C(aryl)-O Bond Formation from Aryl Methanesulfonates via Consecutive Deprotection and SNAr Reactions with Aryl Halides in an Ionic Liquid
Abstract
:Introduction
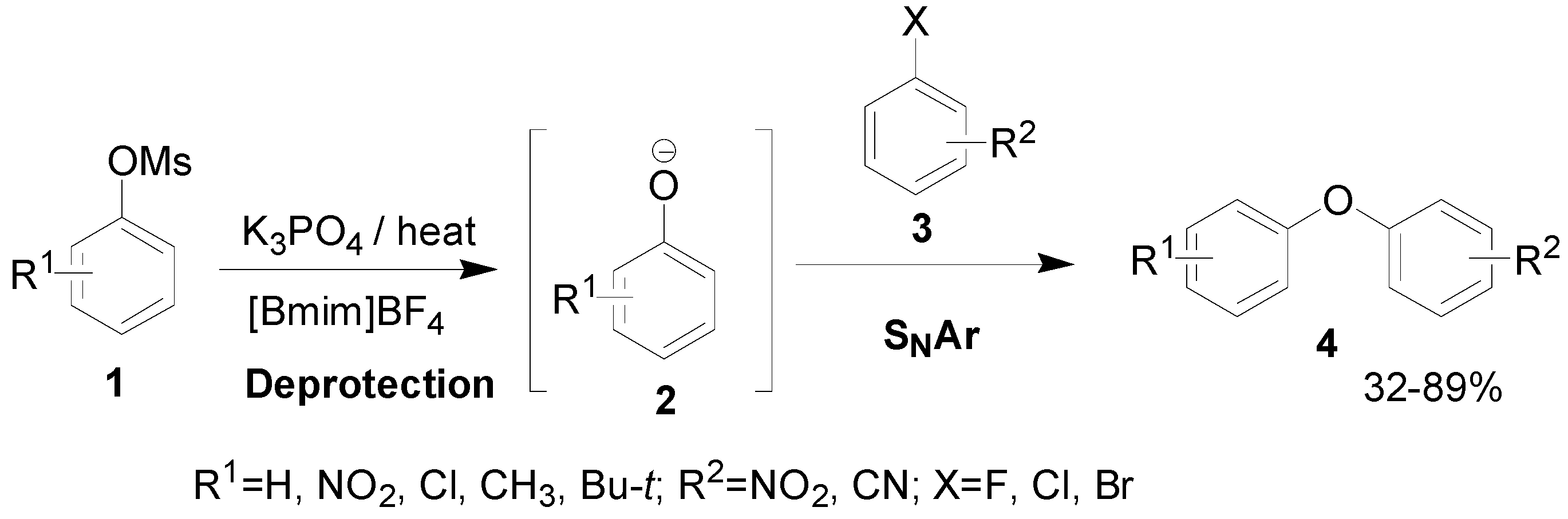
Results and Discussion
| R1 | R2 | X | Temp. (oC) b | Time (h) | Product | Yield (%) c |
|---|---|---|---|---|---|---|
| 4’-Cl | NO2 | 4-F | 100 | 11 | 4a | 70 |
| 3’-CH3 | NO2 | 4-F | 100 | 20 | 4b | 50 |
| 2’-Cl | NO2 | 4-F | 100 | 7 | 4c | 72 |
| 4’-t-Bu | NO2 | 4-F | 114 | 24 | 4d | 52 |
| 4’-NO2 | NO2 | 4-F | 110 | 24.5 | 4e | 42 |
| H | NO2 | 4-F | 110 | 24.5 | 4f | 64 |
| 4’-Cl | NO2 | 2-F | 100 | 12 | 4g | 85 |
| 4’-t-Bu | NO2 | 2-F | 100 | 20 | 4h | 32 |
| 2’-Cl | NO2 | 2-F | 105 | 11 | 4i | 89 |
| 3’-CH3 | NO2 | 2-F | 100 | 20 | 4j | 55 |
| 4’-t-Bu | CN | 2-F | 110 | 25 | 4k | 63 |
| 4’-Cl | CN | 2-F | 110 | 24 | 4l | 57 |
| 2’-Cl | NO2 | 2-Cl | 110 | 29 | 4i | 66 |
| 2’-Cl | NO2 | 4-Br | 110 | 25 | 4c | 88 |
Conclusions
Experimental
General
General procedure for the preparation of unsymmetrical diaryl ethers 4
Acknowledgments
References and Notes
- Huddleston, J. G.; Visser, A. E.; Reichert, W. M.; Willauer, H. D.; Broker, G. A.; Rogers, R. D. Characterization and comparison of hydrophilic and hydrophobic room temperature ionic liquids incorporating the imidazolium cation. Green Chem. 2001, 3, 156–164. [Google Scholar] [CrossRef]
- Lee, S.; Park, J. H.; Kang, J.; Lee, J. K. Lanthanide triflate-catalyzed three-component synthesis of α-amino phosphonates in ionic liquids. A catalyst reactivity and reusability study. Chem. Commun. 2001, 1698–1699. [Google Scholar]
- Csihony, S.; Fischmeister, C.; Bruneau, C.; Horvath, I. T.; Dixneuf, P. H. First ring-opening metathesis polymerization in an ionic liquid. Efficient recycling of a catalyst generated from a cationic ruthenium allenylidene complex. New J. Chem. 2002, 11, 1667–1670. [Google Scholar]
- Fischer, T.; Sethi, A.; Welton, T.; Woolf, J. Diels-alder reactions in room temperature ionic liquids. Tetrahedron Lett. 1999, 40, 793–796. [Google Scholar] Baan, Z.; Finta, Z.; Keglevich, G.; Hermecz, I. Application of ionic liquids in Palladium(II) catalyzed homogeneous transfer hydrogenation. Tetrahedron Lett. 2005, 46, 6203–6204. [Google Scholar]
- Aggarwal, A.; Lancaster, N. L.; Sethi, A. R.; Welton, T. The role of hydrogen bonding in controlling the selectivity of Diels-Alder reactions in room-temperature ionic liquids. Green Chem. 2002, 5, 517–520. [Google Scholar]
- Zhang, F. Y.; Corey, E. J. Highly enantioselective Michael reactions catalyzed by a chiral quaternary ammonium salt. Illustration by asymmetric syntheses of (S)-ornithine and chiral 2-cyclohexenones. Org. Lett. 2000, 2, 1097–1100. [Google Scholar] [CrossRef]
- Vallette, H.; Pican, S.; Boudou, C.; Levillain, J.; Plaquevent, J. C.; Gaumont, A. C. Palladium catalyzed C-P cross-coupling reactions in ionic liquids. Tetrahedron Lett. 2006, 47, 5191–5193. [Google Scholar]
- Guo, S.; Du, Z.Y.; Zhang, S. G.; Li, D. M.; Li, Z. P.; Deng, Y. Q. Clean Beckmann rearrangement of cyclohexanone oxime in caprolactam-base Bronsted acidic ionic liquids. Green Chem. 2006, 3, 296–300. [Google Scholar]
- Evans, D. A.; Wood, M. R.; Trotter, B. W.; Richardson, T. I.; Barrow, J. C.; Katz, J. L. Total synthesis of vancomycin and eremomycin aglycons. Angew. Chem. Int. Ed. 1998, 37, 2700–2704. [Google Scholar] [CrossRef]
- Ullmann, F. Uber eine neue darstellungsweise von Phenylathersalicylsaure. Chem. Ber. 1904, 37, 853–854. [Google Scholar] [CrossRef]
- He, H.; Wu, Y. J. Synthesis of diaryl ethers through the copper-catalyzed arylation of phenols with aryl halides using microwave heating. Tetrahedron Lett. 2003, 44, 3445–3446. [Google Scholar] Sagar, A. D.; Tale, R. H.; Adude, R. N. Synthesis of symmetrical diaryl ethers from arylboronic acids mediated by copper(II) acetate. Tetrahedron Lett. 2003, 44, 7061–7063. [Google Scholar] Decicco, C. P.; Song, S.; Evans, D. A. Intramolecular O-arylation of phenols with phenylboronic acids: Application to the synthesis of macrocyclic metalloproteinase inhibitors. Org. Lett. 2001, 3, 1029–1032. [Google Scholar] Marcoux, J.; Doye, S.; Buchwald, S. L. A general copper-catalyzed synthesis of diaryl ethers. J. Am. Chem. Soc. 1997, 119, 10539–10540. [Google Scholar] Cui, S. L.; Jiang, Z. Y.; Wang, Y. G. A general and efficient protocol for the synthesis of biaryl ethers from aryl silyl ethers using Cs2CO3. Synlett 2004, 1829–1831. [Google Scholar]
- Dinsmore, C. J.; Zartman, C. B. Arylmethanesulfonates are convenient latent phenols in the nucleophilic aromatic substitution reaction. Tetrahedron Lett. 1999, 40, 3989–3990. [Google Scholar] [CrossRef]
- Ritter, T.; Stanek, K., Larrosa; Carreira, E. M. Mild cleavage of aryl mesylates: Methanesulfonate as potent protecting group for phenols. Org. Lett. 2004, 6, 1513–1514. [Google Scholar] [CrossRef]
- Xu, H.; Wang, Y. G. One-pot construction 3,4-dihydropyrimidin-2(1H)-ones catalysed by samarium(III). J. Chem. Research (S) 2003, 6, 377–379. [Google Scholar] Xu, H.; Wang, Y. G. A rapid and efficient Biginelli reaction catalyzed by zinc triflate. Chin. J. Chem. 2003, 21, 327–331. [Google Scholar] Xu, H.; Liao, W. M.; Li, H. F. A mild and efficient ultrasound-assisted synthesis of diaryl ethers without any catalyst. Ultrason. Sonochem. 2007, in press, available online 31 January 2007. [Google Scholar]
- Sample Availability: Samples of the compounds are available from the authors.
© 2007 by MDPI (http://www.mdpi.org). Reproduction is permitted for noncommercial purposes.
Share and Cite
Xu, H.; Chen, Y. C(aryl)-O Bond Formation from Aryl Methanesulfonates via Consecutive Deprotection and SNAr Reactions with Aryl Halides in an Ionic Liquid. Molecules 2007, 12, 861-867. https://doi.org/10.3390/12040861
Xu H, Chen Y. C(aryl)-O Bond Formation from Aryl Methanesulfonates via Consecutive Deprotection and SNAr Reactions with Aryl Halides in an Ionic Liquid. Molecules. 2007; 12(4):861-867. https://doi.org/10.3390/12040861
Chicago/Turabian StyleXu, Hui, and Yang Chen. 2007. "C(aryl)-O Bond Formation from Aryl Methanesulfonates via Consecutive Deprotection and SNAr Reactions with Aryl Halides in an Ionic Liquid" Molecules 12, no. 4: 861-867. https://doi.org/10.3390/12040861
APA StyleXu, H., & Chen, Y. (2007). C(aryl)-O Bond Formation from Aryl Methanesulfonates via Consecutive Deprotection and SNAr Reactions with Aryl Halides in an Ionic Liquid. Molecules, 12(4), 861-867. https://doi.org/10.3390/12040861





