A Review on the Production of Light Olefins Using Steam Cracking of Hydrocarbons
Abstract
:1. Introduction
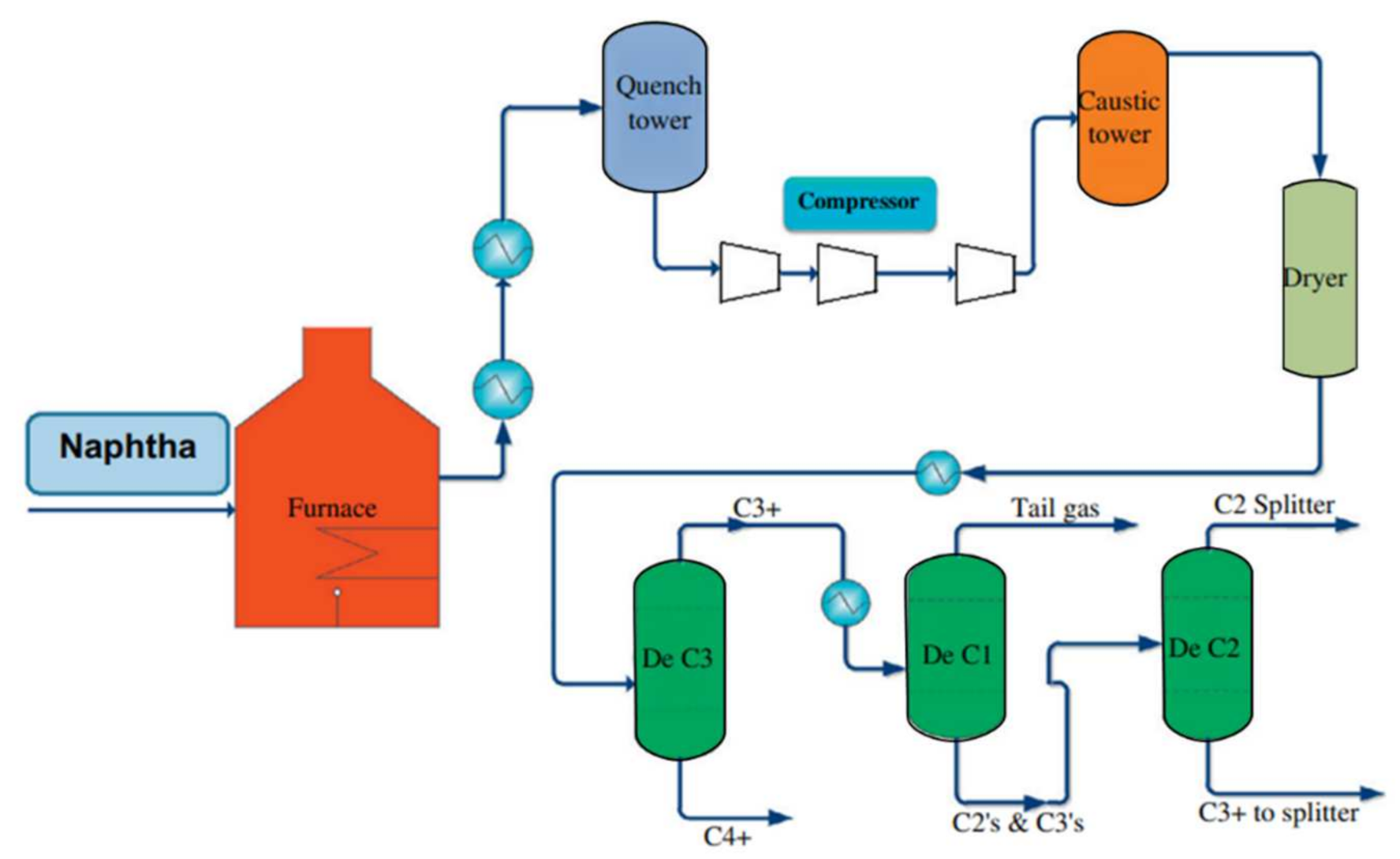
2. Steam Cracking
3. Reaction Mechanism
- Radical reactions
- (1)
- Chain initiation reactions
- (2)
- Chain propagation reactions
- (3)
- Chain termination reactions
- (4)
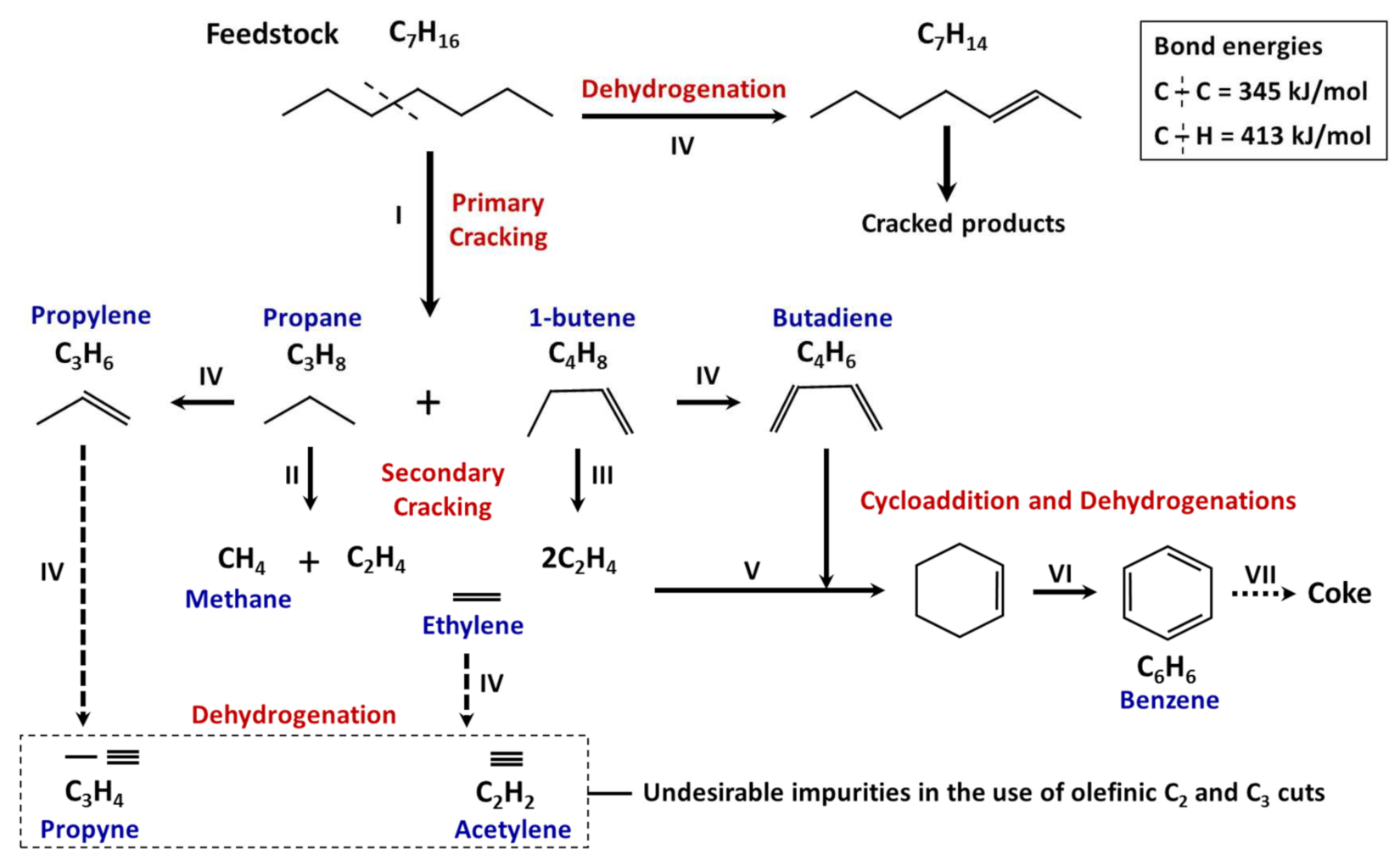
- Secondary reactions
- Molecular reactions
- (1)
- Olefin isomerization
- (2)
- Dehydrogenation reaction
- (3)
- Diels—Alder Molecular reaction
- (4)
- Other molecular reactions
4. Cracking Furnace
4.1. Convection Section
4.2. Radiation Section
4.3. Furnace Draft
5. Effects of Operating Parameters on Olefin Yields
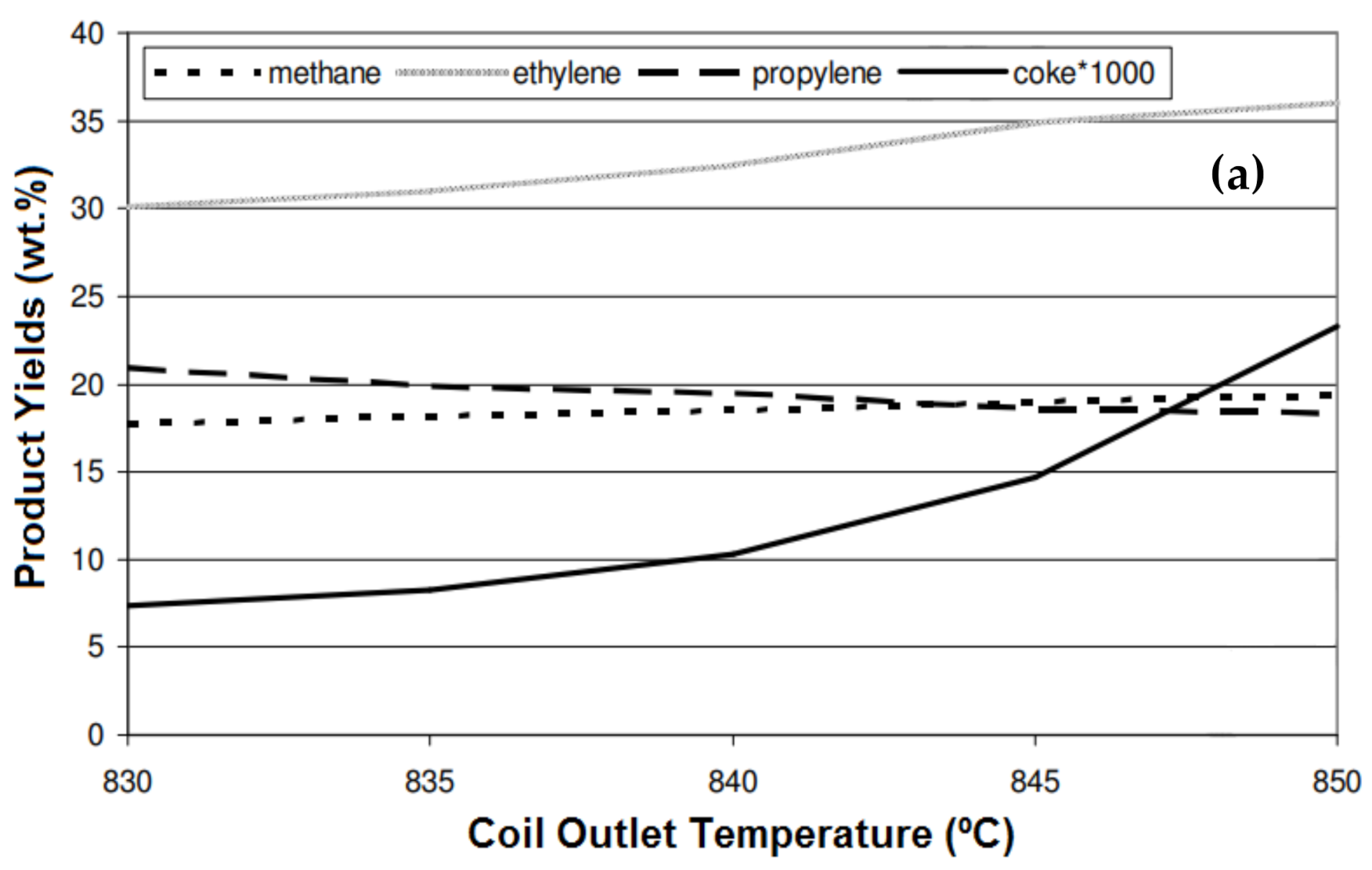
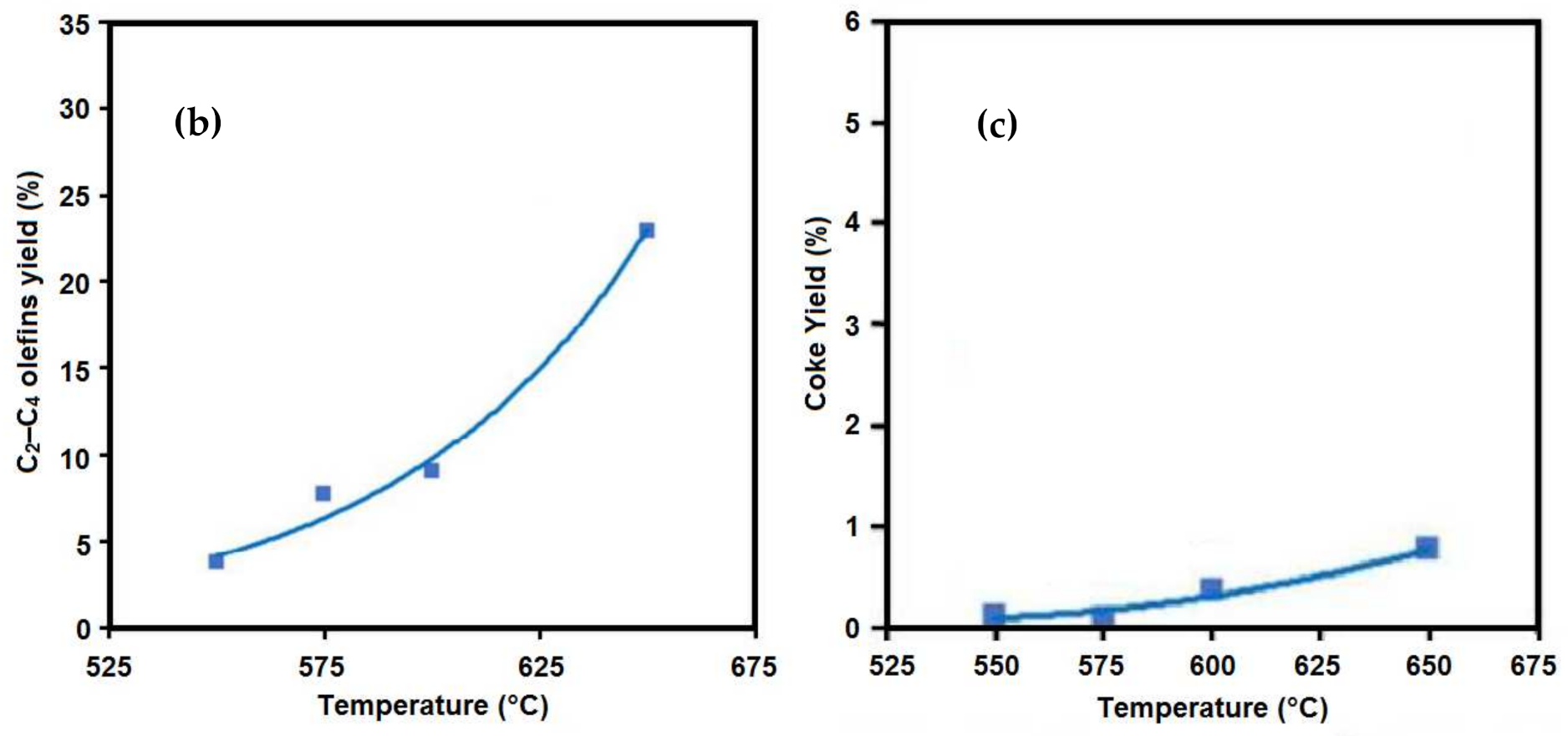
5.1. Temperature
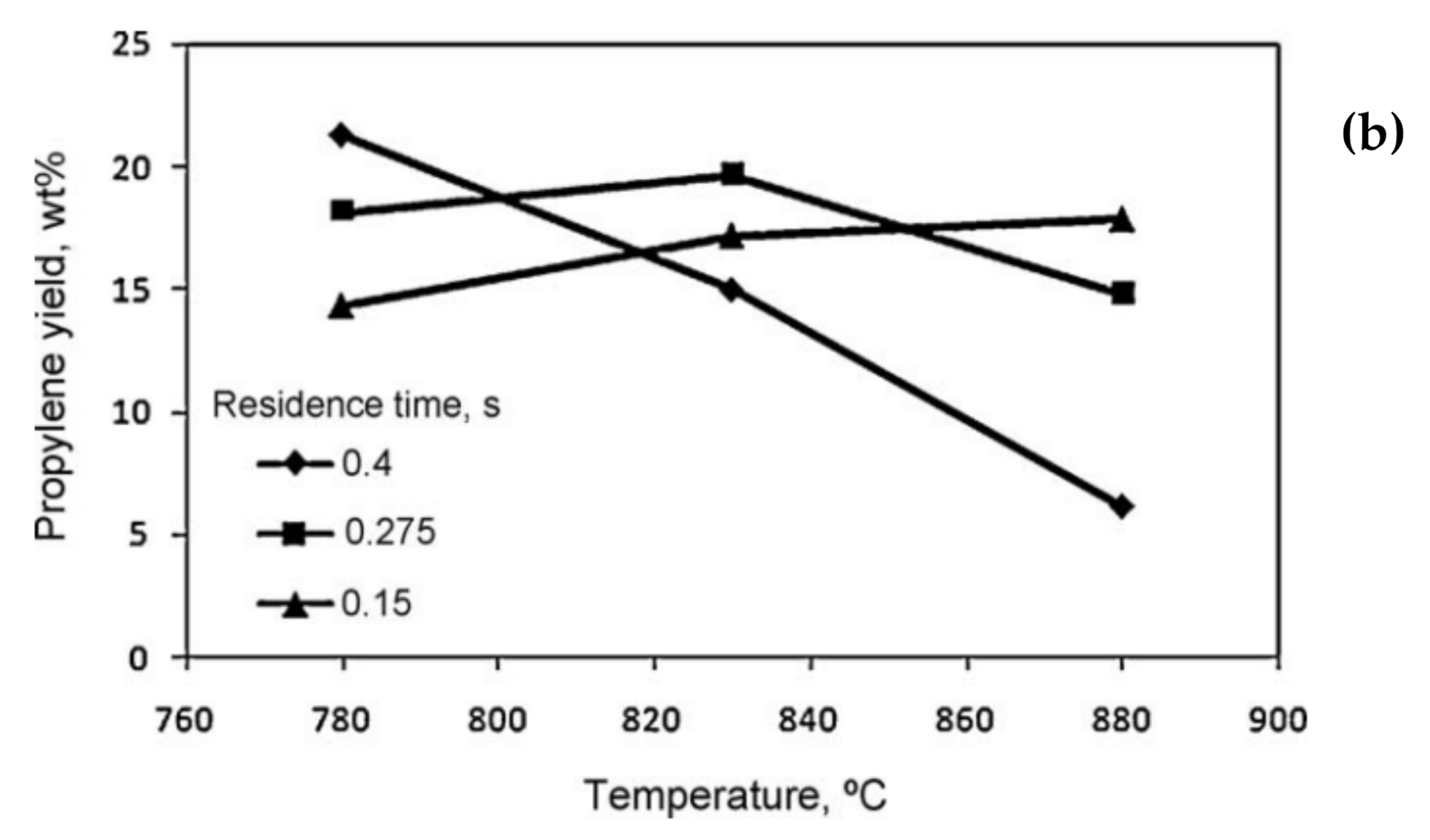
5.2. Residence Time
5.3. Steam-to-Hydrocarbon Ratio
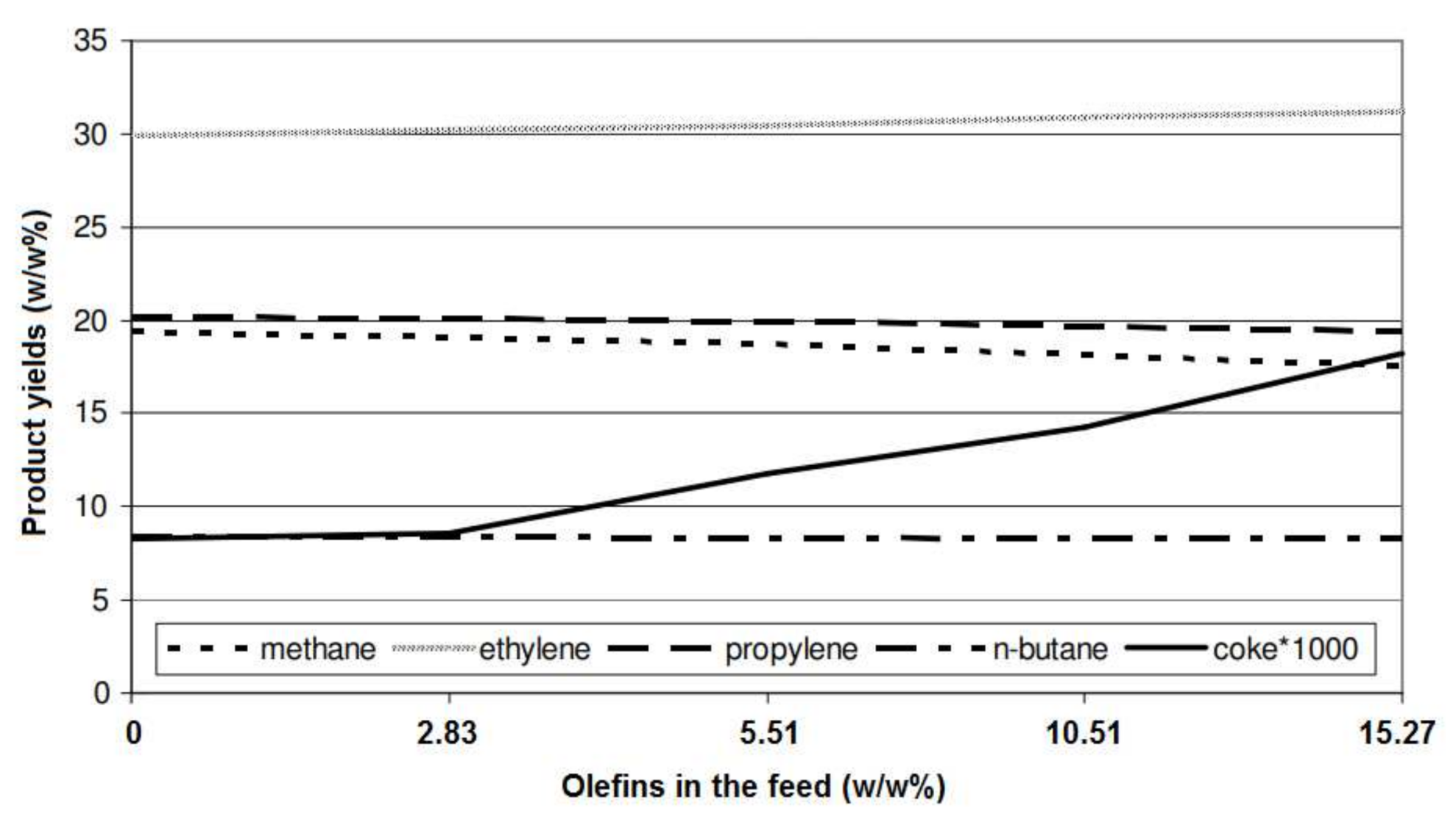
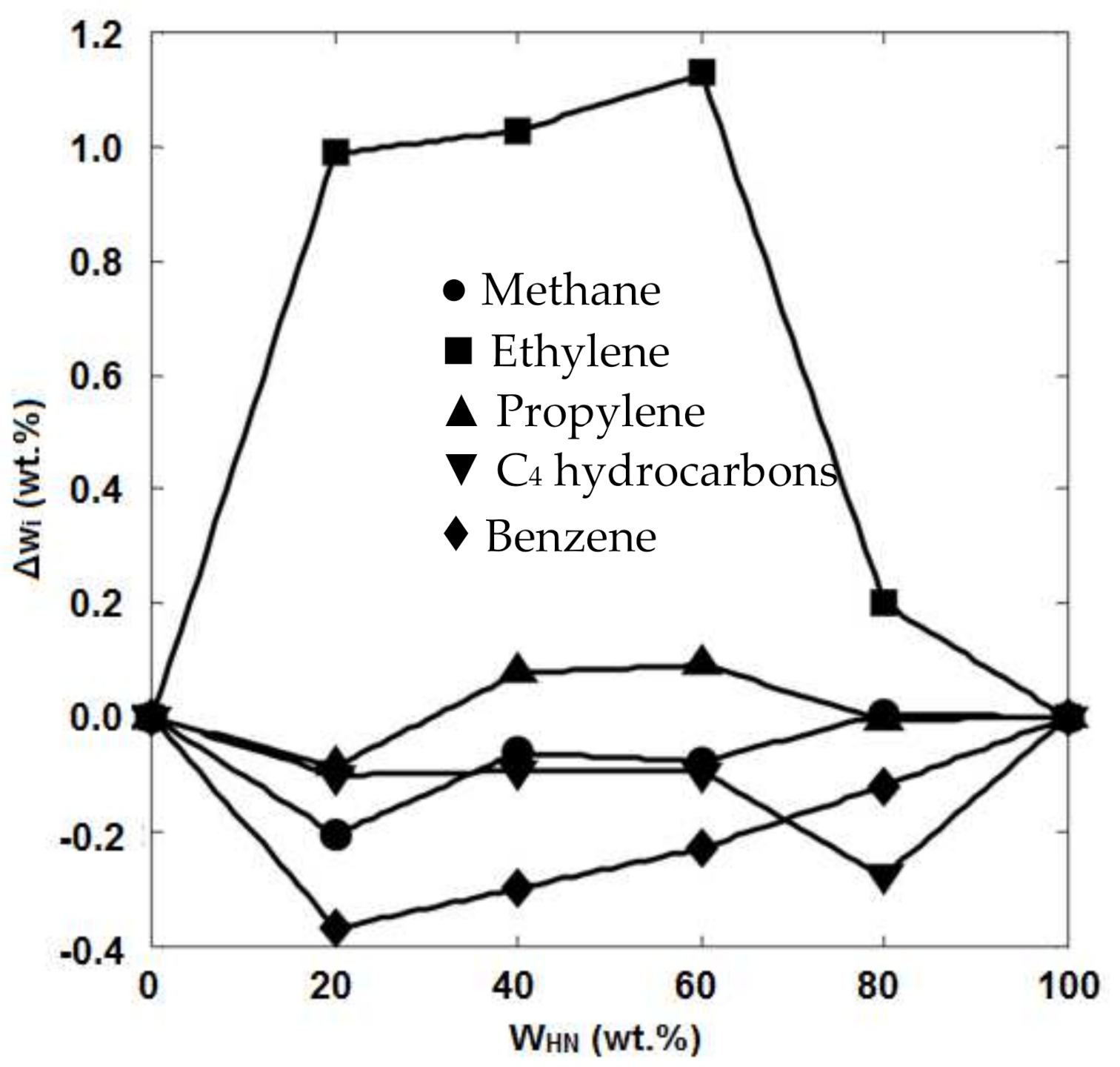
5.4. Feedstock Composition
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Hsu, C.S.; Robinson, P.R. Petroleum Science and Technology; Springer International Publishing: Cham, Switzerland, 2019. [Google Scholar]
- Speight, J.G. (Ed.) Organic Chemistry. In Environmental Organic Chemistry for Engineers; Butterworth-Heinemann: Oxford, UK, 2017; Chapter 2; pp. 43–86. [Google Scholar] [CrossRef]
- Fakhroleslam, M.; Sadrameli, S.M. Thermal Cracking of Hydrocarbons for the Production of Light Olefins; A Review on Optimal Process Design, Operation, and Control. Ind. Eng. Chem. Res. 2020, 59, 12288–12303. [Google Scholar] [CrossRef]
- Deloitte. The Future of Petrochemicals: Growth Surrounded by Uncertainty. 2019. Available online: https://www2.deloitte.com/content/dam/Deloitte/us/Documents/energy-resources/us-the-future-of-petrochemicals.pdf (accessed on 28 June 2021).
- Blay, V.; Louis, B.; Miravalles, R.; Yokoi, T.; Peccatiello, K.A.; Clough, M.; Yilmaz, B. Engineering zeolites for catalytic cracking to light olefins. ACS Catal. 2017, 7, 6542–6566. [Google Scholar] [CrossRef]
- Ren, T.; Patel, M.; Blok, K. Olefins from Conventional and Heavy Feedstocks: Energy use in Steam Cracking and Alternative Processes. Energy 2006, 31, 425–451. [Google Scholar] [CrossRef] [Green Version]
- Amghizar, I.; Vandewalle, L.A.; Van Geem, K.M.; Marin, G.B. New Trends in Olefin Production. Engineering 2017, 3, 171–178. [Google Scholar] [CrossRef]
- Dugkhuntod, P.; Wattanakit, C. A Comprehensive Review of the Applications of Hierarchical Zeolite Nanosheets and Nanoparticle Assemblies in Light Olefin Production. Catalysts 2020, 10, 245. [Google Scholar] [CrossRef] [Green Version]
- Akah, A.; Williams, J.; Ghrami, M. An Overview of Light Olefins Production via Steam Enhanced Catalytic Cracking. Catal. Surv. Asia 2019, 23, 265–276. [Google Scholar] [CrossRef]
- Alotaibi, F.M.; González-Cortés, S.; Alotibi, M.F.; Xiao, T.; Al-Megren, H.; Yang, G.; Edwards, P.P. Enhancing the Production of Light Olefins from Heavy Crude Oils: Turning Challenges into Opportunities. Catal. Today 2018, 317, 86–98. [Google Scholar] [CrossRef]
- Roudgar Saffari, P.; Salarian, H.; Lohrasbi, A.; Salehi, G. The Numerical Simulation of Olefin Production Furnace for Pollution Reduction: Two Case Studies. Gas Process. J. 2021, 9, 15–32. [Google Scholar] [CrossRef]
- Depeyre, D.; Flicoteaux, C.; Chardaire, C. Pure n-hexadecane Thermal Steam Cracking. Ind. Eng. Chem. Process Des. Dev. 1985, 24, 1251–1258. [Google Scholar] [CrossRef]
- Bender, M. An Overview of Industrial Processes for the Production of Olefins—C4 Hydrocarbons. ChemBioEng Rev. 2014, 1, 136–147. [Google Scholar] [CrossRef]
- Zhao, Z.; Jiang, J.; Wang, F. An Economic Analysis of Twenty Light Olefin Production Pathways. J. Energy Chem. 2021, 56, 193–202. [Google Scholar] [CrossRef]
- Gholami, Z.; Gholami, F.; Tišler, Z.; Tomas, M.; Vakili, M. A Review on Production of Light Olefins via Fluid Catalytic Cracking. Energies 2021, 14, 1089. [Google Scholar] [CrossRef]
- Alabdullah, M.; Rodriguez-Gomez, A.; Shoinkhorova, T.; Dikhtiarenko, A.; Chowdhury, A.D.; Hita, I.; Kulkarni, S.R.; Vittenet, J.; Sarathy, S.M.; Castaño, P.; et al. One-Step Conversion of Crude Oil to Light Olefins using a Multi-Zone Reactor. Nat. Catal. 2021, 4, 233–241. [Google Scholar] [CrossRef]
- Zacharopoulou, V.; Lemonidou, A.A. Olefins from biomass intermediates: A review. Catalysts 2018, 8, 2. [Google Scholar] [CrossRef] [Green Version]
- Ren, T.; Patel, M.K. Basic Petrochemicals from Natural Gas, Coal and Biomass: Energy use and CO2 Emissions. Resour. Conserv. Recycl. 2009, 53, 513–528. [Google Scholar] [CrossRef]
- Amghizar, I.; Dedeyne, J.N.; Brown, D.J.; Marin, G.B.; Van Geem, K.M. Sustainable Innovations in Steam Cracking: CO2 Neutral Olefin Production. React. Chem. Eng. 2020, 5, 239–257. [Google Scholar] [CrossRef]
- Seifzadeh Haghighi, S.; Rahimpour, M.R.; Raeissi, S.; Dehghani, O. Investigation of ethylene production in naphtha thermal cracking plant in presence of steam and carbon dioxide. Chem. Eng. J. 2013, 228, 1158–1167. [Google Scholar] [CrossRef]
- Garg, A. Fired Heaters-Key to Efficient Operation of Refineries and Petrochemicals; HarbisonWalker International: Sugar Land, TX, USA, 2017. [Google Scholar]
- Subramanian, R.; Schmidt, L.D. Renewable Olefins from Biodiesel by Autothermal Reforming. Angew. Chem. Int. 2005, 44, 302–305. [Google Scholar] [CrossRef]
- Zimmermann, H.; Walzl, R. Ethylene. In Ullmann’s Encyclopedia of Industrial Chemistry; Wiley: Weinheim, Germany, 2009. [Google Scholar] [CrossRef]
- Geilen, F.M.A.; Stochniol, G.; Peitz, S.; Schulte-Koerne, E. Butenes. In Ullmann’s Encyclopedia of Industrial Chemistry; Wiley: Weinheim, Germany, 2014; pp. 1–13. [Google Scholar] [CrossRef]
- Moreira, J.V. Steam Cracking: Kinetics and Feed Characterisation; Instituto Superior Tecnico: Lisbon, Portugal, 2015; pp. 1–10. Available online: https://fenix.tecnico.ulisboa.pt/downloadFile/1126295043834327/JVM_ExtendedAbstract.pdf (accessed on 3 February 2021).
- Rice, F.O. The Thermal Decomposition of Organic Compounds from the Standpoint of Free Radicals. I. Saturated Hydrocarbons. J. Am. Chem. Soc. 1931, 53, 1959–1972. [Google Scholar] [CrossRef]
- Van Goethem, M.W.M. Next Generation Steam Cracking Reactor Concept. Ph.D. Thesis, Technical University of Delft, Delft, The Netherlands, 2010. [Google Scholar]
- Moulijn, J.A.; Makkee, M.; van Diepen, A.E. Chemical Process Technology, 2nd ed.; John Wiley & Sons: Oxford, UK, 2013. [Google Scholar]
- Depeyre, D.; Flicoteaux, C. Modeling of Thermal Steam Cracking of n-hexadecane. Ind. Eng. Chem. Res. 1991, 30, 1116–1130. [Google Scholar] [CrossRef]
- Sedighi, M.; Keyvanloo, K.; Towfighi, D.J. Olefin Production from Heavy Liquid Hydrocarbon Thermal Cracking: Kinetics and Product Distribution. Iran. J. Chem. Chem. Eng. 2010, 29, 135–147. [Google Scholar] [CrossRef]
- Dente, M.; Ranzi, E.; Goossens, A.G. Detailed Prediction of Olefin Yields from Hydrocarbon Pyrolysis Through a Fundamental Simulation Model (SPYRO). Comput. Chem. Eng. 1979, 3, 61–75. [Google Scholar] [CrossRef]
- Sadrameli, S.M. Thermal/Catalytic Cracking of Hydrocarbons for the Production of Olefins: A State-of-the-Art Review I: Thermal cracking review. Fuel 2015, 140, 102–115. [Google Scholar] [CrossRef]
- Chauvel, A.; Lefebvre, G. Petrochemical Processes 1. In Synthesis-Gas Derivatives and Major Hydrocarbons, 2nd ed.; Editions Technip Paris: Paris, France, 1989. [Google Scholar]
- Van Goethem, M.W.M.; Barendregt, S.; Grievink, J.; Moulijn, J.A.; Verheijen, P.J.T. Model-based, Thermo-Physical Optimisation for High Olefin Yield in Steam Cracking Reactors. Chem. Eng. Res. Des. 2010, 88, 1305–1319. [Google Scholar] [CrossRef]
- Jiang, B.-B.; Liao, Z.-W.; Mohsin, A.; Sun, J.-Y.; Wang, J.-D.; Yang, Y.; Yang, Y.-R. Modeling and Optimization of Ethane Steam Cracking Process in an Industrial Tubular Reactor with Improved Reaction Scheme. China Petrol. Process. Petrochem. Technol. 2020, 22, 117–125. [Google Scholar]
- Karimzadeh, R.; Godini, H.R.; Ghashghaee, M. Flowsheeting of Steam Cracking Furnaces. Chem. Eng. Res. Des. 2009, 87, 36–46. [Google Scholar] [CrossRef]
- Variny, M.; Mierka, O.; Gašparovič, L. Experiences and Future Prospects of GRUCON—SLOVNAFT Cooperation in Energy Auditing Field. In Proceedings of the 42nd International Conference of SSCHE, Tatranské Matliare, Slovakia, 25–29 May 2015; Slovak Society of Chemical Engineering: Bratislava, Slovakia, 2015; pp. 351–358. [Google Scholar]
- Marcos, J.M.M. Modelling of Naphtha Cracking for Olefins Production. Master’s Thesis, Technical University of Lisbon, Lisbon, Portugal, 2016. Available online: https://fenix.tecnico.ulisboa.pt/downloadFile/563345090415270/Thesis_Joao_Marcos_73026.pdf (accessed on 17 April 2021).
- Mahulkar, A.V.; Heynderickx, G.J.; Marin, G.B. Simulation of the Coking Phenomenon in the Superheater of a Steam Cracker. Chem. Eng. Sci. 2014, 110, 31–43. [Google Scholar] [CrossRef]
- Belohlav, Z.; Zamostny, P.; Herink, T. The Kinetic Model of Thermal Cracking for Olefins Production. Chem. Eng. Process. 2003, 42, 461–473. [Google Scholar] [CrossRef]
- Baukal, C.E.; Vaccari, M.; Claxton, M.G. Chapter 20—Burners for Reformers and Cracking Furnaces. Comput. Aided Chem. Eng. 2019, 45, 937–984. [Google Scholar] [CrossRef]
- Wang, Q.; Xu, H.; Pan, L.; Sun, L. Active Disturbance Rejection Control of Boiler Forced Draft System: A Data-Driven Practice. Sustainability 2020, 12, 4171. [Google Scholar] [CrossRef]
- Depeyre, D.; Flicoteaux, C.; Arbabzadeh, F.; Zabaniotou, A. Modeling of Thermal Steam Cracking of an Atomspheric Gas Oil. Ind. Eng. Chem. Res. 1989, 28, 967–976. [Google Scholar] [CrossRef]
- Gál, T.; Lakatos, B.G. Thermal Cracking of Recycled Hydrocarbon Gas-Mixtures for Re-Pyrolysis: Operational Analysis of Some Industrial Furnaces. Appl. Therm. Eng. 2008, 28, 218–225. [Google Scholar] [CrossRef]
- Al-Absi, A.A.; Al-Khattaf, S.S. Conversion of Arabian Light Crude Oil to Light Olefins via Catalytic and Thermal Cracking. Energy Fuels 2018, 32, 8705–8714. [Google Scholar] [CrossRef]
- Che, Y.; Hao, J.; Zhang, J.; Qiao, Y.; Li, D.; Tian, Y. Vacuum Residue Thermal Cracking: Product Yield Determination and Characterization Using Thermogravimetry–Fourier Transform Infrared Spectrometry and a Fluidized Bed Reactor. Energy Fuels 2018, 32, 1348–1357. [Google Scholar] [CrossRef]
- Hulet, C.; Briens, C.; Berruti, F.; Chan, E.W. A Review of Short Residence Time Cracking Processes. Int. J. Chem. React. Eng. 2005, 3, 1–74. [Google Scholar] [CrossRef]
- Keyvanloo, K.; Towfighi, J.; Sadrameli, S.M.; Mohamadalizadeh, A. Investigating the Effect of Key Factors, Their Interactions and Optimization of Naphtha Steam Cracking by Statistical Design of Experiments. J. Anal. Appl. Pyrolysis 2010, 87, 224–230. [Google Scholar] [CrossRef]
- Han, L.; Ding, C.Q.; Lui, H. Studies on Olefin Production by Steam Cracking of Waste Oil Blended with Naphtha. Appl. Mech. Mater. 2013, 291, 738–743. [Google Scholar] [CrossRef]
- Karaba, A.; Rozhon, J.; Patera, J.; Hájek, J.; Zámostný, P. Fischer-Tropsch Wax from Renewable Resources as an Excellent Feedstock for the Steam-Cracking Process. Chem. Eng. Technol. 2021, 44, 329–338. [Google Scholar] [CrossRef]
- Abbasali, S.M.Z.; Farsi, M.; Rahimpour, M.R. Simulation and Dynamic Optimization of an Industrial Naphtha Thermal Cracking Furnace Based on Time Variant Feeding Policy. Chem. Prod. Process Model. 2018, 13, 1–15. [Google Scholar] [CrossRef]
- Abghari, S.Z.; Darian, J.T.; Karimzadeh, R.; Omidkhah, M.R. Determination of Yield Distribution in Olefin Production by Thermal Cracking of Atmospheric Gasoil. Korean J. Chem. Eng. 2008, 25, 681–692. [Google Scholar] [CrossRef]
- Sedighi, M.; Keyvanloo, K.; Towfighi, J. Experimental Study and Optimization of Heavy Liquid Hydrocarbon Thermal Cracking to Light Olefins by Response Surface Methodology. Korean J. Chem. Eng. 2010, 27, 1170–1176. [Google Scholar] [CrossRef]
- Dominov, P.; Gilyazetdinova, R.; Zhirnov, B.; Tarasov, I.; Khlestkin, R. Overview world technologies of pyrolysis and perspective of development. Electron. Sci. J. Oil Gas Bus. 2009, 1, 23–32. Available online: http://ogbus.ru/files/ogbus/eng/authors/Dominov/Dominov_1.pdf (accessed on 29 January 2021).
- Al-Absi, A.A.; Aitani, A.M.; Al-Khattaf, S.S. Thermal and Catalytic Cracking of Whole Crude Oils at High Severity. J. Anal. Appl. Pyrolysis 2020, 145, 104705. [Google Scholar] [CrossRef]
- Geerts, M.; Ristic, N.; Djokic, M.; Ukkandath Aravindakshan, S.; Marin, G.B.; Van Geem, K.M. Crude to Olefins: Effect of Feedstock Composition on Coke Formation in a Bench-Scale Steam Cracking Furnace. Ind. Eng. Chem. Res. 2020, 59, 2849–2859. [Google Scholar] [CrossRef]
- Karaba, A.; Dvořáková, V.; Patera, J.; Zámostný, P. Improving the Steam-Cracking Efficiency of Naphtha Feedstocks by Mixed/Separate Processing. J. Anal. Appl. Pyrolysis 2020, 146, 104768. [Google Scholar] [CrossRef]
- Zámostný, P.; Bělohlav, Z.; Starkbaumová, L.; Patera, J. Experimental Study of Hydrocarbon Structure Effects on the Composition of its Pyrolysis Products. J. Anal. Appl. Pyrolysis 2010, 87, 207–216. [Google Scholar] [CrossRef]
- Hou, X.; Ma, Z.; Chen, B.; Zhang, J.; Ning, Y.; Zhao, L.; Yuan, E. Role of normal/cyclo-alkane in hydrocarbons pyrolysis process and product distribution. J. Anal. Appl. Pyrolysis 2021, 156, 105130. [Google Scholar] [CrossRef]






| No. | Process | Feedstock | Main Products |
|---|---|---|---|
| 1 | Steam cracking of mixed petroleum (PSC) | Naphtha | Ethylene, propylene |
| 2 | Steam cracking of ethane (ESC) | Ethane | Ethylene |
| 3 | Propane dehydrogenation (PDH) | Propane | Propylene |
| 4 | Catalytic pyrolysis process of heavy oil (CPP) | Atmospheric residuum | Ethylene, propylene |
| 5 | Deep catalytic cracking of heavy oil (DCC) | Wax oil | Propylene |
| 6 | Coal gasification produces syngas; methanol is synthesized from syngas and converted into olefins (CMTO) | Coal | Ethylene, propylene |
| 7 | NG reforming produces syngas; methanol is synthesized from syngas and converted into olefins (NMTO) | NG | Ethylene, propylene |
| 8 | Methanol is synthesized from CO2 hydrogenation and converted into olefins (RMTO) | CO2 and H2 | Ethylene, propylene |
| 9 | Biomass gasification produces syngas; methanol is synthesized from syngas and converted into olefins (BMTO) | Bio-waste | Ethylene, propylene |
| 10 | Coal gasification produces syngas; methanol is synthesized from syngas and converted into propylene (CMTP) | Coal | Propylene |
| 11 | NG reforming produce syngas; methanol is synthesized from syngas and converted into propylene (NMTP) | NG | Propylene |
| 12 | Methanol is synthesized from CO2 hydrogenation and converted into propylene (RMTP) | CO2 and H2 | Propylene |
| 13 | Biomass gasification produces syngas; methanol is synthesized from syngas and converted into propylene (BMTP) | Bio-waste | Propylene |
| 14 | Oxidative coupling of methane derived from NG (NOCM) | NG | Ethylene |
| 15 | Oxidative coupling of methane derived from hydrogenation of CO2 (ROCM) | CO2 and H2 | Ethylene |
| 16 | Oxidative coupling of methane derived from biogas (BOCM) | Biomass | Ethylene |
| 17 | Coal gasification produces syngas, and syngas is converted into olefins through FT synthesis (CFTO) | Coal | Ethylene, propylene |
| 18 | NG reforming produces syngas, and syngas is converted into olefins through FT synthesis (NFTO) | NG | Ethylene, propylene |
| 19 | Biomass gasification produces syngas, and syngas is converted into olefins through FT synthesis (BFTO) | Bio-waste | Ethylene, propylene |
| 20 | Ethanol is produced through anaerobic fermentation and converted into ethylene through dehydration (BEDH) | Ethanol | Ethylene |
| Reactor Section | Radiant Coil Type | |||
|---|---|---|---|---|
| A | B | C | D | |
| Radiation zone | 48.1 | 26.3 | 26.8 | 29.2 |
| Adiabatic zone | 48.5 | 27.4 | 29.3 | 30.4 |
| Cooling zone | 47.2 | 27.2 | 29.2 | 31.2 |
| Product | Temperature (°C) | ||
|---|---|---|---|
| 600 | 650 | 700 | |
| Conversion (%) | 90.67 | 91.55 | 93.97 |
| Gas yield (wt.%) | 18.92 | 27.38 | 35.32 |
| Gas composition (wt.%) | |||
| Methane | 2.91 | 5.01 | 9.44 |
| Ethylene | 5.76 | 6.92 | 6.66 |
| Propylene | 4.76 | 6.38 | 6.95 |
| Butenes | 2.19 | 4.02 | 5.12 |
| C2–C4 olefins | 12.71 | 17.32 | 18.73 |
| Olefinicity (%) | 67.16 | 63.26 | 53.03 |
| Coke yield (wt.%) | 8.20 | 8.49 | 9.13 |
| Products Yields (wt.%) | Residence Time (s) | ||
|---|---|---|---|
| 0.22 | 0.30 | 0.51 | |
| Methane | 4.6 | 6.8 | 8.9 |
| Ethane | 1.6 | 1.8 | 2.6 |
| Ethylene | 32.7 | 30.4 | 28.1 |
| Propane | 0.3 | 0.5 | 0.4 |
| Propylene | 15.8 | 15.3 | 13.7 |
| Acetylene | 0.6 | 0.5 | 0.4 |
| i-butane | 0.0 | 0.0 | 0.0 |
| Propadiene | 0.7 | 0.4 | 0.3 |
| n-butane | 0.0 | 0.1 | 0.1 |
| t-2-butene | 0.3 | 0.3 | 0.3 |
| 1-butene | 6.6 | 3.6 | 1.3 |
| i-butene | 1.5 | 1.5 | 1.2 |
| c-2-butene | 0.3 | 0.4 | 0.3 |
| Propyne | 0.5 | 0.5 | 0.4 |
| 1,3-butadiene | 8.7 | 8.1 | 6.3 |
| Cyclopentadiene + isoprene | 3.6 | 3.2 | 2.8 |
| Benzene | 4.2 | 6.6 | 9.8 |
| Toluene | 2.1 | 3.3 | 4.6 |
| Ethylbenzene | 0.4 | 0.4 | 0.3 |
| Xylenes | 1.3 | 1.8 | 2.6 |
| C5–C6 | 4.6 | 3.5 | 2.2 |
| C7–C12 | 2.9 | 2.9 | 3.0 |
| Oil | 6.6 | 8.2 | 10.3 |
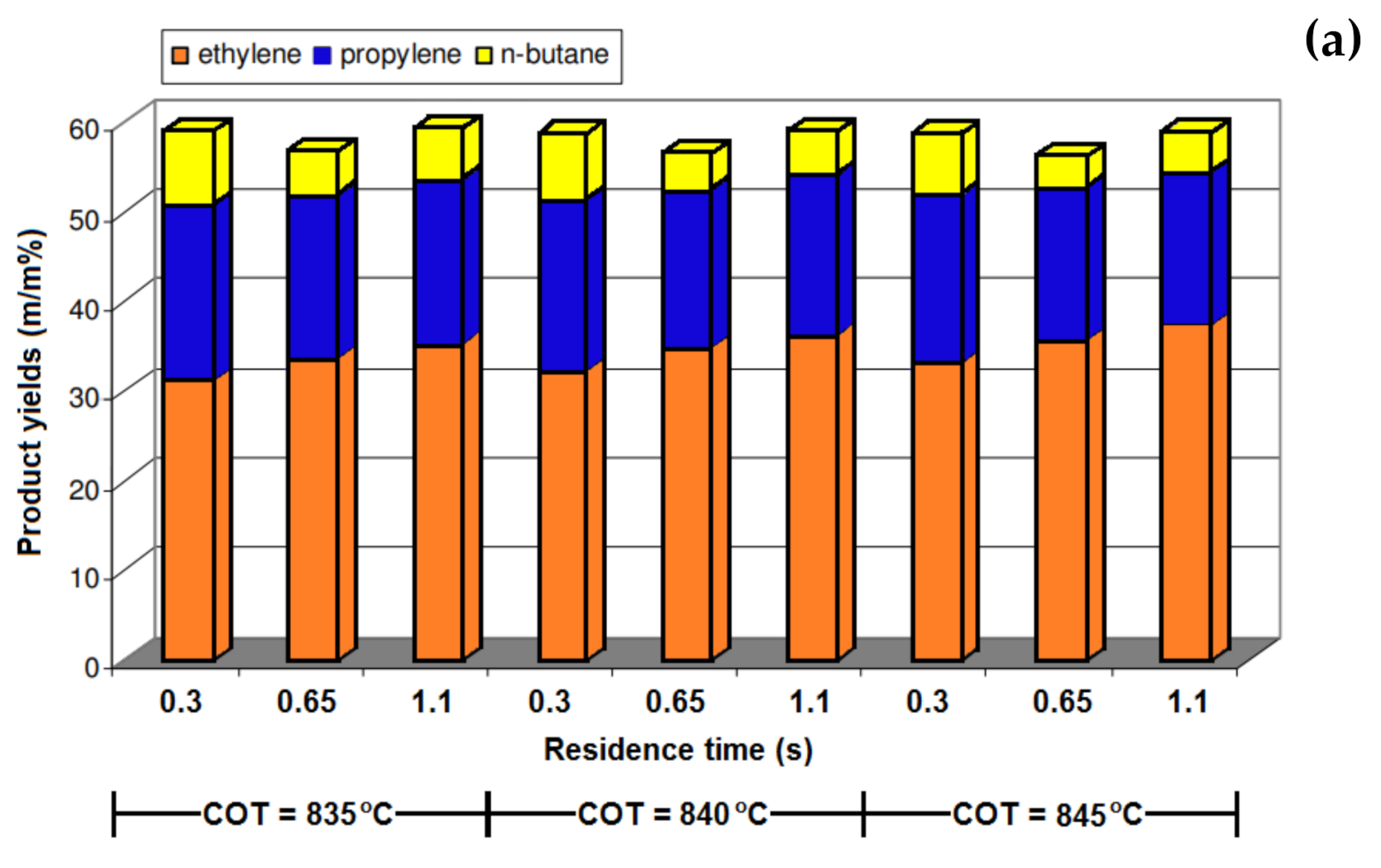
| COT (°C) | 835 | 840 | 835 | 840 | 835 | 840 | 835 | 840 |
|---|---|---|---|---|---|---|---|---|
| Reduction of ST:HC Ratio | 5% | 5% | 10% | 10% | 20% | 20% | 30% | 30% |
| Products | ||||||||
| Methane | 18.22 | 18.75 | 18.32 | 18.72 | 18.53 | 19.06 | 19.27 | 19.89 |
| Ethylene | 32.22 | 33.73 | 32.97 | 33.18 | 31.78 | 32.25 | 30.46 | 30.87 |
| Propylene | 20.63 | 20.15 | 20.68 | 20.34 | 20.79 | 20.32 | 21.14 | 20.87 |
| Butadiene | 3.97 | 3.85 | 3.97 | 3.89 | 3.98 | 3.86 | 3.64 | 3.57 |
| n-butane (Residual) | 8.74 | 7.58 | 8.72 | 7.88 | 8.68 | 7.56 | 8.64 | 7.54 |
| Benzene + Toluene | 1.61 | 1.63 | 1.71 | 1.74 | 1.81 | 1.89 | 1.98 | 2.05 |
| Coke | 0.0084 | 0.0089 | 0.0091 | 0.0097 | 0.0107 | 0.012 | 0.0128 | 0.015 |
| Feed | ASL | AXL | AL | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Temperature (°C) | 600 | 625 | 650 | 600 | 625 | 650 | 600 | 625 | 650 |
| Conversion (%) | 14.5 | 21.1 | 27.6 | 14.0 | 21.6 | 27.0 | 14.2 | 23.3 | 32.8 |
| Product yield (%) | |||||||||
| H2 | 0.1 | 0.1 | 0.1 | 0.1 | 0.1 | 0.1 | 0.1 | 0.1 | 0.2 |
| C1 | 1.5 | 2.5 | 3.6 | 1.7 | 2.8 | 3.5 | 1.8 | 3.1 | 4.6 |
| 2.6 | 4.1 | 6.1 | 3.1 | 5.0 | 6.5 | 2.8 | 5.1 | 7.6 | |
| 3.1 | 5.0 | 6.8 | 3.4 | 5.5 | 6.9 | 3.2 | 5.7 | 8.6 | |
| 3.4 | 4.9 | 6.0 | 3.2 | 4.6 | 5.6 | 3.0 | 4.7 | 6.7 | |
| 9.1 | 14.0 | 18.9 | 9.7 | 15.1 | 19.0 | 9.0 | 15.5 | 22.9 | |
| / | 1.2 | 1.2 | 1.1 | 1.1 | 1.1 | 1.1 | 1.1 | 1.1 | 1.1 |
| Coke | 0.2 | 0.3 | 0.3 | 0.3 | 0.4 | 0.5 | 0.4 | 0.6 | 0.8 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gholami, Z.; Gholami, F.; Tišler, Z.; Vakili, M. A Review on the Production of Light Olefins Using Steam Cracking of Hydrocarbons. Energies 2021, 14, 8190. https://doi.org/10.3390/en14238190
Gholami Z, Gholami F, Tišler Z, Vakili M. A Review on the Production of Light Olefins Using Steam Cracking of Hydrocarbons. Energies. 2021; 14(23):8190. https://doi.org/10.3390/en14238190
Chicago/Turabian StyleGholami, Zahra, Fatemeh Gholami, Zdeněk Tišler, and Mohammadtaghi Vakili. 2021. "A Review on the Production of Light Olefins Using Steam Cracking of Hydrocarbons" Energies 14, no. 23: 8190. https://doi.org/10.3390/en14238190
APA StyleGholami, Z., Gholami, F., Tišler, Z., & Vakili, M. (2021). A Review on the Production of Light Olefins Using Steam Cracking of Hydrocarbons. Energies, 14(23), 8190. https://doi.org/10.3390/en14238190








