Methyl Mercapturate Synthesis: An Efficient, Convenient and Simple Method
Abstract
:Introduction
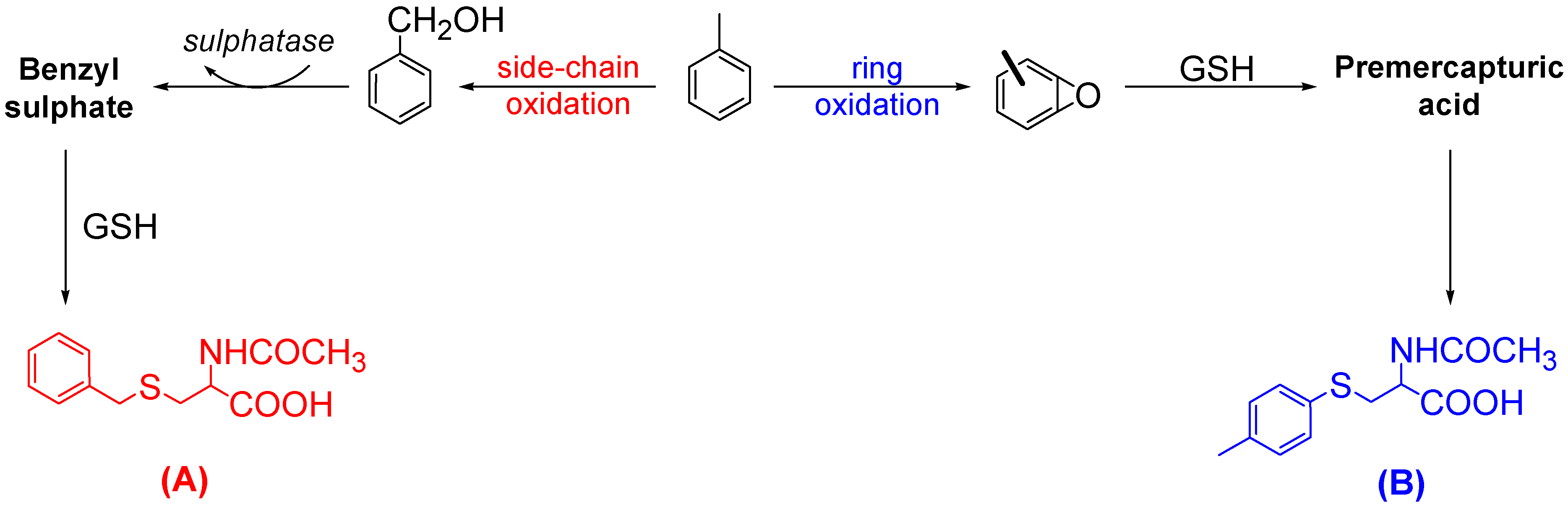


Results and Discussion
Methyl 2-acetamidoacrylate synthesis (MAA; 2)
| Entry | Cs2CO3 (equiv) | CH3I (equiv) | Time (h) | Yield of 2a (%) |
|---|---|---|---|---|
| 1 | 0.5 | 1.2 | 15 | 50 |
| 2 | 0.5 | 2.0 | 15 | 65 |
| 3 | 0.5 | 2.5 | 15 | 76 |
| 4 | 0.5 | 2.5 | 3 | 99.7 |
Methyl S-p-toluylmercapturate (3a) synthesis: optimisation
| Entry | p-Thiocresol (mmol) | Base (mmol) | PTC (mmol) | Solvent | Time (h) | %Yield for 3a |
|---|---|---|---|---|---|---|
| 1 | 1 | NaOH 1M/H2O | TBAHS (0.2) | CH2Cl2/H2O | 48 | 100% of 2 |
| 2 | 1 | NaOH (1.4) | TBAHS (0.2) | THF | 48 | 1 |
| 3 | 1 | KOH (1.2) | TBAHS (0.2) | THF | 18 | 22 |
| 4 | 1 | KOH (1.4) | TBAHS (0.2) | Acetonitrile | 18 | 22 |
| 5 | 1 | KOH (1.2) | TBAHS (0.2) | Toluene | 18 | 33 |
| 6 | 1 | KOH (1.2) | CTAB (0.2) | Toluene | 48 | 31 |
| 7 | 1 | KOH (1.2) | CTAB (0.2) | THF | 18 | 49 |
| 8 | 1 | KOH (1.2) | Aliquat 336 (0.08) | THF | 18 | 6 |
| 9 | 1 | K2CO3 (0.3) | Aliquat 336 (0.08) | THF | 18 | 63 |
| 10 | 1 | K2CO3 (0.3) | Aliquat 336 (0.08) | Toluene | 18 | 61 |
| 11 | 1.4 | K2CO3 (0.3) | Aliquat 336 (0.08) | Toluene | 5 | 99.6 |
Application to the synthesis of various AMEs (3b-m)
| Entry | AMEa | Solvent | Time (h) | RFc | Yieldd (%) | |
|---|---|---|---|---|---|---|
| 1 |  | 3a | Tol | 5 | 0.28 | 99.6 |
| 2 |  | 3b | Tol | 5 | 0.31 | 99 |
| 3 |  | 3c | Tol | 5 | 0.29 | 94 |
| 4 |  | 3d | Tol | 5 | 0.32 | 96 |
| 5 |  | 3e | Tol | 5 | 0.32 | 95 |
| 6 |  | 3f | Tol | 13 | 0.32 | 90 |
| 7 |  | 3g | Tol | 18 | 0.26 | 82 |
| 8 |  | 3h | Tol | 18 | 0.31 | 98 |
| 9 |  | 3i | Tol | 5 | 0.26 | 96 |
| 10 |  | 3j | Tol/THFb | 18 | 0.27 | 76 |
| 11 |  | 3k | Tol | 5 | 0.25 | 88 |
| 12 |  | 3l | Tol | 18 | 0.23 | 76 |
| 13 |  | 3m | Tol/THFb | 18 | 0.26 | 71 |
Conclusions
Experimental
General
Methyl 2-acetamidoacrylate synthesis (MAA; 2)
General procedure for methyl S-arylmercapturate (AME) synthesis: compounds 3a-m
Acknowledgements
References and Notes
- Lauwerys, R.R.; Haufroid, V.; Hoet, P.; Lison, D. Toxicologie industrielle et intoxications professionnelles; Elsevier Masson, S.A.S., Ed.; Issy-les-Moulineaux: (France), 2007; pp. 528–557. [Google Scholar]
- Lataye, R.; Campo, P.; Pouyatos, B.; Cossec, B.; Blachere, V.; Morel, G. Solvent ototoxicity in the rat and guinea pig. Neurotox. Teratol. 2003, 25, 39–50. [Google Scholar] [CrossRef]
- Gagnaire, F.; Marignac, B.; Langlais, C.; Bonnet, P. Ototoxicity in rats exposed to ortho-, meta- and para-xylene vapours for 13 weeks. Pharmacol. Toxicol. 2001, 89, 6–14. [Google Scholar] [CrossRef] [PubMed]
- Korsak, Z.; Rydzynski, K. Neurotoxic effects of acute and subchronic inhalation exposure to trimethylbenzene isomers (pseudocumene, mesitylene, hemimellitene) in rats. Int. J. Occup. Med. Environ. Health. 1996, 9, 341–349. [Google Scholar] [PubMed]
- Vermeulen, N.P.E. Analysis of mercapturic acids as a tool in biotransformation, biomonitoring and toxicological studies. Trends Pharmacol. Sci. 1989, 10, 177–181. [Google Scholar] [CrossRef]
- Nelson, E.D. Determination of mercapturic acid and excretions in exposure control to toxicants. Crit. Rev. Toxicol. 1992, 22, 371–389. [Google Scholar] [CrossRef] [PubMed]
- De Rooij, B.M.; Commandeur, J.N.M.; Vermeulen, N.P.E. Mercapturic acids as biomarkers of exposure to electrophilic chemicals: applications to environmental and industrial chemicals. Biomarkers 1998, 3, 239–303. [Google Scholar]
- Angerer, J.; Ewers, U.; Wilhelm, M. Human biomonitoring: State of the art. Int. J. Hyg. Environ. Health 2007, 210, 201–228. [Google Scholar] [CrossRef] [PubMed]
- Perbellini, L.; Veronese, N.; Princivalle, A. Mercapturic acids in the biological monitoring of occupational exposure to chemicals. J. Chrom. B Analyt. Technol. Biomed. Life Sci. 2002, 781, 269–290. [Google Scholar] [CrossRef]
- Haufroid, V.; Lison, D. Mercapturic acids revisited as biomarkers of exposure to reactive chemicals in occupational toxicology: a minireview. Int. Arch. Occup. Environ. Health 2005, 78, 343–354. [Google Scholar] [CrossRef] [PubMed]
- Van Doorn, R.; Leijdekkers, C.M.; Bos, R.P.; Brouns, R.M.E.; Henderson, P.T. Alcohol and sulphate intermediates in the metabolism of toluene and xylenes to mercapturic acids. J. Appl. Toxicol. 1981, 1, 236–242. [Google Scholar] [CrossRef] [PubMed]
- Zheng, J.; Hanzlik, R.P. Bromo(monohydroxy)phenyl mercapturic acids from bromobenzene-treated rats. Drug Metabol. Disp. 1992, 20, 688–694. [Google Scholar]
- Angerer, J.; Schildbach, M.; Kramer, A. S-p-Toluylmercapturic acid in the urine of workers exposed to toluene: a new biomarker for toluene exposure. Arch. Toxicol. 1998, 72, 119–123. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-Reche, L.M.; Schettgen, T.; Angerer, J. New approaches to the metabolism of xylenes: verification of the formation of phenylmercapturic acid metabolites of xylenes. Arch. Toxicol. 2003, 77, 80–85. [Google Scholar] [PubMed]
- Boogaard, P.J.; Van Sittert, N.J. Biological monitoring of exposure to benzene – A comparison between S-phenylmercapturic acid, trans,trans-muconic acid and phenol. Occup. Environ. Med. 1995, 52, 611–620. [Google Scholar] [CrossRef] [PubMed]
- Inoue, O.; Kanno, E.; Kasai, K.; Ukai, H.; Okamoto, S.; Ikeda, M. Benzylmercapturic acid is superior to hippuric acid and o-cresol as a urinary marker of occupational exposure to toluene. Toxicol. Lett. 2004, 147, 177–186. [Google Scholar] [CrossRef] [PubMed]
- American Conference of Governmental Industrial Hygienists. Threshold Limit Values and Biological Exposure Indices, 1998.
- Deutsche Forschungsgemeinschaft. List of MAK and BAT values. Commission for the investigation of health hazards of chemical compounds in the work area, Report N° 32, VCH: New-York, 1998.
- Van Bladeren, P.J.; Buys, W.; Breimer, D.D.; Van der Gen, A. The Synthesis of Mercapturic Acids and their Esters. Eur. J. Med. Chem. 1980, 15, 495–497. [Google Scholar]
- Hayden, P.; Schaeffer, V.H.; Larsen, G.; Stevens, J.L. Cysteine S-conjugates. Meth. Enzymol. 1987, 143, 228–234. [Google Scholar] [PubMed]
- Buijs, W.; Eid, M.I.A.; Onkenhout, W.; Vermeulen, N.P.E. The use of sulfenyl halides in the synthesis of mercapturic acids and their esters. Recl. Trav. Chim. Pays-Bas 1986, 105, 449–455. [Google Scholar] [CrossRef]
- West, H.D.; Mathura, G.R. Synthesis of some aryl-substituted L-cysteines and their fate in the animal body. J. Biol. Chem. 1954, 208, 315–318. [Google Scholar] [PubMed]
- Parke, D.V.; Williams, R.T. Studies in detoxication 38. The metabolism of benzene: the determination of phenylmercapturic acid in urine; mercapturic acid excretion by rabbits receiving benzene. Biochem. J. 1951, 48, 624–629. [Google Scholar] [CrossRef] [PubMed]
- Ciattini, P.G.; Morera, E.; Ortar, G. A new, palladium-catalyzed synthesis of aromatic mercapturic acid derivatives. Tetrahedron Lett. 1995, 36, 4133–4136. [Google Scholar] [CrossRef]
- Hickman, R.J.S.; Christie, B.J.; Guy, R.W.; White, T.J. Synthesis of aromatic S-substituted derivatives of N-acetyl-L-cysteine. Aust. J. Chem. 1985, 38, 899–904. [Google Scholar] [CrossRef]
- Kondoh, A.; Yorimitsu, H.; Oshima, K. Nucleophilic aromatic substitution reaction of nitroarenes with alkyl- or arylthio groups in dimethyl sulfoxide by means of cesium carbonate. Tetrahedron 2006, 62, 2357–2360. [Google Scholar] [CrossRef]
- Pombrio, J.M.; Giangreco, A.; Li, L.Q.; Wempe, M.F.; Anders, M.W.; Swett, D.H.; Pritchard, J.B.; Ballatori, N. Mercapturic acids (N-acetylcysteine S-conjugates) as endogenous substrates for the renal organic anion transporter-1. Mol. Pharmacol. 2001, 60, 1091–1099. [Google Scholar] [PubMed]
- Saxena, M.; Henderson, G.B. MOAT4, a novel multispecific organic-anion transporter for glucuronides and mercapturates in mouse L1210 cells and human erythrocytes. Biochem. J. 1996, 320, 273–281. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, S.; Goto, K.; Kondo, M.; Naito, S.; Tsuda, Y.; Shishido, K. Enantioselective total synthesis and structure determination of the mercapturic acid sulfoxide conjugate. Bioorg. Med. Chem. Lett. 1997, 7, 2033–2036. [Google Scholar] [CrossRef]
- Behringer, H.; Fackler, E. Eine einfache synthese der racemischen Mercaptursäuren. Justus Liebigs Ann. Chem. 1949, 564, 73–78. [Google Scholar] [CrossRef]
- Hanzlik, R.P.; Weller, P.E.; Desai, J.; Zheng, J.; Hall, L.R.; Slaughter, D.E. Synthesis of mercapturic acid-derivatives of putative toxic metabolites of bromobenzene. J. Org. Chem. 1990, 55, 2736–2742. [Google Scholar] [CrossRef]
- Enders, D.; Lüttgen, K.; Narine, A.A. Asymmetric sulfa-Michael additions. Synthesis 2007, 7, 959–980. [Google Scholar] [CrossRef]
- Montanari, F.; Landini, D.; Rolla, F. Phase transfer catalyzed reactions. Top. Curr. Chem. 1982, 101, 147–200. [Google Scholar]
- Rothstein, E. Experiments in the synthesis of derivatives of α,α-aminoacrylic acid from serine and N-substituted serines. J. Chem. Soc. 1949, 1968–1971. [Google Scholar] [CrossRef]
- Cardillo, G.; Gentilucci, L.; Tolomelli, A.; Tomasini, C. Asymmetric 1,4 addition of Grignard reagents to chiral alpha,beta-unsaturated esters in the presence of Lewis acids. Tetrahedron 1999, 55, 6231–6242. [Google Scholar] [CrossRef]
- Bueno, M.P.; Cativiela, C.; Finol, C.; Mayoral, J.A.; Jaime, C. Diels-Alder reactions of methyl-N-acyl-alpha,beta-dehydroalaninates with cyclopentadiene. Can. J. Chem. 1987, 65, 2182–2186. [Google Scholar] [CrossRef]
- Roper, R.; Ma, T.S. Diazomethane as a reagent for microsynthesis. Microchemical J. 1957, 1, 245–260. [Google Scholar] [CrossRef]
- Kolar, A.J.; Olsen, R.K. Convenient, large-scale preparation of 2-acetamidoacrylic acid and its methyl ester. Synthesis 1977, 457, 457–459. [Google Scholar] [CrossRef]
- Rich, D.H.; Tam, J.P. Synthesis of didehydropeptides from peptides containing 3-alkylthio-amino acid residues. Tetrahedron Lett. 1975, 211–212. [Google Scholar] [CrossRef]
- Srivastava, V.P.; Roberts, M.; Holmes, T.; Stammer, C.H. Synthesis of (+/-) 2,3-methanovaline and (+/-)-2,3-methanoleucine. J. Org. Chem. 1989, 54, 5866–5870. [Google Scholar] [CrossRef]
- Placidi-Rampont, V. Synthèse, séparation et analyse de métabolites soufrés du toluène. PhD Thesis, Henri Poincaré University Nancy 1, Nancy, France, 1997. [Google Scholar]
- Bastien, C. Synthèse et identification de métabolites soufrés du toluène; DEA report; Henri Poincaré University Nancy 1: Nancy, France, 2000. [Google Scholar]
- Wang, S.S.; Gisin, B.F.; Winter, D.P.; Makofske, R.; Kulesha, I.D.; Tzougraki, C.; Meienhofer, J. Facile synthesis of amino-acid and peptide esters under mild conditions via cesium salts. J. Org. Chem. 1977, 42, 1286–1290. [Google Scholar] [CrossRef]
- Chidambaran, M.; Sonavane, S.U.; de la Zerda, J.; Sasson, Y. Didecyldimethylammonium bromide (DDAB): a universal, robust, and highly potent phase-transfer catalyst for diverse organic transformations. Tetrahedron 2007, 63, 7696–7701. [Google Scholar] [CrossRef]
- Makosza, M. Phase-transfer catalysis. A general green methodology in organic synthesis. Pure Appl. Chem. 2000, 72, 1399–1403. [Google Scholar] [CrossRef]
- Sirovski, F.; Reichardt, C.; Garokhova, M.; Ruban, S.; Stoikova, E. Solid liquid phase-transfer catalysis. Some models and solvent influence. Tetrahedron 1999, 55, 6363–6374. [Google Scholar] [CrossRef]
- Yang, H.M.; Lin, C.L. Phase-transfer catalyzed benzylation of sodium benzoate using aliquat 336 as catalyst in liquid-liquid system. J. Mol. Catal. A: Chem. 2003, 206, 67–76. [Google Scholar] [CrossRef]
- Lopez, A.; Moreno-Manas, M.; Pleixats, R.; Roglans, A.; Ezquerra, J.; Pedregal, C. Ethyl N-(diphenylmethylene)glycinate as anionic glycine equivalent. Monoalkylation, dialkylation and Michael additions under solid-liquid phase-transfer catalysis. Tetrahedron 1996, 52, 8385–8386. [Google Scholar] [CrossRef]
- Herriott, A.W.; Picker, D. Phase transfer catalysis – evaluation of catalysts. J. Am. Chem. Soc. 1975, 97, 2345–2349. [Google Scholar] [CrossRef]
- Superchi, S.; Nardiello, M.; Donnoli, M.I.; Scafato, P.; Menicagli, R.; Rosini, C. Enantioselective synthesis of the fragrance trans-magnolione under asymmetric phase transfer catalysis. C.R. Chimie 2005, 8, 867–874. [Google Scholar] [CrossRef]
- Sample Availability: Samples of the compounds mentioned in this paper are available from the corresponding author (B.C.)
© 2008 by the authors. Licensee Molecular Diversity Preservation International, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Cossec, B.; Cosnier, F.; Burgart, M. Methyl Mercapturate Synthesis: An Efficient, Convenient and Simple Method. Molecules 2008, 13, 2394-2407. https://doi.org/10.3390/molecules13102394
Cossec B, Cosnier F, Burgart M. Methyl Mercapturate Synthesis: An Efficient, Convenient and Simple Method. Molecules. 2008; 13(10):2394-2407. https://doi.org/10.3390/molecules13102394
Chicago/Turabian StyleCossec, Benoît, Frédéric Cosnier, and Manuella Burgart. 2008. "Methyl Mercapturate Synthesis: An Efficient, Convenient and Simple Method" Molecules 13, no. 10: 2394-2407. https://doi.org/10.3390/molecules13102394
APA StyleCossec, B., Cosnier, F., & Burgart, M. (2008). Methyl Mercapturate Synthesis: An Efficient, Convenient and Simple Method. Molecules, 13(10), 2394-2407. https://doi.org/10.3390/molecules13102394