Abstract
Reaction of 4-anthracen-9-yl-4-oxo-but-2-enoic acid (1) with indole gave the corresponding butanoic acid 2. Cyclocondensation of 2 with hydrazine hydrate, phenyl hydrazine, semicarbazide and thiosemicarbazide gave the pyridazinone derivatives 3a-d. Reaction of 3a with POCl3 for 30 min gave the chloropyridazine derivative 4a, which was used to prepare the corresponding carbohydrate hydrazone derivatives 5a-d. Reaction of chloropyridazine 4a with some aliphatic or aromatic amines and anthranilic acid gave 6a-f and 7, respectively. When the reaction of the pyridazinone derivative 3a with POCl3 was carried out for 3 hr an unexpected product 4b was obtained. The structure of 4b was confirmed by its reaction with hydrazine hydrate to give hydrazopyridazine derivative 9, which reacted in turn with acetyl acetone to afford 10. Reaction of 4b with methylamine gave 11, which reacted with methyl iodide to give the trimethylammonium iodide derivative 12. The pyridazinone 3a also reacted with benzene- or 4-toluenesulphonyl chloride to give 13a-b and with aliphatic or aromatic aldehydes to give 14a-g. All proposed structures were supported by IR, 1H-NMR, 13C-NMR, and MS spectroscopic data. Some of the new products showed antibacterial activity.
Introduction
In recent years a substantial number of 6-aryl-3-(2H)-pyridazinones have been reported to possess antimicrobial [1,2], potent analgesic [3], anti-inflammatory [3,4,5,6,7], antifeedant [8], herbicidal [9], antihypertensive [10,11,12] and antiplatelet activities [13,14,15], anticancer effects [16] and other anticipated biological [17] and pharmacological properties [18,19]. In particular, a large number of indolylpyridazinone derivatives are well known as antimicrobial agents [1,20], intermediates for drugs and agrochemicals [21,22], antiphlogistics [23], antipyretics [24], inflammation inhibitors [25], blood platelet aggregation inhibitors, cardiovascular and antihypertensive agents [26]. As part of our program aimed at utilizing β-aroylpropionic acid derivatives containing the indole moiety as starting materials for the synthesis of pyridazine and pyridazinone derivatives, these reports of interesting biological activities prompted us to synthesize a new series of 6-anthracenepyridazinones containing indolyl moieties through the nucleophilic addition of indole to 6-anthracene-4-oxo-2-butenoic acid, followed by cyclocondensation of the resulting adduct to give the corresponding dihydropyridazinone and to screen some of these new compounds for antibacterial activity.
Results and Discussion
4-Anthracen-9-yl-4-oxo-but-2-enoic acid (1) was prepared following a reported procedure [1,27]. Reaction of 1 with indole in dry benzene gave 4-anthracen-9-yl-2-(1H-indol-3-yl)-4-oxo-butyric acid (2). Cyclocondensation of 2 with hydrazine hydrate, phenyl hydrazine, semicarbazide and thiosemicarbazide in dry benzene [28,29,30,31] gave 6-anthracen-9-yl-4-(1H-indol-3-yl)-4,5-dihydro-2H-pyridazin-3-one, 6-anthracen-9-yl-4-(1H-indol-3-yl)-2-phenyl-4,5-dihydro-2H-pyridazin-3-one, 3-anthracen-9-yl-5-(1H-indol-3-yl)-6-oxo-5,6-dihydro-4H-pyridazine-1-carboxylic acid amide and 3-anthracen-9-yl-5-(1H-indol-3-yl)-6-oxo-5,6-dihydro-4H-pyridazine-1-carbothioic acid amides 3a-d, respectively (Scheme 1). Physical properties, mass spectral data and elemental analyses for the synthesized compounds 1-3d are given in Table 1.
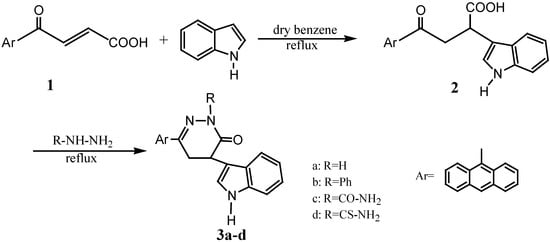
Scheme 1.
Scheme 1.

Table 1.
Physical properties, mass spectral data and elemental analyses for compounds 1-3.
| Compound No | M.P. (°C) Cryst. solvent | Mol. Formula Mol. weight | M.W. from MS | Analysis % Calc./ Found | |||
|---|---|---|---|---|---|---|---|
| C | H | N | S | ||||
| 1 | 215-217 Ethanol | C18H12O3 276.29 | 276.08 | 78.25 78.20 | 4.38 4.35 | ----- | ----- |
Reaction of pyridazinone 3a with POCl3 for 30 min gave the chloropyridazine derivative 4a [32], which reacted with carbohydrate hydrazones of ribose, glucose, galactose and lactose in ethanol to give hydrazonopyradazine derivatives 5a-d [6,12]. Mixing chloropyridazine 4a with aliphatic or aromatic amines, namely methylamine, ethylamine, aniline, sulphanilinic acid, α-naphthylamine or diphenylamine in dry benzene gave pyridazine derivatives 6a-f [7,12,33]. In addition, 4a reacted with anthranilic acid in dry benzene to give 7 (Scheme 2) [1,30,32]. Physical properties, mass spectral data and elemental analyses for all newly synthesized compounds 4a-7 are listed in Table 2.
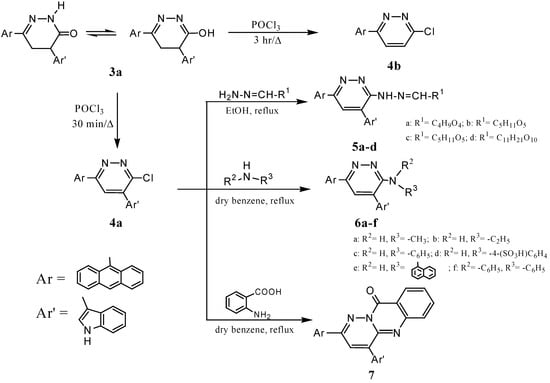
Scheme 2.
Scheme 2.

Table 2.
Physical properties, mass spectral data and elemental analyses for compounds 4-7.
| Compound No | M.P. (°C) Cryst. solvent | Mol. Formula Mol. Weight | M.W. from MS | Analysis % Calc./ Found | |||
|---|---|---|---|---|---|---|---|
| C | H | N | S | ||||
| 4a | 200 Benzene | C26H16ClN3 405.88 | 405.10 | 76.94 76.99 | 3.97 3.94 | 10.35 10.33 | ----- |
Surprisingly, when the reaction of 3a with POCl3 was carried out for 3 hr an unexpected product, 6-anthracen-9-yl-6-chloropyridazine (4b), was obtained via dearylation and substitution of the hydroxyl group by chlorine [20] (Scheme 2). The structure of 4b was proven by the similarity of its melting point to that of an authentic sample which was independently prepared by the reaction of 4-anthracen-9-yl-4-oxo-but-2-enoic acid (1) with hydrazine hydrate in dry benzene and treatment of the resulting pyridazinone 8 with POCl3 for 30 min to give 4b (Scheme 3) [1,5,7,30,31]. Reaction of chloropyridazine 4b with hydrazine hydrate in boiling benzene [34] gave the hydrazinopyridazine derivative 9, whose structure was inferred from its infrared spectrum. The structure of 9 was further confirmed by its reaction with acetyl acetone in boiling methanol that gave 3-anthracen-9-yl-6-(3,5-dimethylpyrazol-1-yl) pyridazine (10) [5,6,14,32]. As a point of interest, it was observed that reaction of 4b with methylamine at 140 °C afforded 11. On the other hand, when compound 11 was reacted with excess CH3I in methanol the quaternary ammonium iodide derivative 12 was formed (Scheme 3). Physical properties, mass spectral data and elemental analyses for all newly synthesized compounds 8-12 are given in Table 3.
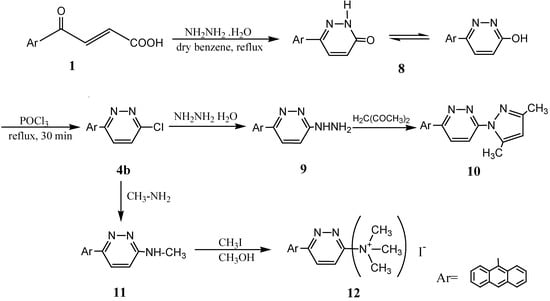
Scheme 3.
Scheme 3.

Table 3.
Physical properties, mass spectral data and elemental analyses for compounds 8-12.
| Compound No | M.P. (°C) Cryst. solvent | Mol. Formula Mol. Weight | M.W. from MS | Analysis % Calc./ Found | |||
|---|---|---|---|---|---|---|---|
| C | H | N | S | ||||
| 8 | 165 Benzene | C18H12N2O 272.30 | 272.30 | 79.39 79.42 | 4.44 4.40 | 10.29 10.32 | ----- |
The reaction of pyridazinone 3a with benzene/4-toluenesulfonyl chloride and anhydrous K2CO3 in dry acetone at reflux for 24 hr gave 6-anthracen-9-yl-4-(1H-indol-3-yl)-2-(benzenesulfonyl or 4-toluenesulfonyl)-4,5-dihydro-2H-pyridazin-3-ones 13a and 13b, respectively. Pyridazinone 3a also reacted with same aliphatic and aromatic aldehydes namely formaldehyde, acetaldehyde, benzaldehyde, 2-hydroxybenzaldehyde, 4-hydroxybenzaldehyde, 2-methoxybenzaldehyde, 4-methoxybenzaldehyde, 2,4-dimethoxybenzaldehyde, 2-hydroxynapthaldehyde and furfuraldehyde to give pyridazinone derivatives 14a-j, respectively (Scheme 4). Physical properties, mass spectral data and elemental analyses for all new compounds 13a-14j are given in Table 4.
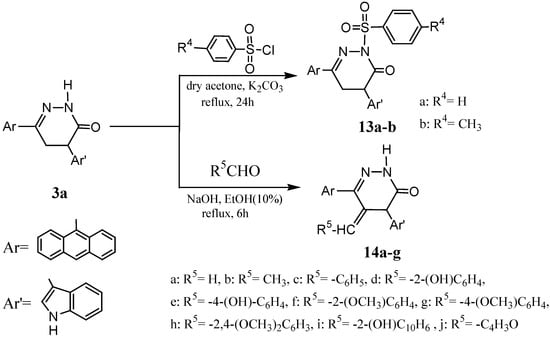
Scheme 4.
Scheme 4.

Table 4.
Physical properties, mass spectral data and elemental analyses for compounds 13-14.
| Compound No | M.P. (°C) Cryst. solvent | Mol. Formula Mol. Weight | M.W. from MS | Analysis % Calc./ Found | |||
|---|---|---|---|---|---|---|---|
| C | H | N | S | ||||
| 13a | 154 Benzene | C32H23N3O3S 526.61 | 529.15 | 72.57 72.50 | 4.38 4.41 | 7.93 7.90 | 6.05 6.00 |
Biological Screening
The activities of some of the prepared compounds against representative Gram positive and negative bacteria were tested by the disk diffusion method [1,8,35]. The results are listed in Table 5. From the data it is clear that compounds 6b, 14i possess high activity against both types of bacteria, while compound 6c displays low activity. Compounds 1 and 6b possess high activity, compounds 2, 3a-3d, 4a, 5a, 5d, 14g, 14i possess moderate activity and compounds 6c and 13a possess less activity against Gram positive strains. As far as Gram negative microorganisms are concerned, compound 14i showed high activity, while compounds 1, 2, 3a, 3b, 3d, 4a, 5a, 5d, 6b, 13a and 14g all displayed moderate activity and 3c and 6c possess less activity against such microorganisms.

Table 5.
Antibacterial activity of select compounds*
| Compound No | Gram positive bacteria | Gram negative bacteria | ||
|---|---|---|---|---|
| Staph. aureus | Staph. epidermis | E. coli | Pr. vulgaris | |
| 1 | +++ | +++ | + | ++ |
* Solvent: DMF, [c] = 250 μg mL-1. Ratings: + = less active (inhibition zone 1-5 mm); ++ = moderately active (inhibition zone 5-10 mm), +++ = more active (inhibition zone 10-15 mm); ++++ = highly active (inhibition zone 15-20 mm); reference substance for Gram positive and Gram negative bacteria: ampicillin.
Conclusions
A novel synthesis of some new indolylpyridazinone derivatives by cyclocondensation of indolylbutyric acid 2 with hydrazine hydrate and its derivatives to give pyridazinone derivatives 3a-d is described. The reactions of pyridazinone 3a with PCl5/POCl3, arylsulphonyl chloride derivatives and aliphatic or aromatic aldehydes were studied, as were the behaviors of chloropyridazine derivatives towards hydrazine hydrate, carbohydrate hydrazones and aliphatic or aromatic amines. The structures of all new synthesized compounds were established from their spectral data and elemental analysis. Additionally, the antimicrobial activity of selected compounds against Gram positive and negative bacteria is reported.
Acknowledgements
I thank Dr. Malka Mehsen in Girls College of Science, Dammam, Saudi Arabia for performing the biological tests
Experimental
General
Melting points were determined on Reichert hot stage microscope and are uncorrected. IR spectra were measured with a Nicolet Magna 520 instrument, using potassium bromide disks; results are given in cm‑1. 1H-NMR and 13C-NMR spectra were recorded at 200 and 90.56 MHz, respectively, in DMSO-d6 on a JEOL JNM-GX270 spectrometer. The chemical shifts are reported in parts per million (ppm) downfield from internal tetramethylsilane (TMS). Electron impact MS spectra were obtained on a JEOL JMS-HX 100 instrument at 70 eV. Elemental microanalysis was done on a Carlo Erba analyzer model 110. Suitable crystals were grown by slow crystallization from methanol, ethanol and benzene.
4-Anthracen-9-yl-4-oxo-but-2-enoic acid (1)
Compound 1 was prepared following the literature procedure [1,20,27]. It was obtained in 85% yield as white crystals; IR (cm-1): 1608 (C=C), 1662 (ketone C=O), 1699 (acid C=O), 3054, 2970, 2927, 2869 (C-H), 4200-3400 (acid OH); 1H-NMR: 7.20-8.50 (m, 11H, Ar-H and vinyl), 11 (br, 1H, COOH); 13C-NMR: 127-144 (16C, Ar and vinyl), 170, 188.10 (2C, acid and ketone C=O). Its physical properties, mass spectral data and elemental analysis are given in Table 1.
4-Anthracen-9-yl-2-(1H-indol-3-yl)-4-oxo-butyric acid (2).
Indole (10 mmol) was added to a solution of 4-anthracen-9-yl-4-oxo-but-2-enoic acid (1, 10 mmol) in dry benzene (10 mL) and the reaction mixture was refluxed for 6 hr. The solid that separated on cooling was recrystallized from benzene to give compound 2 as white crystals, yield 80%; IR (cm-1): 1618 (C=C), 1674 (ketone C=O), 1708 (acid C=O), 3030, 3070, 2926, 2853 (C-H), 3414 (indole N-H); 1H-NMR: 3.40 (d, 2H, CH2), 4.04 (t, 1H, CH), 6.51 (s, 1H, indole CH), 7.02-7.91 (m, 13H, Ar-H), 10.08 (br, 1H, NH), 11.10 (br, 1H, COOH); 13C-NMR: 40-41 (2C, CH2-CH), 112.50-136.50 (21C, Ar), 123 (1C, indole C-NH), 175.30, 198.90 (2C, acid and ketone C=O). Physical properties, mass spectral data and elemental analysis for compound 2 are given in Table 1.
General method for the preparation of 3a-d:
Hydrazine hydrate derivatives (10 mmol) were added to a solution of 2 (10 mmol) in dry benzene (5 mL) and the resulting reaction mixture was refluxed for 6 hr. The solid that separated on cooling was recystallized from benzene to give compounds 3a-d. Their physical properties, mass spectral data and elemental analysis are given in Table 1.
6-Anthracen-9-yl-4-(1H-indol-3-yl)-4,5-dihydro-2H-pyridazin-3-one (3a).
Obtained from hydrazine hydrate and 2 as white needles, yield 81%; IR (cm-1): 1604 (C=C),1635 (C=N), 1671 (pyridazinone C=O), 3080, 3010, 3054, 2957 (C-H), 3100-3272 (-OH), 3411-3375 (pyridazinone and indole N-H); 1H-NMR: 3.35 (d, 2H, CH2), 4.36 (t, 1H, CH), 6.81 (s, 1H, indole CH), 6.90-7.91 (m, 13H, Ar-H), 10.9-12.40 (br, 2H, pyridazinone and indole NH); 13C-NMR: 33.80-40.10 (2C, CH2-CH), 111.20-136 (21C, Ar), 123 (1C, indole C-NH), 153.34 (1C, C=N-N), 170 (1C, C=O).
6-Anthracen-9-yl-4-(1H-indol-3-yl)-2-phenyl-4,5-dihydro-2H-pyridazin-3-one (3b).
Obtained from phenyl hydrazine and 2 as white crystals, yield 79%; IR (cm-1): 1605 (C=C), 1640 (C=N), 1674 (pyridazinone C=O), 3054, 3070, 2923, 2869 (C-H), 3400-3411 (indole N-H); 1H-NMR: 3.35 (d, 2H, CH2), 4.05 (t, 1H, CH), 6.80-7.82 (m, 19H, Ar-H), 11.02 (br, 1H, indole NH); 13C-NMR: 33.80-40.10 (2C, CH2-CH), 111.20-136 (26C, Ar), 123 (1C, indole C-NH), 141.20 (1C, C-N-N), 153.34 (1C, C=N-N), 170 (1C, pyridazinone C=O).
3-Anthracen-9-yl-5-(1H-indol-3-yl)-6-oxo-5,6-dihydro-4H-pyridazine-1-carboxylic acid amide (3c).
Obtained from semicarbazide and 2 as a white solid, yield 75%; IR (cm-1): 1605 (C=C), 1638 (C=N), 1650 (amide C=O), 1678 (pyridazinone C=O), 3070, 3054, 2926(C-H), 3320-3410 (indole and amide N-H, NH2); 1H-NMR: 3.34(d, 2H, CH2), 4.05(t, 1H, CH), 4.50(br, 2H, NH2), 6.81-7.80(m, 14H, Ar-H), 11.02(br, 1H, indole NH); 13C-NMR: 33.80-41.07 (2C, CH2-CH), 112.11-136 (21C, Ar), 123.80 (1C, indole C-NH), 157.36 (1C, C=N-N), 175,189 (2C, pyridazinone and amide C=O).
3-Anthracen-9-yl-5-(1H-indol-3-yl)-6-oxo-5,6-dihydro-4H-pyridazine-1-carbothioic acid amide (3d).
Obtained from thiosemicarbazide and 2 as white needles, yield 87%; IR (cm-1): 1270 (C=S), 1604 (C=C), 1640 (C=N), 1704 (pyridazinone C=O), 3057, 3030, 2959(C-H), 3350-3453 (indole and carbothioic acid amide N-H, NH2); 1H-NMR: 3.34 (d, 2H, CH2), 4.05 (t, 1H, CH), 4.50 (br, 2H, NH2), 7.04-8.30 (m, 14H, Ar-H), 11.02 (br, 1H, indole NH); 13C-NMR: 33.80-41.07 (2C, CH2-CH), 111.56-133.41 (21C, Ar), 122.83 (1C, indole C-NH), 155 (1C, C=N-N), 177 (1C, pyridazinone C=O), 183 (1C, C=S).
3-(6-Anthracen-9-yl-3-chloropyridazin-4-yl)-1H-indole (4a).
POCl3 (5 mL) was added to 3a (10 mmol) and the reaction mixture was heated on oil bath for 30 min, set aside to cool and then poured onto crushed ice (60 g), filtered, washed well with water and recrystallized from benzene to give 4a as a brown solid, yield 81%; IR (cm-1): 756 (C-Cl), 1605 (C=C), 1640 (C=N), 3057, 3047, 2923, 2869 (C-H), 3391 (indole N-H); 1H-NMR: 7.30-8.30 (m, 15H, Ar-H), 10.90 (br, 1H, indole NH); 13C-NMR: 111.10-135 (23C, Ar), 123 (1C, indole C-NH), 152 (1C, N-N=C-Cl), 160 (1C, C=N-N). Physical properties, mass spectral data and elemental analysis for compound 4a are given in Table 2.
3-Anthracen-9-yl-6-chloropyridazine (4b).
Method A: POCl3 (5 mL) was added to 3a (10 mmol) and the reaction mixture was heated on oil bath for 3hr, then set-aside poured on to 60 g crushed ice, filtered, washed well with water and recrystallized from benzene to give 4b as a brown solid, yield 81%.
Method B: POCl3 (5 mL) was added to 8 (10 mmol) and the reaction mixture was heated on oil bath for 30 min, then set-aside poured on to 60 g crushed ice, filtered, washed well with water and recrystallized from benzene to give 4b as a brown solid, yield 87%; IR (cm-1): 752 (C-Cl), 1605 (C=C), 1648 (C=N), 3057, 3028 (C-H); 1H-NMR: 7.32-8.28 (m, 11H, Ar-H); 13C-NMR: 111-132 (16C, Ar), 151 (1C, N-N=C-Cl), 158 (1C, C=N-N). Physical properties, mass spectral data and elemental analysis for compound 4b are given in Table 2.
General procedure for the reaction of chloropyridazine 4a with carbohydrate hydrazones.
The appropriate carbohydrate hydrazone (1 mmol) was added to a mixture of 4a (1 mmol) in ethanol (5 mL) and the reaction mixture was refluxed for 6 hr. The solid that separated on cooling was recystallized from ethanol to give compounds 5a-d. Physical properties, mass spectral data and elemental analysis for compounds 5a-d are given in Table 2.
(2R,3S,4S)-5-{[6-Anthracen-9-yl-4-(1H-indol-3-yl)pyridazin-3-yl]hydrazono}pentane-1,2,3,4,-tetraol (5a).
Ribose hydrazone gave compound 5a as an orange solid, yield 57%; IR (cm-1): 1605 (C=C), 1615 (N=N), 1657 (C=N), 3060, 2967, 2926, 2869 (C-H), 3430-3250 (indole and hydrazone N-H, NH-N=C), 3750-3250 (O-H); 1H-NMR: 3.32-3.50 (m, 3H, aliphatic CH-OH), 3.68 (d, 2H, aliphatic CH2-OH), 3.90 (s, 1H, NH-N), 4.85 (br, 4H, OH), 7- 8.27 (m, 15H, Ar-H), 7.53 (s, 1H, CH=N), 10.90 (br, 1H, indole NH); 13C-NMR: 60.9-74.60 (4C, aliphatic C-O), 111-138.70 (23C, Ar), 122.60 (1C, indole C-NH), 148-154 (2C, C=N-N), 159.20 (1C, N-C=N).
(2R,3R,4R,5S)-6-{[6-Anthracen-9-yl-4-(1H-indol-3-yl)pyridazin-3-yl]-hydrazono}-hexane-1,2,3,4,5-pentaol (5b).
Glucose hydrazone gave compound 5b as a yellow solid, yield 52%; IR (cm-1): 1600 (C=C), 1637 (C=N), 3060, 2960, 2925 (C-H), 3414-3197 (indole and hydrazone N-H), 3625-3620 (O-H); 1H-NMR: 3.30-3.44 (m, 4H, aliphatic CH-OH), 3.68 (m, 2H, aliphatic CH2-OH), 3.90 (s, 1H, NH-N), 4.85 (br, 5H, OH), 6.80-8.50 (m, 15H, Ar-H), 7.53 (s, 1H, CH=N), 10.90 (br, 1H, indole NH); 13C-NMR: 62.10-73.90 (5C, aliphatic C-O), 111-148.70 (23C, Ar), 122.80 (1C, indole C-NH), 148.60-154.7 (2C, C=N-N), 164 (1C, N-C=N).
(2R,3S,4R,5S)-6-{[6-Anthracen-9-yl-4-(1H-indol-3-yl)pyridazin-3-yl]hydrazono}-hexane-1,2,3,4,5-pentaol (5c).
Galactose hydrazone gave compound 5c as a white solid, yield 55%; IR (cm-1): 1565 (HN-N=C), 1605 (C=C), 1640 (C=N), 3057, 2960, 2923, 2890 (C-H), 3460-3412 (indole and hydrazone N-H), 3629-3400 (O-H); 1H-NMR: 2.50-3.48 (m, 4H, aliphatic CH-OH), 3.60 (m, 2H, aliphatic CH2-OH), 3.91 (s, 1H, NH-N), 4.85 (br, 5H, OH), 7.00-8.10 (m, 15H, Ar-H), 7.51 (s, 1H, CH=N), 10.91 (br, 1H, indole NH); 13C-NMR: 61.10-73.90 (5C, aliphatic C-O), 111-148.70 (23C, Ar), 122 (1C, indole C-NH), 148.60-154.70 (2C, C=N-N), 159.20 (1C, N-C=N).
6-{[6-Anthracen-9-yl-4-(1H-indol-3-yl)pyridazin-3-yl]hydrazono}-3-(3,4,5-trihydroxy-6-hydroxy-methyltetrahydropyran-2-yloxy)-hexane-1,2,4,5-tetraol (5d).
Lactose hydrazone gave compound 5d as a white solid, yield 58%; IR (cm-1): 1570 (HN-N=C), 1605 (C=C), 1638 (C=N), 3057, 2960, 2923(C-H), 3412-3412 (indole and hydrazone N-H), 3635-3390 (O-H); 1H-NMR: 2.99-3.76(m, 9H, aliphatic CH-OH), 3.66-3.68 (m,4H, aliphatic CH2-OH), 3.90 (s, 1H, NH-N), 4.85 (br, 8H, OH), 7.00-8.50 (m, 15H, Ar-H), 7.50 (s, 1H, CH=N), 10.90 (br, 1H, indole, NH); 13C-NMR: 62.50-72.10 (10C, aliphatic C-O), 96.50 (1C, O-C-O), 111-148.70 (23C, Ar), 122 (1C, indole C-NH), 148.60-154.70 (2C, C=N-N), 159.20 (1C, N-C=N).
General reaction of chloropyridazine 4a with aliphatic or aromatic amines
The aliphatic or aromatic amine (1 mmol) was added to a mixture of 4a (1 mmol) in dry benzene (5 mL) and the reaction mixture was heated in oil bath for 6 hr. The solid that separated on cooling was recrystallized from benzene to give compounds 6a-f. Their physical properties, mass spectral data and elemental analysis are given in Table 2.
[6-Anthracen-9-yl-4-(1H-indol-3-yl)-pyridazin-3-yl]-methylamine (6a).
Methylamine gave 6a as a white solid, yield 67%; IR (cm-1): 1619 (C=C), 1637 (C=N), 3054, 3030, 2957, 2923 (C-H), 3413-3406 (indole and secondary amine N-H); 1H-NMR: 0.83 (s, 3H, CH3), 3.89 (br, 1H, secondary amine NH), 7.05-7.90 (m, 15H, Ar-H), 11.12 (br, 1H, indole NH); 13C-NMR: 37.50 (1C, N-CH3), 111-136 (23C, Ar), 123 (1C, indole C-NH), 148.60 (1C, N-C=N), 159.2 (1C, C=N-N).
[6-Anthracen-9-yl-4-(1H-indol-3-yl)-pyridazin-3-yl]-ethylamine (6b).
Ethylamine gave 6b as a brown solid, yield 52%; IR (cm-1): 1617 (C=C), 1635 (C=N), 3054, 2967, 2925, 2869 (C-H), 3429-3410 (indole N-H and secondary amine N-H); 1H-NMR: 0.85 (t, 3H, CH3), 1.60 (m, 2H, CH2), 3.89 (br, 1H, secondary amine NH), 7.05-8.00 (m, 15H, Ar-H), 11.10 (br, 1H, indole NH); 13C-NMR: 16.20 (1C, CH3), 44.20 (1C, N-CH2), 111-136 (23C, Ar), 123.41 (1C, indole C-NH), 151.10 (1C, C=N-N), 160.10 (1C, N-C=N).
[6-Anthracen-9-yl-4-(1H-indol-3-yl)-pyridazin-3-yl]-phenylamine (6c).
Aniline gave 6c as white needles, yield 72%; IR (cm-1): 1617 (C=C), 1638 (C=N), 3057, 2982, 2973, 2869 (C-H), 3429-3400 (indole N-H and secondary amine N-H); 1H-NMR: 3.89 (br, 1H, secondary amine NH), 6.80-8.30 (m, 20H, Ar-H), 11.10 (br, 1H, indole NH); 13C-NMR: 111-148 (29C, Ar), 123 (1C, indole C-NH), 150.10 (1C, C=N-N), 160.5 (1C, N-C=N).
4-[6-Anthracen-9-yl-4-(1H-indol-3-yl)-pyridazin-3-ylamino] benzenesulfonic acid (6d).
Sulphanilic acid gave 6d as a gray solid, yield 51%; IR (cm-1): 1160, 1423 (SO2), 1601 (C=C), 1631 (C=N), 3057, 2960, 2920 (C-H), 3390 (indole and secondary amine N-H), 3200-3100 (O-H); 1H-NMR: 3.89 (br, 1H, secondary amine NH), 6.00-8.50 (m, 19H, Ar-H), 11.10 (br, 1H, indole NH), 14.90 (br, 1H, SO3H); 13C-NMR: 111-148.70 (27C, Ar), 133.50 (1C, C-S), 124 (1C, indole C-NH), 148.60 (1C, C=N-N), 150.50 (1C, -NH-Ph), 159 (1C, N-C=N).
[6-Anthracen-9-yl-4-(1H-indol-3-yl)-pyridazin-3-yl]-naphthalen-1-yl amine (6e).
α-Naphthylamine gave 6e as a light brown solid, yield 58%; IR (cm-1): 1616 (C=C), 1640 (C=N), 3054, 2999, 2973 (C-H), 3429-3401 (indole and secondary amine N-H); 1H-NMR: 3.89 (br, 1H, secondary amine NH), 6.70-8.27 (m, 22H, Ar-H), 11.10 (br, 1H, indole NH); 13C-NMR: 109.40-141.60 (33C, Ar), 122 (1C, indole C-NH), 148.60 (1C, C=N-N), 161.20 (1C, N-C=N).
[6-Anthracen-9-yl-4-(1H-indol-3-yl)-pyridazin-3-yl] diphenylamine (6f).
Diphenylamine gave 6f as a dark brown solid, yield 51%; IR: 1617 (C=C), 1635 (C=N), 3057, 2982, 2973 (C-H), 3410 (indole N-H); 1H-NMR: 6.72-8.50 (m, 25H, Ar-H), 11.10 (br, 1H, indole NH); 13C-NMR: 111-136 (33C, Ar), 122.50 (1C, indole C-NH), 142.90 (2C, N-C), 150 (1C, C=N-N), 160 (1C, N-C=N).
2-Anthracen-9-yl-4-(1H-indol-3-yl)-1,9-a,10-triaza-anthracen-9-one (7).
Anthranilic acid (1 mmol) was added to a mixture of 4a (1 mmol) in dry benzene (5 mL) and the reaction mixture was refluxed for 6 hr. The solid that separated on cooling was recystallized from benzene to give 7 as a brown solid, yield 65%; IR: 1600 (C=C), 1638 (C=N), 1665 (C=O), 3050, 2959, 2925, 2869 (C-H), 3410-3290 (indole N-H); 1H-NMR: 7.10-8.60 (m, 19H, Ar-H), 12 (br, 1H, indole NH); 13C-NMR: 111-147.70 (29C, Ar), 122 (1C, indole C-NH), 155.50 (1C, C=N-N), 160 (1C, N-C=N), 170 (1C, N-C=O).
6-Anthracen-9-yl-2H-pyridazin-3-one (8).
Hydrazine hydrate (1 mmol) was added to a solution of 4-anthracen-9-yl-4-oxo-but-2-enoic acid (1, 1 mmol) in dry benzene (10 mL) and the reaction mixture was refluxed for 6 hr. The solid that separated after concentration and cooling was recrystallized from benzene to give 8 as white needles, yield 85%; IR (cm-1): 1604 (C=C), 1630 (C=N), 1670 (C=O), 3070, 3030, 2957 (C-H), 3411-3357 (indole N-H); 1H-NMR: 6.50-8.60 (m, 11H, Ar-H), 10.20 (br, 1H, pyridazinone NH); 13C-NMR: 111-135.70 (16C, Ar), 155 (1C, C=N-N), 168 (1C, C=O).
6-Anthracen-9-yl-pyridazin-3-yl) hydrazine (9).
Hydrazine hydrate (1 mmol) was added to a solution of 4b (1 mmol) in dry benzene (10 mL) and the reaction mixture was refluxed for hr. The solid that separated was recrystallized from dry benzene to give 9 as buff crystals, yield 70%; IR (cm-1): 1605 (C=C), 1181 (C=N), 3054, 3015, 2958 (C-H), 3327-3139 (hydrazine NHNH2); 1H-NMR: 3.89-10 (br, 3H, NH-NH2), 6.59-8.30 (m, 11H, Ar-H); 13C-NMR: 111-137 (16C, Ar), 158 (1C, C=N-N), 160 (1C, N-C=NHNH2).
3-Anthracen-9-yl-6-(3,5-dimethylpyrazol-1-yl)pyridazine (10).
Acetylacetone (1 mmol) was added to a mixture of 9 (1 mmol) in methanol (10 mL) and the reaction mixture was refluxed for 5 hr. The solid that separated after cooling was recrystallized from methanol to give 10 as yellow crystals, yield 62%; IR (cm-1): 1604 (C=C), 1635 (C=N), 3050, 2959, 2925, 2869 (C-H); 1H-NMR: 2.93 (s, 6H, 2CH3), 6.50-8.270 (m, 12H, Ar-H); 13C-NMR: 14.20 (2C, 2CH3), 111-135 (17C, Ar), 148 (2C, C=N), 155 (1C, C=N-N), 160 (1C, N-C=N).
6-Anthracen-9-yl-3-methylamino-pyridazine (11).
Methylamine (1 mmol) was added to a mixture of 4b (1 mmol) and the reaction mixture was heated for 4 hr on an oil-bath at 140 °C; then cooled and triturated with methanol. The solid that separated was recrystallized from methanol to give 11 as white crystals, yield 56%; IR (cm-1): 1604 (C=C), 1635 (C=N), 3050, 2959, 2925, 2869 (C-H), 3412-3412 (indole and hydrazone N-H); 1H-NMR: 2.50 (d, 3H, CH3), 6.30-8.32 (m, 11H, Ar-H), 4.20 (br, 1H, amine NH); 13C-NMR: 35.30 (1C, CH3), 113-135 (16C, Ar), 155.60 (1C, C=N-N), 159 (1C, N-C=N).
(6-Anthracen-9-yl-pyridazin-3-yl)trimethylammonium iodide (12).
Excess methyl iodide (5 mL) was added to a mixture of 11 (1 mmol) in methanol (10 mL) and the reaction mixture was refluxed for 8 hr. After evaporation of all the solvent, the solid residue was recrystallized from methanol to give 12 as white crystals, yield 85%; IR (cm-1): 1604 (C=C), 1635 (C=N), 3050, 2959, 2925, 2869 (C-H), 3412-3412 (N-N); 1H-NMR: 2.89 (s, 9H, 3CH3), 7.39-8.30 (m, 11H, Ar-H); 13C-NMR: 52 (3C, N-CH3), 125.30-135.10 (16C, Ar), 148 (1C, C=N-N), 159 (1C, N-C=N).
6-Anthracen-9-yl-2-benzenesulfonyl-4-(1H-indol-3-yl)-4,5-dihydro-2H-pyridazin-3-one (13a).
Benzenesulfonyl chloride (1 mmol) was added to a mixture of 3a (1 mmol), anhydrous K2CO3 (1 mmol) in dry acetone (5 mL) and the reaction mixture was refluxed for 24 hr. The solid that separated on cooling was recystallized from benzene to give 13a as a white solid, yield 75%; IR (cm-1): 1337-1175 (SO2), 1616 (C=C), 1637 (C=N), 1670 (pyridazinone C=O), 3054, 2957, 2923, 2869 (C-H), 3406 (indole N-H); 1H-NMR: 2.14 (d, 2H, CH2), 3.50 (t, 1H, CH), 6.90-7.69 (m, 19H, Ar-H), 11.02 (br, 1H, indole NH); 13C-NMR: 35.80 (1C, CH2), 43.80 (1C, CH), 111-140.20 (27C, Ar), 123 (1C, indole C-NH), 157 (1C, C=N-N), 176 (1C, pyridazinone C=O).
6-Anthracen-9-yl-4-(1H-indol-3-yl)-2-(toluene-4-sulfonyl)-4,5-dihydro-2H-pyridazin- 3-one (13b).
4-Toluenesulfonyl chloride (1 mmol) was added to a mixture of 3a (1 mmol), anhydrous K2CO3 (1 mmol) in dry acetone (5 mL) and the reaction mixture was refluxed for 24 hr. The solid that separated on cooling was recystallized from benzene to give 13b as a white solid, yield 73%; IR (cm-1): 1337-1173 (SO2), 1616 (C=C), 1637 (C=N), 1670 (pyridazinone C=O), 3054, 2957, 2923, 2869 (C-H), 3406 (indole N-H); 1H-NMR: 2.10 (s, 3H, CH3), 2.30 (t, 1H, CH2), 3.70 (t, 1H, CH), 6.80-8.43 (m, 19H, Ar-H), 10.02 (br, 1H, indole NH); 13C-NMR: 20.90 (1C, CH3), 34.20 (1C, CH2), 43.90 (1C, CH-CO), 111-142 (27C, Ar), 123 (1C, indole C-NH,), 157 (1C, C=N-N), 178 (1C, pyridazinone C=O).
General reaction of pyridazinone 3a with some aliphatic or aromatic aldehydes
Aliphatic or aromatic aldehyde (1 mmol) was added to a mixture of 3a (1 mmol), NaOH (10%) in ethanol (5 mL) and the reaction mixture was refluxed for 6 hr. The solid that separated on cooling was recystallized from benzene to give 14a-j. Their physical properties, mass spectral data and elemental analysis are given in Table 4.
6-Anthracen-9-yl-4-(1H-indol-3-yl)5-methylene-4,5-dihydro-2H-pyridazin-3-one (14a).
Formaldehyde gave 14a as an orange solid, yield 53%; IR (cm-1): 1615 (C=C), 1637 (C=N), 1671 (pyridazinone C=O), 3054, 2957, 2925, 2869 (C-H), 3400-3413 (pyridazinone and indole N-H); 1H-NMR: 3.36 (s, 2H, CH2), 4.40 (s, 1H, pyridazinone CH), 7.04-8.50 (m, 14H, Ar-H), 11.02 (br, 2H, pyridazinone and indole NH); 13C-NMR: 51 (1C, pyridazinone CH), 111-142 (23C, Ar and vinyl), 122 (1C, indole C-NH), 155 (1C, C=N-N), 170 (1C, pyridazinone C=O).
6-Anthracen-9-yl-5-ethylidene-4-(1H-indol-3-yl)-4,5-dihydro-2H-pyridazin-3-one (14b).
Acetaldehyde gave 14b as an orange solid, yield 58%; IR (cm-1): 1617 (C=C), 1637 (C=N), 1667 (pyridazinone C=O), 3054, 2957, 2925, 2869 (C-H), 3400-3414 (pyridazinone and indole N-H); 1H-NMR: 1.70 (d, 3H, CH3), 3.36 (m, 1H, vinyl CH), 4.40 (s, 1H, pyridazinone CH), 6.67-8.22 (m, 14H, Ar-H), 11.02 (br, 2H, pyridazinone and indole NH); 13C-NMR: 12- 52.50 (2C, pyridazinone CH3, CH), 111-140 (23C, Ar and vinyl), 122.80 (1C, indole C-NH), 155.60 (1C, C=N-N), 170 (1C, pyridazinone C=O).
6-Anthracen-9-yl-5-benzylidene-4-(1H-indol-3-yl)-4,5-dihydro-2H-pyridazin-3-one (14c).
Benzaldehyde gave 14c as an orange solid, yield 62%; IR (cm-1): 1616 (C=C), 1637 (C=N), 1671 (pyridazinone C=O), 3054, 2957, 2925, 2869(C-H), 3400-3412 (pyridazinone and indole N-H); 1H-NMR: 3.36 (s, 1H, vinyl CH), 4.40 (s, 1H, pyridazinone CH), 7.20-8.20 (m, 19H, Ar-H), 11.02 (br, 2H, pyridazinone and indole NH); 13C-NMR: 52.40 (1C, pyridazinone CH), 111-137 (29C, Ar and vinyl), 122 (1C, indole C-NH,), 155.60 (1C, C=N-N), 168 (1C, pyridazinone C=O).
6-Anthracen-9-yl-5-(2-hydroxybenzylidene)-4-(1H-indol-3-yl)-4,5-dihydro-2H-pyridazin-3-one (14d).
2-Hydroxybenzaldehyde gave 14d as a brown solid, yield 60%; IR (cm-1): 1619 (C=C), 1637 (C=N), 1681 (pyridazinone C=O), 3059, 2959, 2927, 2870 (C-H), 3250-3500 (O-H), 3400-3411 (pyridazinone and indole N-H); 1H-NMR: 3.37 (s, 1H, vinyl CH), 4.40 (s, 1H, pyridazinone CH), 6.62-8.52 (m, 18H, Ar-H), 5.50 (br, 1H, OH), 11.01 (br, 2H, pyridazinone and indole NH); 13C-NMR: 52 (1C, pyridazinone CH), 111-136.10 (28C, Ar and vinyl), 122.80 (1C, indole C-NH), 155.60 (1C, C=N-N), 156(1C, -C-OH), 168 (1C, pyridazinone C=O).
6-Anthracen-9-yl-5-(4-hydroxybenzylidene)-4-(1H-indol-3-yl)-4,5-dihydro-2H-pyridazin-3-one (14e).
4-Hydroxybenzaldehyde gave 14e as a buff solid, yield 72%; IR (cm-1): 1595 (C=C), 1640 (C=N), 1674 (pyridazinone C=O), 3054, 2957, 2925, 2869 (C-H), 3250-3500 (O-H), 3400-3414 (pyridazinone and indole N-H); 1H-NMR: 3.37 (s, 1H, vinyl CH), 4.40 (s, 1H, pyridazinone CH), 5.67 (br, 1H, OH), 6.44-8.17 (m, 18H, Ar-H), 11.02 (br, 2H, pyridazinone and indole NH); 13C-NMR: 53 (1C, pyridazinone CH), 111-136 (28C, Ar and vinyl), 122.80 (1C, indole C-NH), 155.60 (1C, C=N-N), 157.50 (1C, C-OH), 168 (1C, pyridazinone C=O).
6-Anthracen-9-yl-4-(1H-indol-3-yl)-5-(2-methoxybenzylidene)-4,5-dihydro-2H-pyridazin-3-one (14f).
2-Methoxybenzaldehyde gave 14f as a brown solid, yield 50%; IR (cm-1): 1590 (C=C), 1641 (C=N), 1671 (pyridazinone C=O), 3059, 2958, 2935, 2835 (C-H), 3410-3424 (pyridazinone and indole N-H); 1H-NMR: 3.36 (s, 1H, vinyl CH), 3.70(s, 3H, -OCH3), 4.40 (s, 1H, pyridazinone CH), 6.58-8.28 (m, 18H, Ar-H), 11.02 (br, 2H, pyridazinone and indole NH); 13C-NMR: 52.40 (1C, pyridazinone CH), 56 (1C, O-CH3), 111-138 (28C, Ar and vinyl), 122.80 (1C, indole C-NH,), 155.60 (1C, C=N-N), 159.70 (1C, C-OCH3), 168 (1C, pyridazinone C=O,).
6-Anthracen-9-yl-4-(1H-indol-3-yl)-5-(4-methoxybenzylidene)-4,5-dihydro-2H-pyridazin-3-one (14g).
4-Methoxybenzaldehyde gave 14g as a brown solid, yield 72%; IR (cm-1): 1613 (C=C), 1637 (C=N), 1669 (pyridazinone C=O), 3054, 2957, 2925, 2869 (C-H), 3400-3412 (pyridazinone and indole N-H); 1H-NMR: 3.37 (s, 1H, vinyl CH), 3.74 (s, 3H, OCH3), 4.37 (s, 1H, pyridazinone CH), 6.86-7.80 (m, 18H, Ar-H), 10.02, 11.02 (br, 2H, pyridazinone and indole NH); 13C-NMR: 52 (1C, pyridazinone CH,), 56 (1C, OCH3), 111-136 (28C, Ar and vinyl), 122.80 (1C, indole C-NH), 155.60 (1C, C=N-N), 161 (1C, C-OCH3), 168 (1C, pyridazinone C=O,).
6-Anthracen-9-yl-5-(2,4-dimethoxybenzylidene)-4-(1H-indol-3-yl-4,5-dihydro-2H-pyridazin-3-one (14h).
2,4-Dimethoxybenzaldehyde gave 14h as an orange solid, yield 51%; IR (cm-1): 1601 (C=C), 1636 (C=N), 1669 (pyridazinone C=O), 3059, 2958, 2923, 2855 (C-H), 3410-3424 (pyridazinone and indole N-H); 1H-NMR: 3.36 (s, 1H, CH), 3.75 (s, 6H, -OCH3), 4.40 (s, 1H, pyridazinone CH), 6.48-7.98 (m, 17H, Ar-H), 10.52 (br, 2H, pyridazinone and indole NH); 13C-NMR: 52 (1C, pyridazinone CH), 56.27 (2C, O-CH3), 98-151 (27C, Ar and vinyl), 122 (1C, indole C-NH,), 155.60 (1C, C=N-N), 160-162 (2C, C-O-CH3), 168 (1C, pyridazinone C=O).
6-Anthracen-9-yl-5-(2-hydroxynaphthalen-1-yl-methylene)-4-(1H-indol-3-yl)-4,5-dihydro-2H-pyridazin-3-one (14i).
2-Hydroxynaphthaldehyde gave 14i as a brown solid, yield 58%; IR (cm-1): 1619 (C=C), 1637 (C=N), 1681 (pyridazinone C=O), 3059, 2959, 2927, 2870 (C-H), 3250-3500 (O-H), 3400-3411 (pyridazinone and indole N-H); 1H-NMR: 3.85 (s, 1H, vinyl CH), 4.40 (s, 1H, pyridazinone CH), 6.47-7.95 (m, 20H, Ar-H), 5.62 (br, 1H, OH), 10.21 (br, 2H, pyridazinone and indole NH); 13C-NMR: 52.40 (1C, pyridazinone CH), 111-137 (32C, Ar and vinyl), 122.80 (1C, indole C-NH), 155.60 (1C, C=N-N), 156 (1C, -C-OH), 170 (1C, pyridazinone C=O).
6-Anthracen-9-yl-5-furan-2-yl-methylene-4-(1H-indol-3-yl)-4,5-dihydro-2H-pyridazin-3-one (14j).
Furfuraldehyde gave 14j as a brown solid, yield 59%; IR (cm-1): 1616 (C=C), 1637 (C=N), 1670 (pyridazinone C=O), 3054, 2957, 2925, 2869 (C-H), 3400-3412 (pyridazinone and indole N-H), 1200 (C-O-C); 1H NMR: 3.39 (s, 1H, vinyl CH), 4.37 (s, 1H, pyridazinone CH), 6.30-8.55 (m, 17H, Ar-H), 11.02 (br, 2H, pyridazinone and indole NH); 13C-NMR: 51 (1C, pyridazinone CH, ), 111-136 (25C, Ar and vinyl), 122.80 (1C, indole C-NH), 145-155.3 (2C, furan O-C), 155.60 (1C, C=N-N), 168 (1C, pyridazinone C=O).
References
- Sayed, G.H.; Sayed, M.A.; Mahmoud, M.R.; Shaaban, S.S. Synthesis and Reactions of New Pyidazinone Derivatives of Expected Antimicrobial Activities. Egypt. J. Chem. 2002, 45, 767–776. [Google Scholar]
- Katrusiak, A.; Katrusiak, A.; Baloniak, S. Reactivity of 6-Chloro-4- and 5-Hydrazino-2- Phenyl-3(2H)-Pyridazinones with Vilsmeier Reagent. Tetrahedron 1994, 50, 12933–12940. [Google Scholar] [CrossRef]
- Okcelik, B.; Unlu, S.; Banoglu, E.; Kupeli, E.; Yesilada, E.; Sahin, M.F. Investigation of New Pyridazinone Derivatives for the Synthesis of Potent Analgesic and Anti-Inflammatory Compounds with Cyclooxygenase Inhibitory Activity. Arch. Pharm. Pharm. Med. Chem. 2003, 336, 406–412. [Google Scholar]
- Dogruer, D.S.; Sahin, M.F.; Kupeli, E.; Yesilada, E. Synthesis and Analgesic and Anti-Inflammatory Activity of New Pyridazinones. Turk. J. Chem. 2003, 27, 727–738. [Google Scholar]
- Frolov, E.B.; Lakner, F.J.; Khvat, A.V.; Ivachtchenko, A.V. An Efficient Synthesis of Novel 1,3-oxazolo [4,5-d] Pyridazinones. Tetrahedron Lett. 2004, 45, 4693–4696. [Google Scholar]
- Banoglu, E.; Akoglu, C.; Unlu, S.; Kupeli, E.; Yesilada, E.; Sahin, M.F. Amide Derivatives of [6-(5-Methyl-3-phenylpyrazole-1-yl)-3(2H)-Pyridazinone-2-yl]acetic Acids as Potential Analgesic anti-Inflammatory Compounds. Arch. Pharm. Pharm. Med. Chem. 2004, 337, 7–14. [Google Scholar] [CrossRef]
- Gokce, M.; Dogruer, D.; Sahin, F. Synthesis and Antinociceptive Activity of 6-Substituted-3-pyridazinone Derivatives. Il Farmaco 2001, 56, 233–237. [Google Scholar] [CrossRef]
- Cao, S.; Qian, X.; Song, G.; Chai, B.; Jiang, Z. Synthesis and Antifeedant Activity of New Oxadiazolyl 3(2H)-pyridazinones. J. Agric. Food Chem. 2003, 51, 152–155. [Google Scholar] [CrossRef]
- Piaz, V.D.; Ciciani, G.; Giovannoni, M.P. 5-Acetyl-2-Methyl-4-Nitro-6-Phenyl-3(2H)-Pyriazinone: Versatile Precursor to Hetero-Condensed Pyridazinones. Synthesis 1994, 669–671. [Google Scholar]
- Ogretir, C.; Yarligan, S.; Demirayak, S. Spectroscopic Determination of Acid Dissociation Constants of Some Biologically Active 6-Phenyl-4,5-Dihydro-3(2H)-Pyridazinone Derivatives. J. Chem. Eng. Data 2002, 47, 1396–1400. [Google Scholar] [CrossRef]
- Barbaro, R.; Betti, L.; Botta, M.; Corelli, F.; Giannaccini, G.; Maccari, L.; Manetti, F.; Strappaghetti, G.; Corsano, S. Synthesis Biological Evaluation and Pharmacophore Generation of New Pyridazinone Derivatives with Affinity Toward α1- and α2- Adrenoceptors. J. Med. Chem. 2001, 44, 2118–2132. [Google Scholar]
- Sircar, I. Synthesis of New 1,2,4-Triazolo[4,3-b]pyridazines and Related Compound. J. Hetero-cyclic Chem. 1985, 22, 1045–1048. [Google Scholar]
- Coelho, A.; Sotelo, E.; Fraiz, N.; Yanez, M.; Laguna, R.; Cano, E.; Ravina, E. Pyridazines. Part 36: Synthesis and Antiplatelet Activity of 5-Substituted-6-Phenyl-3(2H)- Pyridazinones. Bioorg. Med. Chem. Lett. 2004, 14, 321–324. [Google Scholar] [CrossRef]
- Sotelo, E.; Centeno, N.B.; Rodrigo, J.; Ravina, E. Pyridazine Derivatives. Part 27: A joint Theoretical and Experimental approach to the Synthesisi of 6-Phenyl-4,5-disubstituted-3(2H)Pyridazinones. Tetrahedron Lett. 2002, 58, 2389–2395. [Google Scholar] [CrossRef]
- Sotelo, E.; Fraiz, N.; Yanez, M.; Terrades, V.; Laguna, R.; Cano, E.; Ravina, E. Pyridazines. Part XXIX: Synthesis and Platelet Aggregation Inhibition Activity of 5-Substituted-6-Phenyl-3(2H)-Pyridazinones Novel Aspects of their Biological Action. Bioorg. Med. Chem. 2002, 10, 2873–2882. [Google Scholar] [CrossRef]
- Malinka, W.; Redzicka, A.; Lozach, O. New Derivatives of Pyrrolo[3,4-d]Pyridazinone and their Anticancer Effects. Il Farmaco 2004, 59, 457–462. [Google Scholar] [CrossRef]
- Youssef, A.S.; Marzouk, M.I.; Madkour, H.M.F.; El-Soll, A.M.A.; El-Hashash, M.A. Synthesis of some heterocyclic systems of anticipated biological activities via 6-aryl-4-pyrazol-1-yl-pyridazin-3-one. Can. J. Chem. 2005, 83, 251–259. [Google Scholar]
- Sotelo, E.; Pita, B.; Ravina, E. Pyridazines. Part 22:1 Highly Efficient Synthesis of Pharmacologically Useful 4-Cyano-6-Phenyl-5-Substituted-3(2H)-Pyridazinones. Tetrahedron Lett. 2000, 41, 2863–2866. [Google Scholar] [CrossRef]
- Sotelo, E.; Coelho, A.; Ravina, E. Pyridazine Derivatives 321): Stille-Based Approaches in the Synthesis of 5-Substituted-6-Phenyl-3(2H)-Pyridazinones. Chem. Pharm. Bull. 2003, 51, 427–430. [Google Scholar] [CrossRef]
- Kassab, R.R. Simple Synthesis and Reactions of Some New Pyridazinono Derivatives and their Antimicrobial Activity. Egypt. J. Chem. 2002, 45, 1055–1073. [Google Scholar]
- Toshiki, S.; Hiroshi, Y. Jpn. Kokai Tokkyo Koho JP 01,100,157, 1989. [Chem Abstr. 1989, 111, 153626 u].
- Masanori, S.; Toshiharu, O.; Hiroko, T. Jpn. Kokai Tokkyo Koho JP 01,31,763, 1989. [Chem Abstr. 1989, 111, 15623 r].
- Tsutomu, F.; Yoichiro, N.; Tomokayu, G.; Amid Kazuimasa, Y. Jpn. Kokai Tokkyo Koho JP 61,148,160, 1987. [Chem Abstr. 1987, 106, 50037 v].
- John, B.E.; James, C.N.; Bayer, A.C. Eur. Pat. Appl. EP 409,027, 1991. [Chem. Abstr. 1991, 114, 228729 c].
- Kamat, G.A.; Joshi, G.R.; Gadaginamath, S.G. Proc. Indian Acad. Sci. Chem. Sci. 1993, 105, 189.
- Halasz, B.D.; Monsieurs, K.; Elias, O.; Karolyhazy, L.; Tapolcsanyi, P.; Maes, B.U.; Riedl, Z.; Hajos, G.; Dommisse, R.A.; Lemiere, G.L.; Kosmrlj, J.; Matyus, P. Synthesis of 5H-Pyridazino[4,5-b]Indoles and their benzofurane analogues utilizing an intramolecular Heck-Type Reaction. Tetrahedron. 2004, 60, 2283–2291. [Google Scholar] [CrossRef]
- Sayed, G.H.; El-Kady, M.Y.; Abd Elhalim, M.S. Synthesis and Reactions of Some α–aryl-(4-bromobenzoyl)propionic acids. Indian J. Chem. 1981, 20, 845–848. [Google Scholar]
- Sayed, G.H.; Hamed, A.A.; Meligi, G.A.; Boraie, W.E.; Shafik, M. The Use of 4-(3,4- Dichlorophenyl)-4-Oxo-2-(4-Antipyrinyl)-Butonoic Acid in the Preparation of Some New Heterocyclic Compounds With Expected Biological Activity. Molecules 2003, 8, 322–332. [Google Scholar] [CrossRef]
- Toth, G.; Molnar, S.; Tamas, T.; Borbely, I. An Efficient Synthesis of 4,5-Dihydro-3(2H)- Pyridazinone Derivative. Synth. Commun. 1997, 27, 3513–3524. [Google Scholar]
- Sayed, G.H.; Sayed, M.A.; Shaaban, S.S.; Mahmoud, M.R. Synthesis and Reactions of 4-(p-Bromophenyl)-4-Oxo-2-(4-Antipyrinyl)Butanoic Acid and Some Unexpected Products. Egypt. J. Chem. 2000, 43, 17–29. [Google Scholar]
- Coates, W.J.; McKillop, A. One Pot Preparation of 6-Substituted 3(2H)-Pyridazinones from Ketones. Synthesis 1993, 334–342. [Google Scholar] [CrossRef]
- Kassab, R.R.; Sayed, G.H.; Radwan, A.M.; Abd El-Azzez, N. Some reactions with (biphenyl)-4-(5-oxo-1,3-diphenyl-2-pyrazolin-4-yl)-4,5-dihydropyridazin-3-(2H)ones. Rev. Roum. Chim. 2001, 46, 649–655. [Google Scholar]
- Jaihne, H.; Sayed, A.; Zaher, H.A.; Sherif, O. Reaction of 3-Chloro- and 3-Hydrazino-6-(p- tolyl)pyridazines. Indian J. Chem. 1977, 250–251. [Google Scholar]
- El-Hashash, M.A.; Amine, M.S.; Soliman, F.M.; Morsi, M.A. Behavior of aroylacyclic acids toward hydrazine hydrate and some on the cyclized products. J.Serb. Chem. Soc. 1992, 57, 563–569. [Google Scholar]
- Chung, K.T.; Chen, S.C.; Wong, T.Y.; Wei, C.I. Effects of Benzidine and Benzidine Analogues on Growth of Bacteria Including Azotobacter Vinelandii. Environ. Toxicol. Chem. 1998, 17, 271–275. [Google Scholar] [CrossRef]
- Sample Availability: Available from the author.
© 2007 by MDPI (http://www.mdpi.org). Reproduction is permitted for noncommercial purposes.
