An Exploration of Current and Perspective Semen Analysis and Sperm Selection for Livestock Artificial Insemination
Abstract
:Simple Summary
Abstract
1. Introduction
1.1. Cattle
1.2. Swine
1.3. Horses
1.4. Small Ruminants
2. Improving AI Technology
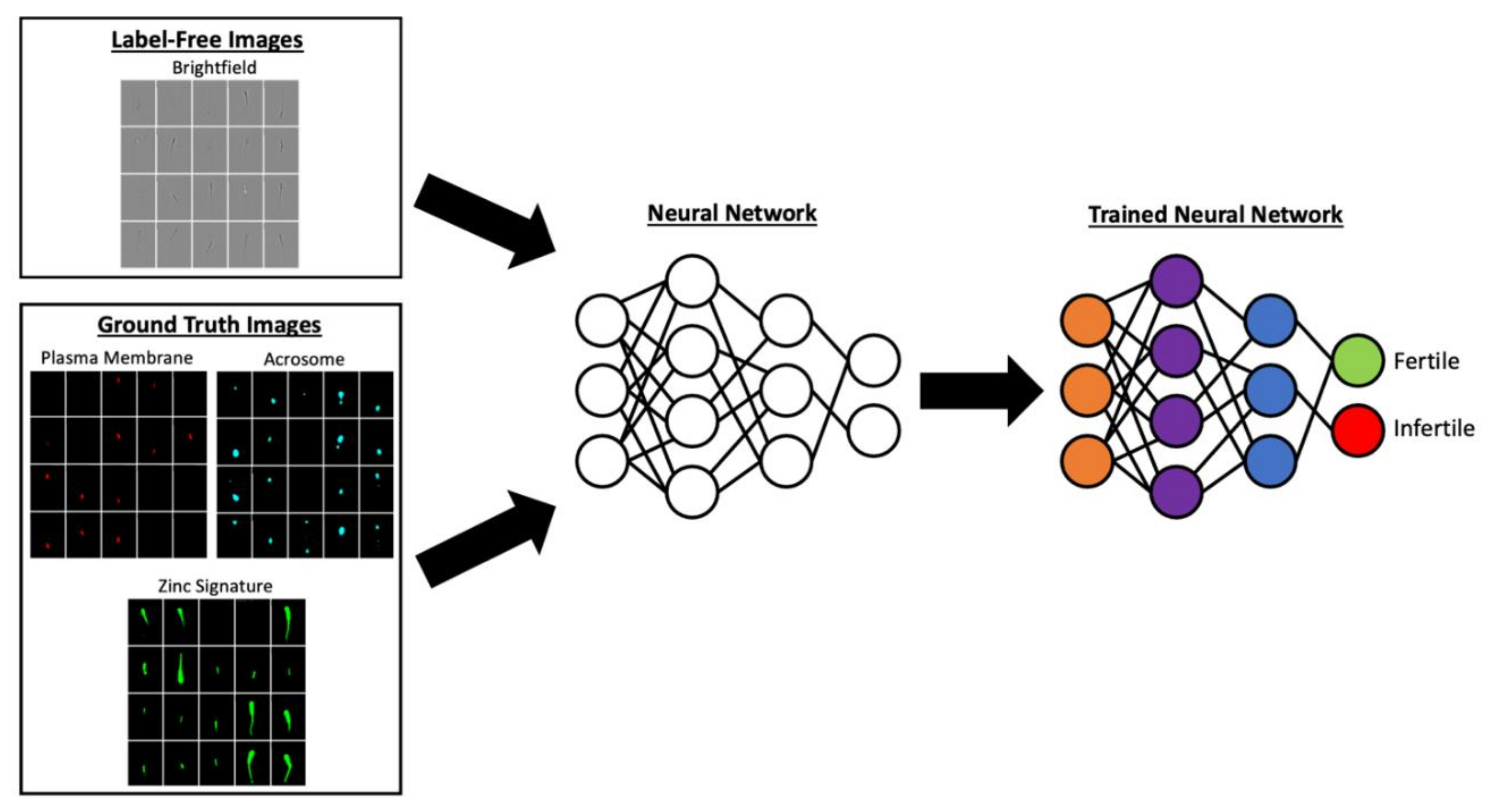
3. Semen Analysis
4. Sperm Selection
5. Sperm Sexing
6. Semen Storage
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ahmed, J.; Lorch, J.; Ong, L.; Wolfgram, J. How the Global Supply Landscape for Meat Protein Will Evolve. Available online: https://www.mckinsey.com/industries/agriculture/our-insights/how-the-global-supply-landscape-for-meat-protein-will-evolve (accessed on 20 December 2020).
- Ombelet, W.; Van Robays, J. Artificial insemination history: Hurdles and milestones. Facts Views Vis. ObGyn 2015, 7, 137–143. [Google Scholar] [PubMed]
- How Many Cows Can a Bull Service in a Normal Breeding Season. Beef Cattle 2019. Available online: https://beef-cattle.extension.org/how-many-cows-can-a-bull-service-in-a-normal-breeding-season/ (accessed on 20 December 2020).
- Norman, H.D.; Hubbard, S.M.; VanRaden, P.M.; Ullrey, D.E.; Baer, C.K.; Pond, W.G. Dairy Cattle: Breeding and Genetics; Taylor & Francis Group: Abingdon, UK, 2011; Volume 1, pp. 262–265. [Google Scholar] [CrossRef]
- Mosbergen, D. RIP Toystory, The Bull Who Was Daddy To 500,000 Offspring. Huffington Post, 27 January 2015. [Google Scholar]
- Perry, E.J. Artificial Insemination of Dairy Cows. Circular 1945, 491, 1–20. [Google Scholar]
- Research, G.V. Veterinary Artificial Insemination Market Size, Share & Trends Analysis Report by Animal Type (Cattle, Swine, Sheep, Canine, Equine, Others), by Product (Normal Semen, Sexed Semen), by End-Use, by Region, and Segment Forecasts, 2020–2027. Available online: https://www.grandviewresearch.com/industry-analysis/veterinary-artificial-insemination-market (accessed on 20 December 2020).
- APHIS. Bull Management Practices on U.S. Beef Cow-calf Operations. Available online: https://www.aphis.usda.gov/animal_health/nahms/beefcowcalf/downloads/beef0708/Beef0708_is_BullMgmt_1.pdf (accessed on 20 December 2020).
- Valergakis, G.E.; Arsenos, G.; Banos, G. Comparison of artificial insemination and natural service cost effectiveness in dairy cattle. Animal 2007, 1, 293–300. [Google Scholar] [CrossRef] [Green Version]
- Yang, D.; Standley, N.; Xu, Z. Application of liquid semen technology under the seasonal dairy production system in New Zealand. Anim. Reprod. Sci. 2018, 194, 2–10. [Google Scholar] [CrossRef]
- Morrell, J.M. Artificial Insemination: Current and Future Trends. In Artificial Insemination in Farm Animals; InTech: London, UK, 2011. [Google Scholar]
- Rodriquez-Martinez, H. Sperm biotechnologies in domestic species: State of the art. Anim. Reprod. 2013, 10, 268–276. [Google Scholar]
- Yeste, M. State-of-the-art of boar sperm preservation in liquid and frozen state. Anim. Reprod. 2017, 14, 69–81. [Google Scholar] [CrossRef]
- Brito, L.; Ramakrishnan, V.; Heuer, C.; Evans, K. Bovine sexed semen production and utilization. Clin. Theriogenol. 2019, 11, 297–315. [Google Scholar]
- Bathgate, R.; Mace, N.; Heasman, K.; Evans, G.; Maxwell, W.; De Graaf, S. Birth of Kids after Artificial Insemination with Sex-Sorted, Frozen-Thawed Goat Spermatozoa. Reprod. Domest. Anim. 2013, 48, 893–898. [Google Scholar] [CrossRef] [PubMed]
- Grossfeld, R.; Klinc, P.; Sieg, B.; Rath, D. Production of piglets with sexed semen employing a non-surgical insemination technique. Theriogenology 2005, 63, 2269–2277. [Google Scholar] [CrossRef]
- Abeydeera, L.; Johnson, L.; Welch, G.; Wang, W.; Boquest, A.; Cantley, T.; Rieke, A.; Day, B. Birth of piglets preselected for gender following in vitro fertilization of in vitro matured pig oocytes by X and Y chromosome bearing spermatozoa sorted by high speed flow cytometry. Theriogenology 1998, 50, 981–988. [Google Scholar] [CrossRef]
- Maes, D.; Rodriguez Alfonso, L.; Tom, R.; Phillip, V.; Soom Ann, V. Artificial Insemination in Pigs; IntechOpen: London, UK, 2011. [Google Scholar]
- Johnson, L.A.; Weitze, K.F.; Fiser, P.; Maxwell, W.M.C. Storage of boar semen. Anim. Reprod. Sci. 2000, 62, 143–172. [Google Scholar] [CrossRef]
- Johnson, L.A.; Rath, D.; Vazquez, J.M.; Maxwell, W.M.; Dobrinsky, J.R. Preselection of sex of offspring in swine for production: Current status of the process and its application. Theriogenology 2005, 63, 615–624. [Google Scholar] [CrossRef] [PubMed]
- Pig Progress. Producing Sex-Sorted Sperm with 99% Accuracy. Available online: https://www.pigprogress.net/Sows/Articles/2017/4/Producing-sex-sorted-sperm-with-99-accuracy-120805E/ (accessed on 14 April 2017).
- Club, T.J. (Ed.) The American Stud Book Principal Rules and Requirements; Arco Publishing: New York, NY, USA, 2013. [Google Scholar]
- Jäkel, H.; Henning, H.; Luther, A.; Rohn, K.; Waberski, D. Assessment of chilling injury in hypothermic stored boar spermatozoa by multicolor flow cytometry. Cytom. Part A 2021, 99, 1033–1041. [Google Scholar] [CrossRef]
- Sathe, S.R. Laparoscopic Artificial Insemination Technique in Small Ruminants—A Procedure Review. Front. Veter-Sci. 2018, 5, 266. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chelucci, S.; Pasciu, V.; Succu, S.; Addis, D.; Leoni, G.G.; Manca, M.E.; Naitana, S.; Berlinguer, F. Soybean lecithin–based extender preserves spermatozoa membrane integrity and fertilizing potential during goat semen cryopreservation. Theriogenology 2015, 83, 1064–1074. [Google Scholar] [CrossRef]
- Cogent Sexed Sheep Semen Service Launched. Available online: https://www.cogentuk.com/news/sexed-sheep-semen-service-launched (accessed on 30 December 2020).
- Countryside Magazine Contributor. RamGo for Your Flock of Sheep. The New, Successful, Non-Surgical Sheep Artifical Insemination. Available online: https://iamcountryside.com/sheep/ramgo-for-your-flock-of-sheep/ (accessed on 30 December 2020).
- Amann, R.P. Weaknesses in reports of “fertility” for horses and other species. Theriogenology 2005, 63, 698–715. [Google Scholar] [CrossRef]
- Flowers, W. Factors Affecting the Efficient Production of Boar Sperm. Reprod. Domest. Anim. 2015, 50, 25–30. [Google Scholar] [CrossRef] [Green Version]
- Sutovsky, P. New Approaches to Boar Semen Evaluation, Processing and Improvement. Reprod. Domest. Anim. 2015, 50 (Suppl. 2), 11–19. [Google Scholar] [CrossRef]
- Farrell, P.; Trouern-Trend, V.; Foote, R.H.; Douglas-Hamilton, D. Repeatability of measurements on human, rabbit, and bull sperm by computer-assisted sperm analysis when comparing individual fields and means of 12 fields. Fertil. Steril. 1995, 64, 208–210. [Google Scholar] [CrossRef]
- Sutovsky, P.; Aarabi, M.; Miranda-Vizuete, A.; Oko, R. Negative biomarker based male fertility evaluation: Sperm phenotypes associated with molecular-level anomalies. Asian J. Androl. 2015, 17, 554–560. [Google Scholar] [CrossRef]
- Kerns, K.; Zigo, M.; Drobnis, E.Z.; Sutovsky, M.; Sutovsky, P. Zinc ion flux during mammalian sperm capacitation. Nat. Commun. 2018, 9, 2061. [Google Scholar] [CrossRef] [Green Version]
- Kerns, K.; Sharif, M.; Zigo, M.; Xu, W.; Hamilton, L.E.; Sutovsky, M.; Ellersieck, M.; Drobnis, E.Z.; Bovin, N.; Oko, R.; et al. Sperm Cohort-Specific Zinc Signature Acquisition and Capacitation-Induced Zinc Flux Regulate Sperm-Oviduct and Sperm-Zona Pellucida Interactions. Int. J. Mol. Sci. 2020, 21, 2121. [Google Scholar] [CrossRef] [Green Version]
- Sutovsky, P.; Lovercamp, K. Molecular markers of sperm quality. Biosci. Proc. 2019. [Google Scholar] [CrossRef] [Green Version]
- Sutovsky, P.; Moreno, R.; Ramalho-Santos, J.; Dominko, T.; Thompson, W.E.; Schatten, G. A putative, ubiquitin-dependent mechanism for the recognition and elimination of defective spermatozoa in the mammalian epididymis. J. Cell Sci. 2001, 114, 1665–1675. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, N.M.; Gilbert, G.R.; Loseth, K.J.; Crabo, B.G. Correlation between clusterin-positive spermatozoa determined by flow cytometry in bull semen and fertility. J. Androl. 2000, 21, 887–894. [Google Scholar]
- Christensen, P.; Hansen, C.; Liboriussen, T.; Lehn-Jensen, H. Implementation of flow cytometry for quality control in four Danish bull studs. Anim. Reprod. Sci. 2005, 85, 201–208. [Google Scholar] [CrossRef] [PubMed]
- Hossain, S.; Johannisson, A.; Wallgren, M.; Nagy, S.; Siqueira, A.P.; Rodriguez-Martinez, H. Flow cytometry for the assessment of animal sperm integrity and functionality: State of the art. Asian J. Androl. 2011, 13, 406–419. [Google Scholar] [CrossRef] [Green Version]
- Petrunkina, A.; Harrison, R. Fluorescence Technologies for Evaluating Male Gamete (Dys) Function. Reprod. Domest. Anim. 2013, 48, 11–24. [Google Scholar] [CrossRef]
- Kerns, K.; Jankovitz, J.; Robinson, J.; Minton, A.; Kuster, C.; Sutovsky, P. Relationship between the Length of Sperm Tail Mitochondrial Sheath and Fertility Traits in Boars Used for Artificial Insemination. Antioxidants 2020, 9, 1033. [Google Scholar] [CrossRef] [PubMed]
- Sutovsky, P.; Kerns, K.; Zigo, M.; Zuidema, D. Boar semen improvement through sperm capacitation management, with emphasis on zinc ion homeostasis. Theriogenology 2019, 137, 50–55. [Google Scholar] [CrossRef]
- Kennedy, C.E.; Krieger, K.B.; Sutovsky, M.; Xu, W.; Vargovič, P.; Didion, B.A.; Ellersieck, M.R.; Hennessy, M.E.; Verstegen, J.; Oko, R.; et al. Protein expression pattern of PAWP in bull spermatozoa is associated with sperm quality and fertility following artificial insemination. Mol. Reprod. Dev. 2014, 81, 436–449. [Google Scholar] [CrossRef]
- Sironen, A.; Uimari, P.; Nagy, S.; Paku, S.; Andersson, M.; Vilkki, J. Knobbed acrosome defect is associated with a region containing the genes STK17b and HECW2 on porcine chromosome 15. BMC Genom. 2010, 11, 699. [Google Scholar] [CrossRef] [Green Version]
- Rubessa, M.; Feugang, J.M.; Kandel, M.E.; Schreiber, S.; Hessee, J.; Salerno, F.; Meyers, S.; Chu, I.; Popescu, G.; Wheeler, M.B. High-throughput sperm assay using label-free microscopy: Morphometric comparison between different sperm structures of boar and stallion spermatozoa. Anim. Reprod. Sci. 2020, 219, 106509. [Google Scholar] [CrossRef]
- Jane, M.; Morrell, H.R.-M. Colloid Centrifugation of Semen: Applications in Assisted Reproduction. Am. J. Anal. Chem. 2016, 7, 597–610. [Google Scholar]
- Sjunnesson, Y.C.B.; Morrell, J.M.; Gonzalez, R. Single layer centrifugation-selected boar spermatozoa are capable of fertilization in vitro. Acta Veter-Scand. 2013, 55, 20. [Google Scholar] [CrossRef] [Green Version]
- Šterbenc, N.; Morrell, J.M.; Kosec, M.; Rath, D.; Klein, S.; Klinc, P. Single layer colloid centrifugation technique improves motility, viability and chromatin integrity of ram spermatozoa after thawing. Cryobiology 2018, 86, 77–83. [Google Scholar] [CrossRef]
- Morrell, J.; Rodriguez-Martinez, H.; Andersson, M. Colloid Centrifugation Selects Normal Spermatozoa from Polymorphic Bull Ejaculates: A Case Study. Reprod. Domest. Anim. 2014, 49, 281–284. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Morrell, J.; Richter, J.; Martinsson, G.; Stuhtmann, G.; Hoogewijs, M.; Roels, K.; Dalin, A.-M. Pregnancy rates after artificial insemination with cooled stallion spermatozoa either with or without Single Layer Centrifugation. Theriogenology 2014, 82, 1102–1105. [Google Scholar] [CrossRef]
- Hoogewijs, M.; Morrell, J.; Van Soom, A.; Govaere, J.; Johannisson, A.; Piepers, S.; De Schauwer, C.; De Kruif, A.; De Vliegher, S. Sperm selection using single layer centrifugation prior to cryopreservation can increase thawed sperm quality in stallions. Equine Veter-J. 2011, 43, 35–41. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Alborcia, M.; Morrell, J.; Parrilla, I.; Barranco, I.; Vazquez, J.M.; Martinez, E.A.; Roca, J. Improvement of boar sperm cryosurvival by using single-layer colloid centrifugation prior freezing. Theriogenology 2012, 78, 1117–1125. [Google Scholar] [CrossRef] [PubMed]
- Odhiambo, J.; DeJarnette, J.; Geary, T.W.; Kennedy, C.E.; Suarez, S.S.; Sutovsky, M.; Sutovsky, P. Increased Conception Rates in Beef Cattle Inseminated with Nanopurified Bull Semen1. Biol. Reprod. 2014, 91, 97. [Google Scholar] [CrossRef]
- Durfey, C.L.; Swistek, S.E.; Liao, S.F.; Crenshaw, M.A.; Clemente, H.J.; Thirumalai, R.V.K.G.; Steadman, C.S.; Ryan, P.L.; Willard, S.T.; Feugang, J.M. Nanotechnology-based approach for safer enrichment of semen with best spermatozoa. J. Anim. Sci. Biotechnol. 2019, 10, 14. [Google Scholar] [CrossRef]
- Feugang, J.M.; Rhoads, C.E.; Mustapha, P.A.; Tardif, S.; Parrish, J.J.; Willard, S.T.; Ryan, P.L. Treatment of boar sperm with nanoparticles for improved fertility. Theriogenology 2019, 137, 75–81. [Google Scholar] [CrossRef]
- MCRM Fertility. Sperm Selection. Available online: https://www.mcrmfertility.com/treatment-options/in-vitro-fertilization-ivf/sperm-selection/#nanobead (accessed on 1 January 2021).
- Morrell, J. Effect of colloid centrifugation on boar sperm quality during storage and function in in vitro fertilization. Theriogenology 2019, 137, 122–126. [Google Scholar] [CrossRef] [PubMed]
- Johnson, L.; Welch, G. Sex preselection: High-speed flow cytometric sorting of X and Y sperm for maximum efficiency. Theriogenology 1999, 52, 1323–1341. [Google Scholar] [CrossRef]
- Thomas, J.; Locke, J.; Vishwanath, R.; Hall, J.; Ellersieck, M.; Smith, M.; Patterson, D. Effective use of SexedULTRA™ sex-sorted semen for timed artificial insemination of beef heifers. Theriogenology 2017, 98, 88–93. [Google Scholar] [CrossRef]
- Joezy-Shekalgorabi, S.; Maghsoudi, A.; Mansourian, M.R. Reproductive performance of sexed versus conventional semen in Holstein heifers in various semiarid regions of Iran. Ital. J. Anim. Sci. 2017, 16, 666–672. [Google Scholar] [CrossRef] [Green Version]
- Gosálvez, J.; Ramirez, M.; López-Fernández, C.; Crespo, F.; Evans, K.; Kjelland, M.; Moreno, J. Sex-sorted bovine spermatozoa and DNA damage: I. Static features. Theriogenology 2011, 75, 197–205. [Google Scholar] [CrossRef]
- Gosálvez, J.; Ramirez, M.; López-Fernández, C.; Crespo, F.; Evans, K.; Kjelland, M.; Moreno, J. Sex-sorted bovine spermatozoa and DNA damage: II. Dynamic features. Theriogenology 2011, 75, 206–211. [Google Scholar] [CrossRef]
- Perry, G.A.; Walker, J.A.; Rich, J.J.; Northrop, E.J.; Perkins, S.D.; Beck, E.E.; Sandbulte, M.D.; Mokry, F.B. Influence of Sexcel™ (gender ablation technology) gender-ablated semen in fixed-time artificial insemination of beef cows and heifers. Theriogenology 2019, 146, 140–144. [Google Scholar] [CrossRef]
- ABS Sexcel. Available online: https://www.absglobal.com/services/sexcel/ (accessed on 30 December 2020).
- Domínguez, E.; Moreno-Irusta, A.; Castex, H.R.; Bragulat, A.F.; Ugaz, C.; Clemente, H.; Giojalas, L.; Losinno, L. Sperm Sexing Mediated by Magnetic Nanoparticles in Donkeys, a Preliminary In Vitro Study. J. Equine Veter-Sci. 2018, 65, 123–127. [Google Scholar] [CrossRef]
- Peter, A.; Markwelder, D.; Asem, E. Phenotypic feminization in a genetic male dog caused by nonfunctional androgen receptors. Theriogenology 1993, 40, 1093–1105. [Google Scholar] [CrossRef]
- Patthanawong, W.; Pongpiachan, P.; Mekchay, S.; Sumretprasong, J. Production of monoclonal antibody against male specific antigen on cell membrane of bovine spermatozoa. Indian J. Anim. Res. 2010, 44, 22–27. [Google Scholar]
- Thongkham, M.; Thaworn, W.; Pattanawong, W.; Teepatimakorn, S.; Mekchay, S.; Sringarm, K. Spermatological parameters of immunologically sexed bull semen assessed by imaging flow cytometry, and dairy farm trial. Reprod. Biol. 2021, 21, 100486. [Google Scholar] [CrossRef]
- Jäkel, H.; Scheinpflug, K.; Mühldorfer, K.; Gianluppi, R.; Lucca, M.S.; Mellagi, A.P.G.; Bortolozzo, F.P.; Waberski, D. In vitro performance and in vivo fertility of antibiotic-free preserved boar semen stored at 5 °C. J. Anim. Sci. Biotechnol. 2021, 12, 9. [Google Scholar] [CrossRef] [PubMed]
- Yeste, M. Sperm cryopreservation update: Cryodamage, markers, and factors affecting the sperm freezability in pigs. Theriogenology 2016, 85, 47–64. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Martinez, H.; Barth, A.D. In vitro evaluation of sperm quality related to in vivo function and fertility. Soc. Reprod. Fertil. Suppl. 2019, 64, 39. [Google Scholar] [CrossRef]
- Rodriguez-Martinez, H. Cryopreservation of Porcine Gametes, Embryos and Genital Tissues: State of the Art; InTech: London, UK, 2012; pp. 231–260. [Google Scholar] [CrossRef] [Green Version]
- Ugur, M.R.; Saber Abdelrahman, A.; Evans, H.C.; Gilmore, A.A.; Hitit, M.; Arifiantini, R.I.; Purwantara, B.; Kaya, A.; Memili, E. Advances in Cryopreservation of Bull Sperm. Front. Vet. Sci. 2019, 6, 268. [Google Scholar] [CrossRef] [Green Version]
- Ryu, D.-Y.; Song, W.-H.; Pang, W.-K.; Yoon, S.-J.; Rahman, S.; Pang, M.-G. Freezability biomarkers in bull epididymal spermatozoa. Sci. Rep. 2019, 9, 12797. [Google Scholar] [CrossRef] [Green Version]
- Vilagran, I.; Yeste, M.; Sancho, S.; Casas, I.; Del Álamo, M.M.R.; Bonet, S. Relationship of sperm small heat-shock protein 10 and voltage-dependent anion channel 2 with semen freezability in boars. Theriogenology 2014, 82, 418–426. [Google Scholar] [CrossRef]
- Llavanera, M.; Delgado-Bermúdez, A.; Fernandez-Fuertes, B.; Recuero, S.; Mateo, Y.; Bonet, S.; Barranco, I.; Yeste, M. GSTM3, but not IZUMO1, is a cryotolerance marker of boar sperm. J. Anim. Sci. Biotechnol. 2019, 10, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Vilagran, I.; Castillo, J.; Bonet, S.; Sancho, S.; Yeste, M.; Estanyol, J.M.; Oliva, R. Acrosin-binding protein (ACRBP) and triosephosphate isomerase (TPI) are good markers to predict boar sperm freezing capacity. Theriogenology 2013, 80, 443–450. [Google Scholar] [CrossRef]
- Wang, P.; Wang, Y.-F.; Wang, H.; Wang, C.-W.; Zan, L.-S.; Hu, J.-H.; Li, Q.-W.; Jia, Y.-H.; Ma, G.-J. HSP90 expression correlation with the freezing resistance of bull sperm. Zygote 2013, 22, 239–245. [Google Scholar] [CrossRef]
- Holt, W.; Del Valle, I.; Fazeli, A. Heat shock protein A8 stabilizes the bull sperm plasma membrane during cryopreservation: Effects of breed, protein concentration, and mode of use. Theriogenology 2015, 84, 693–701. [Google Scholar] [CrossRef]
- Jobim, M.; Oberst, E.; Salbego, C.; Souza, D.; Wald, V.; Tramontina, F.; Mattos, R. Two-dimensional polyacrylamide gel electrophoresis of bovine seminal plasma proteins and their relation with semen freezability. Theriogenology 2003, 61, 255–266. [Google Scholar] [CrossRef]
- Einspanier, R.; Krause, I.; Calvete, J.; Töfper-Petersen, E.; Klostermeyer, H.; Karg, H. Bovine seminal plasma ASFP: Localization of disulfide bridges and detection of three different isoelectric forms. FEBS Lett. 1994, 344, 61–64. [Google Scholar] [CrossRef] [Green Version]
- Safranski, T.; Ford, J.; Rohrer, G.; Guthrie, H. Plenary Contribution to International Conference on Boar Semen Preservation 2011. Genetic Selection for Freezability and its Controversy with Selection for Performance. Reprod. Domest. Anim. 2011, 46, 31–34. [Google Scholar] [CrossRef]
- Fraser, L.; Brym, P.; Pareek, C.S.; Mogielnicka-Brzozowska, M.; Jastrzębski, J.P.; Wasilewska-Sakowska, K.; Mańkowska, A.; Sobiech, P.; Żukowski, K. Transcriptome analysis of boar spermatozoa with different freezability using RNA-Seq. Theriogenology 2019, 142, 400–413. [Google Scholar] [CrossRef] [PubMed]
- Hitit, M.; Ugur, M.R.; Dinh, T.T.N.; Sajeev, D.; Kaya, A.; Topper, E.; Tan, W.; Memili, E. Cellular and Functional Physiopathology of Bull Sperm With Altered Sperm Freezability. Front. Veter-Sci. 2020, 7, 581137. [Google Scholar] [CrossRef]
- Liu, J.-L.; Kusakabe, H.; Chang, C.-C.; Suzuki, H.; Schmidt, D.W.; Julian, M.; Pfeffer, R.; Bormann, C.L.; Tian, X.C.; Yanagimachi, R.; et al. Freeze-Dried Sperm Fertilization Leads to Full-Term Development in Rabbits1. Biol. Reprod. 2004, 70, 1776–1781. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wakayama, T.; Yanagimachi, R. Development of normal mice from oocytes injected with freeze-dried spermatozoa. Nat. Biotechnol. 1998, 16, 639–641. [Google Scholar] [CrossRef] [PubMed]
- Palazzese, L.; Gosalvez, J.; Anzalone, D.A.; Loi, P.; Saragusty, J. DNA fragmentation in epididymal freeze-dried ram spermatozoa impairs embryo development. J. Reprod. Dev. 2018, 64, 393–400. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hara, H.; Abdalla, H.; Morita, H.; Kuwayama, M.; Hirabayashi, M.; Hochi, S. Procedure for Bovine ICSI, not Sperm Freeze-drying, Impairs the Function of the Microtubule-organizing Center. J. Reprod. Dev. 2011, 57, 428–432. [Google Scholar] [CrossRef] [Green Version]
- Ressaissi, Y.; Anzalone, D.A.; Palazzese, L.; Czernik, M.; Loi, P. The impaired development of sheep ICSI derived embryos is not related to centriole dysfunction. Theriogenology 2020, 159, 7–12. [Google Scholar] [CrossRef] [PubMed]
- Grötter, L.G.; Cattaneo, L.; Marini, P.E.; Kjelland, M.E.; Ferré, L.B. Recent advances in bovine sperm cryopreservation techniques with a focus on sperm post-thaw quality optimization. Reprod. Domest. Anim. 2019, 54, 655–665. [Google Scholar] [CrossRef] [PubMed]
- Kuiper, M.; Spencer, M.; Kanyima, B.M.; Ng, C.H.; Newell, M.; Turyahikayo, S.; Makoni, N.; Madan, D.; Lieberman, D.H. Using on-demand dry ice production as an alternative cryogenic cold chain for bovine artificial insemination outreach in low-resource settings1. Transl. Anim. Sci. 2020, 4, 1196–1205. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zuidema, D.; Kerns, K.; Sutovsky, P. An Exploration of Current and Perspective Semen Analysis and Sperm Selection for Livestock Artificial Insemination. Animals 2021, 11, 3563. https://doi.org/10.3390/ani11123563
Zuidema D, Kerns K, Sutovsky P. An Exploration of Current and Perspective Semen Analysis and Sperm Selection for Livestock Artificial Insemination. Animals. 2021; 11(12):3563. https://doi.org/10.3390/ani11123563
Chicago/Turabian StyleZuidema, Dalen, Karl Kerns, and Peter Sutovsky. 2021. "An Exploration of Current and Perspective Semen Analysis and Sperm Selection for Livestock Artificial Insemination" Animals 11, no. 12: 3563. https://doi.org/10.3390/ani11123563





