google Scholar] [CrossRef]
- Williams, S.L.; Strobel, N.A.; Lexis, L.A.; Coombes, J.S. Antioxidant requirements of endurance athletes: Implications for health. Nutr. Rev. 2006, 64, 93–108. [Google Scholar] [CrossRef] [PubMed]
- Timmons, B.W.; Hamadeh, M.J.; Devries, M.C.; Tarnopolsky, M.A. Influence of gender, menstrual phase, and oral contraceptive use on immunological changes in response to prolonged cycling. J. Appl. Physiol. 2005, 99, 979–985. [Google Scholar] [CrossRef] [Green Version]
- Abbasi, A.; de Paula Vieira, R.; Bischof, F.; Walter, M.; Movassaghi, M.; Berchtold, N.C.; Niess, A.M.; Cotman, C.W.; Northoff, H. Sex-specific variation in signaling pathways and gene expression patterns in human leukocytes in response to endotoxin and exercise. J. Neuroinflammation 2016, 10, 289. [Google Scholar] [CrossRef] [Green Version]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Thirupathi, A.; Gu, Y.; Pinho, R.A. Exercise Cuts Both Ways with ROS in Remodifying Innate and Adaptive Responses: Rewiring the Redox Mechanism of the Immune System during Exercise. Antioxidants 2021, 10, 1846. https://doi.org/10.3390/antiox10111846
Thirupathi A, Gu Y, Pinho RA. Exercise Cuts Both Ways with ROS in Remodifying Innate and Adaptive Responses: Rewiring the Redox Mechanism of the Immune System during Exercise. Antioxidants. 2021; 10(11):1846. https://doi.org/10.3390/antiox10111846
Chicago/Turabian StyleThirupathi, Anand, Yaodong Gu, and Ricardo Aurino Pinho. 2021. "Exercise Cuts Both Ways with ROS in Remodifying Innate and Adaptive Responses: Rewiring the Redox Mechanism of the Immune System during Exercise" Antioxidants 10, no. 11: 1846. https://doi.org/10.3390/antiox10111846
APA StyleThirupathi, A., Gu, Y., & Pinho, R. A. (2021). Exercise Cuts Both Ways with ROS in Remodifying Innate and Adaptive Responses: Rewiring the Redox Mechanism of the Immune System during Exercise. Antioxidants, 10(11), 1846. https://doi.org/10.3390/antiox10111846







