Abstract
A selective route for the degradation of the unsaturated side chain of ent-labdanes has been devised, giving two useful synthons: 2β-acetoxy-14,15,17-trinor-ent-labdane-8,13-dione (5) and 2β-acetoxy-14,15-dinor-ent-labd-8(17)-en-13-one (7), the use of which for the preparation of terpenylquinone derivatives shall be reported elsewhere.
Introduction
Diterpenoids with labdanic structures are a very important natural source of hydrocarbon skeletons which have been used in the elaboration of a great number of drimane compounds with remarkable biological properties, among which their olfactory [1,2,3], antifeedant [4], antimicrobial, cytotoxic [5], growth regulator [6], herbicidal and insecticidal activities [7,8,9,10] may be mentioned.
The more common templates used for semisynthetic work are tricyclic diterpenoids such as (+)-abietic acid, (-)-levopimaric acid, podocarpic acid and bicyclic diterpenoids like manool, the communic acids, (-)-sclareol, (+)-cis-abienol, labdanolic acid, glycirretinic acid [11] and zamoranic acid [12,13]. Oxidative cleavage reactions of the side chain of communic acids with ozone and the OsO4-NaIO4 system have been reported previously [14]. The synthesis of sesquiterpenoids from labdane diterpenoids has also been reported [15]. On the other hand, degradations in the side chain of dihydrozamoranic acid were used for the synthesis of drimane [16]. Another important group of chemical reactions which included degradations in the side chain of (-)-sclareol, are those that allowed the preparation of terpenic synthons used in the synthesis of terpenylquinones with antitumoral activities such as (+)-puupehenone [17], chromazonarol and related compounds [18]. A new series of antineoplastic diterpenylquinone/hydroquinones has been prepared by using another route involving Diels-Alder cycloadditions between three labdanic diterpenoids (myrceocommunic acid derivatives) and p-benzoquinone or 1,4-naphthoquinone [19].
In this report we present our results on oxidative degradations of the side chain of natural ent-labdanes isolated from Calceolaria inamoena. Plants of the genus Calceolaria (Scrophulariaceae) are found distributed throughout New Zealand, Central and South America, including Chile, where there are 85 species (C. inamoena is a shrub common in the northern subandean region of Arica, Region I, Chile) [20].
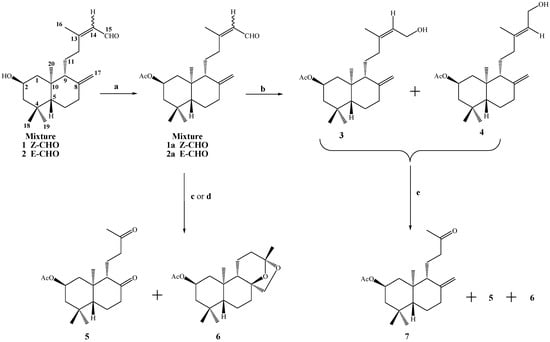
Scheme 1.
Scheme 1.
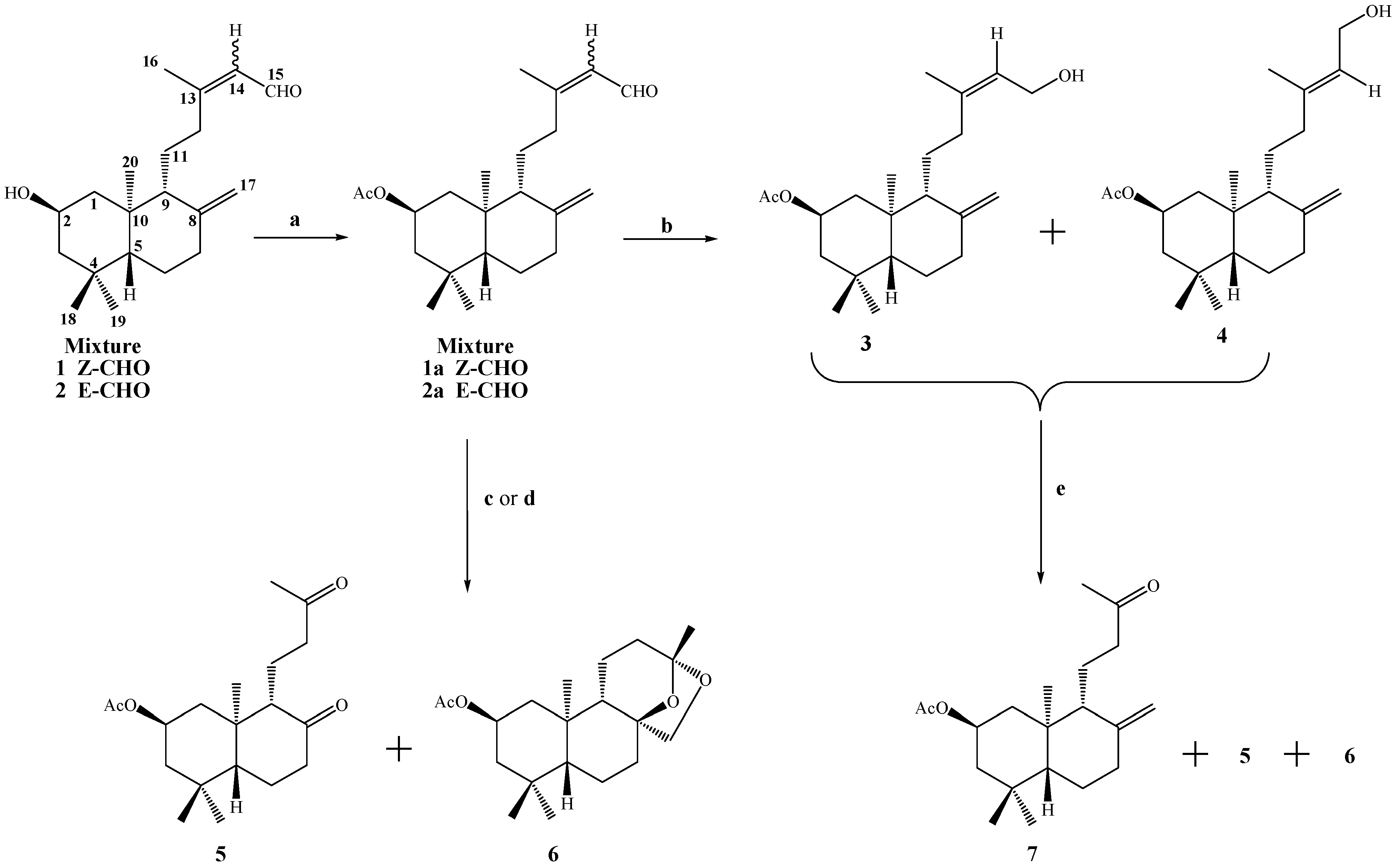
Conditions: a. Ac2O/CH2Cl2/py, DMAP, r.t, 2 h, 98%; b. NaBH4, MeOH, 10°C, 1.8 h, 3, 28%, 4, 43% and 3+4, 29%; c. OsO4/NaIO4, THF/H2O (1:1), r.t, 72 h, 5, 68%, 6, 7.2%; d. RuCl3·3H2O, MeCN/H2O, NaIO4, 40°C, 5 h, 5, 76%, 6, 6.3%; e. KMnO4/MgSO4, Me2CO, r.t, 72 h, 7, 63%, 5, 8.6% and 6, 1.2%.
Results and Discussion
Our main goal in the oxidative degradation of the ent-labdane side chains was to obtain compounds 5 and 7 (Scheme 1), and use these to generate a primary alcohol at the C-12 position. This primary alcohol would then be transformed into a good leaving group, which in turn would lead to suitable terpenic fragments that could be reacted with quinone/hydroquinone moieties in an attempt to synthesize terpenylquinones/hydroquinones with potential anticancerogenic activities. The preparation of the primary alcohols and the synthesis of the terpenylquinones/hydroquinones will be reported elsewhere.
The structural determination of the mixture of compounds 1-2 was accomplished by 1H- and 13C-NMR techniques. The spectral data, when compared with those previously reported for compounds with similar structures containing ent-labdane skeletons [21,22], were consistent with a diterpenic bicyclic labdane structure having a Δ8(17) double bond and a 2β-hydroxyl group. Examination of the 13C-NMR spectra of acetylated derivates 1a-2a, showed that they were a 60/40 mixture of the C-13 E and Z isomers, respectively. After successive purifications of 1a-2a by column chromatography (C.C.) a 10:1 E:Z mixture was isolated (ratio calculated based on the integrals of the Me-16 signals in the 1H-NMR spectrum). This mixture was used for the structural determination of compound 1a, whose 1H-NMR spectrum indicated the existence of a hydrogen at δ 9.96 (1H, d, J=8.1Hz, H-15) corresponding to an aldehyde function, three singlet methyl groups at δ 0.75, 0.86 and 0.91 ppm corresponding to Me-18, Me-19 and Me-20, respectively, as well as two broad singlets at δ 4.49 and 4.87 ppm for the geminal H-17 vinylic protons. A double double triplet at δ 4.99 ppm was also assignable to the hydrogen geminal to an acetyl group (CH3CO δ 2.00 ppm), and with the aid of a gs-sel-1H 1D TOCSY experiment, it was unequivocally determined to be located between C-1 and C-3. No NOE cross peak was observed between H-5 and Me-20, which suggested that the latter is oriented trans to H-5. The relative stereochemistry of 1a was assigned on the basis of both a NOESY correlation and coupling constant data. Main NOESY correlations observed for 1a are shown in Figure 1, which indicated that ring A and B were trans-fused and H-2 was axially oriented. On the other hand, an axial-axial (Jaa=11.8 Hz) and axial-equatorial (Jae=4.2 Hz) coupling constant for H-1, H-2α and H‑3 indicated that the OAc group in C-2 was β-equatorial (see Figure 1a). Furthermore, an allylic methyl group, as indicated by a signal in the 1H-NMR spectrum at δ 2.16 ppm (C-16, δ 17.6 ppm), and long range coupled to a broad singlet corresponding to a vinylic proton at δ 5.86 ppm (see Figure 1b) were characteristic of an unsaturated side chain of a bicyclic diterpenoid having an E configuration at the olefinic bond [23]. Nevertheless, since our plans called for the loss of isomeric relationships at C-13, the 1-2 mixture was used directly as our synthesis.
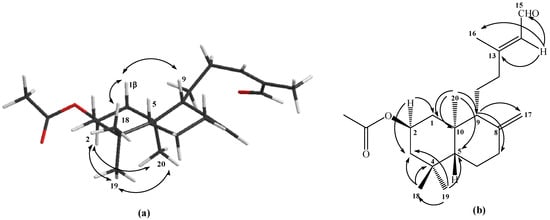
Figure 1.
Structure of compound 1a. (a) NOE correlations. (b) HMBC correlations.
Figure 1.
Structure of compound 1a. (a) NOE correlations. (b) HMBC correlations.
Acetylation of the 1-2 mixture under standard conditions (Ac2O/pyridine/DMAP/CH2Cl2) produced the acetate mixture 1a-2a in 98% yield. Reduction of this acetate mixture with NaBH4/CH3OH after C.C. purification led to the formation of the geometrical isomers 3 (28%) and 4 (43%). The confirmation of the E and Z structures of these geometrically isomeric alcohols, was made by 1H- and 13C-NMR data; specifically the E isomer 4 showed a carbinol methylene group at δ = 4.13 (2H, d, J=6.9Hz, H-15) and a signal at δ = 59.3 (C-15) in its 13C spectrum, whereas the Z isomer 3 showed a carbinol methylene group at δ = 4.02 (2H, dd, J=6.9 and 4.2Hz, H-15) and a 13C signal at δ = 58.9 (C-15). A singlet peak at δ = 1.65 (3H, s, H-16) and δ = 16.3 (C-16) indicated that the stereochemistry of the double bond in the side chain corresponded to E. In the case of the Z isomer, the Me-16 appears at δH = 1.70 and δC = 23.3 ppm, respectively.
Degradation of the side chain of the mixture of compounds 1a-2a using either the OsO4/NaIO4 or RuCl3/NaIO4 systems, a degradation used previously for compounds with similar structures [1,2,3,14], led to the formation of compounds 5 and 6 (Scheme 1). Nevertheless, when the mixture 1a-2a was treated with the RuCl3/NaIO4 system, a greater yield of compound 5 was obtained (76% v/s 68% with OsO4/NaIO4 system). The main spectroscopic data for the confirmation of the structure of 5 was the appearance of a singlet signal at δH = 2.05 (3H, s, H-16) and δC = 29.8 (C-16); in addition, in its 13C spectrum, the presence of two ketone carbonyl signals was observed at δC = 211.3 (C-8) and 208.9 (C-13). The molecular formula for compound 6 (C20H32O4) was deduced from the combined spectra of 1H, 13C, 13C-DEPT-135 and confirmed by MS ([M+ 336] at m/z).
Our calculation of the degree of unsaturation gave a value of five, of which one corresponds to the acetate group, and two are attributed to the A and B rings. In the 13C spectrum, no signals corresponding to C=C double bonds were observed, therefore the formation of a third ring C fused with B, formed by the union between the carbons C-8, C-9, C-11, C-12, C-13-O-C-8, and a fourth ring D fused with C, formed by an ether bridge between C-13 (ketal) and C-17 is suggested.
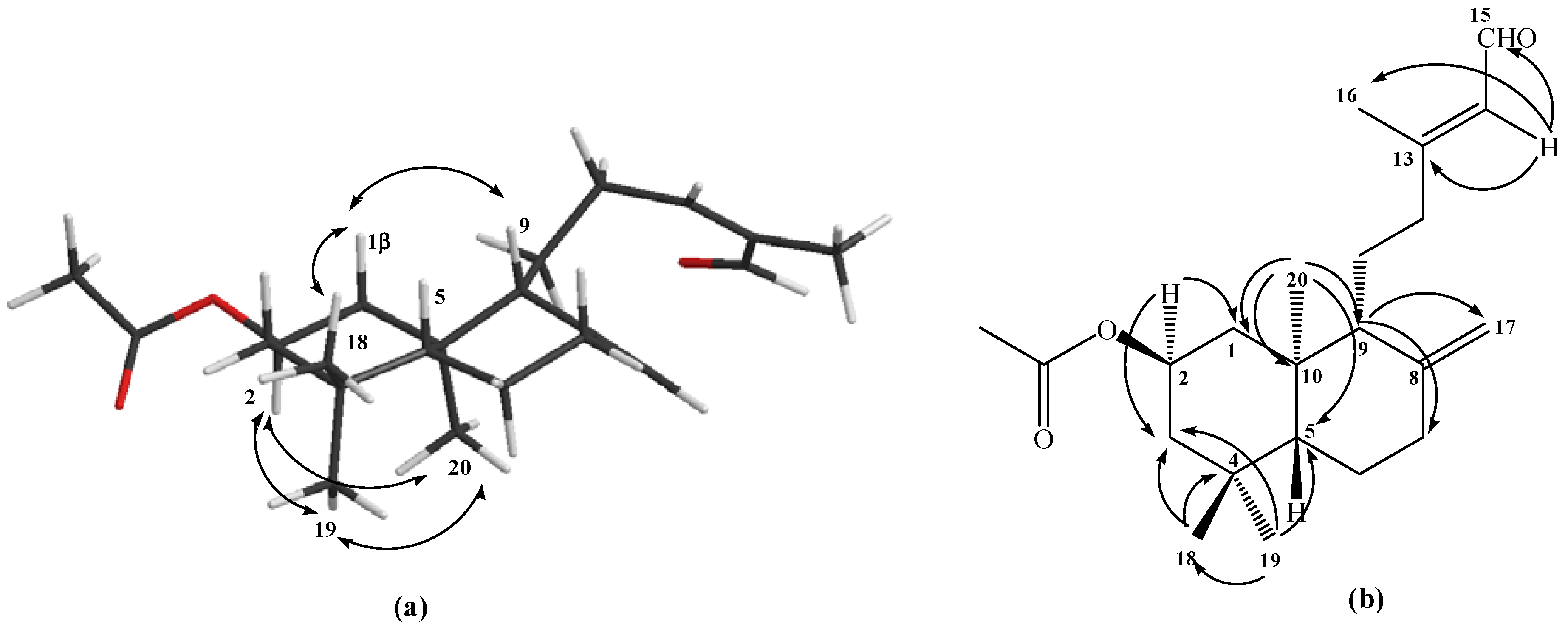
The tetracyclic structure of compound 6 was confirmed mainly by the data obtained from heteronuclear 2D HSQC and HMBC correlations; in the latter, correlations were observed between H‑17β with C-7, C-8 and C-9, whereas H-17α shows correlations with C-9 and C-13, while the Me-16 group also showed correlations with C-13 and C-12 (see Figure 2a). The stereochemistry assigned to the carbons C-8 and C-13 were deduced from 1H sel. 1D NOESY correlations; long range interactions between the Me-16 group with H-12β and H-17β were observed, whereas H-17α showed correlations with Me-20 and H-11α (see Figure 2b). Finally, a tetracyclic structure similar to 6 was reported when labdadienes were treated with O3 or NaIO4/OsO4 system [14].
It is reported that allylic alcohols like (-)-sclareol or lab-13-en-8β-ol-15-oic acid (α,β-unsaturated methyl esters) were selectively oxidized to the corresponding methyl ketones with the KMnO4/MgSO4 in acetone system [24,25]. Under these conditions, when compounds 3, 4 or a mixture of both were treated with KMnO4/MgSO4 in acetone at room temperature, compound 7 was obtained selectively in 63% yield. Specifically, in the 1H-NMR spectrum of 7, the Me-16 appears at δH = 2.03 (s, 3H), whereas in the 13C spectrum, the CH3-CO group showed signals at δC = 29.9 (C-16) and 208.8 ppm (C-13). On the other hand, the exocyclic double bond between C-8 and C-17 showed a signal at δH = 4.79 (1H, bs, H-17a) and δH = 4.40 (1H, bs, H-17b), δC = 147.0 (C-8) and 107.2 (C-17). Combined, all these signals confirm the structure of compound 7 and with it, the selectivity of the side chain oxidation reaction using KMnO4.
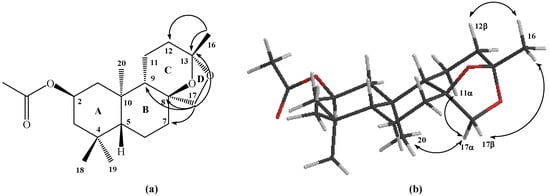
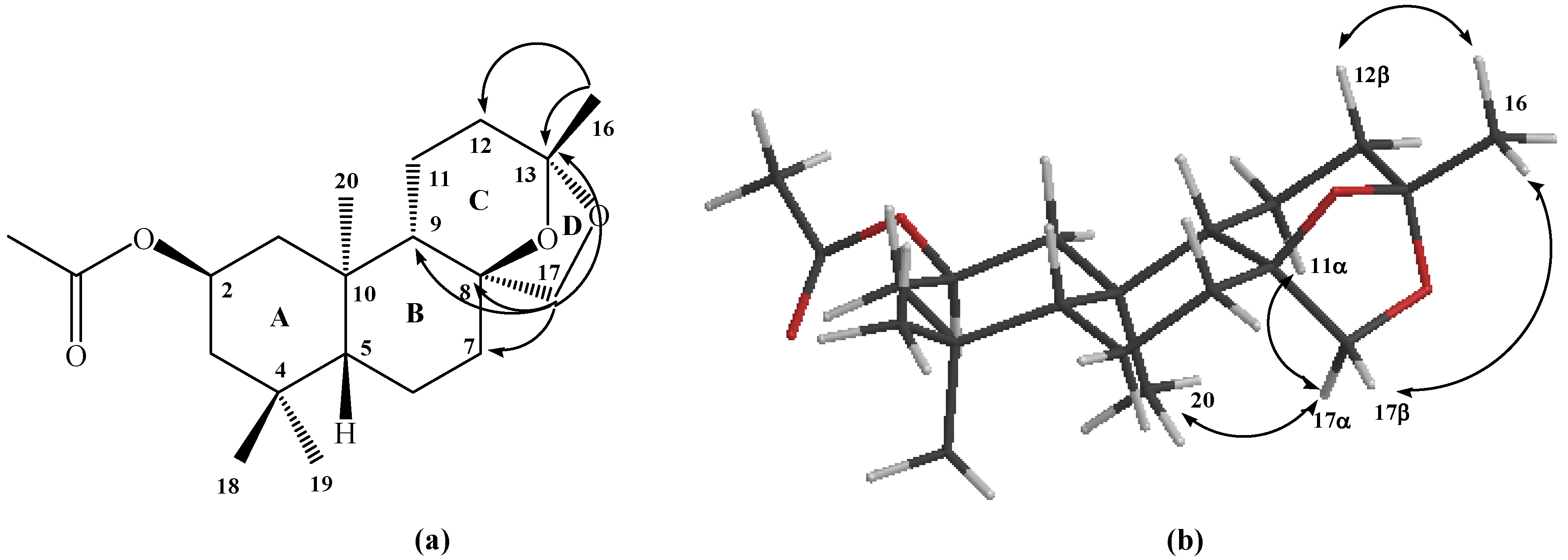
Figure 2.
Structure of compound 6. (a) HMBC correlations. (b) NOE correlations.
Figure 2.
Structure of compound 6. (a) HMBC correlations. (b) NOE correlations.
Experimental
General
Unless otherwise stated, all chemical reagents purchased (Merck or Aldrich) were the highest purity commercially available and were used without previous purification. IR spectra were recorded as thin films in a Nicolet Impact 420 spectrometer and frequencies are reported in cm-1. Optical rotations were measured with a sodium lamp (λ=589 nm, D line) on a Perkin Elmer 241 digital polarimeter equipped with 1 dm cells at the temperature indicated in each case. Low resolution mass spectra were recorded on a Shimadzu QP-2000 spectrometer at 70eV ionising voltage and are given as m/z (% rel. int.) 1H-, 13C- (DEPT 135 and DEPT 90), sel. 1D 1H NOESY, sel. 1D 1H TOCSY, 2D HSQC and 2D HMBC spectra were recorded in CDCl3 solutions and are referenced to the residual peaks of CHCl3 at δ 7.26 ppm and δ 77.0 ppm for 1H- and 13C-, respectively, on a Bruker Avance 400 Digital NMR spectrometer, operating at 400.1MHz for 1H and 100.6MHz for 13C. Chemical shifts are reported in δ ppm and coupling constants (J) are given in Hz. Silica gel (Merck 200-300 mesh) was used for C.C. and silica gel plates HF-254 for TLC. TLC spots were detected by heating after spraying with 25% H2SO4 in H2O.
Plant material
Aerial parts of Calceolaria inamoena Phil. were collected in Arica, Region I, Chile, in January 2006, and authenticated by Professor Eliana Belmonte, Universidad de Tarapacá. A voucher specimen (#98007) was deposited in the Herbarium of the Natural Product Laboratory of the Universidad Técnica Federico Santa María.
Extraction and isolation
Aerial parts of C. inamoena, (2,448 g of dry plant material) were successively extracted at room temperature with three 6.5 L portions of petroleum ether (b.p. 60-80°C) for 72 h each. The solvent was removed in vacuo to yield 110.28 g of a syrupy residue. The crude material was divided into two portions (approx. 55 g each), dissolved in CH2Cl2 and absorbed on silica. Both portions were then each subjected separately to silica gel column chromatography (450 g) eluting with mixtures of petroleum ether and EtOAc of increasing polarity (195:5→50:150). Fractions (47 frs., 10 mL, from a total of 248) were combined based on TLC and 1H-NMR monitoring. After purification by C.C. this produced 53.7 g of the geometric isomer mixture 1-2 (4.5% yield based on dry plant material).
Acetylation of a mixture of 1-2: synthesis of 2β-acetoxy-ent-labda-8(17),13(E)-dien-15-al (1a) and 2β-acetoxy-ent-labda-8(17),13(Z)-dien-15-al (2a).
To a solution of 1-2 mixture (30 g, 98.5 mmol) in dry CH2Cl2 (150 mL), dry pyridine (10 mL), DMAP (70 mg) and Ac2O (9.5 mL) were added and the mixture was stirred at room temperature for 2 h. To this mixture a cooled solution of 10% KHSO4 (approx. 25mL) was then added. The watery layer was discarded and the combined organic layers were washed to neutrality with a saturated solution of NaHCO3 and water, dried over Na2SO4, filtered, evaporated and chromatographed on silica-gel with petroleum ether/EtOAc mixtures of increasing polarity (19.8:0.2→5:15) to give a mixture of 1a-2a (33.4 g, 98%). Compound 1a: viscous oil, 1H-NMR: 9.96 (1H, d, J=8.1Hz, H-15), 5.86 (1H, d, J=8.1Hz, H-14), 4.99 (1H, ddt, J=12.0, 11.8 and 4.2Hz, H-2), 4.87 (1H, br, H-17b), 4.49 (1H, bs, H-17a), 2.16 (3H, s, Me-16), 2.00 (3H, s, OAc), 0.91 (3H, s, Me-18), 0.86 (3H, s, Me-19), 0.75 (3H, s, Me-20); 13C-NMR: 44.0 (C-1), 69.1 (C-2), 46.7 (C-3), 34.9 (C-4), 54.8 (C-5), 23.8 (C-6), 37.8 (C-7), 146.9 (C-8), 56.0 (C-9), 40.9 (C-10), 21.5 (C-11), 39.3 (C-12), 164.5 (C-13), 127.2 (C-14), 191.3 (C-15), 17.6 (C-16), 107.4 (C-17), 33.4 (C-18), 22.3 (C-19), 15.2 (C-20), 170.6 (CH3CO), 21.4 (CH3CO); IR: 2900-2980, 2850, 1720, 1640, 1460, 1395, 1360, 1250, 1140, 1020, 960, 895; MS: 346 ([M+] <1%), 289 (2.0%), 286 (5.3%), 203 (15.1%), 201 (10.5%), 189 (16.9%), 187 (15.7%), 175 (10.2%), 159 (20.3%), 157 (11.1%), 149 (11.7%), 147 (30.3%), 145 (23.8%), 136 (14.2%), 135 (100%), 133 (34.0%) 121 (30.7%). Compound 1b: 13C-NMR: 43.9 (C-1), 69.3 (C-2), 46.7 (C-3), 34.9 (C-4), 54.9 (C-5), 23.8 (C-6), 37.9 (C-7), 146.9 (C-8), 55.6 (C-9), 40.8 (C-10), 21.4 (C-11), 30.9 (C-12), 164.5 (C-13), 129.0 (C-14), 190.9 (C-15), 24.8 (C-16), 107.6 (C-17), 33.5 (C-18), 22.3 (C-19), 15.1 (C-20), 170.6 (CH3CO), 21.4 (CH3CO).
Reduction of the mixture of 1a-2a: synthesis of 2β-acetoxy-15-hydroxy-ent-labda-8(17),13(Z)-diene (3) and 2β-acetoxy-15-hydroxy-ent-labda-8(17),13(E)-diene (4)
NaBH4 (10.1g, 0.266 mol) was added in portions to a solution of 1a-2a mixture (10 g, 28.9 mmol) in MeOH (100 mL), and the mixture stirred at 10°C. After 2 h, and in order to destroy the excess of NaBH4, acetone (20 mL) was added and the mixture stirred at room temperature for 15 min. The solvent was removed until a volume of approximately 10 mL was reached and HCl (5%, 30 mL) was added and the mixture extracted with EtOAc (3 x 30 mL) and the combined organic layers washed successively with 10% NaHCO3 and water, dried over Na2SO4, filtered, evaporated and chromatographed on silica-gel with mixtures of petroleum ether/EtOAc of increasing polarity (19:1→6:14) to give 2.20 g (28%) of 3, 3.37 g (43%) of 4 and 2.28 g (29%) of 3+4 mixture, for a total yield of 7.85 g (78%). Compound 3: viscous oil; 1H-NMR: 5.39 (1H, brt, J=6.9Hz, H-14), 4.98 (1H, ddt, J=11.8, 11.8, 4.1 Hz, H-2), 4.88 (1H, s, H-17b), 4.56 (1H, s, H-17a), 4.02 (2H, dd, J=6.9, 4.2Hz, H2-15), 2.38 (1H, ddd, J=13.0, 3.9 and 2.3Hz, H-7α), 1.99 (1H, m, H-12b), 2.02 (1H, m, H-1α), 2.01 (3H, s, OAc), 1.99 (1H, m, H-12a), 1.93 (1H, ddd, J=13.0, 13.0 and 4.9Hz, H-7β), 1.72 (1H, m, H-3α), 1.70 (3H, s, Me-16), 1.69 (1H, m, H-6β), 1.59 (1H, brd, J=11.3Hz, H-9), 0.91 (3H, s, Me-18), 0.86 (3H, s, Me-19), 0.73 (3H, s, Me-20); 13C-NMR: 43.9 (C-1), 69.3 (C-2), 46.7 (C-3), 34.9 (C-4), 54.9 (C-5), 23.8 (C-6), 37.9 (C-7), 147.3 (C-8), 55.6 (C-9), 40.8 (C-10), 21.7 (C-11), 30.3 (C-12), 139.9 (C-13), 124.7 (C-14), 58.9 (C-15), 23.3 (C-16), 107.4 (C-17), 33.5 (C-18), 22.4 (C-19), 15.2 (C-20), 170.6 (CH3CO), 21.4 (CH3CO); IR: 3451, 3077, 2930, 1730, 1642, 1441, 1369, 1248, 1021; MS: 348 ([M]+ <1%), 288 (16.1%), 273 (72.1%), 225 (55.2%), 202 (33.3%), 175 (53.9%), 135 (86.7%), 121 (67.9%), 55 (66.1%), 41 (100%). Compound 4, viscous oil; 1H-NMR: 5.37 (1H, brt, J=6.8Hz, H-14), 5.00 (1H, ddt, J=11.8, 11.8 and 4.1Hz, H-2), 4.86 (1H, s, H-17b), 4.53 (1H, s, H-17a), 4.13 (2H, d, J=6.9Hz, H2-15), 2.38 (1H, ddd, J=12.9, 3.6 and 2.2Hz, H-7α), 2.14 (1H, ddd, J=14.0, 10.4 and 3.9Hz, H-12b), 2.04 (1H, m, H-1α), 2.02 (3H, s, OAc), 1.95 (1H, ddd, J=13.3, 12.9 and 4.8Hz, H-7β), 1.97 (1H, m, H-12a), 1.72 (1H, m, H-3α), 1.65 (3H, s, Me-16), 1.69 (1H, m, H-6β), 1.62 (1H, brd, J=11.1Hz, H-9), 0.92 (3H, s, Me-18), 0.87 (3H, s, Me-19), 0.75 (3H, s, Me-20); 13C-NMR: 44.1 (C-1), 69.3 (C-2), 46.8 (C-3), 34.9 (C-4), 54.9 (C-5), 23.8 (C-6), 37.9 (C-7), 147.3 (C-8), 56.2 (C-9), 40.9 (C-10), 22.0 (C-11), 38.3 (C-12), 140.1 (C-13), 123.2 (C-14), 59.3 (C-15), 16.3 (C-16), 107.3 (C-17), 33.5 (C-18), 22.4 (C-19), 15.2 (C-20), 170.4 (CH3CO), 21.3 (CH3CO); IR: 3380, 3068, 2945, 1725, 1640, 1445, 1373, 1251, 1028; MS: 348 ([M]+ <1%), 288 (16.1%), 273 (72.1%), 255 (55.2%), 187 (59.4%), 175 (53.9%), 135 (86.7%), 121 (67.9%), 41 (100%).
Oxidative degradation of a 1a-2a mixture with the OsO4-NaIO4 system: synthesis of 2β-acetoxy-14,15, 17-trinor-ent-labdane-8,13-dione (5) and 2β-acetoxy-(13R)-8β,13:13,17-diepoxy-14,15-dinor-ent-labdane (6)
Finely divided NaIO4 (3.1g, 14.5 mmol), was added to a solution of 1a-2a mixture (5g, 14.45 mmol) in 1:1 THF/H2O (80 mL) and the mixture stirred at room temperature under a N2 atmosphere. After 1 h, 4% aq. OsO4 solution (5 mL, 0.787 mmol) was added. The reaction mixture was then stirred at room temperature for 72 h, filtered and the solvent was removed until the volume was reduced to approximately 40 mL; then 5% Na2S2O5 (15 mL) was added, the mixture was extracted with EtOAc (3 x 30 mL) and the combined organic layers were washed successively with 10% NaHCO3 and water, dried over Na2SO4, filtered, evaporated and chromatographed eluting with mixtures of petroleum ether/EtOAc of increasing polarity (19.8:0.2→5:15) to give 3.16 g of compound 5 (68%) and 0.335 g of 6 (7.2%). Compound 5: viscous oil; [α]D23 = +21.1° (c 1.2, CHCl3); 1H-NMR: 4.93 (1H, ddt, J=12.1, 12.1 and 4.0 Hz, H-2), 2.56 (1H, ddd, J=17.7, 7.7 and 5.4Hz, H-12b), 2.39 (1H, ddd, J=13.6, 4.6 and 1.7Hz, H-7α), 2.26 (1H, dd, J=12.8, 7.0Hz, H-7β), 2.11 (1H, brd, J=10.7Hz, H-9), 2.05 (3H, s, Me-16), 1.99 (3H, s, OAc), 1.99 (1H, m, H-12a), 1.72 (1H, m, H-3α), 1.69 (1H, m, H-6β), 0.98 (3H, s, Me-18), 0.90 (3H, s, Me-19), 0.77 (3H, s, Me-20); 13C NMR: 43.8 (C-1), 68.3 (C-2), 46.4 (C-3), 34.8 (C-4), 53.4 (C-5), 23.0 (C-6), 42.1 (C-7), 211.3 (C-8), 62.7 (C-9), 43.6 (C-10), 16.2 (C-11), 42.3 (C-12), 208.9 (C-13), 29.8 (C-16), 33.5 (C-18), 22.3 (C-19), 15.3 (C-20), 170.4 (CH3CO), 21.3 (CH3CO). IR: 2950, 1738, 1723, 1721, 1429, 1368, 1245, 1189, 1025. MS: 322 ([M]+ 6.1%), 249 (12.1%), 247 (100%), 189 (34.5%), 180 (34.2%), 177 (29.7%), 135 (93.9%), 119 (38.2%), 43 (77.6%). Compound 6: viscous oil; [α]D23 = +2.9°(c 2.6, CHCl3); 1H-NMR: 5.02 (1H, ddt, J=12.2, 12.0 and 4.4 Hz, H-2), 4.25 (1H, d, J=7.2Hz, H-17α), 3.34 (1H, d, J=7.2Hz, H-17β), 1.99 (3H, s, OAc), 1.88 (1H, dt, J=12.9, 3.0Hz, H-12β), 1.45 (1H, dd, J=11.7, 4.7Hz, H-9), 1.38 (3H, s, Me-16), 1.00 (1H, dd, J= 15.6, 7.9 Hz, H-5), 0.95 (3H, s, Me-20), 0.93 (3H, s, Me-18), 0.87 (3H, s, Me-19); 13C-NMR: 43.8 (C-1), 68.4 (C-2), 46.5 (C-3), 34.6 (C-4), 55.1 (C-5), 19.5 (C-6), 35.7 (C-7), 82.3 (C-8), 53.2 (C-9), 38.9 (C-10), 17.5 (C-11), 35.9 (C-12), 106.1 (C-13), 24.1 (C-16), 73.4 (C-17), 33.6 (C-18), 22.4 (C-19), 15.5 (C-20), 170.5 (CH3CO), 21.4 (CH3CO); IR: 2945, 1737, 1455, 1393, 1250, 1025; MS: 336 ([M]+ <1%), 306 (6.1%), 215 (13.9%), 187 (41.5%), 173 (33.9%), 134 (27.3%), 107 (24.2%), 55 (19.4%), 43 (100%).
Oxidative degradation of a 1a-2a mixture with the RuCl3-NaIO4 system
To a solution of 1a-2a mixture (5 g, 14.45 mmol) in 3:4 CH3CN/H2O (70 mL) RuCl3·3H2O (50 mg) was added and the mixture stirred at room temperature. After 15 min. finely divided NaIO4 (3.1 g, 14.5 mmol) was added and the mixture was then stirred at 40°C for 5 h. The mixture was filtered and the solvent was removed down to a volume to approximately 40 mL, extracted with EtOAc (3 x 30 mL) and the combined organic layers washed successively with water, dried over Na2SO4, filtered, evaporated and chromatographed eluting with mixtures of petroleum ether/EtOAc of increasing polarity (19.8:0.2→6.4:14.6) to give 3.53 g (76%) of 5 and 0.293 g (6.3%) of 6.
Oxidative degradation of a 3-4 mixture with the KMnO4-MgSO4 system: synthesis of 2β-acetoxy-14, 15-dinor-ent-labd-8(17)-en-13-one (7)
To a solution of 3-4 mixture (2.28 g, 6.54 mmol) in acetone (50 mL), finely divided KMnO4 (1.03 g, 6.54 mmol) and MgSO4 (0.785 g, 6.54 mmol) were added in small portions during 30 minutes and the mixture stirred at room temperature. After 72 hours, the mixture was filtered, evaporated and chromatographed with petroleum ether/EtOAc mixtures of increasing polarity (19.8:0.2→15:5) yielding 1.32 g (63 %) of compound 7, 0.181 g (8.6%) of compound 5, and 0.025 g (1,2%) of compound 6. Compound 7: viscous oil; [α]D23 = -17.6° (c 9.5, CHCl3); 1H-NMR: 4.94 (1H, ddd, J=11.8, 11.8 and 4.1 Hz, H-2), 4.79 (1H, s, H-17b), 4.40 (1H, s, H-17a), 2.51 (1H, ddd, J=17.7, 8.8 and 4.6Hz, H-12b), 2.32 (1H, ddd, J=12.9, 3.9 and 2.3Hz, H-7α), 2.03 (3H, s, Me-16), 1.95 (3H, s, OAc), 1.88 (1H, ddd, J=12.9, 12.9 and 4.9Hz, H-7β), 1.57 (1H, brd, J=11.6Hz, H-9), 1.99 (1H, m, H-12a), 1.72 (1H, m, H-3α), 1.69 (1H, m, H-6β), 0.86 (3H, s, Me-18), 0.81 (3H, s, Me-19), 0.70 (3H, s, Me-20); 13C-NMR: 43.8 (C-1), 69.0 (C-2), 46.6 (C-3), 34.7 (C-4), 54.7 (C-5), 23.7 (C-6), 37.7 (C-7), 147.0 (C-8), 55.8 (C-9), 40.8 (C-10), 17.5 (C-11), 42.4 (C-12), 208.8 (C-13), 29.9 (C-16), 107.2 (C-17), 33.4 (C-18), 22.3 (C-19), 14.8 (C-20), 170.3 (CH3CO), 21.3 (CH3CO); IR: 3062, 2935, 1731, 1715, 1639, 1434, 1363, 1245, 1158, 1020; MS: 320 ([M]+ <1%), 305 (5.5%), 259 (9.7%), 245 (24.8%), 187 (42.4%), 135 (90.9%), 121 (83.0%), 119 (80.9%), 107 (73.9%), 93 (71.5%), 79 (60.1%), 43 (100%).
Acknowledgments
The authors thank the Universidad Técnica Federico Santa María (grant DGIP N° 13.06.26) and Universidad Andrés Bello (grant DI-UNAB 13-04) for financial support.
References and Notes
- Martres, P.; Perfetti, P.; Zahara, J-P.; Weagell, B.; Giraudi, E.; Petrzilka, M. “A Short and Efficient Synthesis of (-)-Ambrox from (-)-Sclareol using a Ruthenium Oxide Catalyzed Key Step”. Tetrahedron Lett. 1993, 34, 629–632. [Google Scholar]
- Martres, P.; Perfetti, P.; Zahara, J-P.; Weagell, B. “Synthesis of Norambracetal: A New Ambergris Derivative”. Tetrahedron Lett. 1993, 34, 3127–3128. [Google Scholar]
- Barrero, A.; Alvarez-Manzaneda, J.; Altarejo, J.; Salido, S.; Ramos, J. M. “Synthesis of Ambrox from (-)-Sclareol and (+)-cis-abietol”. Tetrahedron 1993, 49, 10405–10412. [Google Scholar]
- Ley, S. V.; Toogood, P. L. “Insect Antifeedants”. Chem. Brit. 1990, 26, 31–35. [Google Scholar]
- Anke, H.; Sterner, O. “Comparison of the Antimicrobial and Cytotoxic Activities of twenty Unsaturated Sesquiterpene Dialdehydes from Plants and Mushrooms”. Planta Med. 1991, 57, 344–346. [Google Scholar]
- Asakawa, Y.; Dawson, G. W.; Griffiths, D. C.; Lallemand, J. Y.; Ley, S. V.; Mori, K.; Mudd, A.; Pezechk-Leclaire, M.; Pickett, J. A.; Watanabe, H.; Woodcock, C. M.; Zhong-Ning, Z. “Activity of Drimane Antifeedants and Related Compounds Against Aphids, and Comparative Biological Effects and Chemical Reactivity of (−)- and (+)-polygodial”. J. Chem. Ecol. 1988, 14, 1845–1855. [Google Scholar]
- Butterworth, J. H.; Morgan, E. D. “Isolation of a Substance that Suppresses Feeding in Locusts”. J. Chem. Soc. Chem. Commun. 1968, 23–24. [Google Scholar]
- Anderson, J. C.; Blaney, W. M.; Cole, M. D.; Fellons, L. L.; Ley, S. V.; Shephard, R. N.; Simmons, M. S. J. “The Structure of two new Clerodane Diterpenoid Potent Insect Antifeedants from Scutellaria woronowii (Juz); Jodrellin A & B”. Tetrahedron Lett. 1989, 30, 4737–4740. [Google Scholar]
- Kubo, I.; Lee, Y. W.; Pettei, M. J.; Pilkiewicz, F.; Nakanishi, K. “Potent Army Worm Antifeedants from the East African Warburgia Plants”. J. Chem. Soc., Chem. Commun. 1976, 1013–1014. [Google Scholar]
- Barnes, C. S.; Loder, J. W. “The Structure of Polygodial: A New Sesquiterpene Dialdehyde from Polygonum hydropiper L”. Aust. J. Chem. 1962, 15, 322–327. [Google Scholar]
- Jansen, B. J. M.; Groot, A. “The Synthesis of Drimane Sesquiterpenoinds”. Nat. Prod. Rep. 1991, 8, 319–337. [Google Scholar]
- Urones, J. G.; Marcos, I. S.; Gómez-Pérez, B.; Lithgow, A. M.; Díez, D.; Basabe, P.; Gómez, P. M. “Diastereoselective Ring-Opening of 12-Acetoxy-9α and 9β(11)-Epoxy-7-Drimene: Homochiral Semisynthesis of Poligodial and Warburganal”. Tetrahedron Lett. 1994, 35, 3781–3784. [Google Scholar]
- Urones, J. G.; Marcos, I. S.; Gómez-Pérez, B.; Díez, D.; Lithgow, A. M.; Gómez, P. M.; Basabe, P.; Garrido, N. M. “Chemistry of Zamoranic Acid. Part V. Homochiral Semisynthesis of Active Drimanes: Pereniporin B, Polygodial and Warburganal”. Tetrahedron 1994, 50, 10995–11012. [Google Scholar] [CrossRef]
- Barrero, A. F.; Alvarez-Manzaneda, E. J.; Altarejos, J.; Ramos, J. M.; Salido, S. “Preferential Oxidation Reactions of the Side Chain of Unsaturated Labdanes”. Bull. Soc. Chim. Fr. 1993, 130, 700–707. [Google Scholar]
- Vlad, P. F.; Koltsa, M. N.; Mironov, G. N. “Synthesis of Sesquiterpenoids of the Drimane Group from Labdane Diterpenoids”. Russ. Chem. Bull. 1997, 46, 855–873. [Google Scholar]
- Rodilla, J. M. L.; Díez, D.; Urones, J. G.; Rocha, P. M. “From Labdanes to Drimanes. Degradation of the Side Chain of Dihydrozamorenic Acid”. Molecules 2004, 9, 300–322. [Google Scholar]
- Barrero, A. F.; Alvarez-Manzaneda, E. J.; Chahboun, R. “Enantiospecific Synthesis of (+)-Puupehenone from (-)-Sclareol and Protocatechualdehyde”. Tetrahedron Lett. 1997, 38, 2325–2328. [Google Scholar]
- Barrero, A. F.; Alvarez-Manzaneda, E. J.; Herrador Mar, M.; Chahboun, R.; Galera, P. “Synthesis and Antitumoral Activities of Marine ent-Chromazonarol and Related Compounds”. Bioorg. Med. Chem. Lett. 1999, 9, 2325–2328. [Google Scholar]
- Del Corral, J. Mͣ. M.; Gordaliza, M.; Castro, Mͣ. A.; Mahiques, Mͣ. M.; Chamorro, P.; Molinari, A.; García-Grávalos, Mͣ. D.; Broughton, H. B.; San Feliciano, A. “New Selective Cytotoxic Diterpenylquinones and Diterpenylhydroquinones”. J. Med. Chem. 2001, 44, 1257–1267. [Google Scholar]
- Marticorena, C.; Quezada, M. “Catálogo de la Flora Vascular de Chile”. Gayana Bot. 1985, 42, 68–69. [Google Scholar]
- Bohlmann, F.; Kramp, W.; Grenz, M.; Robinson, H.; King, R. M. “Diterpenes from Baccharis Species”. Phytochemistry 1981, 20, 1907–1913. [Google Scholar]
- Zdero, C.; Bohlmann, F.; King, R. M. “Ent-Labdanes, Manoyloxide and Helipterol Derivatives from Chrysocephalum Ambiguum”. Phytochemistry 1992, 31, 1631–1638. [Google Scholar]
- San Feliciano, A.; Medarde, M.; López, J. L.; Del Corral, J. M. M.; Puebla, P.; Barrero, A. F. “Terpenoids from Leaves of Juniperus Thurifera”. Phytochemistry 1988, 27, 2241–2248. [Google Scholar]
- Khac Manh, D. Do.; Fetizon, M.; Flament, J. P. “Syntheses de Diterpenes Tetracycliques du Type Hibane”. Tetrahedron 1975, 31, 1897–1902. [Google Scholar]
- Leite, F, M-A.; Sarragiotto, M-H.; Imamura, P. M.; Marsaioli, A. J. “Absolute Configuration of Drim-9(11)-en-8-ol from Aspergillus Oryzae.”. J. Org. Chem. 1986, 51, 5409–5410. [Google Scholar]
- Sample Availability: Samples of compounds 1a-7 are available from authors.
© 2007 by MDPI (http://www.mdpi.org). Reproduction is permitted for noncommercial purposes.
