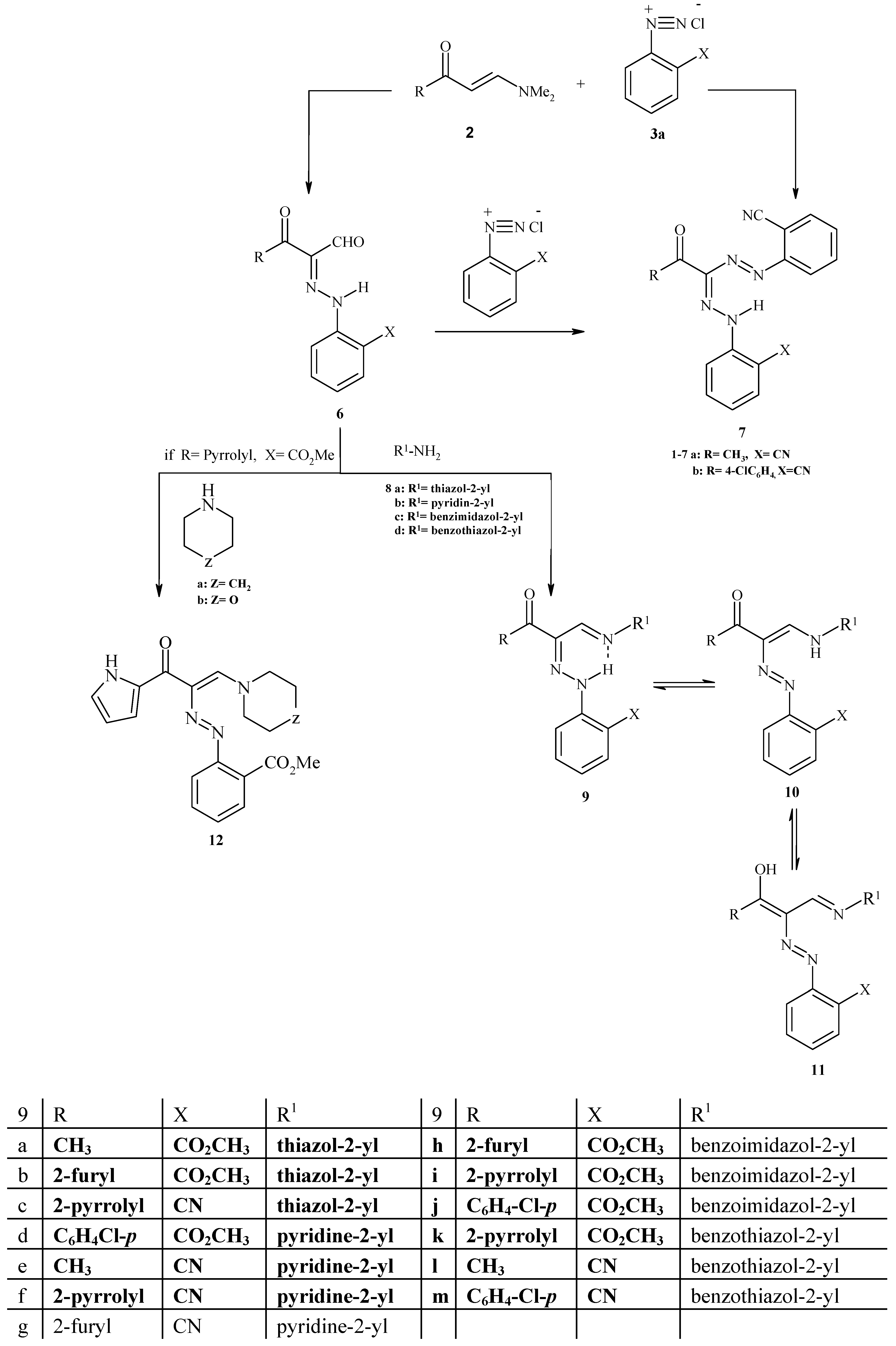
Experimental
General
All melting points were measured on a Gallenkamp Electrothermal melting point apparatus and are uncorrected. The IR absorption spectra (KBr disks) were measured on a Nicolet Magna 520FT IR Spectrophotometer. 1H-NMR and 13C-NMR spectra were recorded in deuterated dimethylsulfoxide (DMSO-d6) or deuterated chloroform (CDCl3) at 200 MHz on a Varian Gemini NMR spectrometer or a Bruker DPX 400 MHz spectrometer using tetramethylsilane (TMS) as an internal reference and results are expressed as δ values (ppm). Mass spectra were recorded on a Shimadzu GCMS-QP 1000 Ex mass spectrometer at 70 eV. Microwave irradiation was carried out using a commercial microwave oven (SGO 390W). Elemental analyses were carried out at the Microanalytical Center of Cairo University, Egypt.
General Procedure for the preparation of enaminones 2a-g
Method I (Δ): Dimethylformamide dimethylacetal (DMFDMA) (0.1 mol) was added to solution of methyl ketone (0.1 mol) in dry xylene (30 mL) or dry toluene (30 mL), and the reaction mixture was refluxed for 8 hours. Removal of the solvent under reduced pressure yielded the crude product, which was recrystallized from xylene.
Method II (Δ without solvent): A mixture of dimethylformamide dimethylacetal (DMFDMA, 0.1 mol) and the corresponding methyl ketone (0.1 mol) was refluxed for 9 hours and was allowed to cool. The solid product formed was collected and recrystallized from xylene.
Method III (μω): Dimethylformamide dimethylacetal (DMFDMA, 0.1 mol) and methyl ketone (0.1 mol) were placed in the microwave oven and irradiated at full power for 1-5 min., left to cool to room temperature and the solid formed was collected and recrystallized from xylene.
Yields and properties of the products are summarized in
Table 1.
Table 1.
| No. | Compound | m.p./οC | Ref. |
|---|
| 2a | 4-Dimethylamino-3-buten-2-one | - | 15 |
| 2b | 3-Dimethylamino-1-(2-furyl)propenone | 92 | 15 |
| 2c | 3-Dimethylamino-1-(2-pyrrolyl)propenone | 94 | 15 |
| 2d | 3-Dimethylamino-1-(2-pyridyl)propenone | 135 | 15 |
| 2e | 3-Dimethylamino-1-(2-hydroxyphenyl) propenone | 123 | - |
| 2f | 3-Dimethylamino-1-(4-hydroxyphenyl) propenone | - | - |
| 2g | 3-Dimethylamino-1-(4-chlorophenyl) propenone | 88 | - |
Preparation of 2-arylhydrazono-3-oxo-3-substituted-propanals 6a-h [15]
A cold solution of aryldiazonium salt (10 mmol) was prepared by adding a solution of sodium nitrite (1 g in 10 mL H
2O) to a cold solution of aryl amine hydrochloride (10 mmol of aryl amine in 5 mL concentrated HCl) with stirring as described earlier [
15]. The resulting solution of the aryldiazonium salt was then added to a cold solution of enaminone in EtOH (50 mL) containing sodium acetate (1g in 10 mL H
2O).The mixture was stirred at room temperature for 1h and the solid product thus formed was collected by filtration and crystallized from the appropriate solvent.
2-(2-methoxycarbonylphenylhydrazono)-3-oxo-butanal (6a). Orange crystals (from ethanol); yield 63%; m.p. 126 ºC; IR νmax cm-1: 3568 (br, NH), 2954 (CH aldehyde), 1695 (C=O ester), 1647 (C=O aldehyde), 1600 (C=O ketone); 1H-NMR: δ = 2.52, 2.65 (s, 3H, CH3), 4.02, 4.03 (s, 3H, OCH3), 7.20-8.15 (m, 4H, Ar-H), 9.59, 10.19 (s, 1H, CHO), 15.57, 15.89 (s, 1H, NH); MS: (M+ +1) 249; Anal. Calcd. for C12H12N2O4 (248.224): C, 58.07; H, 4.67; N, 11.28; Found: C, 58.37; H, 4.71; N, 11.58.
3-(2-furyl)-2-(2-methoxycarbonylphenylhydrazono)-3-oxo-propanal (6b). Dark yellow crystals (from dioxane); yield 90%; m.p. 195 ºC; IR νmax cm-1: 3468 (br, NH), 2837 (CH aldehyde), 1705 (C=O ester), 1652 (C=O aldehyde) and 1615 (C=O ketone); 1H-NMR: δ = 4.05 (s, 3H, CH3), 6.60 (m, 1H, furyl H-4), 7.21-7.74 (m, 4H, Ar-H), 7.95-8.12 (m, 2H, furyl H-3, H-5), 10.23 (s, 1H, CHO) and 15.66 (s, 1H, NH) ppm; MS: (M+) 300; Anal. Calcd. for C15H12N2O5 (300.256): C, 60.00; H, 4.00; N, 9.33 ; Found: C, 59.99; H, 4.01; N, 9.43.
2-(2-methoxycarbonylphenylhydrazono)-3-oxo-3-(2-pyrrolyl)propanal (6c). Pale orange crystals (from dil. dioxane); yield 45%; m.p. 186 ºC; IR νmax cm-1: 3568 (br, NH), 2837 (CH aldehyde), 1720 (C=O ester), 1662 (C=O aldehyde) and 1645 (C=O ketone); MS: (M+ +1) 298; Anal. Calcd. for C15H13N3O4 (299.27): C, 60.20; H, 4.34; N, 14.05; Found: C, 60.31; H, 4.40; N, 14.12.
3-(4-Chlorophenyl)-2-(2-methoxycarbonylphenylhydrazono)-3-oxo-propanal (6d). Yellow crystals (from ethanol); yield 80%; m.p. 189 ºC; IR: νmax cm-1 (this compound shows a complex spectrum due to the to H-bond between O and NH): 3022 (CH aromatic), 1711 (C=O ester), 1650 (C=O aldehyde), 1638 (C=O ketone) and 1586 (C=N); 1H-NMR: δ = 4.05 (s, 3H, CH3), 7.20-8.09 (m, 8H, Ar-H), 10.25 (s, 1H, CHO) and 15.69 (s, 1H, disappeared after D2O exchange, NH); 13C-NMR: δ = 52.90 (COOCH3), 116.19, 116.40, 118.98, 128.36, 133.33, 138.75 (C6H4-CO2M-o), 124.98, 131.59, 131.89, 134.74, 135.50 (C6H4-Cl-p), 143.54 (C=N-N), 166.87 (COOCH3),188.23 (C=O) and 190.49 (CHO); MS: (M+) 344; Anal. Calcd. for C17H13N2O4Cl (344.76): C, 59.23; H, 3.80; N, 8.13; Found: C, 59.33; H, 3.82; N, 8.15.
2-(2-Cyanophenylhydrazono)-3-oxo-butanal (6e). Orange crystals (from ethanol); yield 80%; m.p. 130 ºC; IR: νmax cm-1: 3406 (br, NH), 2221 (CN), 1693 (C=O aldehyde) and 1670 (C=O ketone); 1H-NMR: δ = 2.52, 2.63 (s, 3H, CH3), 7.22-8.02 (m, 4H, Ar-H), 9.58, 10.26 (s, 1H, CHO) and 14.4, 15.4 (s, 1H, NH); MS: (M+) 215; Anal. Calcd. for C11H9N3O2 (215.2): C, 61.39; H, 4.21; N, 19.53; Found: C, 61.48; H, 4.30; N, 19.58.
3-(2-Furyl)-2-(2-cyanophenylhydrazono)-3-oxopropanal (6f). Orange yellowish crystals (from dioxane); yield 78%; m.p. 205 ºC; IR νmax cm-1: 3543 (br, NH), 2221 (CN), 1651 (C=O aldehyde) and 1648 (C=O ketone); 1H-NMR: δ = 6.76 (m, 1H, furyl H-4), 7.34-7.52 (m, 4H, Ar-H), 7.56-8.10 (m, 2H, furyl H-3, H-5), 10.02 (s, 1H, CHO) and 14.49 (s, 1H, NH); 13C- NMR: δ = 112.54, 115.30, 148.22, 149.63 (furoyl carbon), 116.09 (CN), 122.08, 125.49, 133.66, 133.14, 143.70 (C6H4-CN-o), 152 (C=N-N), 176.15 (CHO) and 188.95 (C=O); MS: (M+) 267; Anal. Calcd. for C14H9N3O3 (267.23): C, 62.92; H, 3.37; N, 15.73; Found: C, 62.99; H, 3.40; N, 15.81.
2-(2-Cyanophenylhydrazono)-3-oxo-3(2-pyrrolyl)propanal (6g). Pale brown crystals (from dioxane); yield 65%; m.p. 172 ºC; IR νmax cm-1: 3450 (br, NH), 2216 (CN), 1680 (C=O aldehyde) and 1648 (C=O ketone); 1H-NMR: δ = 6.37 (m, 1H, pyrrolyl H-4), 7.15-7.31 (m, 4H, Ar-H), 7.64-7.91 (m, 2H, pyrrolyl H-3, H-5), 9.85 (br s, 1H, NH), 10.23 (s, 1H, CHO) and 14.98 (s, 1H, NH); MS: (M+) 266; Anal. Calcd. for C14H10N4O2 (266.24): C, 63.16; H, 3.76; N, 21.05; Found: C, 63.23; H, 3.79; N, 21.07.
3-(4-Chlorophenyl)-2-(2-cyanophenylhydrazono)-3-oxo-propanal (6h). Brown crystals (from 1:1 ethanol∕dioxane); yield 88%; m.p. 225 ºC; IR νmax cm-1: 3320 (NH), 3019 (CH aromatic), 2221 (CN), 1675 (C=O aldehyde) and 1640 (C=O ketone); MS: (M+) 311; Anal. Calcd. for C16H10N3O2Cl (311.73): C, 61.65; H, 3.23; N, 13.48; Found: C, 61.70; H, 3.15; N, 13.55.
General procedure for the preparation of bisazo compounds 7a,b
A cold solution of aryldiazonium salt (10 mmol, a slight excess) was prepared by adding a solution of sodium nitrite (1g in 10 mL H2O) with stirring to a cold solution of arylamine hydrochloride (10 mmol of arylamine in 5 mL concentrated HCl) as described earlier. The resulting solution of the aryldiazonium salt was then added to a cold solution of enaminone in EtOH (50 mL) containing sodium acetate (1g in 10 mL H2O). The mixture was stirred at room temperature for 1 h and the solid product thus formed was collected by filtration and crystallized from the appropriate solvent.
3-[(2-cyanophenyl) diazo]-3-[(2-cyanophenyl)hydrazono]propan-2-one (7a). Brown crystals (from ethanol); yield 82%; m.p. 179 ºC; IR νmax cm-1: 3066 (CH aromatic), 2935 (CH aliphatic), 2222 (CN), 1666 (C=O); 1H-NMR: δ = 2.63 (s, 3H, CH3), 7.20-7.81 (m, 8H, Ar-H), and 15.50 (s, 1H, NH); 13C-NMR: δ = 52.26 (CH3), 117.05, 117.46 (2C≡N), 100.67, 108.74, 117.96, 118.86, 125.05, 127.74, 133.39, 133.67, 134.10, 134.19, 134.35, 144.75 (2C6H4-CN-o), 151.75 (C=N-NH) and 197.41 (C=O); MS: (M+-1) 315; Anal. Calcd. for C17H12N6O (316.32): C, 64.55; H, 3.82; N, 26.57; Found: C, 64.60; H, 3.75; N, 26.49.
1-(4-chlorophenyl)-2-[(2-cyanophenyl)diazo]-2-[(2-cyanophenyl)hydrazono]-ethan-1-one (7b). Dark brown crystals (from 1:1 ethanol/dioxane); yield 80%; m.p. 226 ºC; IR νmax cm-1: 3069 (CH aromatic), 2222 (CN), 1646 (C=O); 1H-NMR: δ = 7.18-7.96 (m, 12H, Ar-H) and 15.75 (s, 1H, NH); 13C-NMR: δ = 101.38, 107.99, 117.17, 119.84, 125.17, 127.56, 133.18, 133.54, 133.57, 134.17, 134.34, 145.48 (2C6H4-CN-o), 116.09, 116.36 (2C≡N), 151.74 (C=N-NH) and 190.07 (C=O); MS: (M+-1) 411; Anal. Calcd. for C22H13N6OCl (412.84): C, 64.01; H, 3.17; N, 20.36; Found: C, 64.15; H, 3.20; N, 20.39.
Reaction of 2-Arylhydrazones with heterocyclic amines:
Method I (Δ): A mixture of compounds 6a-h (0.1 mol) and amine (0.1 mol) was refluxed in ethanol (30 mL) for 2 hours, then left to cool to room temperature and the solid was collected and crystallized from the appropriate solvent.
Method II (μω): A mixture of compounds 6a-h (0.1 mol) and amine (0.1 mol) and a few drops of ethanol was placed in the microwave oven and irradiated at 390 w for 5 min., then left to cool to room temperature and the solid was collected and crystallized from the appropriate solvent.
Methyl 2-{N′-[2-Oxo-1-(thiazol-2-yliminomethyl)-propylidene]hydrazino}benzoate (9a). Brown crystals (from methanol); yield 80%; m.p. 145 ºC; IR νmax cm-1: 3450 (br, NH), 3087 (CH aromatic), 2954 (CH aliphatic), 1706 (C=O ester), 1660 (C=O ketone) and 1571 (C=N); MS: (M+) 330; Anal. Calcd. for C15H14N4O3S (330.37C, 54.54; H, 4.27; N, 16.96; Found: C, 54.56; H, 4.20; N, 16.90.
Methyl 2-{N′-[2-Furan-2-yl-2-oxo-1-(thiazol-2-yliminomethyl)ethylidene]hydrazino}benzoate (9b). Light brown crystals (from methanol); m.p. 162 ºC; 1H-NMR δ = 3.97 (s, 3H, CH3O), 6.79 (m, 1H, furyl H- 4), 7.24 (d, 1H, furyl H-3), 7.3, 7.52 (d, 2H, thiazole H-4, H-5), 7.57-8.06 (m, 4H, Ar-H), 7.8 (s, 1H, CH olefinic), 8.13 (d, 1H, furyl H-5) and 15.37 (s, 1H, NH); MS: (M+) 382; Anal. Calcd. for C18H14N4O4S (382.40): C, 56.54; H, 3.69; N, 14.65; Found: C, 56.57; H, 3.70; N, 14.66.
2-{N′-[2-Oxo-2-(1H-pyrrol-2-yl)-1-(thiazol-2-yliminomethyl)ethylidene]hydrazino}benzonitrile (9c). Brown crystals (from ethanol); m.p. 201ºC; IR νmax cm-1: 3490 (br, NH), 3066 (CH aromatic), 2223 (CN), 1665 (C=O) and 1551 (C=N); MS: (M+) 348: Anal. Calcd. for C17H12N6OS (348.39): C, 58.61; H, 3.47; N, 24.12; Found: C, 58.67; H, 3.50; N, 24.15.
Methyl 2-{N′-[2-(4-chlorophenyl)-2-oxo-1-(pyridin-2-yliminomethyl)ethylidene]hydrazino}benzoate (9d). Orange crystals (from 2:1 ethanol/dioxane); m.p. 255 ºC; IR νmax cm-1: 3320 (NH), 3007 (CH aromatic), 1645 (C=O ketone), and 1588 (C=N); 1H-NMR: δ = 3.99 (s, 3H, OCH3), 7.25, 7.47 (m, 2H, pyridyl H-4, H-5), 7.62-8.03 (m, 8H, Ar-H), 8.63 (d, 1H, pyridyl H-3), 8.58 (d, 1H, pyridyl H-6), 9.66 (s, 1H, CH olefinic) and 15.73 (s, 1H, NH); MS: (M+) 420; Anal. Calcd. for C22H17N4O3Cl (420.86): C, 62.79, H, 4.07; N, 13.31; Found: C, 62.70; H, 4.20; N, 13.39.
2-{N′-[2-Oxo-1-(pyridin-2-yliminomethyl)propylidene]hydrazino}benzonitrile (9e). Dark orange crystals (from ethanol); m.p. 198 ºC; IR:νmax cm-1: 3317 (NH), 3065 (CH aromatic), 2920 (CH aliphatic), 2222 (CN), 1664 (C=O ketone) and 1553 (C=N); MS: (M+) 291; Anal. Calcd. for C16H13N5O (291.31): C, 65.97; H, 4.50; N, 24.04; Found: C, 65.87; H, 4.59; N, 24.35.
2-{N′-[2-Oxo-1-(pyridin-2-yliminomethyl)-2-(1H-pyrrol-2-yl)-ethylidene]hydrazino}benzonitrile (9f). Dark orange crystals (from ethanol); m.p. 232 ºC; IR νmax cm-1: 3267 (2 NH), 3050 (CH aromatic), 2222 (CN), 1618 (C=O) and 1539 (C=N); MS: (M+) 342; Anal. Calcd. for C19H14N6O (342.36): C, 66.66; H, 4.12; N, 24.55; Found: C, 66.56; H, 4.20; N, 24.59.
2-{N′-[2-Furan-2-yl-2-oxo-1-(pyridin-2-yliminomethyl)ethylidene]hydrazino}benzonitrile (9g). Brown crystals (from ethanol); m.p. 259 ºC; IR νmax cm-1: 3500 (br, NH), 3105 (CH aromatic), 2220 (CN), 1631 (C=O) and 1549 (C=N); MS: (M+) 343; Anal. Calcd. for C19H13N5O2 (343.35): C, 66.47; H, 3.82; N, 20.40; Found: C, 66.50; H, 3.89; N, 20.35.
Methyl 2-(N′-{1-[(1H-Benzoimidazol-2-ylimino)-methyl]-2-furan-2-yl-2-oxo-ethylidene}hydrazino) benzoate (9h). Brown crystals (from 2:1 ethanol/dioxane); m.p. 240ºC; 1H-NMR: δ = 4.0 (s, 3H, OCH3), 6.83 (dd, 1H, furyl H-4), 7.22-7.25 (m, 4H, imidazole-H), 7.36 (m, 1H, furyl H-3), 7.63-7.99 (m, 4H, Ar-H), 8.08 (d, 1H, furyl H-5), 9.47 (s, 1H, CH olefinic), 12.11 (s, 1H, NH imidazole) and 15.85 (s, 1H, NH hydrazone) ppm; MS: (M+) 415; Anal. Calcd. for C22H17N5O4 (415.41): C, 63.61; H, 4.12; N, 16.86; Found: C, 63.67; H, 4.22; N, 16.90.
Methyl 2-(N′-{1-[(1H-Benzoimidazol-2-ylimino)-methyl]-2-oxo-2-(1H-pyrrol-2-yl)-ethylidene]hydra- zino)benzoate (9i). Dark yellow crystals (from 2:1 ethanol/dioxane); m.p. 281ºC; IR νmax cm-1 (shows complex spectra due to H-bond between O and NH): 3480 (NH pyrrolyl), 3383 (NH imidazole), 3081(CH aromatic),1718 (C=O ester), 1656 (C=O ketone) and 1571 (C=N); 1H-NMR: δ = 3.96 (s, 3H, OCH3), 6.54 (m, 1H, pyrrolyl H-4), 6.95-7.02 (m, 2H, pyrrolyl H-3, H-5), 7.21-7.32 (m, 4H, imidazole H), 7.54-7.97 (m, 4H, Ar-H), 9.22 (s, 1H, CH olefinic), 11.43 (s, 1H, NH pyrrolyl), 11.65 (s, 1H, NH imidazole) and 14.25 (s, 1H, NH hydrazone) ppm; MS: (M+) 414; Anal. Calcd. for C22H18N6O3 (414.43): C, 63.76; H, 4.38; N, 20.28; Found: C, 63.79; H, 4.40; N, 20.30.
Methyl 2-(N′-{1-[(1H-Benzoimidazol-2-ylimino)-methyl]-2-(4-chlorophenyl)-2-oxo-2-ethylidene]-hydrazino} benzoate (9j). Orange crystals (from 2:1 ethanol/dioxane); m.p. 257 ºC; IR νmax cm-1: (shows complex spectra due to H-bond between O and NH) 3354 (NH imidazole), 1690 (C=O ester), 1640 (C=O ketone) and 1586 (C=N); MS: (M+-18) 441; Anal. Calcd. for C24H18N5O3Cl (459.90): C, 62.68; H, 3.95; N, 15.23; Found: C, 62.58; H, 3.90; N, 15.33.
Methyl 2-{N′-[1-(Benzothiazol-2-yliminomethyl)-2-oxo-2-(1H-pyrrol-2-yl)-ethylidene]-hydrazino}- benzoate (9k). Brown crystals (from methanol); m.p. 189 ºC; IR νmax cm-1: 3492, 3224 (2NH), 3023 (CH aromatic), 1720 (C=O ester), 1664 (C=O ketone) and 1575 (C=N); 1H-NMR: δ = 3.96 (s, 3H, OCH3), 6.41 (m, 1H, pyrrolyl H-4), 6.75 (d, 2H, pyrrolyl H-3), 6.89 (s, 1H, CH olefinic), 6.95 (d, 1H, pyrrolyl H-5), 7.22, 7.36 (d, 2H, benzothiazole H-4, H-7), 7.38-7.44 (m, 2H, benzothiazole H-5, H-6), 7.53 (s, 1H, NH pyrrolyl), 7.62-7.69 (m, 2H, Ar H-4, H-5), 7.89, 7.91 (d, 2H, Ar H-3, H-6), and 14.46 (s, 1H, NH hydrazone) ppm; 13C-NMR: δ = 52.46 (OCH3), 110.33, 121.24, 126.57, 131.30 (pyrrolyl carbon), 113.21, 114.03, 116.90, 119.54, 134.21, 136.84 (C6H4-CO2Me-o), 121.58, 123.18, 124.77, 134.32, 137.45 (C6H4NS), 140.50 (C=N-N), 144.82 (N-C-S), 165.27 (HC=N), 167.31 (COOCH3) and 173.42 (C=O) ppm; MS: (M+) 431; Anal. Calcd. for C22H17N5O3S (431.48): C, 61.24; H, 3.97; N, 16.23; Found: C, 61.34; H, 3.87; N, 16.40.
2-{N′-[1-(Benzothiazol-2-yliminomethyl)-2-oxo-propylidene]-hydrazino}-benzonitrile (9l). Brown crystals (from 2:1 ethanol/dioxane); m.p. 223 ºC; IR νmax cm-1: 3350 (NH), 3061 (CH aromatic), 2921 (CH aliphatic), 2216 (C≡N), 1684 (C=O) and 1560 (C=N); 1H-NMR: δ = 2.60 (s, 3H, CH3), 7.24-7.99 (m, 8H, Ar-H), 9.50 (s, 1H, CH olefinic) and 15.29 (s, 1H, NH) ppm, 13C-NMR: δ = 25.10 (CH3CO), 101.32, 115.80, 121.92, 134.23, 134.38, 145.82 (C6H4-CN-o), 116.32 (C≡N), 123.56, 125.45, 125.48, 126.81, 133.12 (C6H4NS), 151.55 (C=N-N), 153.50 (N-C-S), 168.40 (HC=N) and 196.56 (C=O) ppm; MS: (M+) 347; Anal. Calcd. for C18H13N5OS (347.40): C, 62.23; H, 3.77; N, 20.16; Found: C, 62.40; H, 3.97; N, 20.20.
2-{N′-[1-(Benzothiazol-2-yliminomethyl)-2-(4-chlorophenyl)-2-oxo-ethylidene]-hydrazino}-benzo- nitrile (9m). Brown crystals (from 2:1 ethanol/dioxane); m.p. 259 ºC; IR νmax cm-1: 3600 (br, NH), 3070 (CH aromatic), 2219 (C≡N), 1650 (C=O) and 1559 (C=N); 1H-NMR: δ = 6.97-8.35 (m, 12H, Ar-H), 8.13 (s, 1H, CH olefinic) and 15.10 (s, 1H, NH) ppm; MS: (M+) 443; Anal. Calcd. for C23H14N5OClS (443.92): C, 62.23; H, 3.18; N, 15.78; Found: C, 62.40; H, 3.28; N, 15.68.
Methyl 2-[2-piperidin-1-yl-1-(1H-pyrrol-2-carbonyl)-vinylazoethylidene] benzoate (12a). Brown crystals (from ethanol); m.p. 200 ºC; IR νmax cm-1: 3158 (NH pyrrolyl), 3009 (CH aromatic), 2932 (CH aliphatic), 1672 (C=O ester), 1573 (C=O ketone) and 1495 (N=N); 1H-NMR: δ = 1.43-2.75 (m, 10H, piperidin H), 5.57 (s, 1H, NH pyrrolyl), 6.50 (m, 1H, pyrrolyl H-4), 6.82 (d, 1H, pyrrolyl H-3), 6.95-6.99 (m, 1H, Ar H-4), 7.21 (d, 1H, pyrrolyl H-5), 7.49-7.53 (m, 1H, Ar H-5), 7.86, 7.98 (d, 2H, Ar-H-3, H-6) and 8.12 (s, 1H, CH olefinic) ppm; 13C-NMR: δ = 24.54, 26.18, 48.43 (piperidinyl carbons), 52.37 (COOCH3), 109.35, 112.88, 114.03 (pyrrolyl carbons), 116.13, 120.77, 123.43, 131.34, 134.32, 135.38 (C6H4-CO2M-o), 139.45 (HC=C-N), 145.59 (HC=C-N), 167.42 (COOCH3) and 174.40 (C=O) ppm; MS: (M+) 366; Anal. Calcd. for C20H22N4O3 (366.42): C, 65.56; H, 6.05; N, 15.29; Found: C, 65.69; H, 6.25; N, 15.15.
Methyl 2-[2-morpholin-4-yl-1-(1H-pyrrol-2-carbonyl)-vinylazo] benzoate (12b). Brown crystals (from ethanol); m.p. 190 ºC; IR νmax cm-1: 3115 (NH pyrrolyl), 3018 (CH aromatic), 2952 (CH aliphatic), 1697 (C=O ester), 1660 (C=O ketone) and 1495 (N=N); 1H-NMR: δ = 2.47-2.79 (m, 4H, morpholinyl H), 3.58, 3.93 (d, 4H, morpholinyl H), 5.93 (s, 1H, NH pyrrolyl), 6.60 (m, 1H, pyrrolyl H-4), 6.87 (d, 1H, pyrrolyl H-3), 7.20 (d, 1H, pyrrolyl H-5), 7.55-7.97 (m, 4H, Ar-H) and 8.10 (s, 1H, CH olefinic) ppm; MS: (M+) 368; Anal. Calcd. for C19H20N4O4 (368.40): C, 61.95; H, 5.47; N, 15.21; Found: C, 61.92; H, 5.50; N, 15.30.
General procedure for the reaction of 2-arylhydrazono derivatives with active methylene compounds
With benzotriazolacetone
Method I (Δ): A solution of compounds 6e,h (0.1 mol) in ethanol (30 mL) was treated with benzotriazolylacetone (0.1 mol) in the presence of a few drops of piperidine and refluxed for 3 hours. The precipitated material was isolated by filtration and crystallized from the appropriate solvent
Method II (μω): Compounds 6e,h (0.1 mol) and benzotriazolylacetone (0.1 mol) in the presence of a few drops of piperidine was placed in the microwave oven and irradiated at 390 w for 2-15 min., then left to cool to room temperature and the solid was collected and crystallized from the appropriate solvent.
5-Benzotriazolyl-3-(2-cyanophenylhydrazono)-4-hepten-2,6-dione (14a). Brown crystals (from dioxane); m.p. 235 ºC; 1H-NMR: δ = 2.20, 2.30 (s, 3H, 2CH3), 6.64-7.98 (m, 9H, Ar-H + CH olefinic) and 14.12 (s, 1H, NH) ppm; 13C-NMR: δ = 19.77, 19.80 (2CH3), 112.11 (HC), 116.50 (CN), 110.11, 115.82, 119.20, 132.81, 133.65, 150.22 (C6H4CN-o), 128.59, 129.89, 130.23, 131.79 (benzotriazolyl carbons), 144.70 (N-C-CO), 155.39 (C=N-N) and 196.55, 199.75 (2C=O) ppm; MS: (M+) 372; Anal. Calcd. for C20H16N6O2 (372.39): C, 64.51; H, 4.33; N, 22.57; Found: C, 64.65; H, 4.23; N, 22.59.
4-Benzotriazolyl-1-(4-chlorophenyl)-2-(2-cyanophenylhydrazono)-3-hexaen-1,5-dione (14b). Green crystals (from ethanol); m.p. 259 ºC; IR νmax cm-1: 3400 (NH), 3068 (CH aromatic), 2320 (C≡N), 1674 (C=O) and 1646 (C=O ketone); 1H-NMR: δ = 1.99 (s, 3H, CH3), 7.20 (s, 1H, CH olefinic), 7.61-8.11 (m, 12H, Ar-H) and 13.89 (s, 1H, NH) ppm; MS: (M+) 468; Anal. Calcd. for C25H17N6O2Cl (468.91): C, 64.04; H, 3.65; N, 17.92; Found: C, 64.45; H, 3.59; N, 17.99.
With glycine or N-acetylglycine or hippuric acid
Method I (Δ): Each of compounds 6a,b,d,e,f,h (0.1 mol) and glycine or N-acetylglycine or hippuric acid (0.1 mol) was refluxed in acetic anhydride (20 mL) for 1 hour, then left to cool at room temperature and poured into ice-cold water. The solid product so formed was collected by filtration and crystallized from the appropriate solvent.
Method II (μω): Each of compounds 6a,b,d,e,f,h (0.1 mol) and glycine or N-acetylglycine or hippuric acid (0.1 mol) and drops from acetic anhydride (20 mL) was placed in the microwave oven and irradiated at 390 W for 5-15 min., then left to cool to room temperature and the solid was collected and crystallized from the appropriate solvent.
4-Acetylamino-6-acetyl-2-(2-methoxycarbonylphenyl)-2-hydropyridazin-3-one (17a). Brown crystals (from ethanol); m.p. 238 ºC; 1H-NMR: δ = 2.23, 2.43 (s, 3H, 2CH3CO), 3.66 (s, 3H, OCH3), 7.66-8.01 (m, 4H, Ar-H), 8.61 (s, 1H, pyridazinyl H-5) and 10.21 (s, 1H, NH) ppm; 13C-NMR: δ = 24.83 (NHCOCH3), 25.11 (CH3CO), 52.98 (COOCH3), 108.76, 136.98, 143.87 (pyridazine ring), 127.78, 128.99, 130.17, 130.90, 134.02, 140.89 (C6H4-CO2Me-o), 156.25 (C=O ring), 165.54 (COOCH3), 171.98 (NHCOCH3) and 195.66 (CH3CO) ppm; MS: (M+) 329; Anal. Calcd. for C16H15N3O5 (329.32): C, 58.36; H, 4.59; N, 12.76; Found: C, 58.40; H, 4.55; N, 12.86.
4-Acetylamino-6-(2-furylcarbonyl)-2-(2-methoxycarbonylphenyl)-2-hydropyridazin-3-one (17b). Dark brown crystals (from ethanol); m.p. 214 ºC; 1H-NMR: δ = 2.25 (s, 3H, CH3CO), 3.66 (s, 3H, OCH3), 6.73 (m, 1H, furyl H-4), 7.51 (d, 1H, furyl H-3), 7.68-7.89 (m, 4H, Ar H), 8.02 (d, 1H, furyl H-5), 8.69 (s, 1H, pyridazinyl H-5) and 10.26 (s, 1H, NH) ppm; MS: (M+) 381; Anal. Calcd. for C19H15N3O6 (381.35): C, 59.84; H, 3.96; N, 16.08; Found: C, 59.79; H, 3.99; N, 16.25.
4-Acetylamino-6-(4-chlorophenylcarbonyl)-2-(2-methoxycarbonylphenyl)-2-hydropyridazin-3-one (17c). Light yellow crystals (from ethanol); m.p. 246 ºC; IR νmax cm-1: 3285 (NH), 1713 (C=O ester and C=O ketone) and 1644 (C=O amide and C=O pyidazine ring); 1H-NMR: δ = 2.21 (s, 3H, CH3CO), 3.85 (s, 3H, OCH3), 7.53-8.03 (m, 8H, Ar-H), 8.70 (s, 1H, pyridazinyl H-5) and 10.19 (s, 1H, NH) ppm; MS: (M+) 425; Anal. Calcd. for C21H16N3O5Cl (425.83): C, 59.23; H, 3.79; N, 9.87; Found: C, 59.35; H, 3.70; N, 9.96.
4-Acetylamino-6-acethyl-2-(2-cyanophenyl)-2-hydropyridazin-3-one (17d). Dark brown crystals (from ethanol); m.p. 244 ºC; IR νmax cm-1: 3323 (NH), 3107 (CH aromatic), 2900 (CH aliphatic), 2234 (C≡N), 1702 (C=O ketone), 1656 (C=O amide) and 1608 (C=O pyridazine ring); 1H-NMR: δ = 2.26, 2.49 (s, 3H, 2CH3CO), 7.73-8.15 (m, 4H, Ar-H), 8.64 (s, 1H, pyridazinyl H-5) and 10.33 (s, 1H, disappeared after D2O exchange, NH) ppm; MS: (M+) 296; Anal. Calcd. for C15H12N4O3 (296.29): C, 60.81; H, 4.08; N, 18.91; Found: C, 60.91; H, 4.25; N, 18.75.
4-Acetylamino-2-(2-cyanophenyl)-6-(2-furyl carbonyl)-2-hy dropyridazin-3-one (17e). Brown crystals (from ethanol); m.p. 242 ºC; IR νmax cm-1: 3295 (NH), 3037 (CH aromatic), 2236 (C≡N), 1714 (C=O ketone), 1649 (C=O amide) and 1620 (C=O pyridazine ring); 1H-NMR: δ = 2.28 (s, 3H, CH3), 6.76 (m, 1H, furyl H-4), 7.64, 8.13 (d, 2H, furyl H-3, H-5), 7.74-8.05 (m, 4H, Ar-H), 8.70 (s, 1H pyridazinyl H-5) and 10.41 (s, 1H, NH) ppm; MS: (M+) 348; Anal. Calcd. for C18H12N4O4 (348.32): C, 62.07; H, 3.47; N, 16.08; Found: C, 62.25; H, 3.40; N, 16.32.
4-Acetylamino-6-(4-chlorophenylcarbonyl)-2-(2-cyanophenyl)-2-hydropyridazin-3-one (17f). Brown crystals (from 1:1 ethanol/dioxane); m.p. 252 ºC; IR νmax cm-1: 3225 (NH), 3050 (CH aromatic), 2220 (CN), 1718 (C=O ketone), 1640 (C=O amide) and 1635 (C=O pyridazine ring); MS: (M+) 392; Anal. Calcd. for C20H13N4O3Cl (392.80): C, 61.16; H, 3.34; N, 14.26; Found: C, 61.25; H, 3.30; N, 14.29.
6-Acetyl-4-Benzoylamino-2,3-dihydro-2-(2-methoxycarbonylphenyl)pyridazin-3-one (17g). Brown crystals (from ethanol); m.p. 170 ºC; IR νmax cm-1: 3339 (NH), 3077 (CH aromatic), 1799 (C=O ester), 1750 (C=O ketone), 1690 (C=O amide) and 1640 (C=O pyridazine ring); MS: (M+) 391; Anal. Calcd. for C21H17N3O5 (391.39): C, 64.45; H, 4.38; N, 10.74; Found: C, 64.55; H, 4.28; N, 10.80.
General Procedure for the reaction of 2-arylhydrazono derivatives with dimethyl acetylenedicarboxylate (DMAD):
Method I (Δ): To stirred solution of triphenylphosphine (0.1 mol) and each of compound 6b,e (0.1 mol) in dichloroethane (10 mL) was added a few drops of DMAD solution (0.1 mol) in dichloroethane (10 mL) then left at room temperature overnight. The solvent was removed and the residue cooled to deposit a solid, which was crystallized from the appropriate solvent.
Table 2.
Comparison between microwave and conventional heating reactions.
Table 2.
Comparison between microwave and conventional heating reactions.
| Product | Time/min | Yield% |
|---|
| ∆ With Solvent | ∆ Without Solvent | μω | ∆ With Solvent | ∆ Without Solvent | μω |
|---|
| 2a | 2100 | - | - | - | - | - |
| 2b | 420 | 480 | 3 | 79 | 85 | 96 |
| 2c | 420 | 480 | 5 | 89 | 90 | 97 |
| 2d | 420 | 480 | 5 | 60 | 77 | 96 |
| 2e | 420 | 480 | 1 | 75 | 88 | 99 |
| 2f | 420 | 480 | 1 | 80 | 87 | 98 |
| 2g | 420 | 480 | 5 | 61 | 88 | 97 |
| 9a | 120 | - | 5 | 65 | - | 70 |
| 9b | 120 | - | 15 | 70 | - | 75 |
| 9c | 120 | - | 5 | 54 | - | 65 |
| 9d | 120 | - | 7 | 40 | - | 95 |
| 9e | 120 | - | 15 | 60 | - | 99 |
| 9f | 120 | - | 7 | 55 | - | 73 |
| 9g | 120 | - | 15 | 73 | - | 80 |
| 9h | 120 | - | 10 | 60 | - | 99 |
| 9i | 120 | - | 10 | 56 | - | 99 |
| 9j | 120 | - | 10 | 95 | - | 98 |
| 9k | 120 | - | 10 | 46 | - | 60 |
| 9l | 120 | - | 5 | 50 | - | 70 |
| 9m | 120 | - | 15 | 65 | - | 70 |
| 12a | 180 | - | 2 | 30 | - | 70 |
| 12b | 180 | - | 2 | 50 | - | 87 |
| 14a | 180 | - | 2 | 16 | - | 25 |
| 14b | 180 | - | - | 40 | - | - |
| 17a | 60 | - | - | 20 | - | - |
| 17b | 60 | - | - | 80 | - | - |
| 17c | 60 | - | 15 | 25 | - | 35 |
| 17d | 60 | - | 5 | 25 | - | 40 |
| 17e | 60 | - | - | 61 | - | - |
| 17f | 60 | - | - | 25 | - | - |
| 17g | 60 | - | 10 | 35 | - | 50 |
| 22a | 1440 | - | - | 27 | - | - |
| 22b | 1440 | - | 30 | 24 | - | 60 |
Method II (μω): Each of compounds 6b,e (0.1 mol) and triphenylphosphine (0.1 mol) and a few drops of DMAD solution (0.1 mol), was placed in the microwave oven and irradiated at 390 W for 10-30 min., then left to cool to room temperature and the solid was collected and crystallized from the appropriate solvent.
2,3-Dihydro-3,4-dimethoxycarbonyl-6-(2-furylcarbonyl)-2-(2-methoxycarbonylphenyl)pyridazine (22a). Yellow crystals (from ethanol); m.p. 141 ºC; IR: νmax cm-1: 3305 (NH), 3077 (CH aromatic), 2962 (CH aliphatic), 1780, 1750 (3C=O ester) and 1681 (C=O ketone); 1H-NMR: δ = 3.55, 3.70, 3.89 (s, 3H, 3CH3), 6.01 (s, 1H, pyridazine H-3), 6.48 (m, 1H, furyl H-4), 7.36-7.65 (m, 4H, Ar-H), 7.47, 7.77 (d, 2H, furyl H-3, H-5) and 7.83 (s, 1H, pyridazine H-5) ppm; 13C-NMR: δ = 52.24, 52.68, 53.20 (3COOCH3), 57.88, 126.88, 141.01, 149.94 (pyridazine carbons), 112.29, 117.20, 121.94, 131.04, 132.34, 144.40 (C6H4CO2Me-o), 122.35, 123.91, 126.89, 147.20 (furyl carbons), 164.84, 167.03, 168.70 (3COOCH3) and 174.63 (C=O) ppm; MS: (M+) 426; Anal. Calcd. for C21H18N2O8 (426.39): C, 59.16; H, 4.26; N, 6.57; Found: C, 59.10; H, 4.39; N, 6.69.
2-(2-Cyanophenyl)-2,3-dihydro-3,4-dimethoxycarbonyl-6-acetylpyridazine (22b). Yellow crystals (from ethanol); m.p. 175 ºC; IR: νmax cm-1: 3415 (NH), 3013 (CH aromatic), 2959 (CH alephatic), 2221 (C≡N), 1746, 1717 (2C=O ester) and 1673 (C=O ketone); 1H-NMR: δ = 2.20 (s, 3H, CH3), 3.67, 3.76 (s, 3H, 2OCH3), 6.20 (s, 1H, pyridazine H-3), 6.61-7.32 (m, 4H, Ar-H) and 7.80 (s, 1H, pyridazine H-5) ppm; 13C-NMR: δ = 19.80 (CH3), 50.55, 50.81 (2CO-OCH3), 62.80, 126.85, 142.20, 150.45 (pyridazine carbons), 101.32, 113.00, 117.62, 132.81, 133.52, 147.82 (C6H4CN-o), 116.50 (CN), 165.05, 171.20 (2COOCH3) and 196.56 (C=O) ppm; MS: (M++1) 342; Anal. Calcd. For C17H15N3O5 (341.33): C, 59.82; H, 4.43; N, 12.31; Found: C, 59.98; H, 4.35; N, 12.48.