Minimally Invasive Therapies for the Management of Dental Caries—A Literature Review
Abstract
:1. Introduction
2. Methods
3. Chemical Management of Caries Using Fluoride Varnish
4. Silver Diamine Fluoride
4.1. Caries-Preventive and Caries-Arresting Efficacy of SDF
4.2. Limitations of SDF
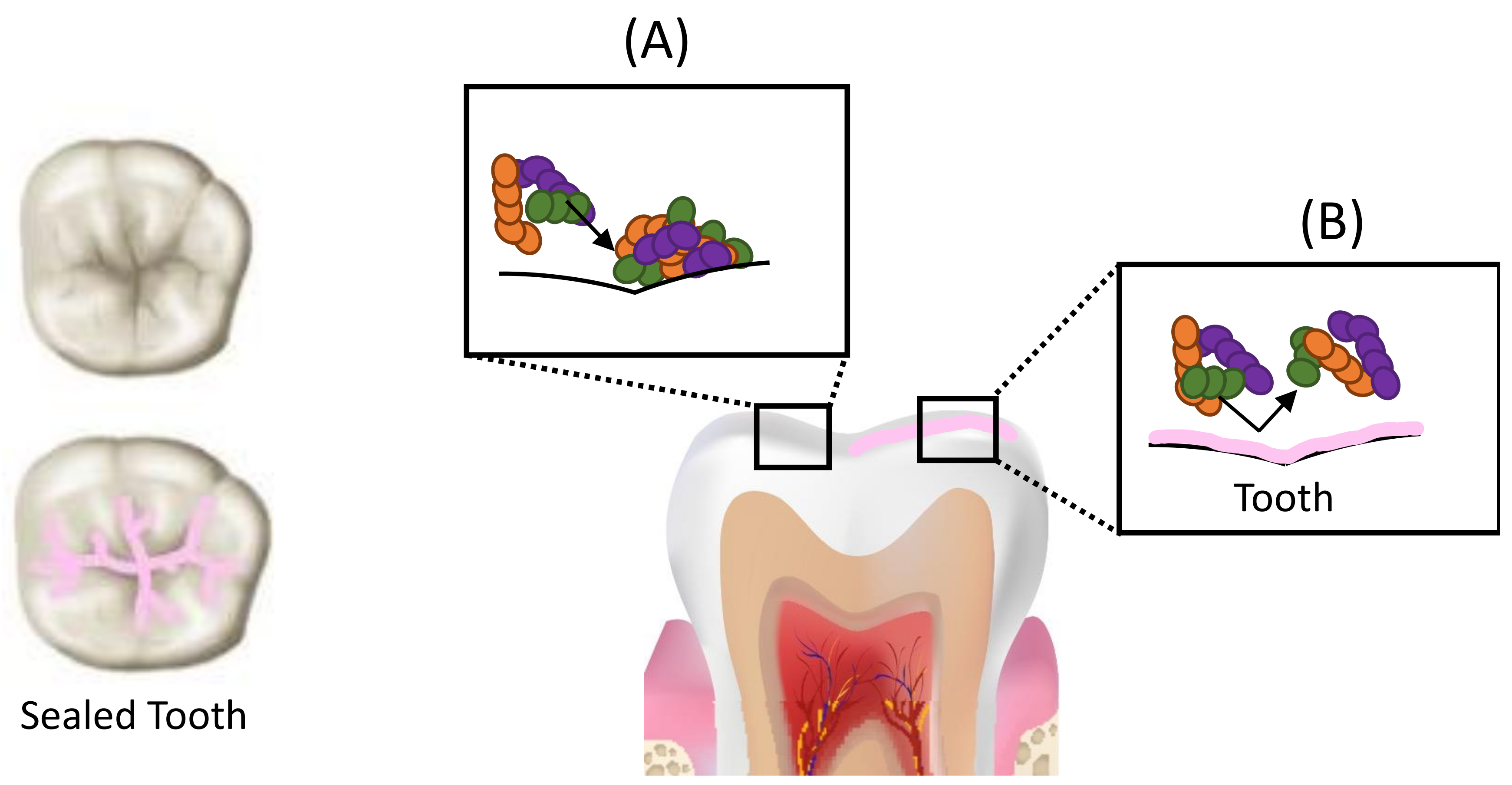
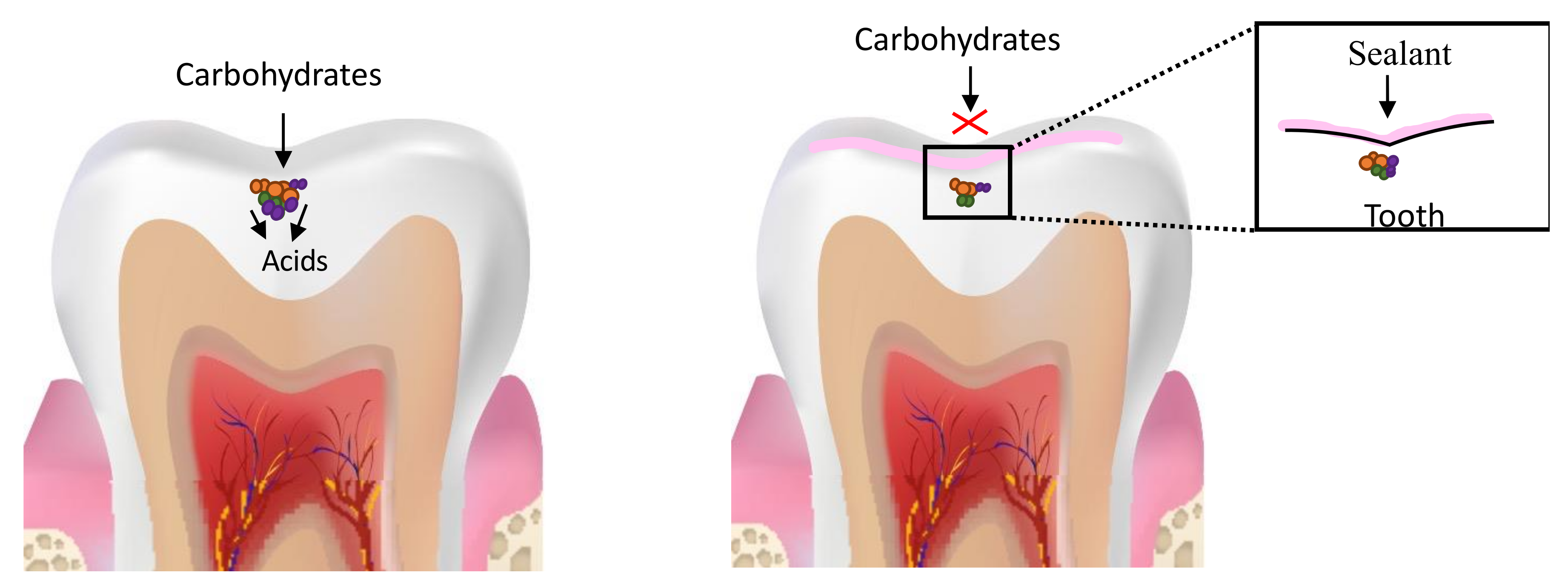
5. Resin-Based Fissure Sealants
Development of Antimicrobial Sealants
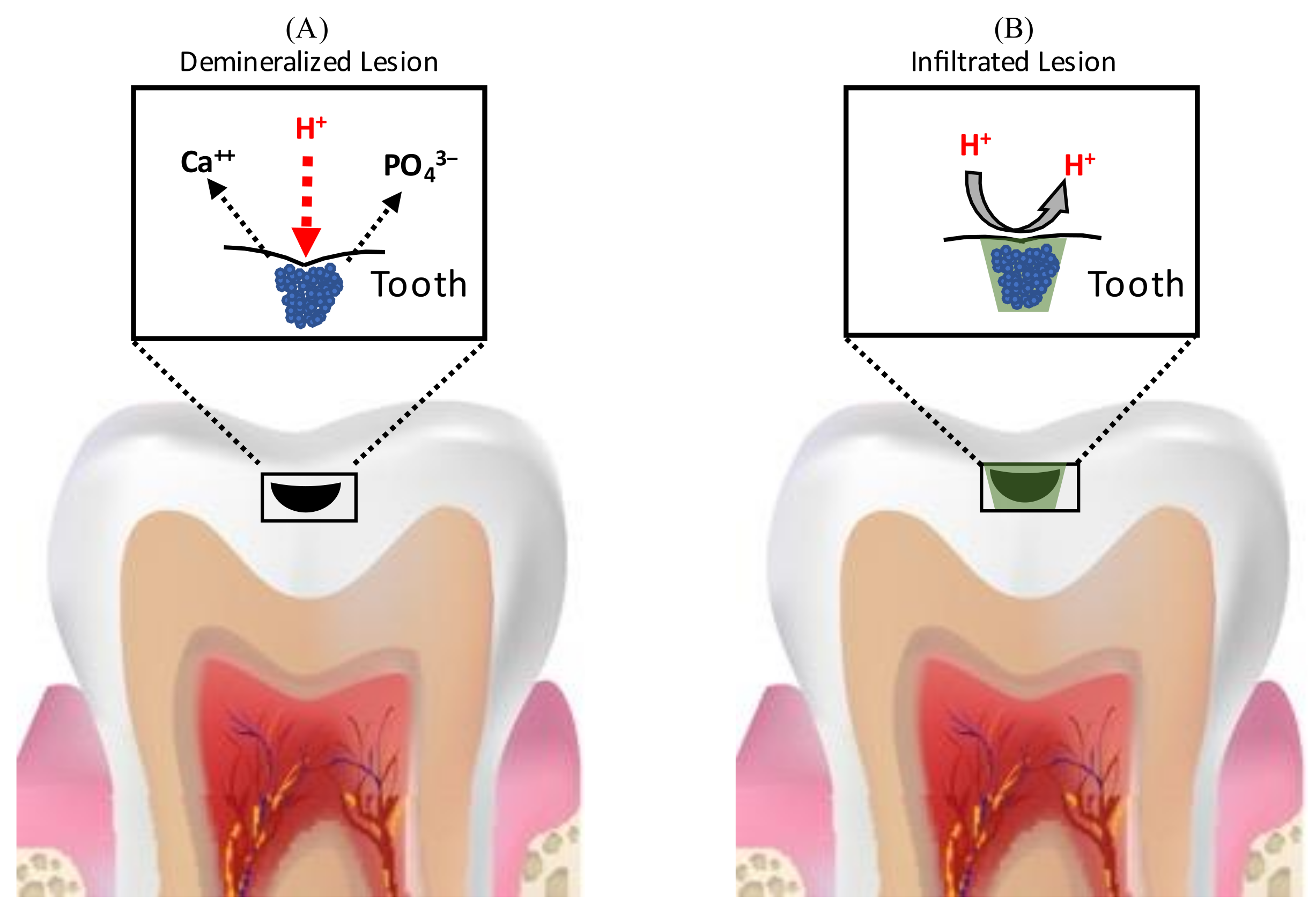
6. Resin Infiltration for the Management of Carious Lesions
6.1. Analysis of Considerations for Use in Non-Cavitated and Cavitated Proximal Lesions
6.2. Micro-Filled Infiltrant Resins (MFIR)
6.2.1. Effect of Fillers on RI Properties
6.2.2. Factors Affecting the Movement of RI from Micro-Filled Infiltrant Resin (MFIR)
6.3. RIs for Arresting Occlusal Carious Lesions
6.4. Limitations of Current Resin Infiltrants
6.4.1. Incomplete Lesion Resin Infiltration/Penetration
6.4.2. Surface Roughness
6.4.3. Polymerization Shrinkage and Microleakage
6.4.4. Leached Monomer Cytoxicity
6.4.5. Methacrylate Resin Degradation
6.5. Improving the Antimicrobial and Anti-Degradative Properties of Resin Infiltrants (RI)
7. Chemomechanical Management of Caries
8. Atraumatic Restorative Treatment (ART)
9. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Banerjee, A.; Frencken, J.E.; Schwendicke, F.; Innes, N.P.T. Contemporary operative caries management: Consensus recommendations on minimally invasive caries removal. Br. Dent. J. 2017, 223, 215–222. [Google Scholar] [CrossRef]
- Going, R.E.; Loesche, W.J.; Grainger, D.A.; Syed, S.A. The viability of microorganisms in carious lesions five years after covering with a fissure sealant. J. Am. Dent. Assoc. 1978, 97, 455–462. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, A.; Yasseri, M.; Munson, M. A method for the detection and quantification of bacteria in human carious dentine using fluorescent in situ hybridisation. J. Dent. 2002, 30, 359–363. [Google Scholar] [CrossRef]
- Paddick, J.S.; Brailsford, S.R.; Kidd, E.A.M.; Beighton, D. Phenotypic and Genotypic Selection of Microbiota Surviving under Dental Restorations. Appl. Environ. Microbiol. 2005, 71, 2467–2472. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ogawa, K.; Yamashita, Y.; Ichijo, T.; Fusayama, T. The Ultrastructure and Hardness of the Transparent of Human Carious Dentin. J. Dent. Res. 1983, 62, 7–10. [Google Scholar] [CrossRef] [PubMed]
- Kreulen, C.M.; De Soet, J.J.; Weerheijm, K.L.; Van Amerongen, W.E. In vivo Cariostatic Effect of Resin Modified Glass lonomer Cement and Amalgam on Dentine. Caries Res. 1997, 31, 384–389. [Google Scholar] [CrossRef]
- Ngo, H.C.; Mount, G.; Mc Intyre, J.; Tuisuva, J.; Von Doussa, R. Chemical exchange between glass-ionomer restorations and residual carious dentine in permanent molars: An in vivo study. J. Dent. 2006, 34, 608–613. [Google Scholar] [CrossRef] [PubMed]
- Li, T.; Zhai, X.; Song, F.; Zhu, H. Selective versus non-selective removal for dental caries: A systematic review and meta-analysis. Acta Odontol. Scand. 2018, 76, 135–140. [Google Scholar] [CrossRef]
- Ali, A.; Koller, G.; Foschi, F.; Andiappan, M.; Bruce, K.; Banerjee, A.; Mannocci, F. Self-Limiting versus Conventional Caries Removal: A Randomized Clinical Trial. J. Dent. Res. 2018, 97, 1207–1213. [Google Scholar] [CrossRef] [Green Version]
- Qvist, V. Longevity of Restorations: The ‘Death Spiral’. Available online: https://scholar.google.com/scholar_lookup?title=Longevity%20of%20restorations%3A%20the%20death%20spiral&publication_year=2008&author=V.%20Qvist (accessed on 8 February 2021).
- Pitts, N.B. Are We Ready to Move from Operative to Non-Operative/Preventive Treatment of Dental Caries in Clinical Practice? Caries Res. 2004, 38, 294–304. [Google Scholar] [CrossRef]
- Splieth, C.; Kanzow, P.; Wiegand, A.; Schmoeckel, J.; Jablonski-Momeni, A. How to intervene in the caries process: Proximal caries in adolescents and adults—A systematic review and meta-analysis. Clin. Oral Investig. 2020, 24, 1623–1636. [Google Scholar] [CrossRef] [PubMed]
- Elamin, F.; Abdelazeem, N.; Salah, I.; Mirghani, Y.; Wong, F. A randomized clinical trial comparing Hall vs conventional technique in placing preformed metal crowns from Sudan. PLoS ONE 2019, 14, e0217740. [Google Scholar] [CrossRef] [PubMed]
- Giacaman, R.A.; Muñoz-Sandoval, C.; Neuhaus, K.W.; Fontana, M.; Chałas, R. Evidence-based strategies for the minimally invasive treatment of carious lesions: Review of the literature. Adv. Clin. Exp. Med. 2018, 27, 1009–1016. [Google Scholar] [CrossRef] [PubMed]
- Frencken, J.E. Atraumatic restorative treatment and minimal intervention dentistry. Br. Dent. J. 2017, 223, 183–189. [Google Scholar] [CrossRef] [PubMed]
- Antonioni, M.B.; Fontana, M.; Salzmann, L.B.; Inglehart, M.R. Pediatric Dentists’ Silver Diamine Fluoride Education, Knowledge, Attitudes, and Professional Behavior: A National Survey. J. Dent. Educ. 2019, 83, 173–182. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Simpson, S.; Waterhouse, P.J. Hall technique: Is it superior in success and savings to conventional restorations? Evid.-Based Dent. 2020, 21, 128–129. [Google Scholar] [CrossRef] [PubMed]
- Jorge, R.; Ammari, M.; Soviero, V.; Souza, I. Randomized controlled clinical trial of resin infiltration in primary molars: 2 years follow-up. J. Dent. 2019, 90, 103184. [Google Scholar] [CrossRef]
- Garcia, R.I.; Gregorich, S.E.; Ramos-Gomez, F.; Braun, P.A.; Wilson, A.; Albino, J.; Tiwari, T.; Harper, M.; Batliner, T.S.; Rasmussen, M.; et al. Absence of Fluoride Varnish–Related Adverse Events in Caries Prevention Trials in Young Children, United States. Prev. Chronic Dis. 2017, 14, E17. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oliveira, B.; Salazar, M.; Carvalho, D.; Falcão, A.; Campos, K.; Nadanovsky, P. Biannual Fluoride Varnish Applications and Caries Incidence in Preschoolers: A 24-month Follow-Up Randomized Placebo-Controlled Clinical Trial. Caries Res. 2014, 48, 228–236. [Google Scholar] [CrossRef] [PubMed]
- Rozier, R.G.; Sutton, B.K.; Bawden, J.W.; Haupt, K.; Slade, G.D.; King, R.S. Prevention of early childhood caries in North Carolina medical practices: Implications for research and practice. J. Dent. Educ. 2003, 67, 876–885. [Google Scholar] [CrossRef]
- Sousa, F.S.D.O.D.; dos Santos, A.P.P.; Nadanovsky, P.; Hujoel, P.; Cunha-Cruz, J.; de Oliveira, B.H. Fluoride Varnish and Dental Caries in Preschoolers: A Systematic Review and Meta-Analysis. Caries Res. 2019, 53, 502–513. [Google Scholar] [CrossRef]
- Li, F.; Jiang, P.; Yu, F.; Li, C.; Wu, S.; Zou, J.; Xu, X.; Ye, L.; Zhou, X.; Zheng, L. Comparison between Fissure Sealant and Fluoride Varnish on Caries Prevention for First Permanent Molars: A Systematic Review and Meta-analysis. Sci. Rep. 2020, 10, 2578. [Google Scholar] [CrossRef] [PubMed]
- Chestnutt, I.G.; Hutchings, S.; Playle, R.; Morgan-Trimmer, S.; Fitzsimmons, D.; Aawar, N.; Angel, L.; Derrick, S.; Drew, C.; Hoddell, C.; et al. Seal or Varnish? A randomised controlled trial to determine the relative cost and effectiveness of pit and fissure sealant and fluoride varnish in preventing dental decay. Health Technol. Assess. 2017, 21, 1–256. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Urquhart, O.; Tampi, M.; Pilcher, L.; Slayton, R.; Araujo, M.; Fontana, M.; Guzmán-Armstrong, S.; Nascimento, M.; Nový, B.; Tinanoff, N.; et al. Nonrestorative Treatments for Caries: Systematic Review and Network Meta-analysis. J. Dent. Res. 2019, 98, 14–26. [Google Scholar] [CrossRef] [PubMed]
- Nelson, T.; Scott, J.; Crystal, Y.O.; Berg, J.H.; Milgrom, P. Silver Diamine Fluoride in Pediatric Dentistry Training Programs: Survey of Graduate Program Directors. Pediatr. Dent. 2016, 38, 212–217. [Google Scholar]
- Slayton, R.L.; Urquhart, O.; Araujo, M.W.; Fontana, M.; Guzmán-Armstrong, S.; Nascimento, M.M.; Nový, B.B.; Tinanoff, N.; Weyant, R.; Wolff, M.S.; et al. Evidence-based clinical practice guideline on nonrestorative treatments for carious lesions. J. Am. Dent. Assoc. 2018, 149, 837–849. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mauro, S.; Robles, E.G.; Cinque, C.; Squassi, A.F.; Bordoni, N.E. Eficiencia de tres fluoruros concentrados para la estabilización de caries de esmalte. Bol. Asoc. Argent Odontol. Niños 2004, 33, 4–11. [Google Scholar]
- Lo, E.; Chu, C.; Lin, H. A community-based caries control program for pre-school children using topical fluorides: 18-month results. J. Dent. Res. 2001, 80, 2071–2074. [Google Scholar] [CrossRef] [PubMed]
- Bijella, M.F.T.B.; Bijella, V.T.; da Silva, M.S.M.B.; Lopes, E.S. Avaliação clínica da aplicação de diamino-fluoreto de prata a 12. Rev. Paul. Odontol. 1991, 13, 28–35. [Google Scholar]
- Nishino, M.; Yoshida, S.; Sobue, S.; Kato, J.; Nishida, M. Effect of topically applied ammoniacal silver fluoride on dental caries in children. J. Osaka Univ. Dent. Sch. 1969, 9, 149–155. [Google Scholar] [PubMed]
- Hu, S.; Meyer, B.; Duggal, M. A silver renaissance in dentistry. Eur. Arch. Paediatr. Dent. 2018, 19, 221–227. [Google Scholar] [CrossRef]
- Llodra, J.; Rodriguez, A.; Ferrer, B.; Menardia, V.; Ramos, T.; Morato, M. Efficacy of Silver Diamine Fluoride for Caries Reduction in Primary Teeth and First Permanent Molars of Schoolchildren: 36-month Clinical Trial. J. Dent. Res. 2005, 84, 721–724. [Google Scholar] [CrossRef]
- American Academy of Pediatric Dentistry. Policy on the Use of Silver Diamine Fluoride for Pediatric Dental Patients. Pediatr. Dent. 2018, 40, 51–54. [Google Scholar]
- Crystal, Y.O.; Niederman, R. Silver Diamine Fluoride Treatment Considerations in Children’s Caries Management. Int. J. Clin. Pediatr. Dent. 2016, 38, 466–471. [Google Scholar]
- Chu, C.; Lo, E.; Lin, H. Effectiveness of Silver Diamine Fluoride and Sodium Fluoride Varnish in Arresting Dentin Caries in Chinese Pre-school Children. J. Dent. Res. 2002, 81, 767–770. [Google Scholar] [CrossRef] [PubMed]
- Yu, O.Y.; Zhao, I.S.; Mei, M.L.; Lo, E.C.; Chu, C. Caries-arresting effects of silver diamine fluoride and sodium fluoride on dentine caries lesions. J. Dent. 2018, 78, 65–71. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Lo, E.; Li, C. Effect of silver and fluoride ions on enamel demineralization: A quantitative study using micro-computed tomography. Aust. Dent. J. 2012, 57, 65–70. [Google Scholar] [CrossRef] [PubMed]
- Chu, C.; Lo, E.C. Microhardness of dentine in primary teeth after topical fluoride applications. J. Dent. 2008, 36, 387–391. [Google Scholar] [CrossRef]
- Mei, M.L.; Ito, L.; Cao, Y.; Li, Q.; Lo, E.C.; Chu, C. Inhibitory effect of silver diamine fluoride on dentine demineralisation and collagen degradation. J. Dent. 2013, 41, 809–817. [Google Scholar] [CrossRef] [PubMed]
- Mei, M.L.; Li, Q.; Chu, C.H.; Yiu, C.K.; Lo, E.C. The inhibitory effects of silver diamine fluoride at different concentrations on matrix metalloproteinases. Dent. Mater. 2012, 28, 903–908. [Google Scholar] [CrossRef] [PubMed]
- Mei, M.L.; Ito, L.; Cao, Y.; Li, Q.; Chu, C.; Lo, E.C. The inhibitory effects of silver diamine fluorides on cysteine cathepsins. J. Dent. 2014, 42, 329–335. [Google Scholar] [CrossRef] [Green Version]
- Mei, M.L.; Chu, C.H.; Lo, E.C.M.; Samaranayake, L.P. Fluoride and silver concentrations of silver diammine fluoride solutions for dental use. Int. J. Paediatr. Dent. 2013, 23, 279–285. [Google Scholar] [CrossRef] [PubMed]
- Mei, M.L.; Ito, L.; Cao, Y.; Lo, E.C.; Li, Q.; Chu, C. An ex vivo study of arrested primary teeth caries with silver diamine fluoride therapy. J. Dent. 2014, 42, 395–402. [Google Scholar] [CrossRef] [Green Version]
- Mei, M.L.; Li, Q.-L.; Chu, C.-H.; Lo, E.-M.; Samaranayake, L.P. Antibacterial effects of silver diamine fluoride on multi-species cariogenic biofilm on caries. Ann. Clin. Microbiol. Antimicrob. 2013, 12, 4–7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mei, M.L.; Chu, C.; Low, K.-H.; Che, C.M.; Lo, E. Caries arresting effect of silver diamine fluoride on dentine carious lesion with S. mutans and L. acidophilus dual-species cariogenic biofilm. Med. Oral Patol. Oral Cir. Bucal 2013, 18, e824–e831. [Google Scholar] [CrossRef]
- Chibinski, A.C.; Wambier, L.M.; Feltrin, J.; Loguercio, A.D.; Wambier, D.S.; Reis, A. Silver Diamine Fluoride Has Efficacy in Controlling Caries Progression in Primary Teeth: A Systematic Review and Meta-Analysis. Caries Res. 2017, 51, 527–541. [Google Scholar] [CrossRef]
- Fung, M.; Duangthip, D.; Wong, M.; Lo, E.; Chu, C. Arresting Dentine Caries with Different Concentration and Periodicity of Silver Diamine Fluoride. JDR Clin. Transl. Res. 2016, 1, 143–152. [Google Scholar] [CrossRef] [PubMed]
- Fung, M.; Duangthip, D.; Wong, M.; Lo, E.; Chu, C. Randomized Clinical Trial of 12% and 38% Silver Diamine Fluoride Treatment. J. Dent. Res. 2018, 97, 171–178. [Google Scholar] [CrossRef]
- Duangthip, D.; Chu, C.; Lo, E. A randomized clinical trial on arresting dentine caries in preschool children by topical fluorides—18 month results. J. Dent. 2016, 44, 57–63. [Google Scholar] [CrossRef] [PubMed]
- Duangthip, D.; Wong, M.; Chu, C.H.; Lo, E. Caries arrest by topical fluorides in preschool children: 30-month results. J. Dent. 2018, 70, 74–79. [Google Scholar] [CrossRef] [PubMed]
- Zhi, Q.H.; Lo, E.C.M.; Lin, H.C. Randomized clinical trial on effectiveness of silver diamine fluoride and glass ionomer in arresting dentine caries in preschool children. J. Dent. 2012, 40, 962–967. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mabangkhru, S.; Duangthip, D.; Chu, C.H.; Phonghanyudh, A.; Jirarattanasopha, V. A randomized clinical trial to arrest dentin caries in young children using silver diamine fluoride. J. Dent. 2020, 99, 103375. [Google Scholar] [CrossRef] [PubMed]
- Horst, J.A.; Heima, M. Prevention of Dental Caries by Silver Diamine Fluoride. Compend. Contin. Educ. Dent. 2019, 40, 158–163. [Google Scholar] [PubMed]
- Oliveira, B.; Rajendra, A.; Veitz-Keenan, A.; Niederman, R. The Effect of Silver Diamine Fluoride in Preventing Caries in the Primary Dentition: A Systematic Review and Meta-Analysis. Caries Res. 2019, 53, 24–32. [Google Scholar] [CrossRef] [PubMed]
- Crystal, Y.O.; Niederman, R. Evidence-Based Dentistry Update on Silver Diamine Fluoride. Dent. Clin. N. Am. 2019, 63, 45–46. [Google Scholar] [CrossRef] [PubMed]
- dos Santos, V.E.; de Vasconcelos, F.M.N.; Ribeiro, A.G.; Rosenblatt, A. Paradigm shift in the effective treatment of caries in schoolchildren at risk. Int. Dent. J. 2012, 62, 47–51. [Google Scholar] [CrossRef]
- Braga, M.M.; Mendes, F.; De Benedetto, M.S.; Imparato, J.C.P. Effect of silver diammine fluoride on incipient caries lesions in erupting permanent first molars: A pilot study. J. Dent. Child. 2009, 76, 28–33. [Google Scholar]
- Rosenblatt, A.; Stamford, T.C.M.; Niederman, R. Silver Diamine Fluoride: A Caries “Silver-Fluoride Bullet”. J. Dent. Res. 2009, 88, 116–125. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Lo, E.; Chu, C.H.; Lin, H. Randomized Trial on Fluorides and Sealants for Fissure Caries Prevention. J. Dent. Res. 2012, 91, 753–758. [Google Scholar] [CrossRef] [Green Version]
- Monse, B.; Heinrich-Weltzien, R.; Mulder, J.; Holmgren, C.; Helderman, W.H.V.P. Caries preventive efficacy of silver diammine fluoride (SDF) and ART sealants in a school-based daily fluoride toothbrushing program in the Philippines. BMC Oral Health 2012, 12, 52. [Google Scholar] [CrossRef] [Green Version]
- Hendre, A.D.; Taylor, G.W.; Chávez, E.M.; Hyde, S. A systematic review of silver diamine fluoride: Effectiveness and application in older adults. Gerodontology 2017, 34, 411–419. [Google Scholar] [CrossRef] [PubMed]
- Raskin, S.E.; Tranby, E.P.; Ludwig, S.; Okunev, I.; Frantsve-Hawley, J.; Boynes, S. Survival of silver diamine fluoride among patients treated in community dental clinics: A naturalistic study. BMC Oral Health 2021, 21, 35. [Google Scholar] [CrossRef]
- Chhokar, S.K.; Laughter, L.; Rowe, D.J. Perceptions of Registered Dental Hygienists in Alternative Practice Regarding Silver Diamine Fluoride. J. Dent. Hyg. JDH 2017, 91, 53–60. [Google Scholar]
- Crystal, Y.O.; Janal, M.N.; Hamilton, D.S.; Niederman, R. Parental perceptions and acceptance of silver diamine fluoride staining. J. Am. Dent. Assoc. 2017, 148, 510–518. [Google Scholar] [CrossRef] [PubMed]
- Hu, S.; Meyer, B.; Lai, B.W.P.; Chay, P.L.; Tong, H.J. Parental acceptance of silver diammine fluoride in children with autism spectrum disorder. Int. J. Paediatr. Dent. 2020, 30, 514–522. [Google Scholar] [CrossRef]
- Alshammari, A.F.; Almuqrin, A.A.; Aldakhil, A.M.; Alshammari, B.H.; Lopez, J.N.J. Parental perceptions and acceptance of silver diamine fluoride treatment in Kingdom of Saudi Arabia. Int. J. Health Sci. 2019, 13, 25–29. [Google Scholar]
- Clemens, J.; Gold, J.; Chaffin, J. Effect and acceptance of silver diamine fluoride treatment on dental caries in primary teeth. J. Public Health Dent. 2017, 78, 63–68. [Google Scholar] [CrossRef] [PubMed]
- Cernigliaro, D.; Kumar, A.; Northridge, M.E.; Wu, Y.; Troxel, A.B.; Cunha-Cruz, J.; Balzer, J.; Okuji, D.M. Caregiver satisfaction with interim silver diamine fluoride applications for their children with caries prior to operating room treatment or sedation. J. Public Health Dent. 2019, 79, 286–291. [Google Scholar] [CrossRef] [PubMed]
- Bagher, S.M.; Sabbagh, H.J.; AlJohani, S.M.; Alharbi, G.; Aldajani, M.; Elkhodary, H. Parental acceptance of the utilization of silver diamine fluoride on their child’s primary and permanent teeth. Patient Prefer. Adherence 2019, 13, 829–835. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huebner, C.E.; Milgrom, P.; Cunha-Cruz, J.; Scott, J.; Spiekerman, C.; Ludwig, S.; Mitchell, M.; Allen, G.; Dysert, J.; Shirtcliff, R.M. Parents’ Satisfaction with Silver Diamine Fluoride Treatment of Carious Lesions in Children. J. Dent. Child. Chic. Ill 2020, 87, 4–11. [Google Scholar]
- Kyoon-Achan, G.; Schroth, R.; Martin, H.; Bertone, M.; Mittermuller, B.; Sihra, R.; Klus, B.; Singh, S.; Moffatt, M. Parents’ Views on Silver Diamine Fluoride to Manage Early Childhood Caries. JDR Clin. Transl. Res. 2021, 6, 251–257. [Google Scholar] [CrossRef] [PubMed]
- Kyoon-Achan, G.; Schroth, R.J.; DeMaré, D.; Sturym, M.; Edwards, J.; Lavoie, J.G.; Sanguins, J.; Campbell, R.; Chartrand, F.; Bertone, M.F.; et al. Indigenous community members’ views on silver diamine fluoride to manage early childhood caries. J. Public Health Dent. 2020, 80, 208–216. [Google Scholar] [CrossRef] [PubMed]
- Wright, J.T.; Tampi, M.P.; Graham, L.; Estrich, C.; Crall, J.J.; Fontana, M.; Gillette, E.J.; Nový, B.B.; Dhar, V.; Donly, K.; et al. Sealants for preventing and arresting pit-and-fissure occlusal caries in primary and permanent molars. J. Am. Dent. Assoc. 2016, 147, 631–645. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ahovuo-Saloranta, A.; Forss, H.; Walsh, T.; Nordblad, A.; Makela, M.; Worthington, H.V. Pit and fissure sealants for preventing dental decay in permanent teeth. Cochrane Database Syst. Rev. 2017, 7, CD001830. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wendt, L.K.; Koch, G. Fissure sealant in permanent first molars after 10 years. Swed. Dent. J. 1988, 12, 181–185. [Google Scholar] [PubMed]
- Kühnisch, J.; Bedir, A.; Lo, Y.-F.; Kessler, A.; Lang, T.; Mansmann, U.; Heinrich-Weltzien, R.; Hickel, R. Meta-analysis of the longevity of commonly used pit and fissure sealant materials. Dent. Mater. 2020, 36, e158–e168. [Google Scholar] [CrossRef] [PubMed]
- Jodkowska, E. Efficacy of pit and fissure sealing: Long-term clinical observations. Quintessence Int. 2008, 39, 593–602. [Google Scholar] [PubMed]
- Crall, J.J.; Donly, K.J. Dental sealants guidelines development: 2002-2014. Pediatr. Dent. 2015, 37, 111–115. [Google Scholar]
- Splieth, C.H.; Ekstrand, K.; Alkilzy, M.; Clarkson, J.; Meyer-Lueckel, H.; Martignon, S.; Paris, S.; Pitts, N.B.; Ricketts, D.; Van Loveren, C. Sealants in Dentistry: Outcomes of the ORCA Saturday Afternoon Symposium 2007. Caries Res. 2010, 44, 3–13. [Google Scholar] [CrossRef]
- Handelman, S.; Washburn, F.; Wopperer, P. Two-year report of sealant effect on bacteria in dental caries. J. Am. Dent. Assoc. 1976, 93, 967–970. [Google Scholar] [CrossRef] [PubMed]
- Oong, E.M.; Griffin, S.O.; Kohn, W.G.; Gooch, B.F.; Caufield, P.W. The Effect of Dental Sealants on Bacteria Levels in Caries Lesions. J. Am. Dent. Assoc. 2008, 139, 271–278. [Google Scholar] [CrossRef] [Green Version]
- Orhan, A.I.; Oz, F.T.; Ozcelik, B.; Orhan, K. A clinical and microbiological comparative study of deep carious lesion treatment in deciduous and young permanent molars. Clin. Oral Investig. 2008, 12, 369–378. [Google Scholar] [CrossRef] [PubMed]
- Fontana, M.; Innes, N. Sealing Carious Tissue Using Resin and Glass-Ionomer Cements. Monogr. Oral Sci. 2018, 27, 103–112. [Google Scholar] [CrossRef] [PubMed]
- Bakhshandeh, A.; Qvist, V.; Ekstrand, K. Sealing occlusal caries lesions in adults referred for restorative treatment: 2–3 years of follow-up. Clin. Oral Investig. 2012, 16, 521–529. [Google Scholar] [CrossRef]
- Qvist, V.; Borum, M.; Møller, K.; Andersen, T.; Blanche, P.; Bakhshandeh, A. Sealing Occlusal Dentin Caries in Permanent Molars. JDR Clin. Transl. Res. 2017, 2, 73–86. [Google Scholar] [CrossRef] [PubMed]
- Borges, B.C.D.; Borges, J.D.S.; Braz, R.; Montes, M.A.J.R.; Pinheiro, I.V.D.A. Arrest of non-cavitated dentinal occlusal caries by sealing pits and fissures: A 36-month, randomised controlled clinical trial. Int. Dent. J. 2012, 62, 251–255. [Google Scholar] [CrossRef] [PubMed]
- Fusayama, T.; Kurosaki, N. Structure and removal of carious dentin. Int. Dent. J. 1972, 22, 401–411. [Google Scholar]
- Pugach, M.; Strother, J.; Darling, C.; Fried, D.; Gansky, S.; Marshall, S.; Marshall, G. Dentin Caries Zones: Mineral, Structure, and Properties. J. Dent. Res. 2009, 88, 71–76. [Google Scholar] [CrossRef] [PubMed]
- Griffin, S.O.; Oong, E.; Kohn, W.; Vidakovic, B.; Gooch, B.F.; CDC Dental Sealant Systematic Review Work Group. The Effectiveness of Sealants in Managing Caries Lesions; Centre for Reviews and Dissemination: York, UK, 2008. Available online: https://www.ncbi.nlm.nih.gov/books/NBK76511/ (accessed on 13 January 2021).
- Mertz-Fairhurst, E.J.; Curtis, J.W.; Ergle, J.W.; Rueggeberg, F.; Adair, S.M. Ultraconservative and Cariostatic Sealed Restorations: Results at Year 10. J. Am. Dent. Assoc. 1998, 129, 55–66. [Google Scholar] [CrossRef] [PubMed]
- Kielbassa, A.M.; Ulrich, I.; Schmidl, R.; Schüller, C.; Frank, W.; Werth, V.D. Resin infiltration of deproteinised natural occlusal subsurface lesions improves initial quality of fissure sealing. Int. J. Oral Sci. 2017, 9, 117–124. [Google Scholar] [CrossRef]
- Hevinga, M.; Opdam, N.; Frencken, J.; Bronkhorst, E.; Truin, G. Can Caries Fissures be Sealed as Adequately as Sound Fissures? J. Dent. Res. 2008, 87, 495–498. [Google Scholar] [CrossRef]
- Hevinga, M.; Opdam, N.; Frencken, J.; Bronkhorst, E.; Truin, G. Microleakage and sealant penetration in contaminated carious fissures. J. Dent. 2007, 35, 909–914. [Google Scholar] [CrossRef]
- Strassler, H.E. Bonding to Sound vs Caries-Affected Dentin Using Photo- and Dual-Cure Adhesives|Inside Dentistry. Available online: https://www.aegisdentalnetwork.com/id/2006/05/bonding-to-sound-vs-caries-affected-dentin-using-photo-and-dual-cure-adhesives (accessed on 25 January 2021).
- Fejerskov, O.; Nyvad, B.; Kidd, E. Dental Caries: The Disease and Its Clinical Management, 3rd ed.; Wiley: Hoboken, NJ, USA, 2015; Available online: https://www.wiley.com/en-ca/Dental+Caries%3A+The+Disease+and+its+Clinical+Management%2C+3rd+Edition-p-9781118935828 (accessed on 25 January 2021).
- Simonsen, R.J.; Neal, R.C. A review of the clinical application and performance of pit and fissure sealants. Aust. Dent. J. 2011, 56, 45–58. [Google Scholar] [CrossRef]
- Li, F.; Li, F.; Wu, D.; Ma, S.; Gao, J.; Li, Y.; Xiao, Y.; Chen, J. The Effect of an Antibacterial Monomer on the Antibacterial Activity and Mechanical Properties of a Pit-and-Fissure Sealant. J. Am. Dent. Assoc. 2011, 142, 184–193. [Google Scholar] [CrossRef]
- Beyth, N.; Domb, A.J.; Weiss, E.I. An in vitro quantitative antibacterial analysis of amalgam and composite resins. J. Dent. 2007, 35, 201–206. [Google Scholar] [CrossRef]
- Wiegand, A.; Buchalla, W.; Attin, T. Review on fluoride-releasing restorative materials—Fluoride release and uptake characteristics, antibacterial activity and influence on caries formation. Dent. Mater. 2007, 23, 343–362. [Google Scholar] [CrossRef]
- Heifetz, S.B.; Yaari, A.; Proskin, H. Anticaries effectiveness of a fluoride and nonfluoride sealant. J. Calif. Dent. Assoc. 2007, 35, 573–577. [Google Scholar]
- Lygidakis, N.A.; Oulis, K.I. A comparison of Fluroshield with Delton fissure sealant: Four year results. Pediatr. Dent. 2000, 21, 429–431. [Google Scholar]
- Williams, B.; Laxton, L.; Holt, R.D.; Winter, G.B. Fissure sealants: A 4-year clinical trial comparing an experimental glass polyalkenoate cement with a bis glycidyl methacrylate resin used as fissure sealants. Br. Dent. J. 1996, 180, 104–108. [Google Scholar] [CrossRef]
- Jensen, O.E.; Billings, R.J.; Featherstone, J.D. Clinical evaluation of Fluroshield pit and fissure sealant. Clin. Prev. Dent. 1990, 12, 24–27. [Google Scholar]
- Yildiz, E.; Dorter, C.; Efes, B.; Koray, F. A comparative study of two fissure sealants: A 2-year clinical follow-up. J. Oral Rehabil. 2004, 31, 979–984. [Google Scholar] [CrossRef] [PubMed]
- Koch, M.J.; García-Godoy, F.; Mayer, T.; Staehle, H.J. Clinical evaluation of Helioseal F fissure sealant. Clin. Oral Investig. 1998, 1, 199–202. [Google Scholar] [CrossRef]
- Fluoride-Releasing Sealants. J. Am. Dent. Assoc. 1985, 110, 90. [CrossRef]
- Imazato, S. Antibacterial properties of resin composites and dentin bonding systems. Dent. Mater. 2003, 19, 449–457. [Google Scholar] [CrossRef]
- Memarpour, M.; Shafiei, F. The Effect of Antibacterial Agents on Fissure Sealant Microleakage: A 6-month In Vitro Study. Oral Health Prev. Dent. 2014, 12, 149–155. [Google Scholar] [CrossRef]
- AlShahrani, S.S.; AlAbbas, M.A.S.; Garcia, I.M.; AlGhannam, M.I.; AlRuwaili, M.A.; Collares, F.M.; Ibrahim, M.S. The Antibacterial Effects of Resin-Based Dental Sealants: A Systematic Review of In Vitro Studies. Materials 2021, 14, 413. [Google Scholar] [CrossRef]
- Yu, F.; Yu, H.; Lin, P.; Dong, Y.; Zhang, L.; Sun, X.; Liu, Z.; Guo, H.; Huang, L.; Chen, J. Effect of an Antibacterial Monomer on the Antibacterial Activity of a Pit-and-Fissure Sealant. PLoS ONE 2016, 11, e0162281. [Google Scholar] [CrossRef] [PubMed]
- Garcia, I.M.; Rodrigues, S.B.; Balbinot, G.D.S.; Visioli, F.; Leitune, V.C.B.; Collares, F.M. Quaternary ammonium compound as antimicrobial agent in resin-based sealants. Clin. Oral Investig. 2019, 24, 777–784. [Google Scholar] [CrossRef]
- Ibrahim, M.S.; Ibrahim, A.S.; Balhaddad, A.A.; Weir, M.D.; Lin, N.J.; Tay, F.R.; Oates, T.W.; Xu, H.H.K.; Melo, M.A.S. A Novel Dental Sealant Containing Dimethylaminohexadecyl Methacrylate Suppresses the Cariogenic Pathogenicity of Streptococcus mutans Biofilms. Int. J. Mol. Sci. 2019, 20, 3491. [Google Scholar] [CrossRef] [Green Version]
- Ibrahim, M.S.; Garcia, I.M.; Vila, T.; Balhaddad, A.A.; Collares, F.M.; Weir, M.D.; Xu, H.H.K.; Melo, M.A.S. Multifunctional antibacterial dental sealants suppress biofilms derived from children at high risk of caries. Biomater. Sci. 2020, 8, 3472–3484. [Google Scholar] [CrossRef]
- Monteiro, J.C.; Stürmer, M.; Garcia, I.M.; Melo, M.A.; Sauro, S.; Leitune, V.C.B.; Collares, F.M. Dental Sealant Empowered by 1,3,5-Tri Acryloyl Hexahydro-1,3,5-Triazine and α-Tricalcium Phosphate for Anti-Caries Application. Polymers 2020, 12, 895. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Anand, V.; Arumugam, S.B.; Manoharan, V.; Kumar, S.A.; Methippara, J.J. Is Resin Infiltration a Microinvasive Approach to White Lesions of Calcified Tooth Structures?: A Systemic Review. Int. J. Clin. Pediatr. Dent. 2019, 12, 53–58. [Google Scholar] [CrossRef]
- Paris, S.; Meyer-Lueckel, H.; Cölfen, H.; Kielbassa, A.M. Resin Infiltration of Artificial Enamel Caries Lesions with Experimental Light Curing Resins. Dent. Mater. J. 2007, 26, 582–588. [Google Scholar] [CrossRef] [Green Version]
- Kielbassa, A.M.; Muller, J.; Gernhardt, C.R. Closing the gap between oral hygiene and minimally invasive dentistry: A review on the resin infiltration technique of incipient (proximal) enamel lesions. Quintessence Int. 2009, 40, 663–681. [Google Scholar]
- Kielbassa, A.; Ulrich, I.; Treven, L.; Mueller, J. An updated review on the resin infiltration technique on incipient proximal enamel lesions. Med. Evol. 2010, 16, 3–15. [Google Scholar]
- Enan, E.T.; Aref, N.S.; Hammad, S.M. Resistance of resin-infiltrated enamel to surface changes in response to acidic challenge. J. Esthet. Restor. Dent. 2019, 31, 353–358. [Google Scholar] [CrossRef]
- Paris, S.; Meyer-Lueckel, H.; Cölfen, H.; Kielbassa, A.M. Penetration coefficients of commercially available and experimental composites intended to infiltrate enamel carious lesions. Dent. Mater. 2007, 23, 742–748. [Google Scholar] [CrossRef] [PubMed]
- Ye, Q.; Spencer, P.; Wang, Y.; Misra, A. Relationship of solvent to the photopolymerization process, properties, and structure in model dentin adhesives. J. Biomed. Mater. Res. Part A 2007, 80, 342–350. [Google Scholar] [CrossRef] [Green Version]
- Araújo, G.S.A.; Sfalcin, R.A.; Araújo, T.G.F.; Alonso, R.C.B.; Puppin-Rontani, R.M. Evaluation of polymerization characteristics and penetration into enamel caries lesions of experimental infiltrants. J. Dent. 2013, 41, 1014–1019. [Google Scholar] [CrossRef] [Green Version]
- Meyer-Lueckel, H.; Paris, S. Infiltration of Natural Caries Lesions with Experimental Resins Differing in Penetration Coefficients and Ethanol Addition. Caries Res. 2010, 44, 408–414. [Google Scholar] [CrossRef] [PubMed]
- Ammari, M.M.; Soviero, V.M.; Fidalgo, T.; Lenzi, M.; Ferreira, D.M.T.; Mattos, C.T.; Souza, I.P.; Maia, L.C. Is non-cavitated proximal lesion sealing an effective method for caries control in primary and permanent teeth? A systematic review and meta-analysis. J. Dent. 2014, 42, 1217–1227. [Google Scholar] [CrossRef] [PubMed]
- Dorri, M.; Dunne, S.M.; Walsh, T.; Schwendicke, F. Micro-invasive interventions for managing proximal dental decay in primary and permanent teeth. Cochrane Database Syst. Rev. 2015, 2015, CD010431. [Google Scholar] [CrossRef] [PubMed]
- Chatzimarkou, S.; Koletsi, D.; Kavvadia, K. The effect of resin infiltration on proximal caries lesions in primary and permanent teeth. A systematic review and meta-analysis of clinical trials. J. Dent. 2018, 77, 8–17. [Google Scholar] [CrossRef] [Green Version]
- Liang, Y.; Deng, Z.; Dai, X.; Tian, J.; Zhao, W. Micro-invasive interventions for managing non-cavitated proximal caries of different depths: A systematic review and meta-analysis. Clin. Oral Investig. 2018, 22, 2675–2684. [Google Scholar] [CrossRef] [PubMed]
- Elrashid, A.H.; Alshaiji, B.S.; Saleh, S.A.; Zada, K.A.; Baseer, M.A. Efficacy of Resin Infiltrate in Noncavitated Proximal Carious Lesions: A Systematic Review and Meta-Analysis. J. Int. Soc. Prev. Community Dent. 2019, 9, 211–218. [Google Scholar] [CrossRef]
- Chen, Y.; Chen, D.; Lin, H. Infiltration and sealing for managing non-cavitated proximal lesions: A systematic review and meta-analysis. BMC Oral Health 2021, 21, 13. [Google Scholar] [CrossRef] [PubMed]
- Peters, M.; Hopkins, A.; Zhu, L.; Yu, Q. Efficacy of Proximal Resin Infiltration on Caries Inhibition: Results from a 3-Year Randomized Controlled Clinical Trial. J. Dent. Res. 2019, 98, 1497–1502. [Google Scholar] [CrossRef]
- Meyer-Lueckel, H.; Bitter, K.; Paris, S. Randomized Controlled Clinical Trial on Proximal Caries Infiltration: Three-Year Follow-Up. Caries Res. 2012, 46, 544–548. [Google Scholar] [CrossRef] [PubMed]
- Paris, S.; Bitter, K.; Krois, J.; Meyer-Lueckel, H. Seven-year-efficacy of proximal caries infiltration—Randomized clinical trial. J. Dent. 2020, 93, 103277. [Google Scholar] [CrossRef]
- Page, L.F.; Beckett, D.; Ahmadi, R.; Schwass, D.; De La Barra, S.L.; Moffat, S.; Meldrum, A.; Thomson, W. Resin Infiltration of Caries in Primary Molars in a Community Setting: 24-Month Randomized Controlled Trial Findings. JDR Clin. Transl. Res. 2017, 2, 287–294. [Google Scholar] [CrossRef]
- Ammari, M.M.; Jorge, R.C.; Souza, I.P.; Soviero, V.M. Efficacy of resin infiltration of proximal caries in primary molars: 1-year follow-up of a split-mouth randomized controlled clinical trial. Clin. Oral Investig. 2017, 22, 1355–1362. [Google Scholar] [CrossRef] [PubMed]
- Ekstrand, K.; Bakhshandeh, A.; Martignon, S. Treatment of Proximal Superficial Caries Lesions on Primary Molar Teeth with Resin Infiltration and Fluoride Varnish versus Fluoride Varnish Only: Efficacy after 1 Year. Caries Res. 2010, 44, 41–46. [Google Scholar] [CrossRef] [PubMed]
- Paris, S.; Bitter, K.; Naumann, M.; Dörfer, C.E.; Meyer-Lueckel, H. Resin infiltration of proximal caries lesions differing in ICDAS codes. Eur. J. Oral Sci. 2011, 119, 182–186. [Google Scholar] [CrossRef] [PubMed]
- Askar, H.; Schwendicke, F.; Lausch, J.; Meyer-Lueckel, H.; Paris, S. Modified resin infiltration of non-, micro- and cavitated proximal caries lesions in vitro. J. Dent. 2018, 74, 56–60. [Google Scholar] [CrossRef] [PubMed]
- Askar, H.; Lausch, J.; Dörfer, C.E.; Meyer-Lueckel, H.; Paris, S. Penetration of micro-filled infiltrant resins into artificial caries lesions. J. Dent. 2015, 43, 832–838. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bourbia, M.; Finer, Y. Biochemical Stability and Interactions of Dental Resin Composites and Adhesives with Host and Bacteria in the Oral Cavity: A Review. J. Can. Dent. Assoc. 2018, 84, i1. [Google Scholar]
- Klapdohr, S.; Moszner, N. New Inorganic Components for Dental Filling Composites. Mon. Chem.-Chem. Mon. 2004, 136, 21–45. [Google Scholar] [CrossRef]
- Lee, J.-H.; Um, C.-M.; Lee, I.-B. Rheological properties of resin composites according to variations in monomer and filler composition. Dent. Mater. 2006, 22, 515–526. [Google Scholar] [CrossRef] [PubMed]
- Beun, S.; Bailly, C.; Dabin, A.; Vreven, J.; Devaux, J.; Leloup, G. Rheological properties of experimental Bis-GMA/TEGDMA flowable resin composites with various macrofiller/microfiller ratio. Dent. Mater. 2009, 25, 198–205. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Pan, J.; Zhang, S.; Malmstrom, H.S.; Ren, Y.-F. Effectiveness of resin-based materials against erosive and abrasive enamel wear. Clin. Oral Investig. 2017, 21, 463–468. [Google Scholar] [CrossRef] [PubMed]
- Lausch, J.; Askar, H.; Paris, S.; Meyer-Lueckel, H. Micro-filled resin infiltration of fissure caries lesions in vitro. J. Dent. 2017, 57, 73–76. [Google Scholar] [CrossRef] [PubMed]
- Paris, S.; Lausch, J.; Selje, T.; Dörfer, C.; Meyer-Lueckel, H. Comparison of sealant and infiltrant penetration into pit and fissure caries lesions in vitro. J. Dent. 2014, 42, 432–438. [Google Scholar] [CrossRef]
- Da Silva, V.B.; De Carvalho, R.N.; Bergstrom, T.G.; Dos Santos, T.M.P.; Lopes, R.T.; Neves, A.D.A. Sealing Carious Fissures with Resin Infiltrant in Association with a Flowable Composite Reduces Immediate Microleakage? Pesqui. Bras. Odontopediatria Clín. Integr. 2020, 20, e5114. [Google Scholar] [CrossRef]
- Bakhshandeh, A.; Ekstrand, K. Infiltration and sealing versus fluoride treatment of occlusal caries lesions in primary molar teeth. 2-3 years results. Int. J. Paediatr. Dent. 2014, 25, 43–50. [Google Scholar] [CrossRef]
- Anauate-Netto, C.; Neto, L.B.; Amore, R.; Di Hipólito, V.; Alpino, P.H.P.D. Caries progression in non-cavitated fissures after infiltrant application: A 3-year follow-up of a randomized controlled clinical trial. J. Appl. Oral Sci. 2017, 25, 442–454. [Google Scholar] [CrossRef] [Green Version]
- Elkwatehy, W.M.A.; Bukhari, O.M. The efficacy of different sealant modalities for prevention of pits and fissures caries: A randomized clinical trial. J. Int. Soc. Prev. Community Dent. 2019, 9, 119–128. [Google Scholar] [CrossRef] [PubMed]
- Schneider, H.; Park, K.-J.; Rueger, C.; Ziebolz, D.; Krause, F.; Haak, R. Imaging resin infiltration into non-cavitated carious lesions by optical coherence tomography. J. Dent. 2017, 60, 94–98. [Google Scholar] [CrossRef] [PubMed]
- Yim, H.-K.; Min, J.-H.; Kwon, H.-K.; Kim, B.-I. Modification of surface pretreatment of white spot lesions to improve the safety and efficacy of resin infiltration. Korean J. Orthod. 2014, 44, 195–202. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Neres, É.Y.; Moda, M.; Chiba, E.K.; Briso, A.; Pessan, J.; Fagundes, T. Microhardness and Roughness of Infiltrated White Spot Lesions Submitted to Different Challenges. Oper. Dent. 2017, 42, 428–435. [Google Scholar] [CrossRef]
- Torres, C.R.G.; Rosa, P.; Ferreira, N.; Borges, A.B. Effect of Caries Infiltration Technique and Fluoride Therapy on Microhardness of Enamel Carious Lesions. Oper. Dent. 2012, 37, 363–369. [Google Scholar] [CrossRef]
- Meyer-Lueckel, H.; Paris, S. Improved Resin Infiltration of Natural Caries Lesions. J. Dent. Res. 2008, 87, 1112–1116. [Google Scholar] [CrossRef] [PubMed]
- De Sousa, F.B.; Soares, J.D.; Vianna, S.S. Natural Enamel Caries: A Comparative Histological Study on Biochemical Volumes. Caries Res. 2012, 47, 183–192. [Google Scholar] [CrossRef] [PubMed]
- Silverstone, L.M. Structure of carious enamel, including the early lesion. Oral Sci. Rev. 1973, 3, 100–160. [Google Scholar]
- Prajapati, D.; Nayak, R.; Pai, D.; Upadhya, N.; Bhaskar, V.K.; Kamath, P. Effect of Resin Infiltration on Artificial Caries: An in vitro Evaluation of Resin Penetration and Microhardness. Int. J. Clin. Pediatr. Dent. 2017, 10, 250–256. [Google Scholar] [CrossRef] [PubMed]
- Kielbassa, A.M.; Leimer, M.R.; Hartmann, J.; Harm, S.; Pasztorek, M.; Ulrich, I.B. Ex vivo investigation on internal tunnel approach/internal resin infiltration and external nanosilver-modified resin infiltration of proximal caries exceeding into dentin. PLoS ONE 2020, 15, e0228249. [Google Scholar] [CrossRef]
- Mueller, J.; Yang, F.; Neumann, K.; Kielbassa, A.M. Surface tridimensional topography analysis of materials and finishing procedures after resinous infiltration of subsurface bovine enamel lesions. Quintessence Int. 2011, 42, 135–147. [Google Scholar] [PubMed]
- Ulrich, I.; Mueller, J.; Wolgin, M.; Frank, W.; Kielbassa, A.M. Tridimensional surface roughness analysis after resin infiltration of (deproteinized) natural subsurface carious lesions. Clin. Oral Investig. 2015, 19, 1473–1483. [Google Scholar] [CrossRef]
- Yazkan, B.; Ermis, R.B. Effect of resin infiltration and microabrasion on the microhardness, surface roughness and morphology of incipient carious lesions. Acta Odontol. Scand. 2018, 76, 473–481. [Google Scholar] [CrossRef] [PubMed]
- Arnold, W.H.; Bachstaedter, L.; Benz, K.; Naumova, E.A. Resin Infiltration into Differentially Extended Experimental Carious Lesions. Open Dent. J. 2014, 8, 251–256. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gurdogan, E.B.; Ozdemir-Ozenen, D.; Sandalli, N. Evaluation of Surface Roughness Characteristics Using Atomic Force Microscopy and Inspection of Microhardness Following Resin Infiltration with Icon ®. J. Esthet. Restor. Dent. 2017, 29, 201–208. [Google Scholar] [CrossRef] [PubMed]
- Meyer-Lueckel, H.; Paris, S.; Mueller, J.; Cölfen, H.; Kielbassa, A. Influence of the application time on the penetration of different dental adhesives and a fissure sealant into artificial subsurface lesions in bovine enamel. Dent. Mater. 2006, 22, 22–28. [Google Scholar] [CrossRef]
- Aziznezhad, M.; Alaghemand, H.; Shahande, Z.; Pasdar, N.; Bijani, A.; Eslami, A.; Dastan, Z. Comparison of the effect of resin infiltrant, fluoride varnish, and nano-hydroxy apatite paste on surface hardness and Streptococcus mutans adhesion to artificial enamel lesions. Electron. Physician 2017, 9, 3934–3942. [Google Scholar] [CrossRef] [Green Version]
- Goldberg, M. In vitro and in vivo studies on the toxicity of dental resin components: A review. Clin. Oral Investig. 2007, 12, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Samuelsen, J.T.; Dahl, J.E.; Karlsson, S.; Morisbak, E.; Becher, R. Apoptosis induced by the monomers HEMA and TEGDMA involves formation of ROS and differential activation of the MAP-kinases p38, JNK and ERK. Dent. Mater. 2007, 23, 34–39. [Google Scholar] [CrossRef] [PubMed]
- Batarseh, G.; Windsor, L.J.; Labban, N.Y.; Liu, Y.; Gregson, K. Triethylene Glycol Dimethacrylate Induction of Apoptotic Proteins in Pulp Fibroblasts. Oper. Dent. 2014, 39, E1–E8. [Google Scholar] [CrossRef] [PubMed]
- Meyer-Lückel, H.; Hartwig, C.; Börner, H.G.; Lausch, J. Elution of Monomers from an Infiltrant Compared with Different Resin-Based Dental Materials. Oral Health Prev. Dent. 2020, 18, 337–341. [Google Scholar] [CrossRef]
- Galler, K.M.; Schweikl, H.; Hiller, K.-A.; Cavender, A.C.; Bolay, C.; D’Souza, R.N.; Schmalz, G. TEGDMA Reduces Mineralization in Dental Pulp Cells. J. Dent. Res. 2010, 90, 257–262. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, M.; Li, J.-Z.; Zuo, Q.-L.; Liu, C.; Jiang, H.; Du, M.-Q. Accelerated aging effects on color, microhardness and microstructure of ICON resin infiltration. Eur. Rev. Med. Pharmacol. Sci. 2019, 23, 7722–7731. [Google Scholar]
- Arslan, S.; Lipski, L.; Dubbs, K.; Elmali, F.; Ozer, F. Effects of different resin sealing therapies on nanoleakage within artificial non-cavitated enamel lesions. Dent. Mater. J. 2018, 37, 981–987. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhao, X.; Ren, Y.-F. Surface Properties and Color Stability of Resin-Infiltrated Enamel Lesions. Oper. Dent. 2016, 41, 617–626. [Google Scholar] [CrossRef]
- Finer, Y.; Santerre, J. Salivary Esterase Activity and Its Association with the Biodegradation of Dental Composites. J. Dent. Res. 2004, 83, 22–26. [Google Scholar] [CrossRef]
- Huang, B.; Siqueira, W.L.; Cvitkovitch, D.G.; Finer, Y. Esterase from a cariogenic bacterium hydrolyzes dental resins. Acta Biomater. 2018, 71, 330–338. [Google Scholar] [CrossRef]
- Stewart, C.A.; Finer, Y. Biostable, antidegradative and antimicrobial restorative systems based on host-biomaterials and microbial interactions. Dent. Mater. 2019, 35, 36–52. [Google Scholar] [CrossRef]
- Singh, J.; Khalichi, P.; Cvitkovitch, D.G.; Santerre, J.P. Composite resin degradation products from BisGMA monomer modulate the expression of genes associated with biofilm formation and other virulence factors in Streptococcus mutans. J. Biomed. Mater. Res. Part A 2009, 88A, 551–560. [Google Scholar] [CrossRef]
- Sadeghinejad, L.; Cvitkovitch, D.G.; Siqueira, W.L.; Santerre, J.P.; Finer, Y. Triethylene Glycol Up-Regulates Virulence-Associated Genes and Proteins in Streptococcus mutans. PLoS ONE 2016, 11, e0165760. [Google Scholar] [CrossRef]
- Khalichi, P. Effect of composite resin biodegradation products on oral streptococcal growth. Biomaterials 2004, 25, 5467–5472. [Google Scholar] [CrossRef]
- Huang, B.; Sadeghinejad, L.; Adebayo, O.I.; Ma, D.; Xiao, Y.; Siqueira, W.L.; Cvitkovitch, D.G.; Finer, Y. Gene expression and protein synthesis of esterase from Streptococcus mutans are affected by biodegradation by-product from methacrylate resin composites and adhesives. Acta Biomater. 2018, 81, 158–168. [Google Scholar] [CrossRef]
- Nedeljkovic, I.; De Munck, J.; Ungureanu, A.-A.; Slomka, V.; Bartic, C.; Vananroye, A.; Clasen, C.; Teughels, W.; Van Meerbeek, B.; Van Landuyt, K.L. Biofilm-induced changes to the composite surface. J. Dent. 2017, 63, 36–43. [Google Scholar] [CrossRef] [PubMed]
- Rai, P.; Pandey, R.K.; Khanna, R. Qualitative and Quantitative Effect of a Protective Chlorhexidine Varnish Layer Over Resin-infiltrated Proximal Carious Lesions in Primary Teeth. Pediatr. Dent. 2016, 38, 6. [Google Scholar]
- Inagaki, L.T.; Dainezi, V.B.; Alonso, R.C.B.; de Paula, A.B.; Garcia-Godoy, F.; Puppin-Rontani, R.M.; Pascon, F.M. Evaluation of sorption/solubility, softening, flexural strength and elastic modulus of experimental resin blends with chlorhexidine. J. Dent. 2016, 49, 40–44. [Google Scholar] [CrossRef] [PubMed]
- Inagaki, L.T.; Alonso, R.C.B.; Araújo, G.A.S.; de Souza-Junior, E.J.C.; Anibal, P.C.; Höfling, J.F.; Pascon, F.M.; Puppin-Rontani, R.M. Effect of monomer blend and chlorhexidine-adding on physical, mechanical and biological properties of experimental infiltrants. Dent. Mater. 2016, 32, e307–e313. [Google Scholar] [CrossRef]
- Flor-Ribeiro, M.D.; Graziano, T.S.; Aguiar, F.H.B.; Stipp, R.N.; Marchi, G.M. Effect of iodonium salt and chitosan on the physical and antibacterial properties of experimental infiltrants. Braz. Oral Res. 2019, 33, e075. [Google Scholar] [CrossRef]
- Cheng, L.; Zhang, K.; Zhang, N.; Melo, M.; Weir, M.; Zhou, X.; Bai, Y.; Reynolds, M.; Xu, H. Developing a New Generation of Antimicrobial and Bioactive Dental Resins. J. Dent. Res. 2017, 96, 855–863. [Google Scholar] [CrossRef] [PubMed]
- Gonçalves, L.S.; Moraes, R.; Ogliari, F.A.; Boaro, L.; Braga, R.R.; Consani, S. Improved polymerization efficiency of methacrylate-based cements containing an iodonium salt. Dent. Mater. 2013, 29, 1251–1255. [Google Scholar] [CrossRef] [PubMed]
- Elsaka, S.E. Antibacterial Activity and Adhesive Properties of a Chitosan-Containing Dental Adhesive. Quintessence Int. 2012, 43, 603–613. [Google Scholar] [PubMed]
- Yu, J.; Huang, X.; Zhou, X.; Han, Q.; Zhou, W.; Liang, J.; Xu, H.H.; Ren, B.; Peng, X.; Weir, M.D.; et al. Anti-caries effect of resin infiltrant modified by quaternary ammonium monomers. J. Dent. 2020, 97, 103355. [Google Scholar] [CrossRef]
- Villegas, N.A.; Compagnucci, M.J.S.; Ajá, M.S.; Rocca, D.M.; Becerra, M.C.; Molina, G.F.; Palma, S.D. Novel Antibacterial Resin-Based Filling Material Containing Nanoparticles for the Potential One-Step Treatment of Caries. J. Health Eng. 2019, 2019, 6367919. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Martinez-Gutierrez, F.; Olive, P.L.; Banuelos, A.; Orrantia, E.; Niño, N.; Sanchez, E.M.; Ruiz, F.; Bach, H.; Av-Gay, Y. Synthesis, characterization, and evaluation of antimicrobial and cytotoxic effect of silver and titanium nanoparticles. Nanomed. Nanotechnol. Biol. Med. 2010, 6, 681–688. [Google Scholar] [CrossRef]
- Sevinç, B.A.; Hanley, L. Antibacterial activity of dental composites containing zinc oxide nanoparticles. J. Biomed. Mater. Res. Part B Appl. Biomater. 2010, 9999B, 22–31. [Google Scholar] [CrossRef] [Green Version]
- Kasraei, S.; Sami, L.; Hendi, S.; AliKhani, M.-Y.; Rezaei-Soufi, L.; Khamverdi, Z. Antibacterial properties of composite resins incorporating silver and zinc oxide nanoparticles on Streptococcus mutans and Lactobacillus. Restor. Dent. Endod. 2014, 39, 109–114. [Google Scholar] [CrossRef] [Green Version]
- Azarsina, M.; Kasraei, S.; Yousefi-Mashouf, R.; Dehghani, N.; Shirinzad, M. The Antibacterial Properties of Composite Resin Containing Nanosilver against Streptococcus mutans and Lactobacillus. J. Contemp. Dent. Pract. 2013, 14, 1014–1018. [Google Scholar] [CrossRef] [PubMed]
- AlHumaid, J.; Al-Harbi, F.; El Tantawi, M.; Elembaby, A. X-ray microtomography assessment of Carisolv and Papacarie effect on dentin mineral density and amount of removed tissue. Acta Odontol. Scand. 2018, 76, 236–240. [Google Scholar] [CrossRef] [PubMed]
- Hegde, S.; Kakti, A.; Bolar, D.R.; Bhaskar, S.A. Clinical Efficiency of Three Caries Removal Systems: Rotary Excavation, Carisolv, and Papacarie. J. Dent. Child. 2016, 83, 22–28. [Google Scholar]
- Santos, T.M.L.; Bresciani, E.; Matos, F.D.S.; Camargo, S.E.A.; Hidalgo, A.P.T.; Rivera, L.M.L.; Bernardino, Í.D.M.; Paranhos, L.R. Comparison between conventional and chemomechanical approaches for the removal of carious dentin: An in vitro study. Sci. Rep. 2020, 10, 8127. [Google Scholar] [CrossRef]
- Deng, Y.; Feng, G.; Hu, B.; Kuang, Y.; Song, J. Effects of Papacarie on children with dental caries in primary teeth: A systematic review and meta-analysis. Int. J. Paediatr. Dent. 2018, 28, 361–372. [Google Scholar] [CrossRef] [PubMed]
- Smales, R.J.; Yip, H.K. The atraumatic restorative treatment (ART) approach for primary teeth: Review of literature. Pediatr. Dent. 2000, 22, 294–298. [Google Scholar]
- Holmgren, C.J.; Roux, D.; Doméjean, S. Minimal intervention dentistry: Part 5. Atraumatic restorative treatment (ART)—A minimum intervention and minimally invasive approach for the management of dental caries. Br. Dent. J. 2013, 214, 11–18. [Google Scholar] [CrossRef] [PubMed]
- Tyas, M. Cariostatic effect of glass ionomer cement: A five-year clinical study. Aust. Dent. J. 1991, 36, 236–239. [Google Scholar] [CrossRef]
- Tam, L.E.; Chan, G.P.; Yim, D. In vitro caries inhibition effects by conventional and resin-modified glass-ionomer restorations. Oper. Dent. 1997, 22, 4–14. [Google Scholar]
- Forsten, L. Fluoride release and uptake by glass-ionomers and related materials and its clinical effect. Biomaterials 1998, 19, 503–508. [Google Scholar] [CrossRef]
- Donly, K.J.; Nelson, J.J. Fluoride release of restorative materials exposed to a fluoridated dentifrice. ASDC J. Dent. Child. 1997, 64, 249–250. [Google Scholar] [PubMed]
- Crystal, Y.O.; Marghalani, A.A.; Ureles, S.D.; Wright, J.T.; Sulyanto, R.; Divaris, K.; Fontana, M.; Graham, L. Use of Silver Diamine Luoride for Dental Caries Management in Children and Adolescents, Including Those with Special Health Care Needs. Pediatr. Dent. 2017, 39, 135–145. [Google Scholar] [PubMed]
- Kemoli, A.M.; Van Amerongen, W.E. Effects of oral hygiene, residual caries and cervical Marginal-gaps on the survival of proximal atraumatic restorative treatment approach restorations. Contemp. Clin. Dent. 2011, 2, 318–323. [Google Scholar] [CrossRef]
- Da Franca, C.; Colares, V.; Van Amerongen, E. Two-year evaluation of the atraumatic restorative treatment approach in primary molars class I and II restorations. Int. J. Paediatr. Dent. 2011, 21, 249–253. [Google Scholar] [CrossRef] [PubMed]
- De Amorim, R.G.; Frencken, J.E.; Raggio, D.P.; Chen, X.; Hu, X.; Leal, S.C. Survival percentages of atraumatic restorative treatment (ART) restorations and sealants in posterior teeth: An updated systematic review and meta-analysis. Clin. Oral Investig. 2018, 22, 2703–2725. [Google Scholar] [CrossRef] [PubMed]
- Faccin, E.S.; Ferreira, S.H.; Kramer, P.F.; Ardenghi, T.; Feldens, C.A. Clinical Performance of Art Restorations in Primary Teeth: A Survival Analysis. J. Clin. Pediatr. Dent. 2009, 33, 295–298. [Google Scholar] [CrossRef]
- Joshi, J.S.; Roshan, N.M.; Sakeenabi, B.; Poornima, P.; Nagaveni, N.B.; Subbareddy, V.V. Inhibition of Residual Cariogenic Bacteria in Atraumatic Restorative Treatment by Chlorhexidine: Disinfection or Incorporation. Pediatr. Dent. 2017, 39, 308–312. [Google Scholar]
- Kabil, N.S.; Badran, A.; Wassel, M.O. Effect of the addition of chlorhexidine and miswak extract on the clinical performance and antibacterial properties of conventional glass ionomer: An in vivo study. Int. J. Paediatr. Dent. 2016, 27, 380–387. [Google Scholar] [CrossRef] [PubMed]





Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Desai, H.; Stewart, C.A.; Finer, Y. Minimally Invasive Therapies for the Management of Dental Caries—A Literature Review. Dent. J. 2021, 9, 147. https://doi.org/10.3390/dj9120147
Desai H, Stewart CA, Finer Y. Minimally Invasive Therapies for the Management of Dental Caries—A Literature Review. Dentistry Journal. 2021; 9(12):147. https://doi.org/10.3390/dj9120147
Chicago/Turabian StyleDesai, Hetal, Cameron A. Stewart, and Yoav Finer. 2021. "Minimally Invasive Therapies for the Management of Dental Caries—A Literature Review" Dentistry Journal 9, no. 12: 147. https://doi.org/10.3390/dj9120147
APA StyleDesai, H., Stewart, C. A., & Finer, Y. (2021). Minimally Invasive Therapies for the Management of Dental Caries—A Literature Review. Dentistry Journal, 9(12), 147. https://doi.org/10.3390/dj9120147





