Abstract
2–Amino–4-phenyl–5,6,7,8–tetrahydroquinoline–3–carbonitrile (3) was synthesized by treating cyclohexanone (1) with 2–benzylidenemalononitrile (2) in the presence of ammonium acetate. The reactivity of compound 3 towards dimethylformamide dimethyl acetal (DMF-DMA), carbon disulfide, urea, thiourea, formamide, formic acid, acetyl chloride and isothiocyanate were studied. In addition, the antimicrobial activity of some selected derivatives is reported.
Introduction
Pyrimidoquinolines are important compounds because of their biological properties, which are known to depend mainly on the nature and position of substituents, and include antimalarial [1], anticancer [2], antimicrobial [3,4], and anti-inflammatory activities [5,6]. Recently there has also been considerable interest in the synthesis and chemistry of tetrahydroquinolines and their fused derivatives [7,8,9,10,11]. Our aim in the work presented herein was to synthesize pyrimido[4,5-b]quinolines using tetrahydroqinolinecarbonitriles as building blocks. Such a synthesis of condensed azines is of biological interest due to the formal isoelectronic relationship that exists between the pyrimidine ring and tetrahydroquinoline [12,13,14,15,16,17,18,19,20,21,22,23].
Results and Discussion
Treatment of cyclohexanone (1) with the α,β-unsaturated nitrile derivative 2 in the presence of ammonium acetate afforded the tetrahydroquinoline derivative 3. The structure of 3 was unambiguously confirmed by X-ray crystallography (cf. Figure 1 and Table 1). On the other hand, compounds 4a,b could be obtained upon reaction of 1 with 2 in absolute ethanol in the presence of p-chloroaniline or p-hydroxyaniline (Scheme 1).
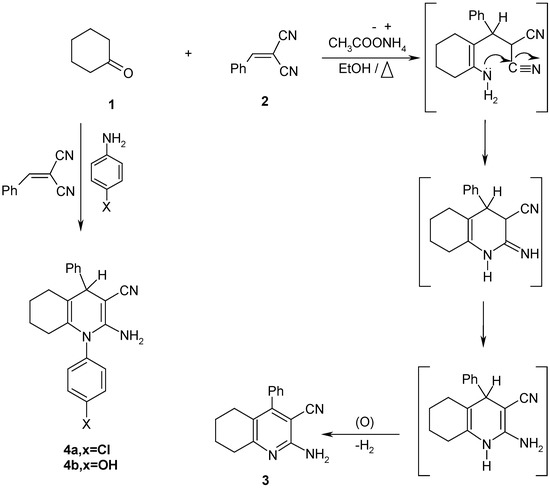
Scheme 1.
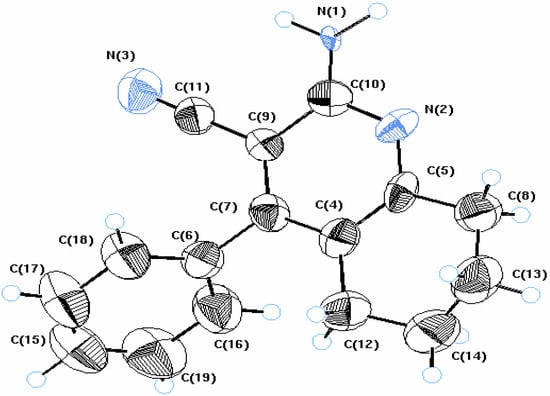
Figure 1.
ORTEP diagram of compound 3.

Table 1.
Crystal data and structure refinement for compound 3.
| Empirical formula | C16H15N3 |
| Formula weight | 249.317 |
| Temperature | 289 K |
| Wavelength | 0.71073A |
| Crystal system, space group | Monoclinic, P21/c |
| Unit cell dimensions | a = 12.7076 (11)Å |
| b = 5.8985 (5)Å | |
| c = 18.227 (2)Å | |
| α = 90.00° | |
| β = 101.395 (3)° | |
| Volume | 1339.3(2) A3 |
| Z, Calculated density | 4, 1.237 Mg/m3 |
| Absorption coefficient | 0.08 mm-1 |
| F(000) | 236 |
| Crystal size | 1.00 x 0.22 x 0.16 mm |
| Diffract meter | Kappa CCD |
| Θ Rang (0) | 2.910—19.211 ° |
| Limiting indices | -11<=h<=11, -5<=k<=5, -16<=l<=16 |
| Reflections collected / unique | 2106 / 1242 [R(int) = 0.044] |
| Absorption correction | None |
| Refinement method | Full-matrix least-squares on F2 |
| Data / restraints / parameters | 1242 / 0 / 172 |
| Goodness-of-fit on F2 | 2.291 |
| Final R indices [I>3sigma(I)] | R1 = 0.055, wR2 = 0.103 |
| R indices (all data) | R1 = 0.1261, wR2 = 0.117 |
| Extinction coefficient | 0.047(2) |
| Largest diff. peak and hole | 0.37 and -0.30 e. Å3 |
Compound 3 reacted with DMF-DMA in dioxane to afford compound 5, whose structure was also unambiguously confirmed by X-ray crystallography (Figure 2 and Table 2). When compound 5 was refluxed with hydrazine hydrate in absolute ethanol it yielded the corresponding 3–amino–4(3H) imino–5-phenyl–6,7,8,9–tetrahydropyrimido[4, 5-b]quinoline (7) through the intermediate 6 (Scheme 2).
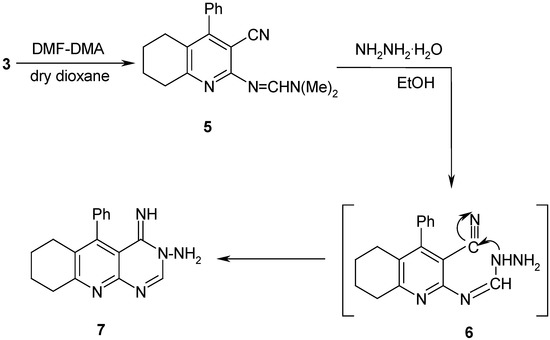
Scheme 2.
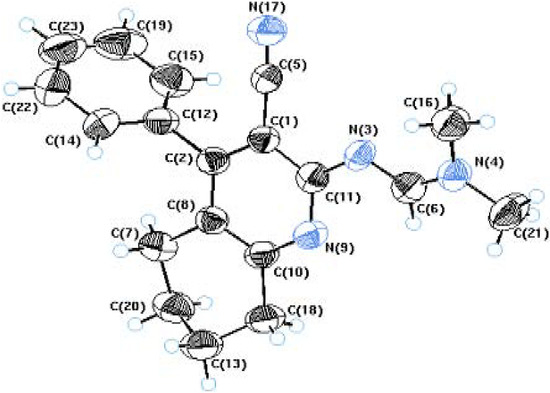
Figure 2.
ORTEP diagram of Compound 5.

Table 2.
Crystallographic and experimental data of compound 5.
| Empirical formula | C19H20N4 |
| Formula weight | 304.359 |
| Temperature | 298 K |
| Wavelength | 0.71073A |
| Crystal system, space group | Monoclinic, P21/c |
| Unit cell dimensions | a = 7.3256 (4)Å |
| b = 11.4319 (6)Å | |
| c = 20.2817 (11)Å | |
| α = 90.00° | |
| β = 98.810 (3) ° | |
| Volume | 1678.5 (2)Å3 |
| Z, Calculated density | 4, 1.493 Mg/m3 |
| Absorption coefficient | 0.10 mm-1 |
| F(000) | 236 |
| Crystal size | 1.00 x 0.22 x 0.16 mm |
| Diffract meter | Kappa CCD |
| Θ Rang (0) | 2.910—26.733 ° |
| Limiting indices | -9<=h<=9, -13<=k<=14, -25<=l<=24 |
| Reflections collected / unique | 5914 / 4177 [R(int) = 0.048] |
| Absorption correction | None |
| Refinement method | Full-matrix least-squares on F2 |
| Data / restraints / parameters | 1395 / 0 / 208 |
| Goodness-of-fit on F2 | 2.768 |
| Final R indices [I>3sigma(I)] | R1 = 0.093, wR2 = 0.223 |
| R indices (all data) | R1 = 0.381, wR2 = 0.218 |
| Extinction coefficient | 0.030(2) |
| Largest diff. peak and hole | 0.68 and -0.67 e. Å3 |
Compound 3 reacted with thiourea or urea in an ethanol/sodium ethoxide mixture for 6 h to afford the 4–amino–10–phenyl–6, 7, 8, 9–tetrahydropyrimido[4,5-b]quinoline–2(1H)–thione/one derivatives 9a and 9b (Scheme 3). The reaction products 9 were formed via the intermediate 8 by loss of an ammonia molecule, followed by an intramolecular addition to the cyano function to give the final isolated products 9a,b.
The target ring system 12 was synthesized by reaction of 3 with carbon disulfide through the intermediates 10 and 11, whose subsequent rearrangement leads to the fused pyrimidinedithione 12.
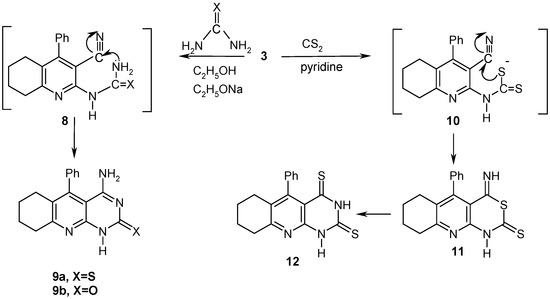
Scheme 3.
Compound 3 reacted with formic acid to yield 13, which was treated with alkaline hydrogen peroxide to give the corresponding cyclized pyrimidoquinoline derivative 15 via the intermediate 14. The reaction proceeds by initial hydration of the nitrile group to give a carboxamide, which then undergoes cyclization in the alkaline medium. The structures of both 13 and 15 were confirmed by elemental and spectral analyses. Meanwhile, acylation of 3 with acid chloride gave 2–methyl–5–phenyl–6,7,8,9–tetrahydropyrimido[4,5-b]quinolin–4–(3H)one (18) through the intermediates 16 and 17 (Scheme 4).
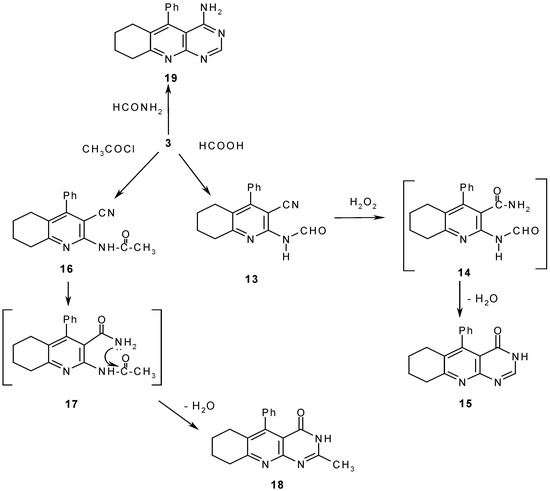
Scheme 4.
In addition, compound 3 was refluxed with excess formamide to afford 4–amino–5-phenyl–6,7,8,9–tetrahydropyrimido[4,5-b]quinoline (19) (Scheme 4). The structure of the reaction product was established based on its elemental analysis and spectra data. Both isocyanates and isothiocyanates reacted with 3 to give initially the corresponding substituted ureido or thioureido derivatives 20a,b, which readily cyclized to give the corresponding fused heterocyclic systems 21a,b (Scheme 5). The same reaction products 21a,b could be obtained in a one step reaction through prolonged heating of 3 with phenylisocyanate or phenyl isothiocyanate in DMF containing a catalytic amount of TEA. Finally, compound 3 reacted with benzoylisothiocyanate to yield 22, which then cyclized into 23 which undergoes a Dimroth rearrangement to give 24 (Scheme 5).
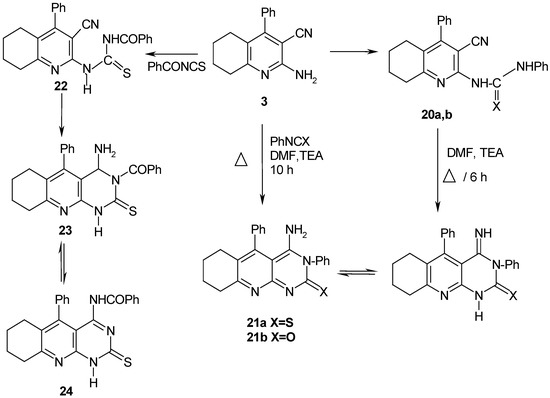
Scheme 5.
Biological Activity
Screening of antimicrobial activity was performed at the Microbiology Lab of the National Research Center. Representative derivatives 3, 4a, 4b, 7, 9a, 12, 13, 15, 18, 20, 22 and 24 were selected and tested for their antimicrobial activity against three yeasts (Candida albicans, Aspergillus niger and Saccharomyces cervisiae) and two bacteria (Staphylococcus aureus and Esherichia coli ) using the modified agar diffusion cylinder method [24]. The results are given in Table 3.

Table 3.
The antimicrobial activity of select derivatives at 1000 ppm concentration.
| Compd. | Species tested | ||||
|---|---|---|---|---|---|
| C. albicans | A. niger | S. cerevisiae | S. aureus Gram (+ve ) | E. coli Gram (- ve ) | |
| 3 | - | + | - | + | + |
| 4a | - | ++ | ++ | ++ | - |
| 4b | + | - | ++ | - | + |
| 7 | + | - | - | ++ | - |
| 9a | + | +++ | + | - | - |
| 12 | ++ | - | + | + | - |
| 13 | - | + | +++ | - | + |
| 15 | + | ++ | - | + | - |
| 18 | - | ++ | + | + | - |
| 20a | ++ | + | + | - | + |
| 22 | ++ | + | ++ | - | + |
| 24 | ++ | + | ++ | - | + |
+++: Strong activity, ++: Moderate activity, +: Weak activity, - : No activity
Conclusions
The varied biological activities of tetrahydropyrimidoquinoline derivatives prompted us to synthesize some new compounds and study their antimicrobial activities. The antifungal activity assays showed that compounds 12, 20a, 22 and 24 display moderate activity against C. albicans, while compound 9a shows strong activity against A. niger and compound 13 shows strong activity against S. cerevisiae. The bactericidal activity studies revealed that compounds 4a and 7 show moderate activities against S. aureus.
Experimental
General
All melting points are uncorrected. Elemental analyses were carried out in the Microanalytical Center, Cairo University, Giza, Egypt. IR spectra (KBr) were recorded on Pye Unicam SP 1200 Spectrophotometer. 1H-NMR spectra were recorded in CDCl3 or DMSO- d6 on a 90 MHz Varian NMR Spectrometer using TMS as an internal standard and chemical shifts are expressed as δ ppm units. The mass spectra were determined with HP model MS-5988 at electron energy 70 eV. The homogeneity of all compounds synthesized was checked by TLC on 2.0cm x 6.0cm aluminum sheets recoated with silica gel 60 containing a fluorescent indicator, to a thickness of 0.25. Characterization data of the various compounds prepared are given in Table 4 and Table 5.
X-ray crystallography [25]
2–Amino–4–phenyl–5,6,7,8–tetrahydroquinoline–3–carbonitrile (3).
A solution of cyclohexanone (1, 0.01 mol) in absolute ethanol (30 mL) containing excess ammonium acetate and the arylidene derivative 2 (0.01 mol) was heated under reflux for 3-5 h. The solid material which separated during heating was collected by filtration and recrystallized from ethanol to yield the tetrahydroquinoline derivative 3.
2–Amino–3–cyano–1,4–diphenyl–1,4,5,6,7,8–hexahydroquinoline derivatives 4a,b.
A solution of 2 (0.01 mol) and absolute ethanol (100 mL) was placed in a conical flask, cyclohexanone (1, 0.01 mol) and p-chloroaniline or p-hydroxylaniline (0.01 mol) were added and the reaction mixture was refluxed for 10-12 h. and then left to cool to room temperature overnight. The solids obtained were recrystallized from ethanol to give the title compounds 4a,b.
2–Dimethylaminomethelenimino–3–cyano–4–phenyl–5,6,7,8–tetrahydroquinoline (5)
A solution of 3 (0.005 mol) in dry dioxane (20 mL) and DMF-DMA (0.005 mol) was refluxed for 4 hr. and the reaction mixture was then cooled to room temperature and poured into ice/cold water to complete precipitation. The solid was filtered off and recrystallized from ethanol to give compound 5.
3–Amino–4–(3H)imino–5–phenyl–6,7,8,9–tetrahydropyrimido[4,5–b]quinoline (7).
A mixture of 5 (0.005 mol) and hydrazine hydrate (0.005 mol) in absolute ethanol (30 mL) was refluxed for 4 hr. and the reaction mixture was left at room temperature overnight and then poured into ice/cold water to complete precipitation. The product was filtered off and recrystallized from dry benzene to give compound 7.
4–Amino–5–phenyl–6,7,8,9–tetrahydropyrimido[4,5–b]quinoline–2(1H)–thione/one derivatives 9a,b.
A mixture of 3 (0.005 mol) and thiourea (0.005 mol) or urea (0.005 mol) in absolute ethanol (20 mL) containing sodium ethoxide (0.005 mol) was refluxed for 6 hr. The reaction mixture was left to cool to room temperature, then poured into ice cold water (50 mL) and neutralized with dilute hydrochloric acid; the separated material was filtered off and recrystallized from ethanol to give compounds 9a,b.
5–Phenyl–1,3,6,7,8,9–hexahydropyrimido[4,5–b]quinolin–2,4–dithione (12).
To a solution of 3 (0.005 mol) in dry pyridine (30 mL) carbon disulphide (0.005 mol) was added and the reaction mixture was refluxed on a water bath for 6 hr., then left to cool to room temperature, poured into cold water and neutralized with diluted hydrochloric acid to complete precipitation. The solid obtained was filtered off, washed with water, dried well and recrystallized from methanol to give compound 12.
2–Formylamino–3–cyano–4–phenyl–5,6,7,8–tetrahydroquinoline (13).
An equimolar amount of 3 (0.005 mol) and formic acid (0.005 mol) in absolute ethanol (30 mL) was refluxed for 2 hr. The reaction mixture was then concentrated and left to cool overnight to room temperature for complete precipitation. The precipitated solid was filtered off, dried well and recrystallized from aqueous ethanol to give compound 13.
5–Phenyl–6,7,8,9–tetrahydropyrimido[4,5–b]quinolin–4(3H)–one (15).
A solution of 13 (0.002 mol) in potassium carbonate (10 %, 10 mL) and hydrogen peroxide (30 %, 5 mL) was refluxed for 1 hr. The reaction mixture was left to cool at room temperature for complete precipitation. The precipitated solid was collected by filtration and recrystallized from aqueous ethanol to give compound 15.
2–Methyl–5–phenyl–6,7,8,9–tetrahydropyrimido[4,5–b]quinolin –4–(3H)one (18).
Acetyl chloride (0.005 mol) was added to a solution of 3 (0.005 mol) in dry pyridine (30 mL) and the mixture was refluxed on a water bath for 3 hr., then left to cool to room temperature and poured into ice cold water and neutralized by diluted hydrochloric acid for complete precipitation. The separated material was collected by filtration, washed with water, dried well and recrystallized from acetic acid to yield compound 18.
4–Amino–5–phenyl–6,7,8,9–tetrahydropyrimido[4,5–b]quinoline (19).
Compound 3 (0.005 mol) and an excess of formamide was placed in a conical flask and the reaction mixture was refluxed for 4 hr., then left to cool for complete precipitation. The separated solid product was filtered and recrystallized from ethanol to give compound 19.
2–(Phenylthioureido)–4–phenyl–5,6,7,8–tetrahydroquinoline–3–carbonitrile (20a,b).
A mixture of 3 (0.005 mol) and phenylisothiocyanate (0.005 mol) or phenylisocyanate (0.005 mol) in dimethylformamide containing a catalytic amount of triethylamine (4 drops) was refluxed for 6 hr. and then left to cool to room temperature. The reaction mixture was poured into cold water for complete precipitation, then filtered off washed with water dried well and recrystallized from aqueous methanol to give compounds 20a,b
4–Amino–5–phenyl–6,7,8,9–tetrahydropyrimido[4,5–b]quinoline2–(3H) thione/one derivatives (21a,b).
Method A: A few drops of triethylamine were added to a solution of 20a,b (0.005 mol) in dimethylformamide and the reaction mixture was refluxed for 6 hr., then left to cool. The product was filtered off, washed with water, dried well and recrystallized from ethanol to give compounds 21a,b.
Method B: An equimolar mixture of 3 (0.005 mol) and phenylisocyanate (0.005 mol) or phenyl–isothiocyanate (0.005 mol) in dimethylformamide (30 mL) in the presence of a few drops of triethylamine (4 drops) was refluxed for 10 hr. The reaction mixture was left to cool and poured into cold water for complete precipitation. The separated solid was filtered off, washed with water, dried well and recrystallized from ethanol to give compounds 21a, b.
2–(Phenylthioureido)–4–phenyl–5,6,7,8–tetrahydroquinoline–3–carbonitrile (22).
A mixture of 3 (0.005 mol) and benzoylisothiocyanate (0.005 mol) in dimethylformamide (30 mL) containing a catalytic amount of triethylamine (4 drops) was refluxed for 6 hr. and left to cool to room temperature. The reaction mixture was poured into cold water for complete precipitation, and then filtered off, washed with water dried well and recrystallized from aqueous methanol to give compound 22.
3–Benzoylamino–5–phenyl–6,7,8,9–tetrahydropyrimido[4,5–b]quinolin–2–(1H)thione (24).
Method A: To a solution of 22 (0.005 mol) in dimethylformamide was added few drops of triethylamine. The reaction mixture was refluxed 6 hr., then left to cool, the solids filtered off, washed with water, dried well and recrystallized from ethanol to give compound 24.
Method B: An equimolar mixture of 3 (0.005 mol) and benzoylisothiocyanate (0.005 mol) was refluxed for 10 hr. in dimethylformamide (30 mL) containing four drops of triethylamine. The reaction mixture was left to cool and poured into cold water for complete precipitation. The separated solid was filtered off, washed with water, dried well and recrystallized from ethanol to give compound 24.

Table 4.
Physical properties and elemental analyses of the new compounds.
| Compd. | M.P.°C | Formula (mw) | Analysis % Calcd. (Found) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| C | H | N | S | Cl | |||||||||
| 3 | 240 | C16H15N3 | 77.08 | 6.06 | 16.86 | – | – | ||||||
| (249.326) | (77.02) | (6.01) | (16.86) | ||||||||||
| 4a | 270 | C22H20N3Cl | 73.02 | 5.57 | 11.61 | – | 9.8 | ||||||
| 361.87 | (73.00) | (5.46) | (11.49) | (9.74) | |||||||||
| 4b | 260 | C22H21N3O | 76.94 | 6.16 | 12.24 | – | – | ||||||
| (343.43) | (76.89) | (6.04) | (12.15) | ||||||||||
| 5 | 184 | C19H20N4 (304.373) | 74.98 (74.89) | 6.62 (6.56) | 18.14 (18.20) | ||||||||
| 7 | 210 | C17H17N5 | 70.33 | 5.55 | 24.12 | – | – | ||||||
| (291.323) | (70.24) | (5.47) | (24.04) | ||||||||||
| 9a | 290 | C17H16N4S | 66.21 | 5.22 | 18.17 | 10.4 | – | ||||||
| (308.413) | (66.07) | (5.09) | (18.07) | (10.34) | |||||||||
| 9b | 213 | C17H16N4O | 69.85 | 5.51 | 19.17 | – | – | ||||||
| (292.342) | (69.74) | (5.43) | (19.11) | ||||||||||
| 12 | 207~209 | C17H15N3S2 | 62.74 | 4.64 | 12.91 | 19.70 | – | ||||||
| (325.464) | (62.79) | (4.62) | (12.94) | (19.79) | |||||||||
| 13 | 195~196 | C17H15N3O | 73.63 | 5.45 | 15.15 | – | – | ||||||
| (277.332) | (73.74) | (5.40) | (15.21) | ||||||||||
| 15 | 187~188 | C17H15N3O | 73.63 | 5.45 | 15.15 | – | – | ||||||
| (277.332) | (73.83) | (5.14) | (15.27) | ||||||||||
| 18 | 187~188 | C18H17N3O | 74.21 | 5.88 | 14.42 | – | – | ||||||
| (291.364) | (74.03) | (5.73) | (14.27) | ||||||||||
| 19 | 205 | C17H16N4 | 73.89 | 5.83 | 20.27 | – | – | ||||||
| (276.343) | (73.84) | (5.78) | (20.18) | ||||||||||
| 20a | 172 | C23H20N4S | 71.84 | 5.24 | 14.57 | 8.34 | – | ||||||
| (384.51) | (72.01) | (5.29) | (14.59) | (8.29) | |||||||||
| 20b | 160 | C23H20N4O | 74.98 | 5.47 | 15.21 | – | – | ||||||
| (368.444) | (74.67) | (5.63) | (15.04) | ||||||||||
| 21a | 218 | C23H20N4S | 71.84 | 5.24 | 14.57 | 8.34 | |||||||
| (384.51) | (72.00) | (5.29) | (14.52) | (8.29) | |||||||||
| 21b | 190 | C23H20N4O | 74.98 | 5.47 | 15.21 | – | – | ||||||
| (368.444) | (74.71) | (5.67) | (15.11) | ||||||||||
| 22 | 174 | C24H20N4OS | 69.88 | 4.88 | 13.58 | 7.77 | – | ||||||
| (412.527) | (69.74) | (4.73) | (13.42) | (7.71) | |||||||||
| 24 | 225 | C24H20N4OS | 69.88 | 4.88 | 13.58 | 7.77 | – | ||||||
| 412.527 | (69.79) | (4.81) | (13.52) | (7.71) | |||||||||

Table 5.
IR, 1H-NMR, and MS of the new compounds.
| Compd. | IR (cm-1) | 1H-NMR (δ, ppm) and/or MS | |
|---|---|---|---|
| 3 | 3420-3305 (NH2), 2212 (CN), 1645 (C=N). | 1.6-2.8 (m, 8H, 4CH2); 5.3(s, 2H, NH2); 7.2-7.6 (m, 5H, Ar-H). MS: m/e = 249 (M+, 100%). | |
| 4a | 3424-3345 (NH2), 2211 (CN), 1650 (C=N). | 1.6-2.8 (m, 8H, 4CH2); 4.8 (s, 1H, quinoline H-4), 5.8 (s, 2H, NH2), 7.2-7.4 (m, 9H, Ar-H). MS: m/e = 361 (M+, 18%), 362 (M+1, 6%). | |
| 4b | 3421 (OH), 3306 (NH2), 2215 (CN), 1622 (C=N). | 1.6-2.8 (m, 8H, 4CH2); 4.8 (s, 1H, quinoline H-4); 5.3 (s, 2H, NH2); 7.2-7.6 (m, 9H, Ar-H); 9.2 (s, 1H, OH). MS: m/e = 343 (M+, 75 %). | |
| 5 | 2216 (CN), 1623 (C=N). | 1.6-2.8 (m, 8H, 4CH2); 3.2 (s, 6H, 2CH3); 7.2-7.4 (m, 5H, Ar-H); 8.4 (s, 1H, vinyl H). MS: m/e =304 (M+, 100%). | |
| 7 | 3421-3360 (NH2), 3148 (NH), 1645 (C=N). | 1.6-2.8 (m, 8H, 4CH2); 5.4 (s, 2H, NH2); 7.2-7.4 (m, 5H, Ar-H); 7.50 (s, 1H, pyrimidine H); 8.01 (s, 1H, NH). MS: m/e = 291 (M+, 15%). | |
| 9a | 3408-3320 (NH2), 3226 (NH), 1337 (C=S). | 1.6-2.8 (m, 8H, 4CH2); 5.4 (s, 2H, NH2); 7.1-7.5 (m, 5H, Ar-H); 8.2 (s, 1H, NH). MS: m/e = 308 (M+, 15 %). | |
| 9b | 3421 (NH2), 3426 (NH), 1717 (C=O), 1646 (C=N). | MS: m/e = 292 (M+, 50%). | |
| 12 | 3426 (NH), 1643 (C=N), 1344 (C=S). | 1.62-2.88 (m, 8H, 4CH2); 5.8 (s, 1H exchangeable, NH); 7.12- 7.64 (m, 5H, Ar-H); 9.08 (bs, 1H, exchangeable, NH).MS: m/e = 325 (M+, 10%). | |
| 13 | 3228 (NH), 2211 (CN), 1692 (C=O), 1581 (C=N). | MS: m/e = 277 (M+, 30%). | |
| 15 | 3146 (NH), 1707 (C=O), 1645 (C=N). | 1.34-1.98 (m, 8H, 4CH2); 7.13-7.35 (m, 5H, Ar-H); 7.50 (s, 1H, pyrimidine H); 8.01 (s, 1H, NH). | |
| 18 | 3146 (NH), 1707 (C=O), 1645 (C=N). | 0.9 (s, 3H, CH3); 1.34-1.96 (m, 8H, 4CH2); 7.14-7.30 (m, 5H, Ar-H); 8.01 (s, 1H, NH). MS: m/e = 291 (M+, 15%). | |
| 19 | 3319-3322 (NH2), 1661 (C=N). | 1.35-2.68 (m, 8H, 4CH2); 5.4 (s, 2H, NH2); 4.8 (s, 1H, pyrimidine H); 7.13-7.46 (m, 5H, Ar-H). MS: m/e = 276 (M+, 55 %). | |
| 20a | 3148 (NH), 3059 (NH), 2211(CN), 645 (C=N), 1346 (C=S). | MS: m/e = 384 (M+, 10%). | |
| 20b | 3179 (NH), 3149 (NH), 2228 (CN), 1707 (C=O), 1631 (C=N). | MS: m/e = 368(M+, 40%). | |
| 21a | 3421 – 3307 (NH2), 1645 (C=N), 1346 (C=S). | MS: m/e = 384 (M+, 10%). | |
| 21b | 3419-3303 (NH2), 1645 (C=N), 1707 (C=O). | MS: m/e = 368 (M+, 40%). | |
| 22 | 3148 (NH), 3059 (NH), 2211 (CN), 1677 (C=O), 1645(C=N), 1346 (C=S). | MS: m/e = 412 (M+, 10%). | |
| 24 | 3182 (NH), 3120 (NH), 1672 (C=O), 1605 (C=N), 1363 (C=S ) . | MS: m/e = 412 (M+, 10%). | |
References
- Joshi, A.A.; Viswanathan, C. L. Recent developments in antimalarials drug discovery. Anti-Infect. Agent. Med. Chem. 2006, 5, 105–122. [Google Scholar]
- Dlugosz, A.; Dus, D. Synthesis and anticancer properties of pyrimido[4,5-b]quinolines. Farmaco 1996, 51, 364–374. [Google Scholar]
- El-Sayed, O. A.; El-Bieh, F. M.; Al-Bassam, B.A. Novel 4-aminopyrimido[4,5-b]quinoline derivatives as potential antimicrobial agents. Boll. Chim. Farm. 2002, 141, 461–465. [Google Scholar]
- El-Sayed, O. A.; Al-Bassam, B. A.; Hussein, M. E. Synthesis of some novel quinoline-3-carboxylic acids and pyrimidoquinoline derivatives as potential antimicrobial agents. Arch. Pharm. (Weinheim) 2002, 335, 403–410. [Google Scholar]
- Gavrilov, M. Yu.; Mardanova, L.G.; Kolla, V. E.; Konshin, M. E. Synthesis, Anti-inflammatory and analgesic activities of tetrahydroquini\oline-3-carboxamides. Pharm. Chem. J. 1988, 22, 554–556. [Google Scholar]
- El-Sayed, O. A.; Al-Turki, T. M.; Al-Daffiri, H. M.; Al-Bassam, B. A.; Hussein, M. E. Pyrimidoquinoline derivatives as anti-inflammatory and antimicrobial agents. Boll. Chim. Farm. 2004, 143, 227–238. [Google Scholar]
- Al-Mousawi, S. M.; Elkholy, Y.M.; Mohammed, M. A.; Elnagdi, M. H. Synthesis of new condensed 2-amino-4H-pyran-3-carbonitriles and of 2-Aminoquinoline-3-carbonitriles. Org. Prep. Proceed. Int. 1999, 31, 205–214. [Google Scholar]
- Elassar, A. A.; Elkholy, Y. M. Synthesis of 3,4,7-triazaacenaphthylene and pyrido[3,4,5-de]-cinnoline derivatives. Heteroatom Chem . 2003, 14, 427–433. [Google Scholar]
- Elkholy, Y. M. Studies with polyfunctionally substituted heterocycles. Chem. Heterocycl. Comp. 2002, 38, 1342–1347. [Google Scholar] [CrossRef]
- Elkholy, Y. M. Synthesis and antimicrobial of new polyfunctionally substituted pyridazines and their derivatives. Heterocycl. Commun. 2005, 11, 89–96. [Google Scholar]
- Asolkar, R. N.; Schroder, D.; Heckmann, R.; Land, S.; Wagner-Dobler, I.; Laatsch, H. Helquinoline, a new tetrahydroquinoline antibiotic from Janibacter limosus Hel. J. Antibiotics 2004, 57, 17–23. [Google Scholar]
- Schoenwald, R. D. Tetrahydroquinoline analogs for use in glaucoma treatment. U.S. Pat. 5776482, 1988. [Google Scholar]
- Dimon, D. B.; Robert, W. Preparation of 4-carboxyamino-2-substituted tetrahydroquinoline derivatives as cholesteryl ester transfer protein inhibitors. JP 2001163859, 2001. [Google Scholar]
- Shaaban, M. A.; Ghoneim, K.M.; Kalifa, M. Synthesis of certain 4-oxo-tetrahydroquinoline and benzauocine derivatives likely to possess analgesic activity. Pharmazie 1977, 32, 90–92. [Google Scholar] [PubMed]
- Hashimoto, K.; Okaichi, Y.; Nomi, D.; Bando, M.; Minamikawa, J. A practical synthesis of (S)-(−)-nadifloxacin: Novel acid-catalyzed racemization of tetrahydroquinoline derivatives. Chem. Pharm. Bull. 1996, 44, 642–645. [Google Scholar] [CrossRef]
- Chakaravorty, P.K.; Grelnlee, W. P.C.T. Int. Appl WO 92,20,687,156, 1992. [C.A. 1993, 118, 213104d].
- Shujiang, T. U.; Fang, F.; Tuanjie, L.; Songlei, Z.; Xiaojing, Z. An efficient one-pot synthesis of novel pyrimidoquinoline derivative under microwave irradiation without catalyst. J Heterocycl. Chem 2005, 42, 707–710. [Google Scholar]
- Shimamure, H.; Terajima, K.; and Kawase, Y. Jpn. Kokai Tokyo Koho JP 05,112,559, 1993.
- Gavrilov, M. Yu.; Konshin, M. E. Synthesis of Octahydroquinoline[4,5-b]quinoline-2,4-dione. Chem. Heterocycl. Comp. 1989, 25, 932–935. [Google Scholar] [CrossRef]
- Abdel-Gawad, S. M.; El-Gagy, M. S. A.; Heiba, H. I.; Aly, H. M.; Ghorab, M. M. Synthesis and radiation stability of some new biologically active hydroquinoline and pyrimido[4,5-b]quinoline derivatives. J. Chin. Chem. Soc. 2005, 52, 1227–1236. [Google Scholar]
- Abu-Shanab, F. A.; Elkholy, Y. M.; Elnagdi, M. H. Enaminones as building blocks in organic Synthesis. Synth. Commun. 2002, 32, 3493–3502. [Google Scholar]
- Leoncini, G.; Signorello, M. G.; Grossi, G. C.; Di Braccio, M. Mechanism of action of two new pyrimidoquinoline and isoquinoline derivatives in human platelets. Thromb. Res. 1997, 87, 483–492. [Google Scholar] [CrossRef] [PubMed]
- Omura, S.; Nakagawa, A. Structure of virantmycin, a novel antiviral antibiotic. Tetrahedron Lett. 1981, 2199–2202. [Google Scholar]
- Nene, Y. L.; Thapliyal, P.N. Fungicides in plant disease control; Oxford & IBH Publ.: New Delhi, 1982; pp. 192–198. [Google Scholar]
- CCDC 626536 and 626537 contain the supplementary crystallographic data for this paper. These data can be obtained free of charge from The Cambridge Crystallographic Data Centre via www.ccdc.cam.ac.uk/data_request/cif.
- Mackay, S.; Gilmore, C. J.; Edwards, C.; Stewart, N.; Shankland, K. maXus Computer Program for the Solution and Refinement of Crystal Structures. Bruker Nonius: Delft, The Netherlands and Mac Science: Japan & The University of Glasgow. 1999. [Google Scholar]
- Johnson, C. K. ORTEP--II. A Fortran Thermal--Ellipsoid Plot Program. Report ORNL-5138. Oak Ridge National Laboratory: Oak Ridge, Tennessee, USA, 1976. [Google Scholar]
- Otwinowski, Z.; Minor, W. Methods in Enzymology; Carter, Jr. C. W., Sweet, R. M., Eds.; Academic Press: New York, 1997; Vol. 276, pp. 307–326. [Google Scholar]
- Altomare, A.; Cascarano, G.; Giacovazzo, C.; Guagliardi, A.; Burla, M. C.; Polidori, G.; Camalli, M. J. Appl. Cryst 1994, 27, 435.
- Waasmaier, D.; Kirfel, A. Acta Cryst. 1995, A51, 416–431.
- Sample Availability: Available from the authors.
© 2006 by MDPI (http://www.mdpi.org) Reproduction is permitted for noncommercial purposes.