Injectable Human Hair Keratin–Fibrinogen Hydrogels for Engineering 3D Microenvironments to Accelerate Oral Tissue Regeneration
Abstract
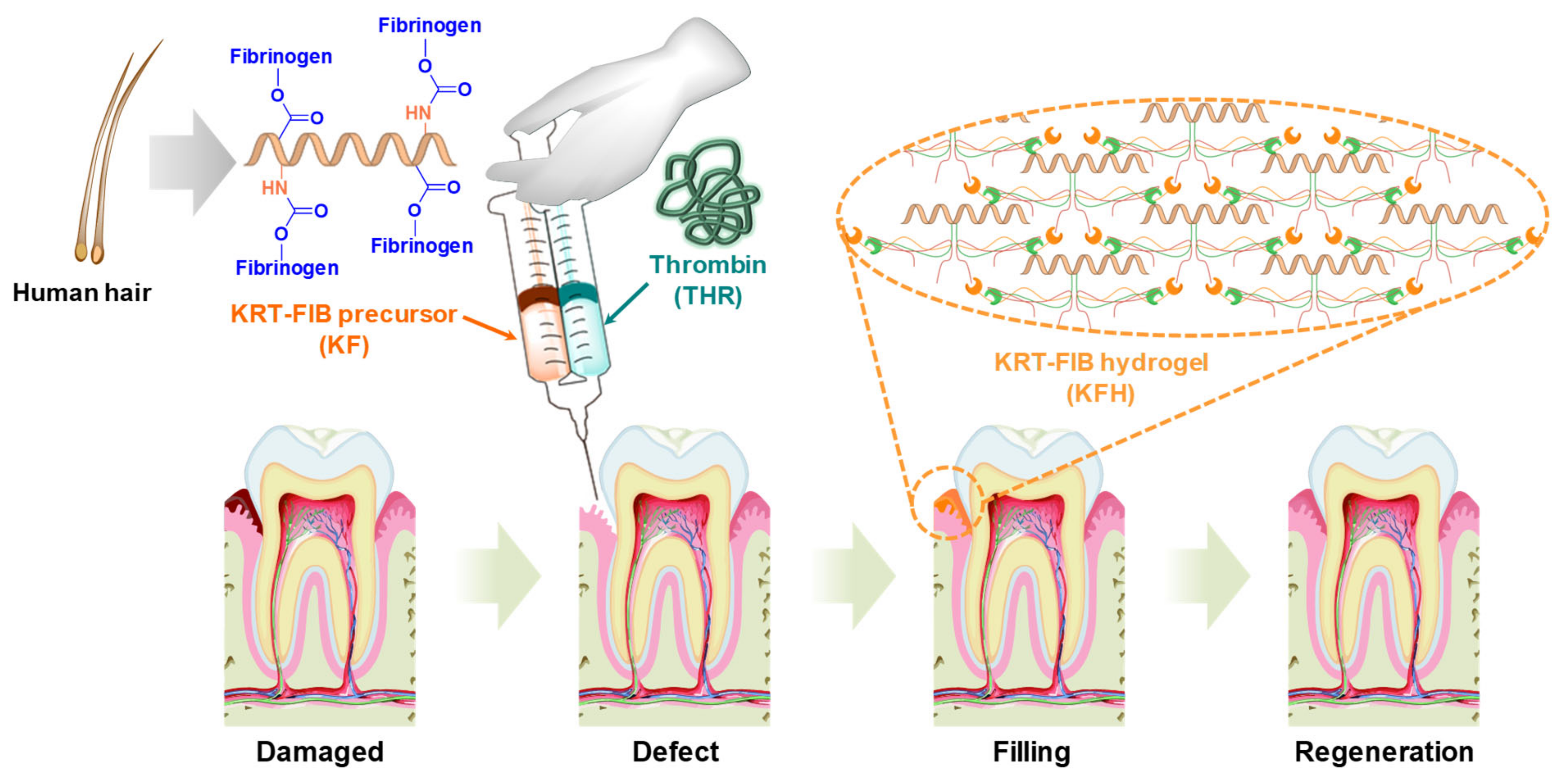
:1. Introduction
2. Results and Discussion
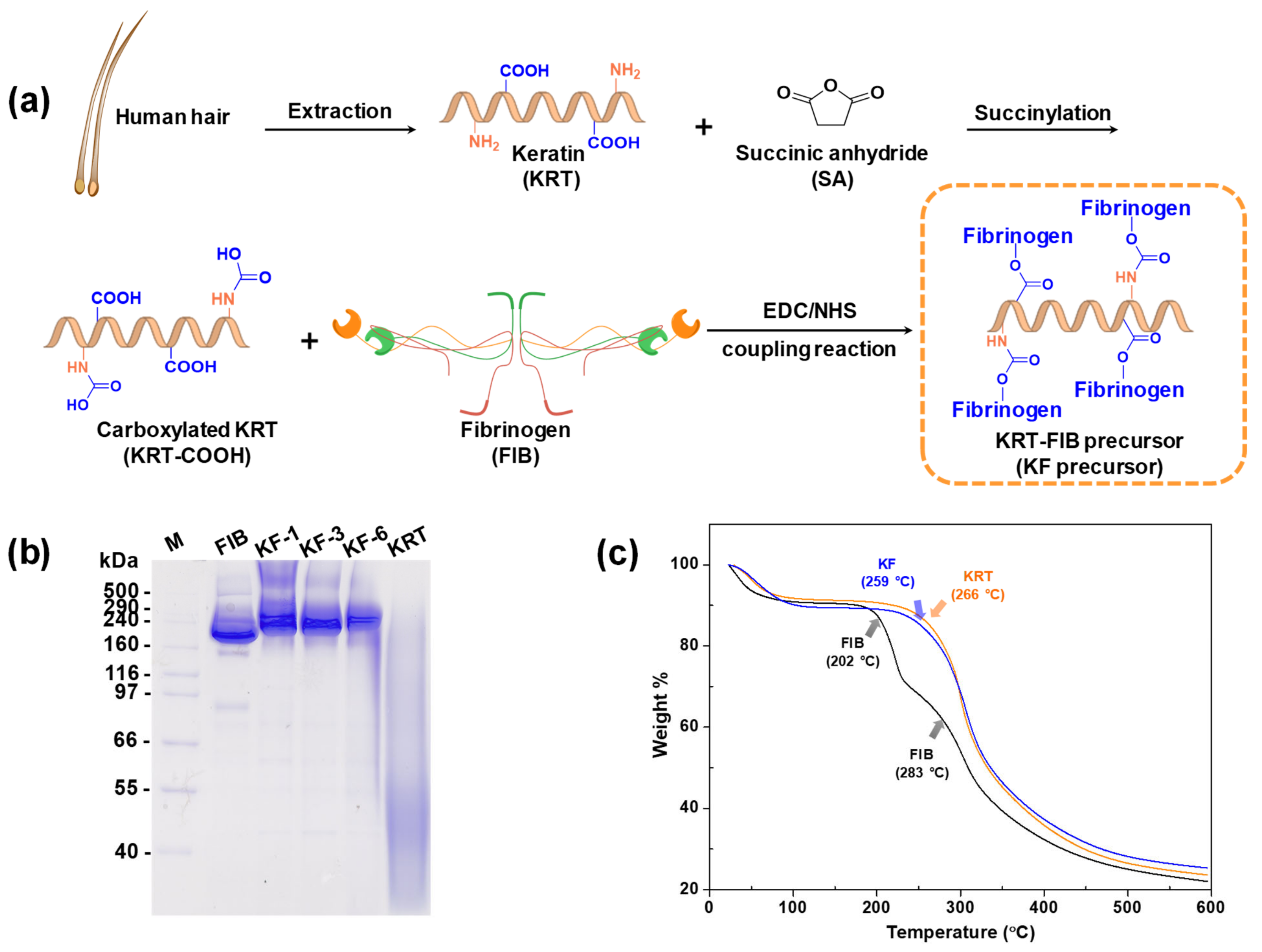
2.1. Synthesis of KRT-FIB Precursors
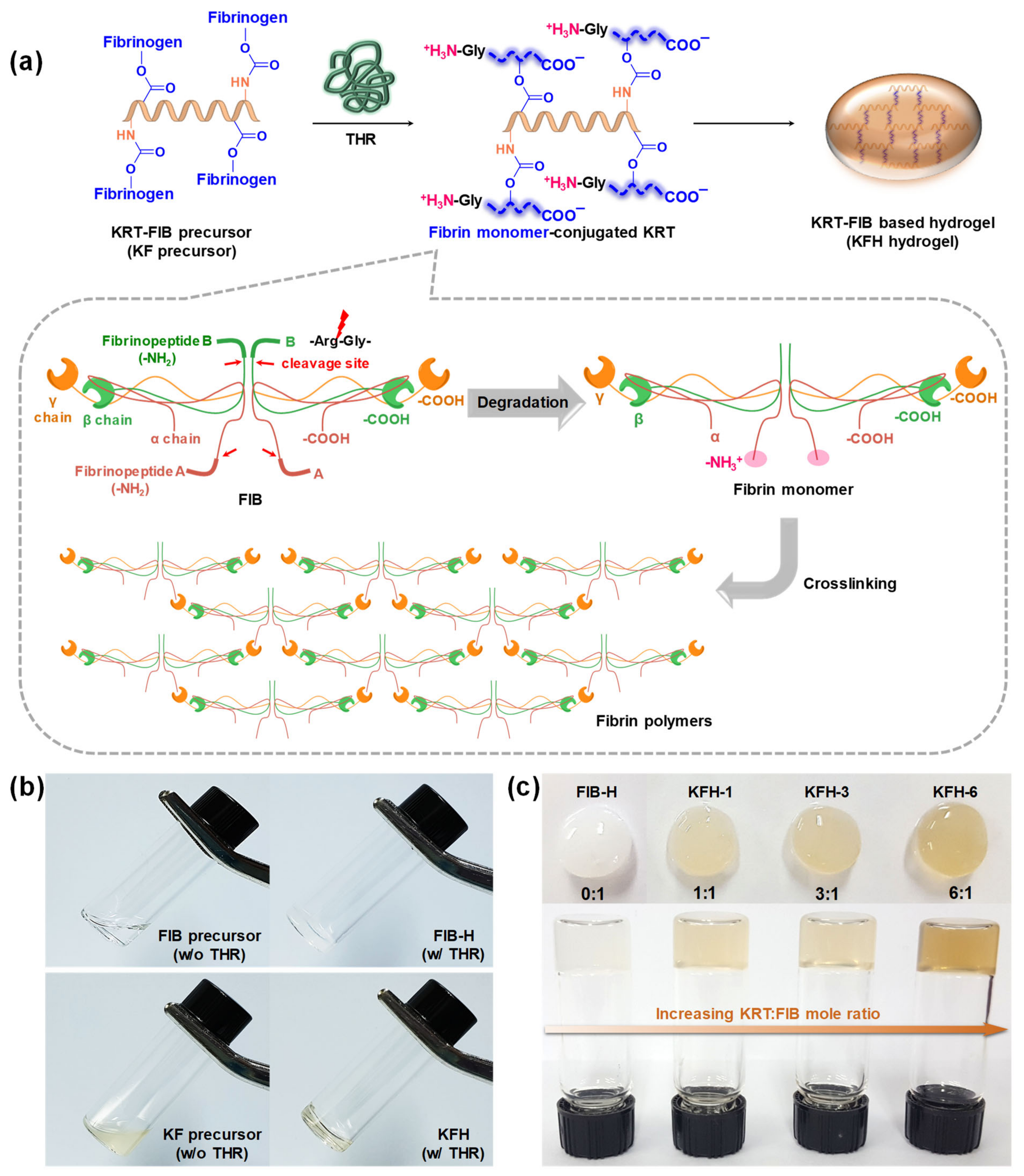
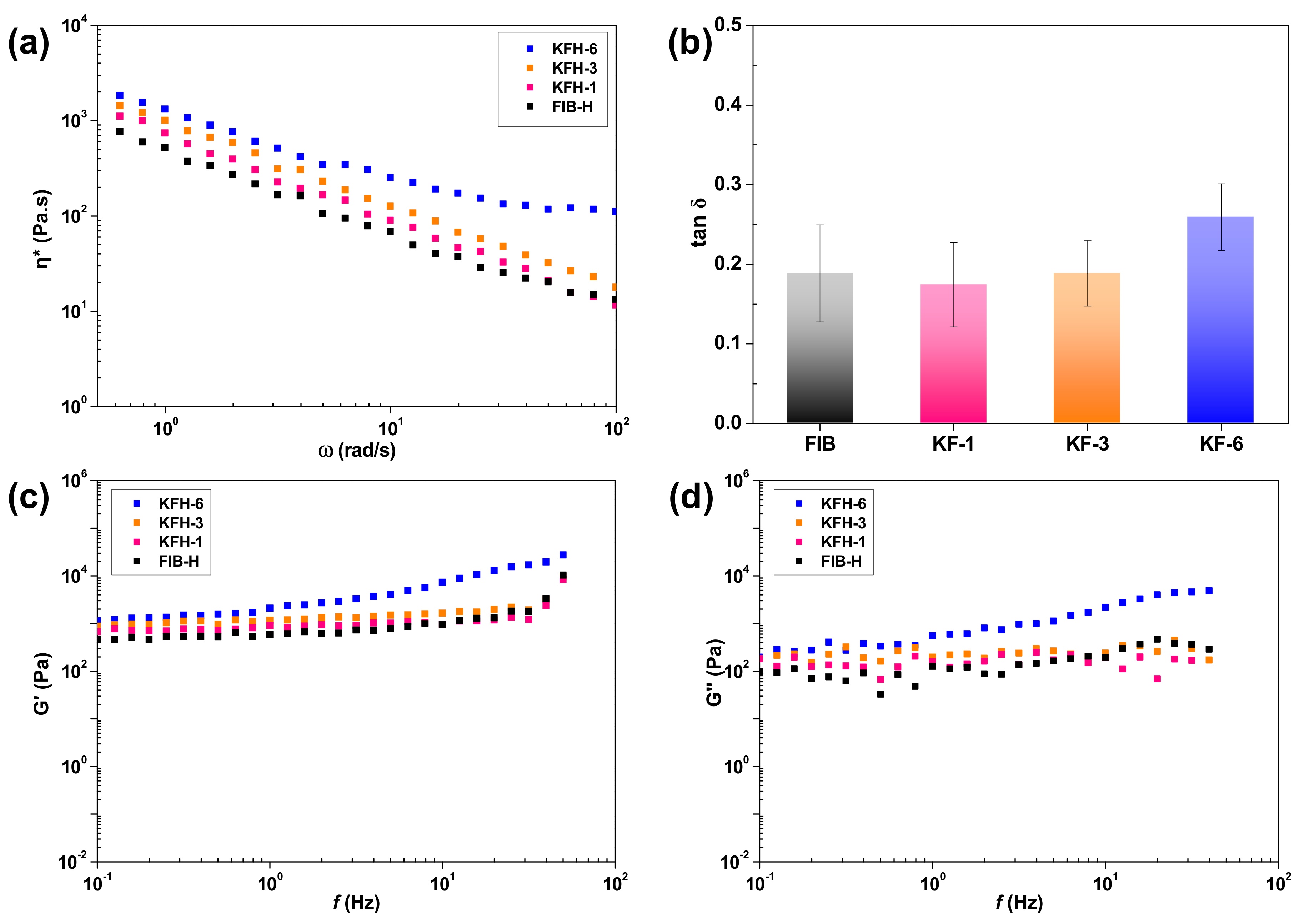
2.2. Preparation and Rheological Studies of KFHs
2.3. Injectable Performance, Swelling, and Degradation Behavior of KFH-3
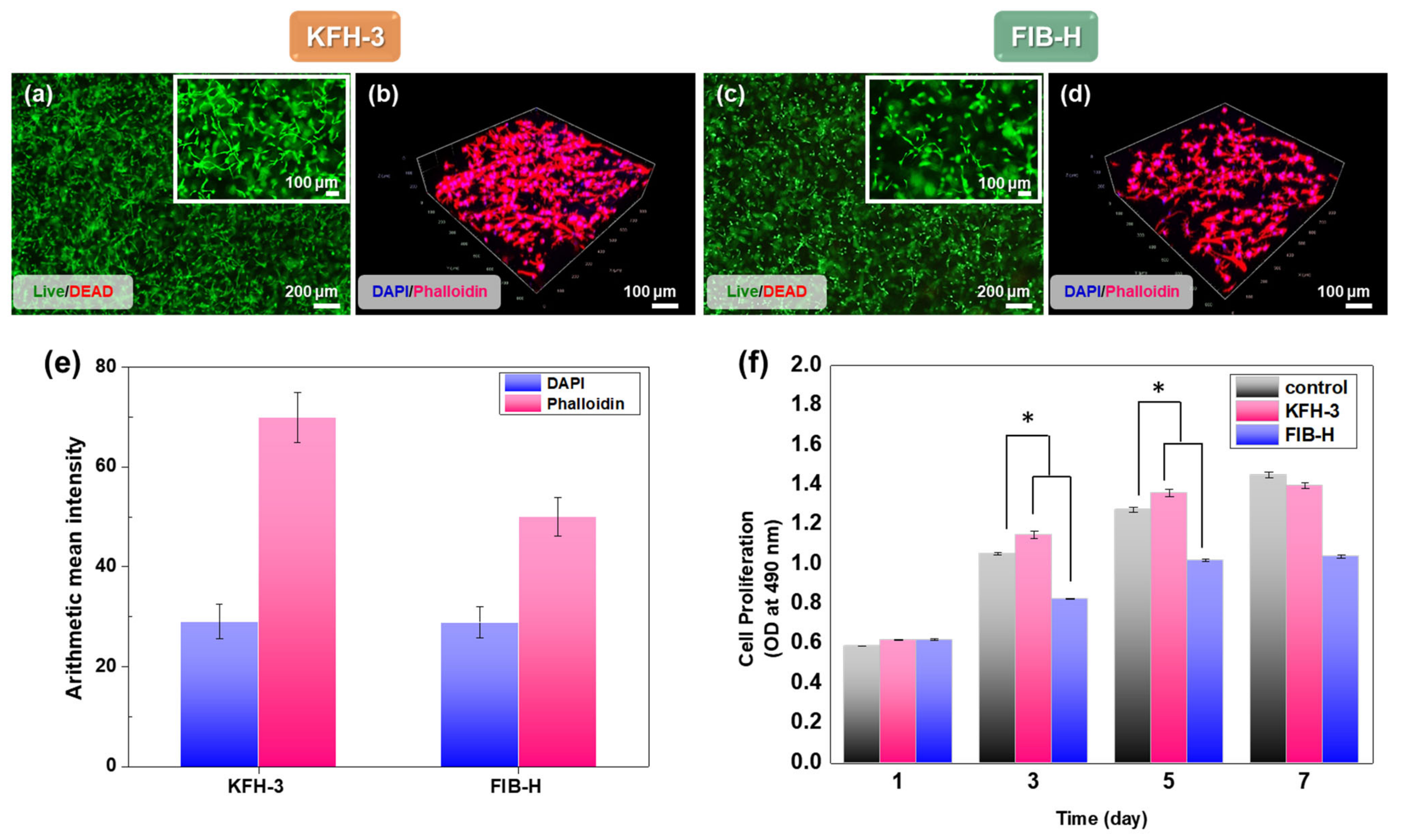
2.4. HGF Viability in KRT-Based Fibrin Hydrogels: Cytotoxicity Studies and 3D Cell Encapsulation in KRT-Based Fibrin Hydrogels
3. Materials and Methods
3.1. Materials
3.2. Succinylation of KRT Protein (KRT-COOH)
3.3. Synthesis of KRT-FIB Precursors via An EDC/NHS Coupling Reaction
3.4. SDS-PAGE
3.5. Preparation of KFHs and FIB-H Hydrogels
3.6. Characterization of Injectable Hydrogels
3.7. Swelling and Degradation Studies
3.8. Cell Culture
3.9. Cell Encapsulation
3.10. Cell Viability
3.11. Confocal Laser Scanning Microscopy
3.12. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Moll, R.; Divo, M.; Langbein, L. The human keratins: Biology and pathology. Histochem. Cell Biol. 2008, 129, 705. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marceau, N.; Loranger, A.; Gilbert, S.; Daigle, N.; Champetier, S. Keratin-mediated resistance to stress and apoptosis in simple epithelial cells in relation to health and disease. Biochem. Cell Biol. 2001, 79, 543–555. [Google Scholar] [CrossRef] [PubMed]
- Sierpinski, P.; Garrett, J.; Ma, J.; Apel, P.; Klorig, D.; Smith, T.; Koman, L.A.; Atala, A.; Van Dyke, M. The use of keratin biomaterials derived from human hair for the promotion of rapid regeneration of peripheral nerves. Biomaterials 2008, 29, 118–128. [Google Scholar] [CrossRef] [PubMed]
- Richter, J.R.; De Guzman, R.C.; Greengauz-Roberts, O.K.; Van Dyke, M. Structure-property relationships of meta-kerateine biomaterials derived from human hair. Acta Biomater. 2012, 8, 274–281. [Google Scholar] [CrossRef]
- Rahmany, M.B.; Hantgan, R.R.; Van Dyke, M. A mechanistic investigation of the effect of keratin-based hemostatic agents on coagulation. Biomaterials 2013, 34, 2492–2500. [Google Scholar] [CrossRef]
- Rouse, J.G.; Van Dyke, M.E. A review of keratin-based biomaterials for biomedical applications. Materials 2010, 3, 999–1014. [Google Scholar] [CrossRef] [Green Version]
- Verma, V.; Verma, P.; Ray, P.; Ray, A.R. Preparation of scaffolds from human hair proteins for tissue-engineering applications. Biomed. Mater. 2008, 3, 025007. [Google Scholar] [CrossRef]
- Hsieh, J.Y.; Smith, T.D.; Meli, V.S.; Tran, T.N.; Botvinick, E.L.; Liu, W.F. Differential regulation of macrophage inflammatory activation by fibrin and fibrinogen. Acta Biomater. 2017, 47, 14–24. [Google Scholar] [CrossRef] [Green Version]
- Horbett, T.A. Fibrinogen adsorption to biomaterials. J. Biomed. Mater. Res. A 2018, 106, 2777–2788. [Google Scholar] [CrossRef]
- Solovieva, E.V.; Fedotov, A.Y.; Mamonov, V.E.; Komlev, V.S.; Panteleyev, A.A. Fibrinogen-modified sodium alginate as a scaffold material for skin tissue engineering. Biomed. Mater. 2018, 13, 025007. [Google Scholar] [CrossRef] [Green Version]
- Litvinov, R.I.; Weisel, J.W. Fibrin mechanical properties and their structural origins. Matrix Biology 2017, 60, 110–123. [Google Scholar] [CrossRef]
- Yu, Z.; Li, H.; Xia, P.; Kong, W.; Chang, Y.; Fu, C.; Wang, K.; Yang, X.; Qi, Z. Application of fibrin-based hydrogels for nerve protection and regeneration after spinal cord injury. J. Biol. Eng. 2020, 14, 1–15. [Google Scholar] [CrossRef]
- Cao, Y.; Yao, Y.; Li, Y.; Yang, X.; Cao, Z.; Yang, G. Tunable keratin hydrogel based on disulfide shuffling strategy for drug delivery and tissue engineering. J. Colloid Interface Sci. 2019, 544, 121–129. [Google Scholar] [CrossRef]
- Lou, J.; Liu, F.; Lindsay, C.D.; Chaudhuri, O.; Heilshorn, S.C.; Xia, Y. Dynamic hyaluronan hydrogels with temporally modulated high injectability and stability using a biocompatible catalyst. Adv. Mater. 2018, 30, 1705215. [Google Scholar] [CrossRef]
- Han, C.; Zhang, H.; Wu, Y.; He, X.; Chen, X. Dual-crosslinked hyaluronan hydrogels with rapid gelation and high injectability for stem cell protection. Sci. Rep. 2020, 10, 1–7. [Google Scholar]
- Zhu, Y.; Luo, Q.; Zhang, H.; Cai, Q.; Li, X.; Shen, Z.; Zhu, W. A shear-thinning electrostatic hydrogel with antibacterial activity by nanoengineering of polyelectrolytes. Biomater. Sci. 2020, 8, 1394–1404. [Google Scholar] [CrossRef]
- Slaughter, B.V.; Khurshid, S.S.; Fisher, O.Z.; Khademhosseini, A.; Peppas, N.A. Hydrogels in regenerative medicine. Adv. Mater. 2009, 21, 3307–3329. [Google Scholar] [CrossRef] [Green Version]
- Yan, C.; Pochan, D.J. Rheological properties of peptide-based hydrogels for biomedical and other applications. Chem. Soc. Rev. 2010, 39, 3528–3540. [Google Scholar] [CrossRef] [Green Version]
- Mendez, J.J.; Ghaedi, M.; Sivarapatna, A.; Dimitrievska, S.; Shao, Z.; Osuji, C.O.; Steinbacher, D.M.; Leffell, D.J.; Niklason, L.E. Mesenchymal stromal cells form vascular tubes when placed in fibrin sealant and accelerate wound healing in vivo. Biomaterials 2015, 40, 61–71. [Google Scholar] [CrossRef] [Green Version]
- Dai, Y.; Liu, G.; Ma, L.; Wang, D.; Gao, C. Cell-free macro-porous fibrin scaffolds for in situ inductive regeneration of full-thickness cartilage defects. J. Mater. Chem. B 2016, 4, 4410–4419. [Google Scholar] [CrossRef]
- Sauerwein, M.; Steeb, H. Modeling of dynamic hydrogel swelling within the pore space of a porous medium. Int. J. Eng. Sci. 2020, 155, 103353. [Google Scholar] [CrossRef]
- Reinertsen, E.; Skinner, M.; Wu, B.; Tawil, B. Concentration of fibrin and presence of plasminogen affect proliferation, fibrinolytic activity, and morphology of human fibroblasts and keratinocytes in 3D fibrin constructs. Tissue Eng. Part A 2014, 20, 2860–2869. [Google Scholar] [CrossRef] [Green Version]
- Tsai, T.-Y.; Shen, K.-H.; Chang, C.-W.; Jovanska, L.; Wang, R.; Yeh, Y.-C. In situ formation of nanocomposite double-network hydrogels with shear-thinning and self-healing properties. Biomater. Sci. 2021, 9, 985–999. [Google Scholar] [CrossRef]
- Rahman, M.S.; Islam, M.M.; Islam, M.S.; Zaman, A.; Ahmed, T.; Biswas, S.; Sharmeen, S.; Rashid, T.U.; Rahman, M.M. Morphological Characterization of Hydrogels. In Cellulose-Based Superabsorbent Hydrogels; Mondal, M.I.H., Ed.; Springer International Publishing: Cham, Switzerland, 2019; pp. 819–863. [Google Scholar]
- Gao, L.; Chen, J.; Feng, W.; Song, Q.; Huo, J.; Yu, L.; Liu, N.; Wang, T.; Li, P.; Huang, W. A multifunctional shape-adaptive and biodegradable hydrogel with hemorrhage control and broad-spectrum antimicrobial activity for wound healing. Biomater. Sci. 2020, 8, 6930–6945. [Google Scholar] [CrossRef]
- Akther, F.; Little, P.; Li, Z.; Nguyen, N.-T.; Ta, H.T. Hydrogels as artificial matrices for cell seeding in microfluidic devices. RSC Adv. 2020, 10, 43682–43703. [Google Scholar] [CrossRef]
- Annabi, N.; Mithieux, S.M.; Weiss, A.S.; Dehghani, F. Cross-linked open-pore elastic hydrogels based on tropoelastin, elastin and high-pressure CO2. Biomaterials 2010, 31, 1655–1665. [Google Scholar] [CrossRef]
- Rosser, J.; Calvo, I.; Schlager, M.; Purtscher, M.; Jenner, F.; Ertl, P. Recent advances of biologically inspired 3D microfluidic hydrogel cell culture systems. J. Cell Biol. Cell Metab. 2015, 2, 1–14. [Google Scholar]
- Lee, J.-Y.; Tan, B.; Cooper, A.I. CO2-in-water emulsion-templated poly (vinyl alcohol) hydrogels using poly (vinyl acetate)-based surfactants. Macromolecules 2007, 40, 1955–1961. [Google Scholar] [CrossRef]
- Murphy, C.M.; O’Brien, F.J. Understanding the effect of mean pore size on cell activity in collagen-glycosaminoglycan scaffolds. Cell Adhes. Migr. 2010, 4, 377–381. [Google Scholar] [CrossRef] [Green Version]
- Gun’Ko, V.M.; Mikhalovska, L.I.; Savina, I.N.; Shevchenko, R.V.; James, S.L.; Tomlins, P.E.; Mikhalovsky, S.V. Characterisation and performance of hydrogel tissue scaffolds. Soft Matter 2010, 6, 5351–5358. [Google Scholar] [CrossRef]
- Rivero, R.E.; Capella, V.; Liaudat, A.C.; Bosch, P.; Barbero, C.A.; Rodríguez, N.; Rivarola, C.R. Mechanical and physicochemical behavior of a 3D hydrogel scaffold during cell growth and proliferation. RSC Adv. 2020, 10, 5827–5837. [Google Scholar] [CrossRef]
- Kim, S.Y.; Park, B.J.; Lee, Y.; Park, N.J.; Park, K.M.; Hwang, Y.-S.; Park, K.D. Human hair keratin-based hydrogels as dynamic matrices for facilitating wound healing. J. Ind. Eng. Chem. 2019, 73, 142–151. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kang, H.J.; Ko, N.; Oh, S.J.; An, S.Y.; Hwang, Y.-S.; Kim, S.Y. Injectable Human Hair Keratin–Fibrinogen Hydrogels for Engineering 3D Microenvironments to Accelerate Oral Tissue Regeneration. Int. J. Mol. Sci. 2021, 22, 13269. https://doi.org/10.3390/ijms222413269
Kang HJ, Ko N, Oh SJ, An SY, Hwang Y-S, Kim SY. Injectable Human Hair Keratin–Fibrinogen Hydrogels for Engineering 3D Microenvironments to Accelerate Oral Tissue Regeneration. International Journal of Molecular Sciences. 2021; 22(24):13269. https://doi.org/10.3390/ijms222413269
Chicago/Turabian StyleKang, Hyeon Jeong, Nare Ko, Seung Jun Oh, Seong Yeong An, Yu-Shik Hwang, and So Yeon Kim. 2021. "Injectable Human Hair Keratin–Fibrinogen Hydrogels for Engineering 3D Microenvironments to Accelerate Oral Tissue Regeneration" International Journal of Molecular Sciences 22, no. 24: 13269. https://doi.org/10.3390/ijms222413269
APA StyleKang, H. J., Ko, N., Oh, S. J., An, S. Y., Hwang, Y.-S., & Kim, S. Y. (2021). Injectable Human Hair Keratin–Fibrinogen Hydrogels for Engineering 3D Microenvironments to Accelerate Oral Tissue Regeneration. International Journal of Molecular Sciences, 22(24), 13269. https://doi.org/10.3390/ijms222413269





