An Outbreak in Pigeons Caused by the Subgenotype VI.2.1.2 of Newcastle Disease Virus in Brazil
Abstract
:1. Introduction
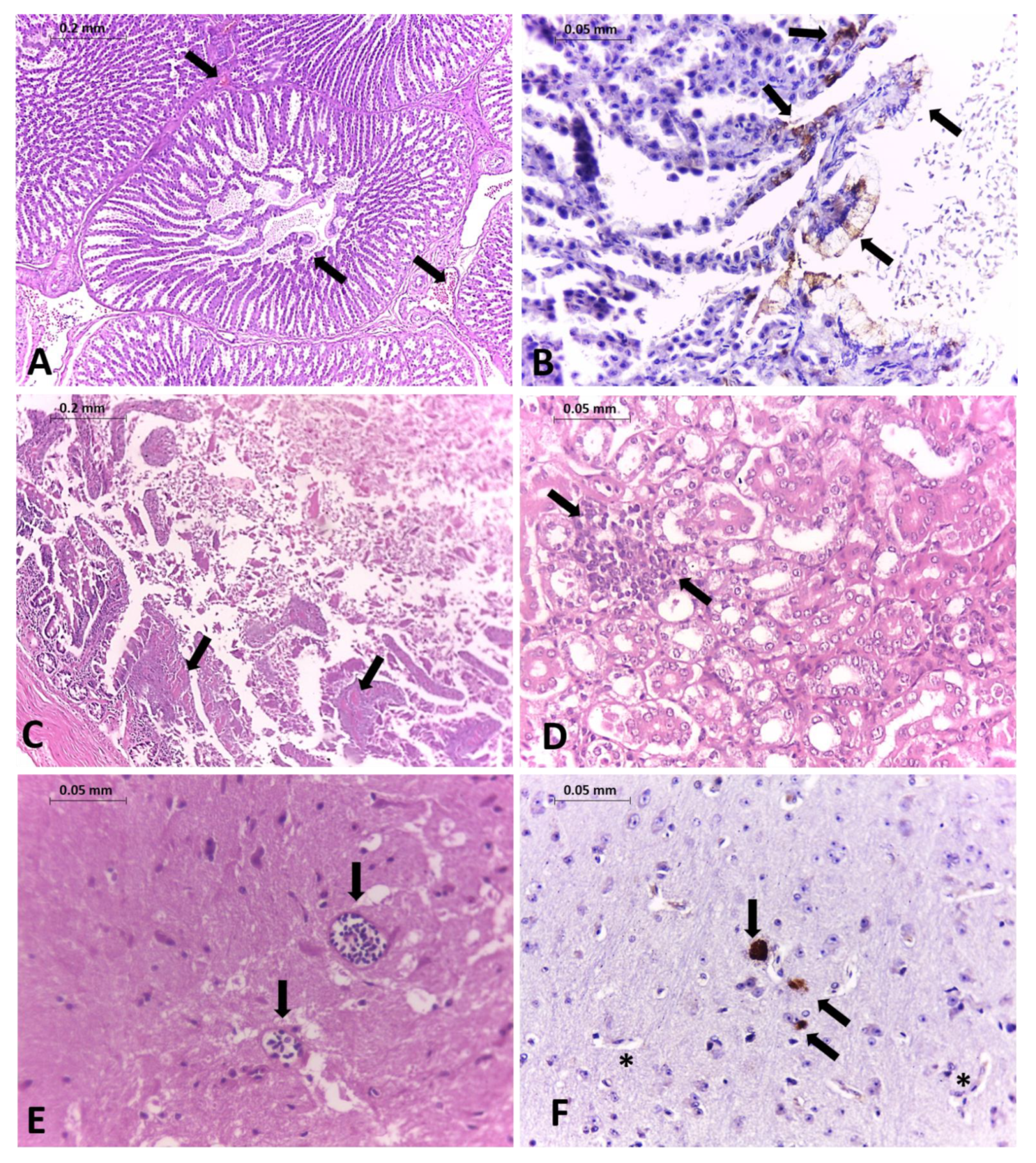
2. Case Report
3. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Amarasinghe, G.K.; Ayllon, M.A.; Bao, Y.; Basler, C.F.; Bavari, S.; Blasdell, K.R.; Briese, T.; Brown, P.A.; Bukreyev, A.; Balkema-Buschmann, A.; et al. Taxonomy of the order Mononegavirales: Update 2019. Arch. Virol. 2019, 164, 1967–1980. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Czegledi, A.; Ujvari, D.; Somogyi, E.; Wehmann, E.; Werner, O.; Lomniczi, B. Third genome size category of avian paramyxovirus serotype 1 (Newcastle disease virus) and evolutionary implications. Virus Res. 2006, 120, 36–48. [Google Scholar] [CrossRef]
- Dimitrov, K.M.; Abolnik, C.; Afonso, C.L.; Albina, E.; Bahl, J.; Berg, M.; Briand, F.X.; Brown, I.H.; Choi, K.S.; Chvala, I.; et al. Updated unified phylogenetic classification system and revised nomenclature for Newcastle disease virus. Infect. Genet. Evol. 2019, 74, 103917. [Google Scholar] [CrossRef]
- Miller, P.J.; Koch, G. Newcastle Disease. In Diseases of Poultry, 13th ed.; Swayne, D., Ed.; John Wiley & Sons, Inc.: Ames, IA, USA, 2013; pp. 89–107, 120–130. [Google Scholar]
- OIE (Ed.) Chapter 3.3.14 Newcastle Disease (infection with Newcastle disease virus). In Manual of Diagnostic Tests and Vaccines for Terrestrial Animals; Biological Standards Commission, World Organization for Animal Health: Paris, France, 2019. [Google Scholar]
- Dimitrov, K.M.; Afonso, C.L.; Yu, Q.; Miller, P.J. Newcastle disease vaccines-A solved problem or a continuous challenge? Vet. Microbiol. 2017, 206, 126–136. [Google Scholar] [CrossRef]
- Collins, M.S.; Alexander, D.J.; Brockman, S.; Kemp, P.A.; Manvell, R.J. Evaluation of mouse monoclonal antibodies raised against an isolate of the variant avian paramyxovirus type 1 responsible for the current panzootic in pigeons. Arch. Virol. 1989, 104, 53–61. [Google Scholar] [CrossRef] [PubMed]
- Ujvari, D.; Wehmann, E.; Kaleta, E.F.; Werner, O.; Savic, V.; Nagy, E.; Czifra, G.; Lomniczi, B. Phylogenetic analysis reveals extensive evolution of avian paramyxovirus type 1 strains of pigeons (Columba livia) and suggests multiple species transmission. Virus Res. 2003, 96, 63–73. [Google Scholar] [CrossRef]
- Meulemans, G.; van den Berg, T.P.; Decaesstecker, M.; Boschmans, M. Evolution of pigeon Newcastle disease virus strains. Avian Pathol. 2002, 31, 515–519. [Google Scholar] [CrossRef] [PubMed]
- Schuler, K.L.; Green, D.E.; Justice-Allen, A.E.; Jaffe, R.; Cunningham, M.; Thomas, N.J.; Spalding, M.G.; Ip, H.S. Expansion of an exotic species and concomitant disease outbreaks: Pigeon paramyxovirus in free-ranging Eurasian collared doves. EcoHealth 2012, 9, 163–170. [Google Scholar] [CrossRef]
- Bari, F.D.; Gelaye, E.; Tekola, B.G.; Harder, T.; Beer, M.; Grund, C. Antigenic and Molecular Characterization of Virulent Newcastle Disease Viruses Circulating in Ethiopia Between 1976 and 2008. Vet. Med. 2021, 12, 129–140. [Google Scholar] [CrossRef]
- Dodovski, A.; Cvetkovikj, I.; Krstevski, K.; Naletoski, I.; Savic, V. Characterization and Epidemiology of Pigeon Paramyxovirus Type-1 Viruses (PPMV-1) Isolated in Macedonia. Avian Dis. 2017, 61, 146–152. [Google Scholar] [CrossRef] [Green Version]
- Ferreira, H.L.; Taylor, T.L.; Dimitrov, K.M.; Sabra, M.; Afonso, C.L.; Suarez, D.L. Virulent Newcastle disease viruses from chicken origin are more pathogenic and transmissible to chickens than viruses normally maintained in wild birds. Vet. Microbiol. 2019, 235, 25–34. [Google Scholar] [CrossRef] [PubMed]
- Kommers, G.D.; King, D.J.; Seal, B.S.; Brown, C.C. Pathogenesis of chicken-passaged Newcastle disease viruses isolated from chickens and wild and exotic birds. Avian Dis. 2003, 47, 319–329. [Google Scholar] [CrossRef]
- Prajna, N.V.; Lalitha, P.; Chen, C.; Zhong, L.; Lietman, T.M.; Doan, T.; Seitzman, G.D. Acute Keratoconjunctivitis Resulting from Coinfection with Avian Newcastle Virus and Human Adenovirus. Cornea 2021. [Google Scholar] [CrossRef]
- Kuiken, T.; Breitbart, M.; Beer, M.; Grund, C.; Hoper, D.; van den Hoogen, B.; Kerkhoffs, J.H.; Kroes, A.C.M.; Rosario, K.; van Run, P.; et al. Zoonotic Infection With Pigeon Paramyxovirus Type 1 Linked to Fatal Pneumonia. J. Infect. Dis. 2018, 218, 1037–1044. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Goebel, S.J.; Taylor, J.; Barr, B.C.; Kiehn, T.E.; Castro-Malaspina, H.R.; Hedvat, C.V.; Rush-Wilson, K.A.; Kelly, C.D.; Davis, S.W.; Samsonoff, W.A.; et al. Isolation of avian paramyxovirus 1 from a patient with a lethal case of pneumonia. J. Virol. 2007, 81, 12709–12714. [Google Scholar] [CrossRef] [Green Version]
- Wise, M.G.; Suarez, D.L.; Seal, B.S.; Pedersen, J.C.; Senne, D.A.; King, D.J.; Kapczynski, D.R.; Spackman, E. Development of a real-time reverse-transcription PCR for detection of newcastle disease virus RNA in clinical samples. J. Clin. Microbiol. 2004, 42, 329–338. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sanchez-Seco, M.P.; Rosario, D.; Quiroz, E.; Guzman, G.; Tenorio, A. A generic nested-RT-PCR followed by sequencing for detection and identification of members of the alphavirus genus. J. Virol. Methods 2001, 95, 153–161. [Google Scholar] [CrossRef]
- Johnson, N.; Wakeley, P.R.; Mansfield, K.L.; McCracken, F.; Haxton, B.; Phipps, L.P.; Fooks, A.R. Assessment of a novel real-time pan-flavivirus RT-polymerase chain reaction. Vector Borne Zoonotic Dis. 2010, 10, 665–671. [Google Scholar] [CrossRef] [PubMed]
- Eiden, M.; Vina-Rodriguez, A.; Hoffmann, B.; Ziegler, U.; Groschup, M.H. Two new real-time quantitative reverse transcription polymerase chain reaction assays with unique target sites for the specific and sensitive detection of lineages 1 and 2 West Nile virus strains. J. Vet. Diagn. Investig. 2010, 22, 748–753. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Z.; Liu, D.; Sun, W.; Liu, J.; He, L.; Hu, J.; Gu, M.; Wang, X.; Liu, X.; Hu, S.; et al. Multiplex one-step Real-time PCR by Taqman-MGB method for rapid detection of pan and H5 subtype avian influenza viruses. PLoS ONE 2017, 12, e0178634. [Google Scholar] [CrossRef]
- Nei, M.; Kumar, S. Molecular Evolution and Phylogenetics; Oxford University Press: New York, NY, USA, 2000; p. 333. [Google Scholar]
- Kumar, S.; Stecher, G.; Tamura, K. MEGA7: Molecular Evolutionary Genetics Analysis Version 7.0 for Bigger Datasets. Mol. Biol. Evol. 2016, 33, 1870–1874. [Google Scholar] [CrossRef] [Green Version]
- Tamura, K.; Nei, M.; Kumar, S. Prospects for inferring very large phylogenies by using the neighbor-joining method. Proc. Natl. Acad. Sci. USA 2004, 101, 11030–11035. [Google Scholar] [CrossRef] [Green Version]
- Ferreira, H.L.; Taylor, T.L.; Absalon, A.E.; Dimitrov, K.M.; Cortes-Espinosa, D.V.; Butt, S.L.; Marin-Cruz, J.L.; Goraichuk, I.V.; Volkening, J.D.; Suarez, D.L.; et al. Presence of Newcastle disease viruses of sub-genotypes Vc and VIn in backyard chickens and in apparently healthy wild birds from Mexico in 2017. Virus Genes 2019, 55, 479–489. [Google Scholar] [CrossRef]
- Xiang, B.; Chen, L.; Cai, J.; Liang, J.; Lin, Q.; Xu, C.; Ding, C.; Liao, M.; Ren, T. Insights into Genomic Epidemiology, Evolution, and Transmission Dynamics of Genotype VII of Class II Newcastle Disease Virus in China. Pathogens 2020, 9, 837. [Google Scholar] [CrossRef] [PubMed]
- Nooruzzaman, M.; Barman, L.R.; Mumu, T.T.; Chowdhury, E.H.; Dimitrov, K.M.; Islam, M.R. A Pigeon-Derived Sub-Genotype XXI.1.2 Newcastle Disease Virus from Bangladesh Induces High Mortality in Chickens. Viruses 2021, 13, 1520. [Google Scholar] [CrossRef]
- Souza, S.P.; Fredo, G.; Dupont, P.; Leite-Filho, R.; Pavarini, S.C.C.; Driemeier, D. Pathological and molecular findings of avian avulavirus Type 1 outbreak in pigeons (Columba livia) of southern Brazil. Pesqui. Vet. Bras. 2018, 38, 2254–2261. [Google Scholar] [CrossRef]
- Haag-Wackernagel, D.; Moch, H. Health hazards posed by feral pigeons. J. Infect. 2004, 48, 307–313. [Google Scholar] [CrossRef] [PubMed]

| Sample ID | Type of Sample | Collection Date | Sent to | Ct Values NDV M Gene | Ct Values NDV F Gene |
|---|---|---|---|---|---|
| CCZ001 | CS | 24/07/2019 | LVCM-USP | 26.5 | 24.7 |
| CCZ002 | CS | 24/07/2019 | LVCM-USP | 18.4 | 29.4 |
| CCZ002 | OS | 24/07/2019 | LVCM-USP | 15.1 | 35.3 |
| CCZ003 | CS | 31/07/2019 | LVCM-USP | 19.5 | 27.1 |
| CCZ004 | CS | 05/08/2019 | LVCM-USP | 21.6 | Negative |
| CCZ005 | CS | 05/08/2019 | LVCM-USP | 22.1 | 30.9 |
| CCZ006 | CS | 05/08/2019 | LVCM-USP | 35.1 | Negative |
| CCZ007 | CS | 06/08/2019 | LVCM-USP | 20.5 | 28.8 |
| CCZ0030 | LI | 08/08/2019 | FMVZ/LVCM-USP | 23.9 | 29.2 |
| CCZ0031 | CS | 12/08/2019 | FMVZ/LVCM-USP | 24.7 | 30.4 |
| CCZ0031 | LI | 12/08/2019 | FMVZ/LVCM-USP | 27.4 | 39.9 |
| CCZ0031 | OS | 12/08/2019 | FMVZ/LVCM-USP | 28.6 | 34.5 |
| CCZ0031 | TR | 12/08/2019 | FMVZ/LVCM-USP | 36.8 | Negative |
| CCZ0032 | CS | 12/08/2019 | FMVZ/LVCM-USP | 26.7 | 32.6 |
| CCZ0032 | OS | 12/08/2019 | FMVZ/LVCM-USP | Negative | Negative |
| CCZ0033 | CS | 12/08/2019 | FMVZ/LVCM-USP | 22.6 | 28.2 |
| CCZ0033 | OS | 12/08/2019 | FMVZ/LVCM-USP | 26.8 | 32.1 |
| CCZ0034 | CS | 13/08/2019 | FMVZ/LVCM-USP | Negative | Negative |
| CCZ0034 | OS | 13/08/2019 | FMVZ/LVCM-USP | Negative | Negative |
| CCZ0035 | CS | 14/08/2019 | FMVZ/LVCM-USP | 23.4 | 28.8 |
| CCZ0035 | SI | 14/08/2019 | FMVZ/LVCM-USP | 28.9 | 33.9 |
| CCZ0038 | CS | 17/08/2019 | LVCM-USP | Negative | Negative |
| CCZ0038 | LI | 17/08/2019 | LVCM-USP | Negative | Negative |
| CCZ0041 | CS | 20/08/2019 | LVCM-USP | 27.2 | 32.2 |
| CCZ0041 | SI | 20/08/2019 | LVCM-USP | 27.5 | 32.6 |
| CCZ0043 | CS | 20/08/2019 | LVCM-USP | 22.0 | 27.9 |
| CCZ0043 | LI | 20/08/2019 | LVCM-USP | 22.1 | 27.1 |
| LF1 | CS | 10/09/2019 | LFDA-SP | Negative | 35.6 |
| LF2 | CS | 10/09/2019 | LFDA-SP | 19.5 | 32.1 |
| LF3 | CS | 10/09/2019 | LFDA-SP | 22.9 | 34.5 |
| LF4 | CS | 10/09/2019 | LFDA-SP | Negative | Negative |
| LF5 | CS | 10/09/2019 | LFDA-SP | 24.2 | 34.8 |
| LF6 | CS | 10/09/2019 | LFDA-SP | 36.9 | Negative |
| LF7 | CS | 10/09/2019 | LFDA-SP | 23.1 | 35 |
| ID | Strain Name | ID | |||||
|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | ||
| 1 | NDV/pigeon/SP-Brazil/CCZ002/2019 | ||||||
| 2 | KX097024_Pigeon/RS-Brazil/1065/2014 | 97.9 | |||||
| 3 | AY734536_pigeon/Argentina/Capital_3/1997 | 94.3 | 94.9 | ||||
| 4 | JX518532_laughingdove/Kenya/B2/Isiolo/2012 | 92.0 | 92.7 | 94.3 | |||
| 5 | HG424627 pigeon/Nigeria/NIE13/92/2013 | 92.7 | 93.4 | 93.7 | 93.8 | ||
| 6 | HG326604 pigeon/Nigeria/NIE09/1898/2009 | 91.9 | 92.6 | 93.6 | 97.3 | 93.7 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Thomazelli, L.M.; Sinhorini, J.A.; Oliveira, D.B.L.; Knöbl, T.; Bosqueiro, T.C.M.; Sano, E.; Costa, G.C.V.; Monteiro, C.; Dorlass, E.G.; Utecht, N.; et al. An Outbreak in Pigeons Caused by the Subgenotype VI.2.1.2 of Newcastle Disease Virus in Brazil. Viruses 2021, 13, 2446. https://doi.org/10.3390/v13122446
Thomazelli LM, Sinhorini JA, Oliveira DBL, Knöbl T, Bosqueiro TCM, Sano E, Costa GCV, Monteiro C, Dorlass EG, Utecht N, et al. An Outbreak in Pigeons Caused by the Subgenotype VI.2.1.2 of Newcastle Disease Virus in Brazil. Viruses. 2021; 13(12):2446. https://doi.org/10.3390/v13122446
Chicago/Turabian StyleThomazelli, Luciano M., Juliana A. Sinhorini, Danielle B. L. Oliveira, Terezinha Knöbl, Tatiana C. M. Bosqueiro, Elder Sano, Gladyston C. V. Costa, Cairo Monteiro, Erick G. Dorlass, Nathalia Utecht, and et al. 2021. "An Outbreak in Pigeons Caused by the Subgenotype VI.2.1.2 of Newcastle Disease Virus in Brazil" Viruses 13, no. 12: 2446. https://doi.org/10.3390/v13122446
APA StyleThomazelli, L. M., Sinhorini, J. A., Oliveira, D. B. L., Knöbl, T., Bosqueiro, T. C. M., Sano, E., Costa, G. C. V., Monteiro, C., Dorlass, E. G., Utecht, N., Scagion, G. P., Meneguin, C., Silva, L. M. N., Moraes, M. V. S., Bueno, L. M., Reischak, D., Carrasco, A. O. T., Arns, C. W., Ferreira, H. L., & Durigon, E. L. (2021). An Outbreak in Pigeons Caused by the Subgenotype VI.2.1.2 of Newcastle Disease Virus in Brazil. Viruses, 13(12), 2446. https://doi.org/10.3390/v13122446









