Recent Advancement in Chitosan-Based Nanoparticles for Improved Oral Bioavailability and Bioactivity of Phytochemicals: Challenges and Perspectives
Abstract
:1. Introduction
2. Physicochemical Properties of Phytochemicals and Challenges in Oral Delivery
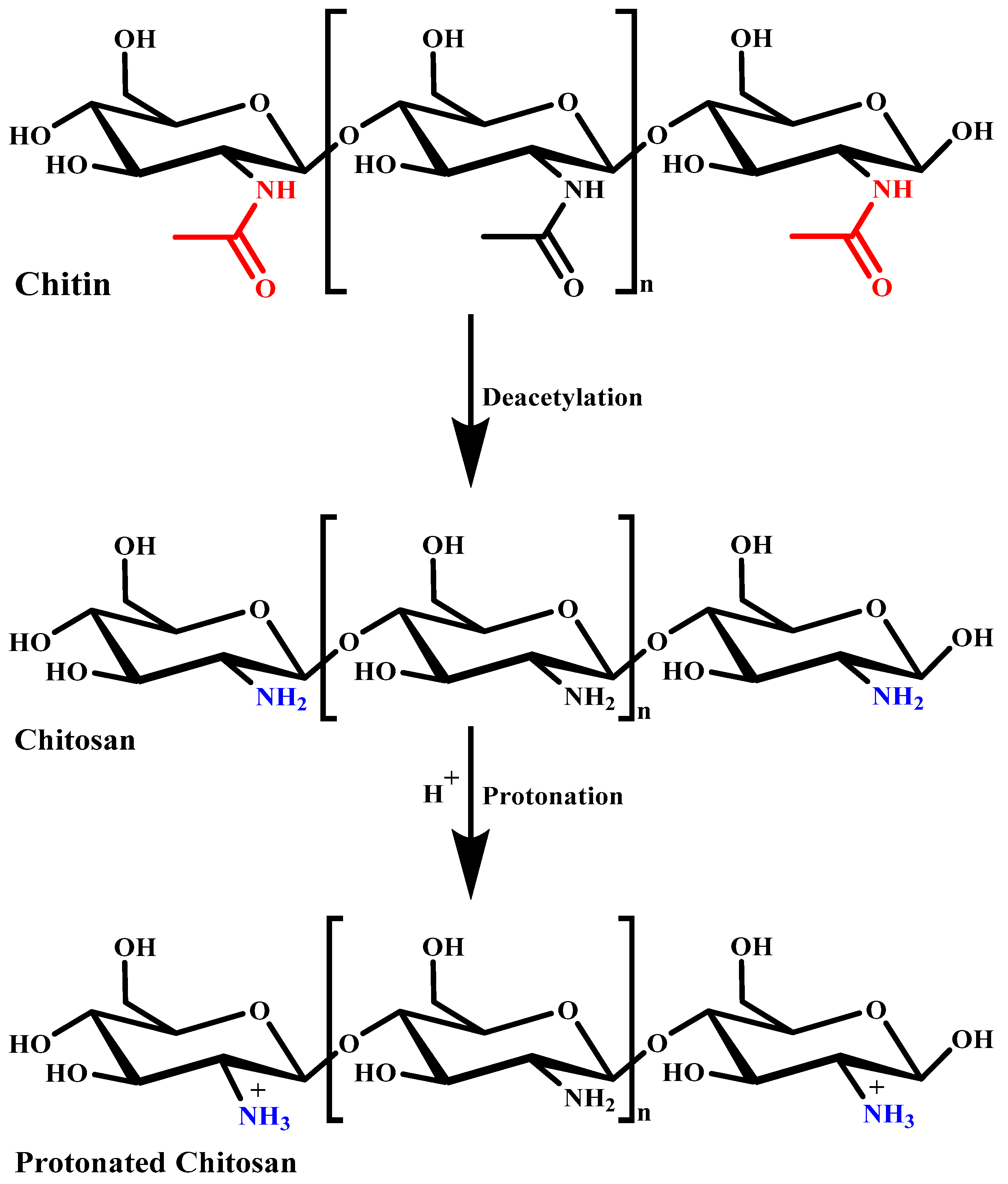
3. Chitosan: Source and Structure
4. Basic Characteristics of Chitosan
4.1. Aqueous Solubility
4.2. Mucoadhesion
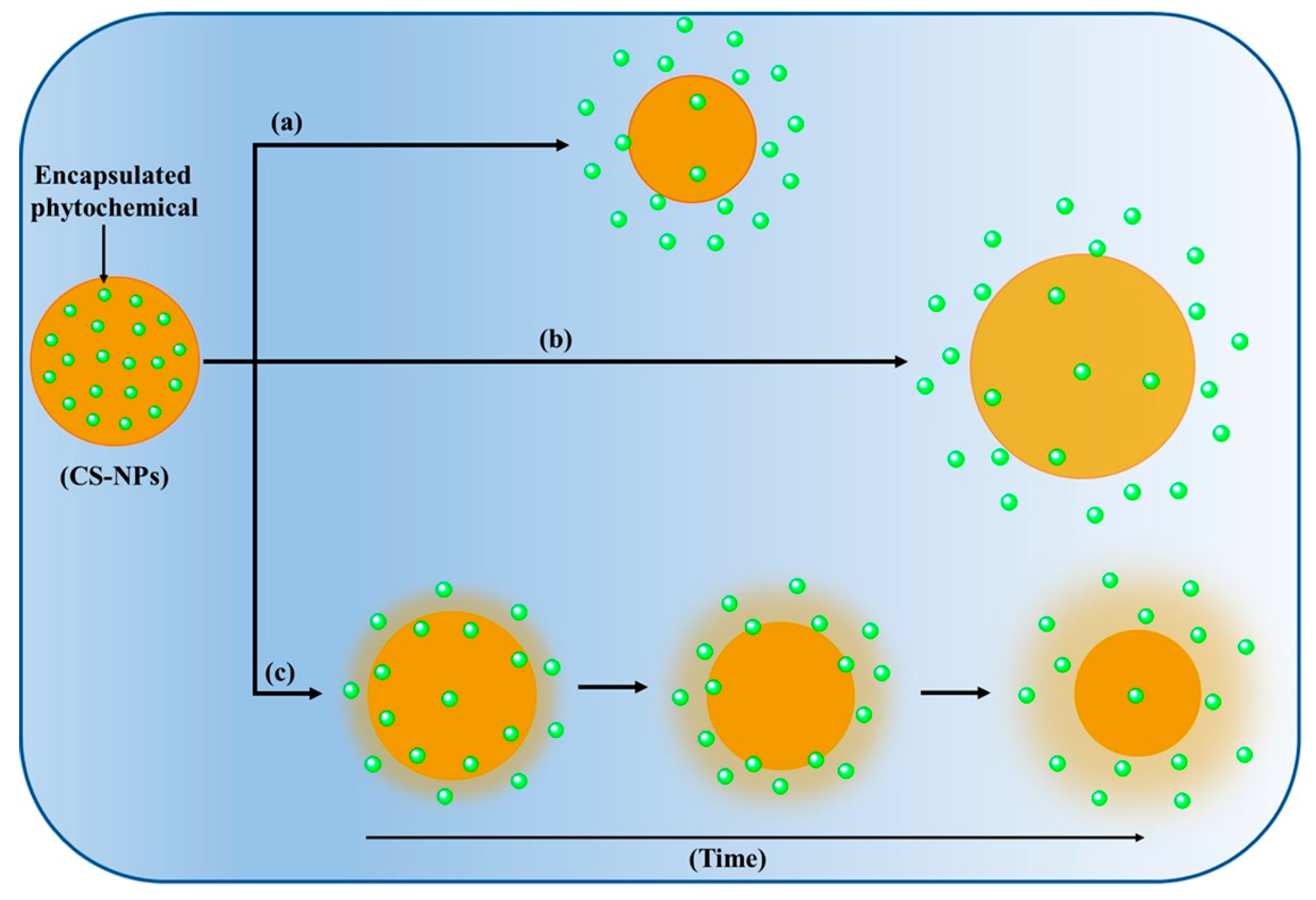
4.3. Controlled Release
4.4. Intestinal Permeation Enhancement
4.5. Biodegradability and Safety
5. Chitosan Nanoparticles for Oral Delivery
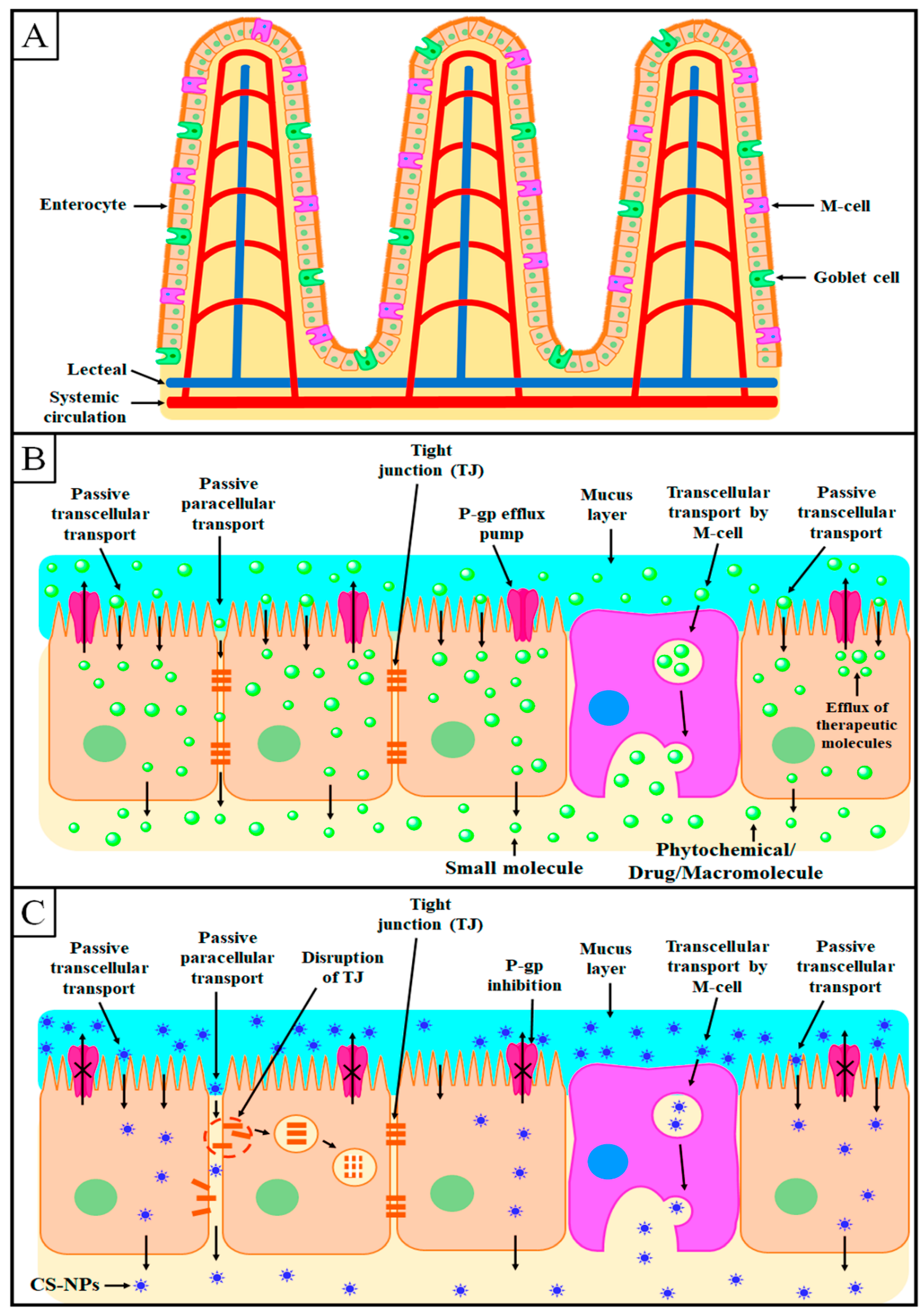
6. Mechanism of Intestinal Absorption of Chitosan Nanoparticles
6.1. Transcellular Transport
6.2. Paracellular Transport
7. Phytochemical-Loaded CS-NPs for Improved Oral Bioavailability and Bioactivity
7.1. Curcumin
7.2. Quercetin
7.3. Resveratrol
7.4. Thymoquinone
7.5. Epigallocatechin-3-Gallate
7.6. Ursolic Acid
7.7. Ferulic Acid
7.8. 10-Hydroxycamptothecin
7.9. Apocynin
7.10. Astaxanthin
7.11. Berberine
7.12. Piperine
7.13. Lutein
7.14. Silymarin
7.15. Naringenin
| Phytochemical | Main Excipients | PS (nm) | ZP (mV) | EE (%) | Major Outcome | Ref |
|---|---|---|---|---|---|---|
| Curcumin | Chitosan, acrylonitrile, arginine | 218.3 ± 7.2 | 40.1 ± 2.81 | 76.53 ± 3.58 |
| [119] |
| Chitosan | 332.4 ± 9.4 | 42.1 ± 3.0 | 77.2 ± 3.6 |
| [120] | |
| Chitosan, eudragit | 236 ± 3.2 | –29.8 ± 2.2 | 42 ± 1.9 |
| [121] | |
| N-trimethyl chitosan, palmitic acid, TPGS | 311.9 ± 67.7 | 35.7 ± 1.03 | 93.12 ± 0.08 |
| [122] | |
| Quercetin | Chitosan, caseinate, zein | ~550 | 55 | >78 |
| [132] |
| Chitosan, zein | ~100 nm | ~60 | >90 |
| [133] | |
| Resveratrol | Carboxymethyl chitosan | 155.3 ± 15.2 | –10.2 ± 6.4 | 44.5 ± 2.2 |
| [141] |
| N-trimethyl chitosan, palmitic acid | 258.2 ± 18.7 | 20.7 ± 0.63 | 95.45 ± 2.18 |
| [142] | |
| Thymoquinone | Chitosan, glyceryl monostearate | 166.5 ± 5.83 | 12.5 ± 1.21 | 82.66 ± 3.47 |
| [148] |
| Chitosan, phospholipid | 372.2 ± 3.11 | 13.12 ± 2.3 | 81.38 ± 3.85 |
| [150] | |
| Epigallocatechin-3-gallate | Chitosan | <200 | - | - |
| [162] |
| Chitosan, polyaspartic acid | 102.4 ± 5.6 | - | 25.0 ± 2.1 |
| [164] | |
| Ursolic acid | Chitosan, poly (lactic acid) | 329.3 ± 37.2 | 27.80 ± 9.4 | 97.47 ± 1.3 |
| [171] |
| Chitosan, compritol® 888 ATO | 103.7 ± 2.8 | −24.1 ± 1.6 | 88.63 ± 2.7 |
| [172] | |
| Ferulic acid | Chitosan, phospholipid | 123.2 ± 1.11 | 32 ± 1.28 | >90 |
| [180] |
| Chitosan, poly lactic-co-glycolic acid | 242 ± 19 | 32 ± 5 | 50 ± 4 |
| [181] | |
| 10-Hydroxycamptothecin | Chitosan, hyaluronic acid | 226.7 | - | 89.23 |
| [185] |
| Chitosan, poly lactic-co-glycolic acid | 209.9 ± 1.6 | 6.76 ± 0.27 | 71.83 ± 2.77 |
| [186] | |
| Apocynin | Chitosan, glycerol tristearate | 265.3 ± 7.64 | 40.57 ± 1.0 | 45.30 ± 2.52 |
| [190] |
| Chitosan oligosaccharide, | 436.2 ± 24.4 | 38.2 ± 1.47 | 35.06 ± 1.89 |
| [191] | |
| Astaxanthin | Chitosan, poly (ethylene glycol) | 122.1 ± 6.4 | 37.53 ± 2.7 | >85 |
| [195] |
| Chitosan, caseinate, dextran | 91.7–148.8 | 0.11–0.2 | - |
| [196] | |
| Berberine | Chitosan, lecithin, dihexadecylphosphate | 264 ± 8 | 29.3 ± 0.5 | 78.4 ± 0.5 |
| [201] |
| Chitosan, fucoidan | 187.4 ± 6.2 | +7.6 ± 0.5 | 50.1 ± 2.5 |
| [202] | |
| Piperine | Chitosan, glyceryl monostearate | 175.3 ± 2.54 | −25.24 | 80.65 ± 1.23 |
| [207] |
| Lutein | Chitosan, oleic acid, sodium alginate | 125 ± 30 | 45 ± 5 | - |
| [213] |
| Silymarin | Chitosan, poly(lactic-co-glycolic acid), DSPEPEG2000 | 286.5 ± 23.8 | 45.3 ± 8.9 | 97.05 ± 0.01 |
| [219] |
| Naringenin | Chitosan, sodium alginate | 216.44 ± 6 | −36 ± 2.7 | 91.4 |
| [226] |
8. Associated Challenges and Future Outlook
9. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Xiao, J.; Cao, Y.; Huang, Q. Edible nanoencapsulation vehicles for oral delivery of phytochemicals: A perspective paper. J. Agric. Food Chem. 2017, 65, 6727–6735. [Google Scholar] [CrossRef] [PubMed]
- Holst, B.; Williamson, G. Nutrients and phytochemicals: From bioavailability to bioefficacy beyond antioxidants. Curr. Opin. Biotechnol. 2008, 19, 73–82. [Google Scholar] [CrossRef]
- Williamson, G.; Manach, C. Bioavailability and bioefficacy of polyphenols in humans. II. Review of 93 intervention studies. Am. J. Clin. Nutr. 2005, 81, 243S–255S. [Google Scholar] [CrossRef]
- Rein, M.J.; Renouf, M.; Cruz-Hernandez, C.; Actis-Goretta, L.; Thakkar, S.K.; da Silva Pinto, M. Bioavailability of bioactive food compounds: A challenging journey to bioefficacy. Br. J. Clin. Pharmacol. 2013, 75, 588–602. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fatima, M.; Iqubal, M.K.; Iqubal, A.; Kaur, H.; Gilani, S.J.; Rahman, M.H.; Ahmadi, A.; Rizwanullah, M. Current insight into the therapeutic potential of phytocompounds and their nanoparticle-based systems for effective management of lung cancer. Anticancer Agents Med. Chem. 2021, 22, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, R.; Srivastava, S.; Ghosh, S.; Khare, S.K. Phytochemical delivery through nanocarriers: A review. Colloids Surf. B Biointerfaces 2021, 197, 111389. [Google Scholar] [CrossRef]
- Martínez-Ballesta, M.; Gil-Izquierdo, Á.; García-Viguera, C.; Domínguez-Perles, R. Nanoparticles and controlled delivery for bioactive compounds: Outlining challenges for new “smart-foods” for health. Foods 2018, 7, 72. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McClements, D.J. Advances in nanoparticle and microparticle delivery systems for increasing the dispersibility, stability, and bioactivity of phytochemicals. Biotechnol. Adv. 2020, 38, 107287. [Google Scholar] [CrossRef]
- Rizwanullah, M.; Amin, S.; Mir, S.R.; Fakhri, K.U.; Rizvi, M.M.A. Phytochemical based nanomedicines against cancer: Current status and future prospects. J. Drug Target. 2018, 26, 731–752. [Google Scholar] [CrossRef] [PubMed]
- Aqil, F.; Munagala, R.; Jeyabalan, J.; Vadhanam, M.V. Bioavailability of phytochemicals and its enhancement by drug delivery systems. Cancer Lett. 2013, 334, 133–141. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lopes, V.F.; Giongo, C.N.; Campos, L.D.; Abraham, W.R.; Mainardes, R.M.; Khalil, N.M. Chitosan nanoparticles potentiate the in vitro and in vivo effects of curcumin and other natural compounds. Curr. Med. Chem. 2021, 28, 4935–4953. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, M.Z.; Rizwanullah, M.; Ahmad, J.; Alasmary, M.Y.; Akhter, M.H.; Abdel-Wahab, B.A.; Warsi, M.H.; Haque, A. Progress in nanomedicine-based drug delivery in designing of chitosan nanoparticles for cancer therapy. Int. J. Polym. Mater. Polym. Biomater. 2021, 71, 1–22. [Google Scholar] [CrossRef]
- Chen, M.C.; Mi, F.L.; Liao, Z.X.; Hsiao, C.W.; Sonaje, K.; Chung, M.F.; Hsu, L.W.; Sung, H.W. Recent advances in chitosan-based nanoparticles for oral delivery of macromolecules. Adv. Drug Deliv. Rev. 2013, 65, 865–879. [Google Scholar] [CrossRef] [PubMed]
- Di Santo, M.C.; D’Antoni, C.L.; Rubio, A.P.; Alaimo, A.; Pérez, O.E. Chitosan-tripolyphosphate nanoparticles designed to encapsulate polyphenolic compounds for biomedical and pharmaceutical applications—A review. Biomed. Pharmacother. 2021, 142, 111970. [Google Scholar] [CrossRef] [PubMed]
- Ganesan, P.; Ramalingam, P.; Karthivashan, G.; Ko, Y.T.; Choi, D.K. Recent developments in solid lipid nanoparticle and surface-modified solid lipid nanoparticle delivery systems for oral delivery of phyto-bioactive compounds in various chronic diseases. Int. J. Nanomed. 2018, 13, 1569–1583. [Google Scholar] [CrossRef] [Green Version]
- da Costa, J.P. A current look at nutraceuticals - key concepts and future prospects. Trends Food Sci. Technol. 2017, 62, 68–78. [Google Scholar] [CrossRef]
- Espin, J.C.; Garcia-Conesa, M.T.; Tomas-Barberan, F.A. Nutraceuticals: Facts and fiction. Phytochemistry 2007, 68, 2986–3008. [Google Scholar] [CrossRef]
- McClements, D.J.; Xiao, H. Excipient foods: Designing food matrices that improve the oral bioavailability of pharmaceuticals and nutraceuticals. Food Funct. 2014, 5, 1320–1333. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Davidov-Pardo, G.; McClements, D.J. Nutraceutical delivery systems: Resveratrol encapsulation in grape seed oil nanoemulsions formed by spontaneous emulsification. Food Chem. 2015, 167, 205–212. [Google Scholar] [CrossRef]
- Gleeson, J.P. Diet, food components and the intestinal barrier. Nutr. Bull. 2017, 42, 123–131. [Google Scholar] [CrossRef]
- McClements, D.J.; Xiao, H. Designing food structure and composition to enhance nutraceutical bioactivity to support cancer inhibition. Semin. Cancer Biol. 2017, 46, 215–226. [Google Scholar] [CrossRef] [PubMed]
- Oehlke, K.; Adamiuk, M.; Behsnilian, D.; Gräf, V.; Mayer-Miebach, E.; Walz, E.; Greiner, R. Potential bioavailability enhancement of bioactive compounds using food-grade engineered nanomaterials: A review of the existing evidence. Food Funct. 2014, 5, 1341–1359. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nie, X.; Chen, Z.; Pang, L.; Wang, L.; Jiang, H.; Chen, Y.; Zhang, Z.; Fu, C.; Ren, B.; Zhang, J. Oral Nano drug delivery systems for the treatment of type 2 diabetes mellitus: An available administration strategy for antidiabetic phytocompounds. Int. J. Nanomed. 2020, 15, 10215–10240. [Google Scholar] [CrossRef] [PubMed]
- Available online: https://go.drugbank.com/drugs/DB11672 (accessed on 29 October 2021).
- Available online: https://go.drugbank.com/drugs/DB04216 (accessed on 29 October 2021).
- Available online: https://go.drugbank.com/drugs/DB02709 (accessed on 29 October 2021).
- Available online: https://go.drugbank.com/drugs/DB16447 (accessed on 29 October 2021).
- Available online: https://go.drugbank.com/drugs/DB03823 (accessed on 29 October 2021).
- Available online: https://go.drugbank.com/drugs/DB15588 (accessed on 29 October 2021).
- Available online: https://go.drugbank.com/drugs/DB07767 (accessed on 29 October 2021).
- Available online: https://go.drugbank.com/drugs/DB12385 (accessed on 29 October 2021).
- Available online: https://go.drugbank.com/drugs/DB12618 (accessed on 29 October 2021).
- Available online: https://go.drugbank.com/drugs/DB06543 (accessed on 29 October 2021).
- Available online: https://go.drugbank.com/drugs/DB04115 (accessed on 29 October 2021).
- Available online: https://go.drugbank.com/drugs/DB12582 (accessed on 29 October 2021).
- Available online: https://go.drugbank.com/drugs/DB00137 (accessed on 29 October 2021).
- Available online: https://go.drugbank.com/drugs/DB09298 (accessed on 29 October 2021).
- Available online: https://go.drugbank.com/drugs/DB03467 (accessed on 29 October 2021).
- Kong, M.; Chen, X.G.; Xing, K.; Park, H.J. Antimicrobial properties of chitosan and mode of action: A state of the art review. Int. J. Food Microbiol. 2010, 144, 51–63. [Google Scholar] [CrossRef] [PubMed]
- Wiśniewska-Wrona, M.; Niekraszewicz, A.; Ciechańska, D.; Pospieszny, H.; Orlikowski, L.B. Biological properties of chitosan degradation products. In Progress on Chemistry and Application of Chitin and Its Derivatives; Jaworska, M., Ed.; Polish Chitin Society: Łódź, Poland, 2007; Volume 7, pp. 149–156. [Google Scholar]
- Gzyra-Jagieła, K.; Pęczek, B.; Wiśniewska-Wrona, M.; Gutowska, N. Physicochemical properties of chitosan and its degradation products. In Chitin and Chitosan: Properties and Applications; Van den Broek, L.A., Boeriu, C.G., Eds.; John Wiley and Sons: Hoboken, NJ, USA, 2019; Volume 1, pp. 61–80. [Google Scholar]
- Tikhonov, V.E.; Stepnova, E.A.; Babak, V.G.; Yamskov, I.A.; Palma-Guerrero, J.; Jansson, H.B.; Lopez-Llorca, L.V.; Salinas, J.; Gerasimenko, D.V.; Avdienko, I.D.; et al. Bactericidal and antifungal activities of a low molecular weight chitosan and its N-/2 (3)-(dodec-2-enyl) succinoyl/-derivatives. Carbohydr. Polym. 2006, 64, 66–72. [Google Scholar] [CrossRef]
- Feng, P.; Luo, Y.; Ke, C.; Qiu, H.; Wang, W.; Zhu, Y.; Hou, R.; Xu, L.; Wu, S. Chitosan-based functional materials for skin wound repair: Mechanisms and applications. Front. Bioeng. Biotechnol. 2021, 9, 650598. [Google Scholar] [CrossRef] [PubMed]
- Maeda, Y.; Kimura, Y. Antitumor effects of various low-molecular-weight chitosans are due to increased natural killer activity of intestinal intraepithelial lymphocytes in sarcoma 180-bearing mice. J. Nutr. 2004, 134, 945–950. [Google Scholar] [CrossRef] [PubMed]
- Torzsas, T.; Kendall, C.; Sugano, M.; Iwamoto, Y.; Rao, A. The influence of high and low molecular weight chitosan on colonic cell proliferation and aberrant crypt foci development in CF1 mice. Food Chem. Toxicol. 1996, 34, 73–77. [Google Scholar] [CrossRef]
- Zhou, S.H.; Hong, Y.; Fang, G.J. Preparation, characterization and anticancer effect of chitosan nanoparticles. J. Clin. Rehabil. Tissue Eng. Res. 2007, 11, 9688–9691. [Google Scholar]
- Friedman, A.J.; Phan, J.; Schairer, D.O.; Champer, J.; Qin, M.; Pirouz, A.; Blecher-Paz, K.; Oren, A.; Liu, P.T.; Modlin, R.L.; et al. Antimicrobial and anti-inflammatory activity of chitosan–alginate nanoparticles: A targeted therapy for cutaneous pathogens. J. Investig. Dermatol. 2013, 133, 1231–1239. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, X.; Xing, R.; Xu, C.; Liu, S.; Qin, Y.; Li, K.; Yu, H.; Li, P. Immunostimulatory effect of chitosan and quaternary chitosan: A review of potential vaccine adjuvants. Carbohydr. Polym. 2021, 264, 118050. [Google Scholar] [CrossRef] [PubMed]
- Rampino, A.; Borgogna, M.; Blasi, P.; Bellich, B.; Cesaro, A. Chitosan nanopraticles: Preparation, size evolution and stability. Int. J. Pharm. 2013, 455, 219–228. [Google Scholar] [CrossRef] [PubMed]
- Fu, S.; Xia, J.; Wu, J. Functional chitosan nanoparticles in cancer treatment. J. Biomed. Nanotechnol. 2016, 12, 1585–1603. [Google Scholar] [CrossRef] [PubMed]
- Bellich, B.; D’Agostino, I.; Semeraro, S.; Gamini, A.; Cesàro, A. “The good, the bad and the ugly” of chitosans. Mar. Drugs 2016, 14, 99. [Google Scholar] [CrossRef] [Green Version]
- Roy, S.G.; Shirsat, N.S.; Mishra, A.C.; Waghulde, S.O.; Kale, M.K. A review on chitosan nanoparticles applications in drug delivery. J. Pharm. Phytochem. 2018, 7, 1–4. [Google Scholar] [CrossRef]
- Bernkop-Schnürch, A.; Dünnhaupt, S. Chitosan-based drug delivery systems. Eur. J. Pharm. Biopharm. 2012, 81, 463–469. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Vimal, A.; Kumar, A. Why Chitosan? From properties to perspective of mucosal drug delivery. Int. J. Biol. Macromol. 2016, 91, 615–622. [Google Scholar] [CrossRef] [PubMed]
- Bowman, K.; Leong, K.W. Chitosan nanoparticles for oral drug and gene delivery. Int. J. Nanomed. 2006, 1, 117–128. [Google Scholar] [CrossRef] [PubMed]
- Jin, M.X.; Hu, Q.H. Characterization and application in bioadhesive drug delivery system of chitosan. Cent. South Pharm. 2008, 6, 324–327. [Google Scholar]
- Parhi, R. Drug delivery applications of chitin and chitosan: A review. Environ. Chem. Lett. 2020, 18, 577–594. [Google Scholar] [CrossRef]
- Safdar, R.; Omar, A.A.; Arunagiri, A.; Regupathi, I.; Thanabalan, M. Potential of Chitosan and its derivatives for controlled drug release applications–A review. J. Drug Deliv. Sci. Technol. 2019, 49, 642–659. [Google Scholar] [CrossRef]
- Jhaveri, J.; Raichura, Z.; Khan, T.; Momin, M.; Omri, A. Chitosan nanoparticles-insight into properties, functionalization and applications in drug delivery and theranostics. Molecules 2021, 26, 272. [Google Scholar] [CrossRef] [PubMed]
- Jones, R.A.; Cheung, C.Y.; Black, F.E.; Zia, J.K.; Stayton, P.S.; Hoffman, A.S.; Wilson, M.R. Poly (2-alkylacrylic acid) polymers deliver molecules to the cytosol by pH-sensitive disruption of endosomal vesicles. Biochem. J. 2003, 372, 65–75. [Google Scholar] [CrossRef] [Green Version]
- McCarthy, P.C.; Zhang, Y.; Abebe, F. Recent applications of dual-stimuli responsive chitosan hydrogel nanocomposites as drug delivery tools. Molecules 2021, 26, 4735. [Google Scholar] [CrossRef]
- Available online: https://wayback.archive-it.org/7993/20171031005742/https://www.fda.gov/Food/IngredientsPackagingLabeling/GRAS/NoticeInventory/ucm347791.htm (accessed on 29 October 2021).
- Kean, T.; Thanou, M. Biodegradation, biodistribution and toxicity of chitosan. Adv. Drug Deliv. Rev. 2010, 62, 3–11. [Google Scholar] [CrossRef] [PubMed]
- Wedmore, I.; McManus, J.; Pusateri, A.; Holcomb, J. A special report on the chitosan-based hemostatic dressing: Experience in current combat operations. J. Trauma 2006, 60, 655–658. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mohammed, M.A.; Syeda, J.; Wasan, K.M.; Wasan, E.K. An overview of chitosan nanoparticles and its application in non-parenteral drug delivery. Pharmaceutics 2017, 9, 53. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thanou, M.; Verhoef, J.; Junginger, H. Oral drug absorption enhancement by chitosan and its derivatives. Adv. Drug Deliv. Rev. 2001, 52, 117–126. [Google Scholar] [CrossRef]
- Sonia, T.; Sharma, C. Chitosan and its derivatives for drug delivery perspective. Adv. Polym. Sci. 2011, 243, 23–54. [Google Scholar]
- Alshehri, S.; Imam, S.S.; Rizwanullah, M.; Fakhri, K.U.; Rizvi, M.M.; Mahdi, W.; Kazi, M. Effect of chitosan coating on PLGA nanoparticles for oral delivery of thymoquinone: In vitro, ex vivo, and cancer cell line assessments. Coatings 2021, 11, 6. [Google Scholar] [CrossRef]
- Amidi, M.; Mastrobattista, E.; Jiskoot, W.; Hennink, W.E. Chitosan-based delivery systems for protein therapeutics and antigens. Adv. Drug Deliv. Rev. 2010, 62, 59–82. [Google Scholar] [CrossRef] [PubMed]
- Sonaje, K.; Lin, K.J.; Tseng, M.T.; Wey, S.P.; Su, F.Y.; Chuang, E.Y.; Hsu, C.W.; Chen, C.T.; Sung, H.W. Effects of chitosan-nanoparticle-mediated tight junction opening on the oral absorption of endotoxins. Biomaterials 2011, 32, 8712–8721. [Google Scholar] [CrossRef] [PubMed]
- Mikušová, V.; Mikuš, P. Advances in chitosan-based nanoparticles for drug delivery. Int. J. Mol. Sci. 2021, 22, 9652. [Google Scholar] [CrossRef] [PubMed]
- Bravo-Osuna, I.; Vauthier, C.; Farabollini, A.; Palmieri, G.F.; Ponchel, G. Mucoadhesion mechanism of chitosan and thiolated chitosan-poly (isobutyl cyanoacrylate) core-shell nanoparticles. Biomaterials 2007, 28, 2233–2243. [Google Scholar] [CrossRef] [PubMed]
- Sadeghi, A.M.; Dorkoosh, F.A.; Avadi, M.R.; Weinhold, M.; Bayat, A.; Delie, F.; Gurny, R.; Larijani, B.; Rafiee-Tehrani, M.; Junginger, H.E. Permeation enhancer effect of chitosan and chitosan derivatives: Comparison of formulations as soluble polymers and nanoparticulate systems on insulin absorption in Caco-2 cells. Eur. J. Pharm. Biopharm. 2008, 70, 270–278. [Google Scholar] [CrossRef] [PubMed]
- Singla, A.; Chawla, M. Chitosan: Some pharmaceutical and biological aspects-an update. J. Pharm. Pharmacol. 2001, 53, 1047–1067. [Google Scholar] [CrossRef] [PubMed]
- Hejazi, R.; Amiji, M. Chitosan-based gastrointestinal delivery systems. J. Control. Release 2003, 89, 151–165. [Google Scholar] [CrossRef]
- Thanou, M.; Kotze, A.; Scharringhausen, T.; Lueßen, H.; De Boer, A.; Verhoef, J.; Junginger, H. Effect of degree of quaternization of N-trimethyl chitosan chloride for enhanced transport of hydrophilic compounds across intestinal Caco-2 cell monolayers. J. Control. Release 2000, 64, 15–25. [Google Scholar] [CrossRef]
- Roldo, M.; Hornof, M.; Caliceti, P.; Bernkop-Schnürch, A. Mucoadhesive thiolated chitosans as platforms for oral controlled drug delivery: Synthesis and in vitro evaluation. Eur. J. Pharm. Biopharm. 2004, 57, 115–121. [Google Scholar] [CrossRef]
- Bernkop-Schnürch, A.; Guggi, D.; Pinter, Y. Thiolated chitosans: Development and in vitro evaluation of a mucoadhesive, permeation enhancing oral drug delivery system. J. Control Release 2004, 94, 177–186. [Google Scholar] [CrossRef] [PubMed]
- Salama, N.N.; Eddington, N.D.; Fasano, A. Tight junction modulation and its relationship to drug delivery. Adv. Drug Deliv. Rev. 2006, 58, 15–28. [Google Scholar] [CrossRef]
- Balcerzak, S.P.; Lane, W.C.; Bullard, J.W. Surface structure of intestinal epithelium. Gastroenterology 1970, 58, 49–55. [Google Scholar] [CrossRef]
- Cheng, H.; Leblond, C.P. Origin, differentiation and renewal of the four main epithelial cell types in the mouse small intestine I. Columnar cell. Am. J. Anat. 1974, 141, 461–479. [Google Scholar] [CrossRef] [PubMed]
- Yun, Y.; Cho, Y.W.; Park, K. Nanoparticles for oral delivery: Targeted nanoparticles with peptidic ligands for oral protein delivery. Adv. Drug Deliv. Rev. 2013, 65, 822–832. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Neutra, M.; Grand, R.; Trier, J. Glycoprotein synthesis, transport, and secretion by epithelial cells of human rectal mucosa: Normal and cystic fibrosis. Lab. Investig. 1977, 36, 535–546. [Google Scholar]
- Neutra, M.; Leblond, C. Synthesis of the carbohydrate ofmucus in the Golgi complex as shown by electron microscope radioautography of goblet cells fromrats injected with glucose-H3. J. Cell Biol. 1966, 30, 119–136. [Google Scholar] [CrossRef] [PubMed]
- Kiyono, H.; Fukuyama, S. NALT- versus Peyer’s-patch-mediated mucosal immunity. Nat. Rev. Immunol. 2004, 4, 699–710. [Google Scholar] [CrossRef] [PubMed]
- Bourdet, D.L.; Pollack, G.M.; Thakker, D.R. Intestinal absorptive transport of the hydrophilic cation ranitidine: A kinetic modeling approach to elucidate the role of uptake and efflux transporters and paracellular vs. transcellular transport in Caco-2 cells. Pharm. Res. 2006, 23, 1178–1187. [Google Scholar] [CrossRef]
- Lin, Y.H.; Mi, F.L.; Chen, C.T.; Chang, W.C.; Peng, S.F.; Liang, H.F.; Sung, H.W. Preparation and characterization of nanoparticles shelled with chitosan for oral insulin delivery. Biomacromolecules 2007, 8, 146–152. [Google Scholar] [CrossRef]
- Angelova, N.; Hunkeler, D. Effect of preparation conditions on properties and permeability of chitosansodium hexametaphosphate capsules. J. Biomater. Sci. Polym. Ed. 2001, 12, 1317–1337. [Google Scholar] [CrossRef] [PubMed]
- Behrens, I.; Pena, A.I.V.; Alonso, M.J.; Kissel, T. Comparative uptake studies of bioadhesive and non-bioadhesive nanoparticles in human intestinal cell lines and rats: The effect of mucus on particle adsorption and transport. Pharm. Res. 2002, 19, 1185–1193. [Google Scholar] [CrossRef] [PubMed]
- Pridgen, E.M.; Alexis, F.; Farokhzad, O.C. Polymeric nanoparticle technologies for oral drug delivery. Clin. Gastroenterol. Hepatol. 2014, 12, 1605–1610. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Florence, A.T. Nanoparticle uptake by the oral route: Fulfilling its potential? Drug Discov. Today 2005, 2, 75–81. [Google Scholar] [CrossRef] [PubMed]
- Desai, M.P.; Labhasetwar, V.; Amidon, G.L.; Levy, R.J. Gastrointestinal uptake of biodegradable microparticles: Effect of particle size. Pharm. Res. 1996, 13, 1838–1845. [Google Scholar] [CrossRef]
- Pridgen, E.M.; Alexis, F.; Farokhzad, O.C. Polymeric nanoparticle drug delivery technologies for oral delivery applications. Expert Opin. Drug Deliv. 2015, 12, 1459–1473. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, M.C.; Sonaje, K.; Chen, K.J.; Sung, H.W. A review of the prospects for polymeric nanoparticle platforms in oral insulin delivery. Biomaterials 2011, 32, 9826–9838. [Google Scholar] [CrossRef] [PubMed]
- Takeuchi, H.; Yamamoto, H.; Kawashima, Y. Mucoadhesive nanoparticulate systems for peptide drug delivery. Adv. Drug Deliv. Rev. 2001, 47, 39–54. [Google Scholar] [CrossRef]
- Yin, L.; Ding, J.; He, C.; Cui, L.; Tang, C.; Yin, C. Drug permeability and mucoadhesion properties of thiolated trimethyl chitosan nanoparticles in oral insulin delivery. Biomaterials 2009, 30, 5691–5700. [Google Scholar] [CrossRef] [PubMed]
- Soane, R.; Frier, M.; Perkins, A.; Jones, N.; Davis, S.; Illum, L. Evaluation of the clearance characteristics of bioadhesive systems in humans. Int. J. Pharm. 1999, 178, 55–65. [Google Scholar] [CrossRef]
- Yeh, T.H.; Hsu, L.W.; Tseng, M.T.; Lee, P.L.; Sonjae, K.; Ho, Y.C.; Sung, H.W. Mechanism and consequence of chitosan-mediated reversible epithelial tight junction opening. Biomaterials 2011, 32, 6164–6173. [Google Scholar] [CrossRef] [PubMed]
- Taipaleenmäki, E.; Städler, B. Recent advancements in using polymers for intestinal mucoadhesion and mucopenetration. Macromol. Biosci. 2020, 20, e1900342. [Google Scholar] [CrossRef] [PubMed]
- Cheng, X.; Tian, X.; Wu, A.; Li, J.; Tian, J.; Chong, Y.; Chai, Z.; Zhau, Y.; Chen, C.; Ge, C. Protein corona influences cellular uptake of gold nanoparticles by phagocytic and nonphagocytic cells in a size-dependent manner. ACS Appl. Mater. Interfaces 2015, 37, 20568–20575. [Google Scholar] [CrossRef] [PubMed]
- Malhaire, H.; Gimel, J.; Roger, E.; Benoît, J.; Lagarce, F. How to design the surface of peptide-loaded nanoparticles for efficient oral bioavailability? Adv. Drug Deliv. Rev. 2016, 106, 320–336. [Google Scholar] [CrossRef]
- Zhao, Z.; Ukidve, A.; Krishnan, V.; Mitragotri, S. Effect of physicochemical and surface properties on in vivo fate of drug nanocarriers. Adv. Drug Deliv. Rev. 2019, 143, 3–21. [Google Scholar] [CrossRef] [PubMed]
- Rizwanullah, M.; Perwez, A.; Mir, S.R.; Rizvi, M.M.; Amin, S. Exemestane encapsulated polymer-lipid hybrid nanoparticles for improved efficacy against breast cancer: Optimization, in vitro characterization and cell culture studies. Nanotechnology 2021, 32, 415101. [Google Scholar] [CrossRef] [PubMed]
- Du, Q.; Chen, J.; Yan, G.; Lyu, F.; Huang, J.; Ren, J.; Di, L. Comparison of different aliphatic acid grafted N-trimethyl chitosan surface-modified nanostructured lipid carriers for improved oral kaempferol delivery. Int. J. Pharm. 2019, 568, 118506. [Google Scholar] [CrossRef]
- Smith, J.; Wood, E.; Dornish, M. Effect of chitosan on epithelial cell tight junctions. Pharm. Res. 2004, 21, 43–49. [Google Scholar] [CrossRef] [PubMed]
- Naskar, S.; Kuotsu, K.; Sharma, S. Chitosan-based nanoparticles as drug delivery systems: A review on two decades of research. J. Drug Target. 2019, 27, 379–393. [Google Scholar] [CrossRef] [PubMed]
- Shahab, M.S.; Rizwanullah, M.; Alshehri, S.; Imam, S.S. Optimization to development of chitosan decorated polycaprolactone nanoparticles for improved ocular delivery of dorzolamide: In vitro, ex vivo and toxicity assessments. Int. J. Biol. Macromol. 2020, 163, 2392–2404. [Google Scholar] [CrossRef] [PubMed]
- Soni, K.; Rizwanullah, M.; Kohli, K. Development and optimization of sulforaphane-loaded nanostructured lipid carriers by the Box-Behnken design for improved oral efficacy against cancer: In vitro, ex vivo and in vivo assessments. Artif. Cells Nanomed. Biotechnol. 2018, 46, 15–31. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sanna, V.; Roggio, A.M.; Siliani, S.; Piccinini, M.; Marceddu, S.; Mariani, A.; Sechi, M. Development of novel cationic chitosan-and anionic alginate–coated poly (d, l-lactide-co-glycolide) nanoparticles for controlled release and light protection of resveratrol. Int. J. Nanomed. 2012, 7, 5501–5516. [Google Scholar]
- de Souza, M.P.; Sábio, R.M.; de Cassia Ribeiro, T.; Dos Santos, A.M.; Meneguin, A.B.; Chorilli, M. Highlighting the impact of chitosan on the development of gastroretentive drug delivery systems. Int. J. Biol. Macromol. 2020, 159, 804–822. [Google Scholar] [CrossRef] [PubMed]
- Abd El Hady, W.E.; Mohamed, E.A.; Soliman, O.A.; El-Sabbagh, H.M. In vitro–in vivo evaluation of chitosan-PLGA nanoparticles for potentiated gastric retention and anti-ulcer activity of diosmin. Int. J. Nanomed. 2019, 14, 7191–7213. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bonifácio, B.V.; da Silva, P.B.; dos Santos Ramos, M.A.; Negri, K.M.; Bauab, T.M.; Chorilli, M. Nanotechnology-based drug delivery systems and herbal medicines: A review. Int. J. Nanomed. 2014, 9, 1–15. [Google Scholar]
- Xie, Y.; Ma, C.; Yang, X.; Wang, J.; Long, G.; Zhou, J. Phytonanomaterials as therapeutic agents and drug delivery carriers. Adv. Drug Deliv. Rev. 2021, 176, 113868. [Google Scholar] [CrossRef] [PubMed]
- Maheshwari, R.K.; Singh, A.K.; Gaddipati, J.; Srimal, R.C. Multiple biological activities of curcumin: A short review. Life Sci. 2006, 78, 2081–2087. [Google Scholar] [CrossRef] [PubMed]
- Sharma, R.A.; Gescher, A.J.; Steward, W.P. Curcumin: The story so far. Eur. J. Cancer 2005, 41, 1955–1968. [Google Scholar] [CrossRef]
- Lopresti, A.L.; Hood, S.D.; Drummond, P.D. Multiple antidepressant potential modes of action of curcumin: A review of its anti-inflammatory, monoaminergic, antioxidant, immune-modulating and neuroprotective effects. J. Psychopharmacol. 2012, 26, 1512–1524. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fan, X.; Zhang, C.; Liu, D.B.; Yan, J.; Liang, H.P. The clinical applications of curcumin: Current state and the future. Curr. Pharm. Des. 2013, 19, 2011–2031. [Google Scholar] [PubMed]
- Garcea, G.; Jones, D.J.; Singh, R.; Dennison, A.R.; Farmer, P.B.; Sharma, R.A.; Steward, W.P.; Gescher, A.J.; Berry, D.P. Detection of curcumin and its metabolites in hepatic tissue and portal blood of patients following oral administration. Br. J. Cancer 2004, 90, 1011–1015. [Google Scholar] [CrossRef] [PubMed]
- Raja, M.A.; Zeenat, S.; Arif, M.; Liu, C. Self-assembled nanoparticles based on amphiphilic chitosan derivative and arginine for oral curcumin delivery. Int. J. Nanomed. 2016, 11, 4397–4412. [Google Scholar] [CrossRef] [Green Version]
- Ng, S.W.; Selvarajah, G.T.; Hussein, M.Z.; Yeap, S.K.; Omar, A.R. In vitro evaluation of curcumin-encapsulated chitosan nanoparticles against feline infectious peritonitis virus and pharmacokinetics study in cats. BioMed Res. Int. 2020, 2020, 3012198. [Google Scholar] [PubMed]
- Khatik, R.; Mishra, R.; Verma, A.; Dwivedi, P.; Kumar, V.; Gupta, V.; Paliwal, S.K.; Mishra, P.R.; Dwivedi, A.K. Colon-specific delivery of curcumin by exploiting Eudragit-decorated chitosan nanoparticles in vitro and in vivo. J. Nanopart. Res. 2013, 15, 1893. [Google Scholar]
- Ramalingam, P.; Ko, Y.T. Enhanced oral delivery of curcumin from N-trimethyl chitosan surface-modified solid lipid nanoparticles: Pharmacokinetic and brain distribution evaluations. Pharm. Res. 2015, 32, 389–402. [Google Scholar] [CrossRef]
- Baek, J.S.; Cho, C.W. Surface modification of solid lipid nanoparticles for oral delivery of curcumin: Improvement of bioavailability through enhanced cellular uptake, and lymphatic uptake. Eur. J. Pharm. Biopharm. 2017, 117, 132–140. [Google Scholar] [CrossRef] [PubMed]
- Knekt, P.; Kumpulainen, J.; Järvinen, R.; Rissanen, H.; Heliövaara, M.; Reunanen, A.; Hakulinen, T.; Aromaa, A. Flavonoid intake and risk of chronic diseases. Am. J. Clin. Nutr. 2002, 76, 560–568. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen-yu, G.; Chun-fen, Y.; Qi-lu, L.; Qi, T.; Yan-wei, X.; Wei-na, L.; Guang-Xi, Z. Development of a quercetin-loaded nanostructured lipid carrier formulation for topical delivery. Int. J. Pharm. 2012, 430, 292–298. [Google Scholar] [CrossRef] [PubMed]
- Boots, A.W.; Haenen, G.R.; Bast, A. Health effects of quercetin: From antioxidant to nutraceutical. Eur. J. Pharmacol. 2008, 585, 325–337. [Google Scholar] [CrossRef] [PubMed]
- Tran, T.H.; Guo, Y.; Song, D.; Bruno, R.S.; Lu, X. Quercetin-containing self-nanoemulsifying drug delivery system for improving oral bioavailability. J. Pharm. Sci. 2014, 103, 840–852. [Google Scholar] [CrossRef]
- Patel, A.R.; Heussen, P.C.; Hazekamp, J.; Drost, E.; Velikov, K.P. Quercetin loaded biopolymeric colloidal particles prepared by simultaneous precipitation of quercetin with hydrophobic protein in aqueous medium. Food Chem. 2012, 133, 423–439. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Wang, Y.; Ma, Y.; Yu, A.; Cai, F.; Shao, W.; Zhai, G. Formulation optimization and in situ absorption in rat intestinal tract of quercetin-loaded microemulsion. Colloids Surf. B Biointerfaces 2009, 71, 306–314. [Google Scholar] [CrossRef]
- Moon, Y.J.; Wang, L.; DiCenzo, R.; Morris, M.E. Quercetin pharmacokinetics in humans. Biopharm. Drug Dispos. 2008, 29, 205–217. [Google Scholar] [CrossRef]
- Barbosa, A.I.; Lima, S.A.C.; Reis, S. Application of pH-responsive fucoidan/chitosan nanoparticles to improve oral quercetin delivery. Molecules 2019, 24, 346. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhou, J.F.; Zheng, G.D.; Wang, W.J.; Yin, Z.P.; Chen, J.G.; Li, J.E.; Zhang, Q.F. Physicochemical properties and bioavailability comparison of two quercetin loading zein nanoparticles with outer shell of caseinate and chitosan. Food Hydrocol. 2021, 120, 106959. [Google Scholar] [CrossRef]
- Ma, J.J.; Huang, X.N.; Yin, S.W.; Yu, Y.G.; Yang, X.Q. Bioavailability of quercetin in zein-based colloidal particles-stabilized Pickering emulsions investigated by the in vitro digestion coupled with Caco-2 cell monolayer model. Food Chem. 2021, 360, 130152. [Google Scholar] [CrossRef]
- Mukhopadhyay, P.; Maity, S.; Mandal, S.; Chakraborti, A.S.; Prajapati, A.K.; Kundu, P.P. Preparation, characterization and in vivo evaluation of pH sensitive, safe quercetin-succinylated chitosan-alginate core-shell-corona nanoparticle for diabetes treatment. Carbohydr. Polym. 2018, 182, 42–51. [Google Scholar] [CrossRef] [PubMed]
- Tzankova, V.; Aluani, D.; Kondeva-Burdina, M.; Yordanov, Y.; Odzhakov, F.; Apostolov, A.; Yoncheva, K. Hepatoprotective and antioxidant activity of quercetin loaded chitosan/alginate particles in vitro and in vivo in a model of paracetamol-induced toxicity. Biomed. Pharmacother. 2017, 92, 569–579. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.L.; Sun, Z.J.; Yu, L.; Meng, K.W.; Qin, X.L.; Pan, C.E. Effect of resveratrol and in combination with 5-FU on murine liver cancer. World J. Gastroenterol. 2004, 10, 3048–3052. [Google Scholar] [CrossRef] [PubMed]
- Meyer, A.S.; Yi, O.S.; Pearson, D.A.; Waterhouse, A.L.; Frankel, E.N. Inhibition of human low density lipoprotein oxidation in relation to composition of phenolic antioxidants in grapes (Vitis vinifera). J. Agric. Food Chem. 1997, 45, 1638–1643. [Google Scholar] [CrossRef]
- Pace-Asciak, C.R.; Hahn, S.; Diamandis, E.P.; Soleas, S.; Goldberg, D.M. The red wine phenolics trans-resveratrol and quercetin block human platelet aggregation and eicosanoid synthesis: Implications for protection against coronary heart disease. Clin. Chim. Acta 1995, 235, 207–219. [Google Scholar] [CrossRef]
- Baur, J.A.; Sinclair, D.A. Therapeutic potential of resveratrol: The in vivo evidence. Nat. Rev. Drug Discov. 2006, 5, 493–506. [Google Scholar] [CrossRef] [PubMed]
- Wenzel, E.; Somoza, V. Metabolism and bioavailability of transresveratrol. Mol. Nutr. Food Res. 2005, 49, 472–481. [Google Scholar] [CrossRef] [PubMed]
- Zu, Y.; Zhang, Y.; Wang, W.; Zhao, X.; Han, X.; Wang, K.; Ge, Y. Preparation and in vitro/in vivo evaluation of resveratrol-loaded carboxymethyl chitosan nanoparticles. Drug Deliv. 2016, 23, 971–981. [Google Scholar] [CrossRef]
- Ramalingam, P.; Ko, Y.T. Improved oral delivery of resveratrol from N-trimethyl chitosan-g-palmitic acid surface-modified solid lipid nanoparticles. Colloids Surf. B Biointerfaces 2016, 139, 52–61. [Google Scholar] [CrossRef]
- Du, S.; Lv, Y.; Li, N.; Huang, X.; Liu, X.; Li, H.; Wang, C.; Jia, Y.F. Biological investigations on therapeutic effect of chitosan encapsulated nano resveratrol against gestational diabetes mellitus rats induced by streptozotocin. Drug Deliv. 2020, 27, 953–963. [Google Scholar] [CrossRef]
- Pauluk, D.; Padilha, A.K.; Khalil, N.M.; Mainardes, R.M. Chitosan-coated zein nanoparticles for oral delivery of resveratrol: Formation, characterization, stability, mucoadhesive properties and antioxidant activity. Food Hydrocoll. 2019, 94, 411–417. [Google Scholar] [CrossRef]
- Elmowafy, M.; Samy, A.; Raslan, M.A.; Salama, A.; Said, R.A.; Abdelaziz, A.E.; El-Eraky, W.; El Awdan, S.; Viitala, T. Enhancement of bioavailability and pharmacodynamic effects of thymoquinone via nanostructured lipid carrier (NLC) formulation. AAPS PharmSciTech 2016, 17, 663–672. [Google Scholar] [CrossRef]
- Darakhshan, S.; Pour, A.B.; Colagar, A.H.; Sisakhtnezhad, S. Thymoquinone and its therapeutic potentials. Pharmacol. Res. 2015, 95–96, 138–158. [Google Scholar] [CrossRef] [PubMed]
- Kalam, M.A.; Raish, M.; Ahmed, A.; Alkharfy, K.M.; Mohsin, K.; Alshamsan, A.; Al-Jenoobi, F.I.; Al-Mohizea, A.M.; Shakeel, F. Oral bioavailability enhancement and hepatoprotective effects of thymoquinone by self-nanoemulsifying drug delivery system. Mater. Sci. Eng. C Mater. Biol. Appl. 2017, 76, 319–329. [Google Scholar] [CrossRef] [PubMed]
- Rahat, I.; Rizwanullah, M.; Gilani, S.J.; Bin-Jummah, M.N.; Imam, S.S.; Kala, C.; Asif, M.; Alshehri, S.; Sharma, S.K. Thymoquinone loaded chitosan-Solid lipid nanoparticles: Formulation optimization to oral bioavailability study. J. Drug Deliv. Sci. Tech. 2021, 64, 102565. [Google Scholar] [CrossRef]
- Rahat, I.; Imam, S.S.; Rizwanullah, M.; Alshehri, S.; Asif, M.; Kala, C.; Taleuzzaman, M. Thymoquinone-entrapped chitosan-modified nanoparticles: Formulation optimization to preclinical bioavailability assessments. Drug Deliv. 2021, 28, 973–984. [Google Scholar] [CrossRef]
- Fakhria, A.; Gilani, S.J.; Imam, S.S. Formulation of thymoquinone loaded chitosan nano vesicles: In-vitro evaluation and in-vivo anti-hyperlipidemic assessment. J. Drug Deliv. Sci. Tech. 2019, 50, 339–346. [Google Scholar] [CrossRef]
- Wang, H.; Provan, G.J.; Helliwell, K. Tea flavonoids: Their functions, utilisation and analysis. Trends Food Sci. Tech. 2000, 11, 152–160. [Google Scholar] [CrossRef]
- Hackman, R.M.; Polagruto, J.A.; Zhu, Q.Y.; Sun, B.; Fujii, H.; Keen, C.L. Flavanols: Digestion, absorption and bioactivity. Phytochem. Rev. 2008, 7, 195. [Google Scholar] [CrossRef]
- Lambert, J.D.; Yang, C.S. Mechanisms of cancer prevention by tea constituents. J. Nutr. 2003, 133, 3262S–3267S. [Google Scholar] [CrossRef] [PubMed]
- Nagle, D.G.; Ferreira, D.; Zhou, Y.D. Epigallocatechin-3-gallate (EGCG): Chemical and biomedical perspectives. Phytochemistry 2006, 67, 1849–1855. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zaveri, N.T. Green tea and its polyphenolic catechins: Medicinal uses in cancer and noncancer applications. Life Sci. 2006, 78, 2073–2080. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Q.Y.; Zhang, A.; Tsang, D.; Huang, Y.; Chen, Z.Y. Stability of green tea catechins. J. Agric. Food Chem. 1997, 45, 4624–4628. [Google Scholar] [CrossRef]
- Sang, S.; Lambert, J.D.; Ho, C.T.; Yang, C.S. The chemistry and biotransformation of tea constituents. Pharmacol. Res. 2011, 64, 87–99. [Google Scholar] [CrossRef] [PubMed]
- Zhu, M.; Chen, Y.; Li, R.C. Oral absorption and bioavailability of tea catechins. Planta Med. 2000, 66, 444–447. [Google Scholar] [CrossRef]
- Lin, L.C.; Wang, M.N.; Tseng, T.Y.; Sung, J.S.; Tsai, T.H. Pharmacokinetics of (–)-epigallocatechin-3-gallate in conscious and freely moving rats and its brain regional distribution. J. Agric. Food Chem. 2007, 55, 1517–1524. [Google Scholar] [CrossRef]
- Lu, H.; Meng, X.; Yang, C.S. Enzymology of methylation of tea catechins and inhibition of catechol-O-methyltransferase by (-)-epigallocatechin gallate. Drug Metab. Dispos. 2003, 31, 572–579. [Google Scholar] [CrossRef] [Green Version]
- Hong, J.; Lambert, J.D.; Lee, S.H.; Sinko, P.J.; Yang, C.S. Involvement of multidrug resistance-associated proteins in regulating cellular levels of (-)-epigallocatechin-3-gallate and its methyl metabolites. Biochem. Biophys. Res. Commun. 2003, 310, 222–227. [Google Scholar] [CrossRef]
- Khan, N.; Bharali, D.J.; Adhami, V.M.; Siddiqui, I.A.; Cui, H.; Shabana, S.M.; Mousa, S.A.; Mukhtar, H. Oral administration of naturally occurring chitosan-based nanoformulated green tea polyphenol EGCG effectively inhibits prostate cancer cell growth in a xenograft model. Carcinogenesis 2014, 35, 415–423. [Google Scholar] [CrossRef] [PubMed]
- Siddiqui, I.A.; Bharali, D.J.; Nihal, M.; Adhami, V.M.; Khan, N.; Chamcheu, J.C.; Khan, M.I.; Shabana, S.; Mousa, S.A.; Mukhtar, H. Excellent anti-proliferative and pro-apoptotic effects of (−)-epigallocatechin-3-gallate encapsulated in chitosan nanoparticles on human melanoma cell growth both in vitro and in vivo. Nanomedicine 2014, 10, 1619–1626. [Google Scholar] [CrossRef] [PubMed]
- Hong, Z.; Xu, Y.; Yin, J.F.; Jin, J.; Jiang, Y.; Du, Q. Improving the effectiveness of (−)-epigallocatechin gallate (EGCG) against rabbit atherosclerosis by EGCG-loaded nanoparticles prepared from chitosan and polyaspartic acid. J. Agric. Food Chem. 2014, 62, 12603–12609. [Google Scholar] [CrossRef] [PubMed]
- Dube, A.; Nicolazzo, J.A.; Larson, I. Chitosan nanoparticles enhance the plasma exposure of (−)-epigallocatechin gallate in mice through an enhancement in intestinal stability. Eur. J. Pharm. Sci. 2011, 44, 422–426. [Google Scholar] [CrossRef] [PubMed]
- Ikeda, Y.; Murakami, A.; Ohigashi, H. Ursolic acid: An anti-and pro-inflammatory triterpenoid. Mol. Nutr. Food Res. 2008, 52, 26–42. [Google Scholar] [CrossRef] [PubMed]
- Seo, D.Y.; Lee, S.R.; Heo, J.W.; No, M.H.; Rhee, B.D.; Ko, K.S.; Kwak, H.B.; Han, J. Ursolic acid in health and disease. Korean J. Physiol. Pharmacol. 2018, 22, 235–248. [Google Scholar] [CrossRef] [Green Version]
- Kashyap, D.; Tuli, H.S.; Sharma, A.K. Ursolic acid (UA): A metabolite with promising therapeutic potential. Life Sci. 2016, 146, 201–213. [Google Scholar] [CrossRef] [PubMed]
- López-Hortas, L.; Pérez-Larrán, P.; González-Muñoz, M.J.; Falqué, E.; Domínguez, H. Recent developments on the extraction and application of ursolic acid. A review. Food Res. Int. 2018, 103, 130–149. [Google Scholar] [CrossRef]
- Zhang, H.; Li, X.; Ding, J.; Xu, H.; Dai, X.; Hou, Z.; Zhang, K.; Sun, K.; Sun, W. Delivery of ursolic acid (UA) in polymeric nanoparticles effectively promotes the apoptosis of gastric cancer cells through enhanced inhibition of cyclooxygenase 2 (COX-2). Int. J. Pharm. 2013, 441, 261–268. [Google Scholar] [CrossRef] [PubMed]
- Antonio, E.; Junior, O.D.; Marcano, R.G.; Diedrich, C.; da Silva Santos, J.; Machado, C.S.; Khalil, N.M.; Mainardes, R.M. Chitosan modified poly (lactic acid) nanoparticles increased the ursolic acid oral bioavailability. Int. J. Biol. Macromol. 2021, 172, 133–142. [Google Scholar] [CrossRef]
- Das, S.; Ghosh, S.; De, A.K.; Bera, T. Oral delivery of ursolic acid-loaded nanostructured lipid carrier coated with chitosan oligosaccharides: Development, characterization, in vitro and in vivo assessment for the therapy of leishmaniasis. Int. J. Biol. Macromol. 2017, 102, 996–1008. [Google Scholar] [CrossRef]
- Wang, M.; Zhao, T.; Liu, Y.; Wang, Q.; Xing, S.; Li, L.; Wang, L.; Liu, L.; Gao, D. Ursolic acid liposomes with chitosan modification: Promising antitumor drug delivery and efficacy. Mater. Sci. Eng. C Mater. Biol. Appl. 2017, 71, 1231–1240. [Google Scholar] [CrossRef] [PubMed]
- Mancuso, C.; Santangelo, R. Ferulic acid: Pharmacological and toxicological aspects. Food Chem. Toxicol. 2014, 65, 185–195. [Google Scholar] [CrossRef]
- Chaudhary, A.; Jaswal, V.S.; Choudhary, S.; Sharma, A.; Beniwal, V.; Tuli, H.S.; Sharma, S. Ferulic acid: A promising therapeutic phytochemical and recent patents advances. Recent Pat. Inflamm. Allergy Drug Discov. 2019, 13, 115–123. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Rui, Y.X.; Guo, S.D.; Luan, F.; Liu, R.; Zeng, N. Ferulic acid: A review of its pharmacology, pharmacokinetics and derivatives. Life Sci. 2021, 284, 119921. [Google Scholar] [CrossRef]
- Kumar, N.; Pruthi, V. Potential applications of ferulic acid from natural sources. Biotechnol. Rep. 2014, 4, 86–93. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zdunska, K.; Dana, A.; Kolodziejczak, A.; Rotsztejn, H. Antioxidant properties of ferulic acid and its possible application. Skin Pharmacol. Physiol. 2018, 31, 332–336. [Google Scholar] [CrossRef] [PubMed]
- Yan, N.; Tang, Z.; Xu, Y.; Li, X.; Wang, Q. Pharmacokinetic study of ferulic acid following transdermal or intragastric administration in rats. AAPS PharmSciTech 2020, 21, 1–7. [Google Scholar] [CrossRef]
- Telange, D.R.; Jain, S.P.; Pethe, A.M.; Kharkar, P.S.; Rarokar, N.R. Use of combined nanocarrier system based on chitosan nanoparticles and phospholipids complex for improved delivery of ferulic acid. Int. J. Biol. Macromol. 2021, 171, 288–307. [Google Scholar] [CrossRef]
- Lima, I.A.; Khalil, N.M.; Tominaga, T.T.; Lechanteur, A.; Sarmento, B.; Mainardes, R.M. Mucoadhesive chitosan-coated PLGA nanoparticles for oral delivery of ferulic acid. Artif. Cells Nanomed. Biotechnol. 2018, 46, 993–1002. [Google Scholar] [CrossRef] [Green Version]
- Guo, Q.; Yuan, Q. A novel 10-hydroxycamptothecin-glucoside from the fruit of Camptotheca acuminata. Nat. Prod. Res. 2016, 30, 1053–1059. [Google Scholar] [CrossRef] [PubMed]
- Kolhatkar, R.B.; Swaan, P.; Ghandehari, H. Potential oral delivery of 7-ethyl-10-hydroxy-camptothecin (SN-38) using poly(amidoamine) dendrimers. Pharm. Res. 2008, 25, 1723–1729. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tagen, M.; Zhuang, Y.; Zhang, F.; Harstead, K.E.; Shen, J.; Schaiquevich, P.; Fraga, C.H.; Panetta, J.C.; Waters, C.M.; Stewart, C.F. P-glycoprotein, but not multidrug resistance protein 4, plays a role in the systemic clearance of irinotecan and SN-38 in mice. Drug Metab. Lett. 2010, 4, 195–201. [Google Scholar] [CrossRef] [PubMed]
- Sharifi, F.; Jahangiri, M.; Ebrahimnejad, P. Synthesis of novel polymeric nanoparticles (methoxy-polyethylene glycol-chitosan/hyaluronic acid) containing 7-ethyl-10-hydroxycamptothecin for colon cancer therapy: In vitro, ex vivo and in vivo investigation. Artif. Cells Nanomed. Biotechnol. 2021, 49, 367–380. [Google Scholar] [CrossRef] [PubMed]
- Guo, M.; Rong, W.T.; Hou, J.; Wang, D.F.; Lu, Y.; Wang, Y.; Yu, S.Q.; Xu, Q. Mechanisms of chitosan-coated poly (lactic-co-glycolic acid) nanoparticles for improving oral absorption of 7-ethyl-10-hydroxycamptothecin. Nanotechnology 2013, 24, 245101. [Google Scholar] [CrossRef] [PubMed]
- Stefanska, J.; Pawliczak, R. Apocynin: Molecular aptitudes. Mediat. Inflamm. 2008, 2008, 691–698. [Google Scholar] [CrossRef] [Green Version]
- de Oliveira, J.K.; Ronik, D.F.V.; Ascari, J.; Mainardes, R.M.; Khalil, N.M. A stability-indicating high performance liquid chromatography method to determine apocynin in nanoparticles. J. Pharm. Anal. 2017, 7, 129–133. [Google Scholar] [CrossRef] [PubMed]
- de Oliveira, J.K.; Ronik, D.F.V.; Ascari, J.; Mainardes, R.M.; Khalil, N.M. Nanoencapsulation of apocynin in bovine serum albumin nanoparticles: Physicochemical characterization. Nanosci. Nanotechnol. Asia 2018, 8, 90–99. [Google Scholar] [CrossRef]
- Aman, R.M.; Hashim, I.I.A.; Meshali, M.M. Novel chitosan-based solid-lipid nanoparticles to enhance the bio-residence of the miraculous phytochemical “Apocynin”. Eur. J. Pharm. Sci. 2018, 124, 304–318. [Google Scholar] [CrossRef]
- Anter, H.M.; Hashim, I.I.A.; Awadin, W.; Meshali, M.M. Novel chitosan oligosaccharide-based nanoparticles for gastric mucosal administration of the phytochemical “apocynin”. Int. J. Nanomed. 2019, 14, 4911–4929. [Google Scholar] [CrossRef] [Green Version]
- Fakhri, S.; Abbaszadeh, F.; Dargahi, L.; Jorjani, M. Astaxanthin: A mechanistic review on its biological activities and health benefits. Pharmacol. Res. 2018, 136, 1–20. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Liu, Z.; Sun, X.; Zhang, S.; Wang, S.; Feng, F.; Wang, D.; Xu, Y. Fabrication and characterization of β-lactoglobulin-based nanocomplexes composed of chitosan oligosaccharides as vehicles for delivery of astaxanthin. J. Agric. Food Chem. 2018, 66, 6717–6726. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Zhang, S.; McClements, D.J.; Wang, D.; Xu, Y. Design of astaxanthin-loaded core–shell nanoparticles consisting of chitosan oligosaccharides and poly (lactic-co-glycolic acid): Enhancement of water solubility, stability, and bioavailability. J. Agric. Food Chem. 2019, 67, 5113–5121. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Gu, Z.; Liao, Y.; Li, S.; Xue, Y.; Firempong, M.A.; Xu, Y.; Yu, J.; Smyth, H.D.; Xu, X. Improved intestinal absorption and oral bioavailability of astaxanthin using poly (ethylene glycol)-graft-chitosan nanoparticles: Preparation, in vitro evaluation, and pharmacokinetics in rats. J. Sci. Food Agric. 2021, 101, 1–4. [Google Scholar] [CrossRef] [PubMed]
- Hu, Q.; Hu, S.; Fleming, E.; Lee, J.Y.; Luo, Y. Chitosan-caseinate-dextran ternary complex nanoparticles for potential oral delivery of astaxanthin with significantly improved bioactivity. Int. J. Biol. Macromol. 2020, 151, 747–756. [Google Scholar] [CrossRef]
- Hou, Q.; He, W.J.; Wu, Y.S.; Hao, H.J.; Xie, X.Y.; Fu, X.B. Berberine: A traditional natural product with novel biological activities. Altern. Ther. Health Med. 2020, 26, 20–27. [Google Scholar] [PubMed]
- Song, D.; Hao, J.; Fan, D. Biological properties and clinical applications of berberine. Front. Med. 2020, 14, 564–582. [Google Scholar] [CrossRef]
- Zuo, F.; Nakamura, N.; Akao, T.; Hattori, M. Pharmacokinetics of berberine and its main metabolites in conventional and pseudo germ-free rats determined by liquid chromatography/ion trap mass spectrometry. Drug Metab. Dispos. 2006, 34, 2064–2072. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tan, W.; Li, Y.; Chen, M.; Wang, Y. Berberine hydrochloride: Anticancer activity and nanoparticulate delivery system. Int. J. Nanomed. 2011, 6, 1773–1777. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nguyen, T.X.; Huang, L.; Liu, L.; Abdalla, A.M.; Gauthier, M.; Yang, G. Chitosan-coated nano-liposomes for the oral delivery of berberine hydrochloride. J. Mater. Chem. B 2014, 2, 7149–7159. [Google Scholar] [CrossRef]
- Wu, S.J.; Don, T.M.; Lin, C.W.; Mi, F.L. Delivery of berberine using chitosan/fucoidan-taurine conjugate nanoparticles for treatment of defective intestinal epithelial tight junction barrier. Mar. Drugs 2014, 12, 5677–5697. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Haq, I.U.; Imran, M.; Nadeem, M.; Tufail, T.; Gondal, T.A.; Mubarak, M.S. Piperine: A review of its biological effects. Phytother. Res. 2021, 35, 680–700. [Google Scholar] [CrossRef] [PubMed]
- Meghwal, M.; Goswami, T.K. Piper nigrum and piperine: An update. Phytother. Res. 2013, 27, 1121–1130. [Google Scholar] [CrossRef]
- Sahu, P.K.; Sharma, A.; Rayees, S.; Kour, G.; Singh, A.; Khullar, M.; Magotra, A.; Paswan, S.K.; Gupta, M.; Ahmad, I.; et al. Pharmacokinetic study of piperine in Wistar rats after oral and intravenous administration. Int. J. Drug Deliv. 2014, 6, 82–88. [Google Scholar]
- Hashimoto, K.; Yaoi, T.; Koshiba, H.; Yoshida, T.; Maoka, T.; Fujiwara, Y.; Yamamoto, Y.; Mori, K. Photochemical isomerization of piperine, a pungent constituent in pepper. Food Sci. Technol. Int. 1996, 2, 24–29. [Google Scholar] [CrossRef] [Green Version]
- Zafar, A.; Alruwaili, N.K.; Imam, S.S.; Alsaidan, O.A.; Alharbi, K.S.; Yasir, M.; Elmowafy, M.; Mohammed, E.F.; Al-Oanzi, Z.H. Formulation of chitosan-coated piperine NLCs: Optimization, in vitro characterization, and in vivo preclinical assessment. AAPS PharmSciTech 2021, 22, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Giordano, E.; Quadro, L. Lutein, zeaxanthin and mammalian development: Metabolism, functions and implications for health. Arch. Biochem. Biophys. 2018, 647, 33–40. [Google Scholar] [CrossRef]
- Arathi, B.P.; Sowmya, P.R.; Vijay, K.; Baskaran, V.; Lakshminarayana, R. Metabolomics of carotenoids: The challenges and prospects–A review. Trends Food Sci. Technol. 2015, 45, 105–117. [Google Scholar] [CrossRef] [Green Version]
- Landrum, J.T.; Bone, R.A. Lutein, zeaxanthin, and the macular pigment. Arch. Biochem. Biophys. 2001, 385, 28–40. [Google Scholar] [CrossRef] [PubMed]
- Lakshminarayana, R.; Aruna, G.; Sangeetha, R.K.; Baskar, N.; Divakar, S.; Baskaran, V. Possible degradation/biotransformation of lutein in vitro and in vivo. Isolation and structural elucidation of lutein metabolites by HPLC and LC-MS (APCI). Free Radic. Biol. Med. 2008, 45, 982–993. [Google Scholar] [CrossRef] [PubMed]
- Van het Hof, K.H.; West, C.E.; Weststrate, J.A.; Hautvast, J.G. Dietary factors that affect the bioavailability of carotenoids. J. Nutr. 2000, 130, 503–506. [Google Scholar] [CrossRef] [PubMed]
- Toragall, V.; Jayapala, N.; Vallikannan, B. Chitosan-oleic acid-sodium alginate a hybrid nanocarrier as an efficient delivery system for enhancement of lutein stability and bioavailability. Int. J. Biol. Macromol. 2020, 150, 578–594. [Google Scholar] [CrossRef] [PubMed]
- Shwetha, H.J.; Shilpa, S.; Mukherjee, M.B.; Ambedkar, R.; Raichur, A.M.; Lakshminarayana, R. Fabrication of chitosan nanoparticles with phosphatidylcholine for improved sustain release, basolateral secretion, and transport of lutein in Caco-2 cells. Int. J. Biol. Macromol. 2020, 163, 2224–2235. [Google Scholar] [CrossRef]
- Camini, F.C.; Costa, D.C. Silymarin: Not just another antioxidant. J. Basic Clin. Physiol. Pharmacol. 2020, 31, 20190206. [Google Scholar] [CrossRef] [PubMed]
- Javed, S.; Kohli, K.; Ali, M. Patented bioavailability enhancement techniques of silymarin. Recent Pat. Drug Deliv. Formul. 2010, 4, 145–152. [Google Scholar] [CrossRef]
- Di Costanzo, A.; Angelico, R. Formulation strategies for enhancing the bioavailability of silymarin: The state of the art. Molecules 2019, 24, 2155. [Google Scholar] [CrossRef] [Green Version]
- Wu, J.W.; Lin, L.C.; Hung, S.C.; Chi, C.W.; Tsai, T.H. Analysis of silibinin in rat plasma and bile for hepatobiliary excretion and oral bioavailability application. J. Pharm. Biomed. Anal. 2007, 45, 635–641. [Google Scholar] [CrossRef]
- Liang, J.; Liu, Y.; Liu, J.; Li, Z.; Fan, Q.; Jiang, Z.; Yan, F.; Wang, Z.; Huang, P.; Feng, N. Chitosan-functionalized lipid-polymer hybrid nanoparticles for oral delivery of silymarin and enhanced lipid-lowering effect in NAFLD. J. Nanobiotechnol. 2018, 16, 64. [Google Scholar] [CrossRef] [PubMed]
- Aboshanab, M.H.; El-Nabarawi, M.A.; Teaima, M.H.; El-Nekeety, A.A.; Abdel-Aziem, S.H.; Hassan, N.S.; Abdel-Wahhab, M.A. Fabrication, characterization and biological evaluation of silymarin nanoparticles against carbon tetrachloride-induced oxidative stress and genotoxicity in rats. Int. J. Pharm. 2020, 587, 119639. [Google Scholar] [CrossRef] [PubMed]
- Salehi, B.; Fokou, P.V.; Sharifi-Rad, M.; Zucca, P.; Pezzani, R.; Martins, N.; Sharifi-Rad, J. The therapeutic potential of naringenin: A review of clinical trials. Pharmaceuticals 2019, 12, 11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bhia, M.; Motallebi, M.; Abadi, B.; Zarepour, A.; Pereira-Silva, M.; Saremnejad, F.; Santo, A.C.; Zarrabi, A.; Melero, A.; Jafari, S.M.; et al. Naringenin nano-delivery systems and their therapeutic applications. Pharmaceutics 2021, 13, 291. [Google Scholar] [CrossRef] [PubMed]
- Rani, N.; Bharti, S.; Krishnamurthy, B.; Bhatia, J.; Sharma, C.; Kamal, M.A.; Ojha, S.; Arya, D.S. Pharmacological properties and therapeutic potential of naringenin: A citrus flavonoid of pharmaceutical promise. Curr. Pharm. Des. 2016, 22, 4341–4359. [Google Scholar] [CrossRef] [PubMed]
- Kanaze, F.I.; Bounartzi, M.I.; Georgarakis, M.; Niopas, I. Pharmacokinetics of the citrus flavanone aglycones hesperetin and naringenin after single oral administration in human subjects. Eur. J. Clin. Nutr. 2007, 61, 472–477. [Google Scholar] [CrossRef] [Green Version]
- Yen, F.L.; Wu, T.H.; Lin, L.T.; Cham, T.M.; Lin, C.C. Naringenin-loaded nanoparticles improve the physicochemical properties and the hepatoprotective effects of naringenin in orally-administered rats with CCl4-induced acute liver failure. Pharm. Res. 2009, 26, 893–902. [Google Scholar] [CrossRef] [PubMed]
- Maity, S.; Mukhopadhyay, P.; Kundu, P.P.; Chakraborti, A.S. Alginate coated chitosan core-shell nanoparticles for efficient oral delivery of naringenin in diabetic animals—An in vitro and in vivo approach. Carbohydr. Polym. 2017, 170, 124–132. [Google Scholar] [CrossRef]
- Kumar, S.P.; Birundha, K.; Kaveri, K.; Devi, K.R. Antioxidant studies of chitosan nanoparticles containing naringenin and their cytotoxicity effects in lung cancer cells. Int. J. Biol. Macromol. 2015, 78, 87–95. [Google Scholar] [CrossRef] [PubMed]
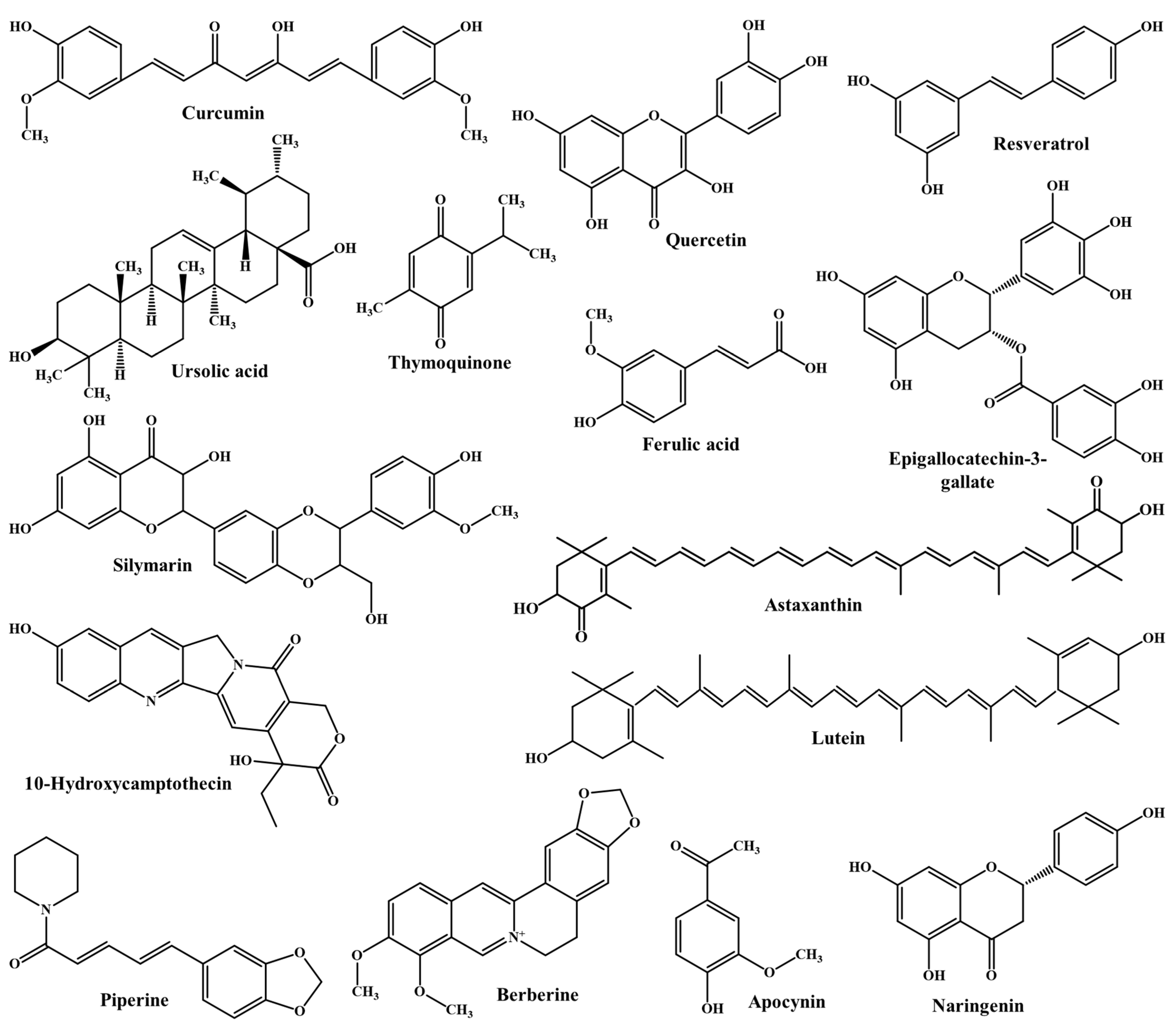

| Phytochemicals | Molecular Weight (g/mol) | Aqueous Solubility (mg/mL) | logP Value | pKa Value (Strongest Acidic) | pKa Value (Strongest Basic) | Ref. |
|---|---|---|---|---|---|---|
| Curcumin | 368.4 | 0.00575 | 4.12 | 9.06 | –4.4 | [24] |
| Quercetin | 302.236 | 0.261 | 2.16 | 6.44 | –4 | [25] |
| Resveratrol | 228.25 | 0.0688 | 3.4 | 8.49 | –6.2 | [26] |
| Thymoquinone | 164.201 | <1 | 2.55 | - | –7.7 | [27] |
| Epigallocatechin-3-gallate | 458.372 | 0.871 | 3.08 | 8.73 | –3.3 | [28] |
| Ursolic acid | 456.7 | 0.00059 | 6.58 | 4.74 | –0.84 | [29] |
| Ferulic acid | 194.18 | 0.906 | 1.67 | 3.77 | –4.9 | [30] |
| 10-Hydroxycamptothecin | 392.404 | 0.331 | 1.69 | 9.65 | 3.17 | [31] |
| Apocynin | 166.174 | 3.04 | 1.62 | 8.27 | –4.9 | [32] |
| Astaxanthin | 596.841 | 0.000667 | 8.05 | 13.07 | –3.5 | [33] |
| Berberine | 336.3612 | 0.000354 | 3.6 | 15 | –4.4 | [34] |
| Piperine | 285.35 | 0.149 | 3.38 | 12.21 | –0.13 | [35] |
| Lutein | 568.871 | 0.000732 | 8.55 | 18.22 | –0.91 | [36] |
| Silymarin | 482.44 | 0.0926 | 2.63 | 7.75 | –3 | [37] |
| Naringenin | 272.257 | 0.214 | 2.84 | 7.91 | –3.9 | [38] |
| Biological Activity | Discussion | Ref. |
|---|---|---|
| Antibacterial | CS shows strong antibacterial activity against Gram-positive bacteria (such as Staphylococcus aureus, Corynebacterium, Staphylococcus epidermidis, Enterococcus faecalis) as well as Gram-negative bacteria (such as Escherichia coli, Pseudomonas aeruginosa, Proteus mirabilis, Salmonella enteritidis, Enterobacter aerogenes), as a result of its polycationic structure. | [39] |
| Antiviral | The soluble degraded product of CS can effectively inhibit Lucerne mosaic virus and tobacco mosaic virus. | [40,41] |
| Antifungal | CS derivatives with a large charge density can effectively inhibit different fungi such as Candida albicans and Candida parapsilosis. | [39,42] |
| Wound healing | Pure CS is widely used as a wound dressing material due to its excellent wound healing activity. | [39,43] |
| Anticancer | Low-molecular weight CS and chito-olegosaccharide could significantly reduce tumor growth. | [44,45,46] |
| Anti-inflammatory | CS shows anti-inflammatory activity by inhibiting the production of cytokines and keratinocytes. | [47] |
| Immunostimulatory | CS and CS derivatives effectively activate antigen-presenting cells by different mechanisms and induce cytokine stimulation to produce an effective immune response. | [48] |
| Advantages of CS-NPs | Limitations of CS-NPs |
|---|---|
|
|
|
|
|
|
|
|
|
|
|
|
| |
| |
|
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Imam, S.S.; Alshehri, S.; Ghoneim, M.M.; Zafar, A.; Alsaidan, O.A.; Alruwaili, N.K.; Gilani, S.J.; Rizwanullah, M. Recent Advancement in Chitosan-Based Nanoparticles for Improved Oral Bioavailability and Bioactivity of Phytochemicals: Challenges and Perspectives. Polymers 2021, 13, 4036. https://doi.org/10.3390/polym13224036
Imam SS, Alshehri S, Ghoneim MM, Zafar A, Alsaidan OA, Alruwaili NK, Gilani SJ, Rizwanullah M. Recent Advancement in Chitosan-Based Nanoparticles for Improved Oral Bioavailability and Bioactivity of Phytochemicals: Challenges and Perspectives. Polymers. 2021; 13(22):4036. https://doi.org/10.3390/polym13224036
Chicago/Turabian StyleImam, Syed Sarim, Sultan Alshehri, Mohammed M. Ghoneim, Ameeduzzafar Zafar, Omar Awad Alsaidan, Nabil K. Alruwaili, Sadaf Jamal Gilani, and Md. Rizwanullah. 2021. "Recent Advancement in Chitosan-Based Nanoparticles for Improved Oral Bioavailability and Bioactivity of Phytochemicals: Challenges and Perspectives" Polymers 13, no. 22: 4036. https://doi.org/10.3390/polym13224036
APA StyleImam, S. S., Alshehri, S., Ghoneim, M. M., Zafar, A., Alsaidan, O. A., Alruwaili, N. K., Gilani, S. J., & Rizwanullah, M. (2021). Recent Advancement in Chitosan-Based Nanoparticles for Improved Oral Bioavailability and Bioactivity of Phytochemicals: Challenges and Perspectives. Polymers, 13(22), 4036. https://doi.org/10.3390/polym13224036









