Pimarane-type Diterpenes: Antimicrobial Activity against Oral Pathogens
Abstract
:Introduction
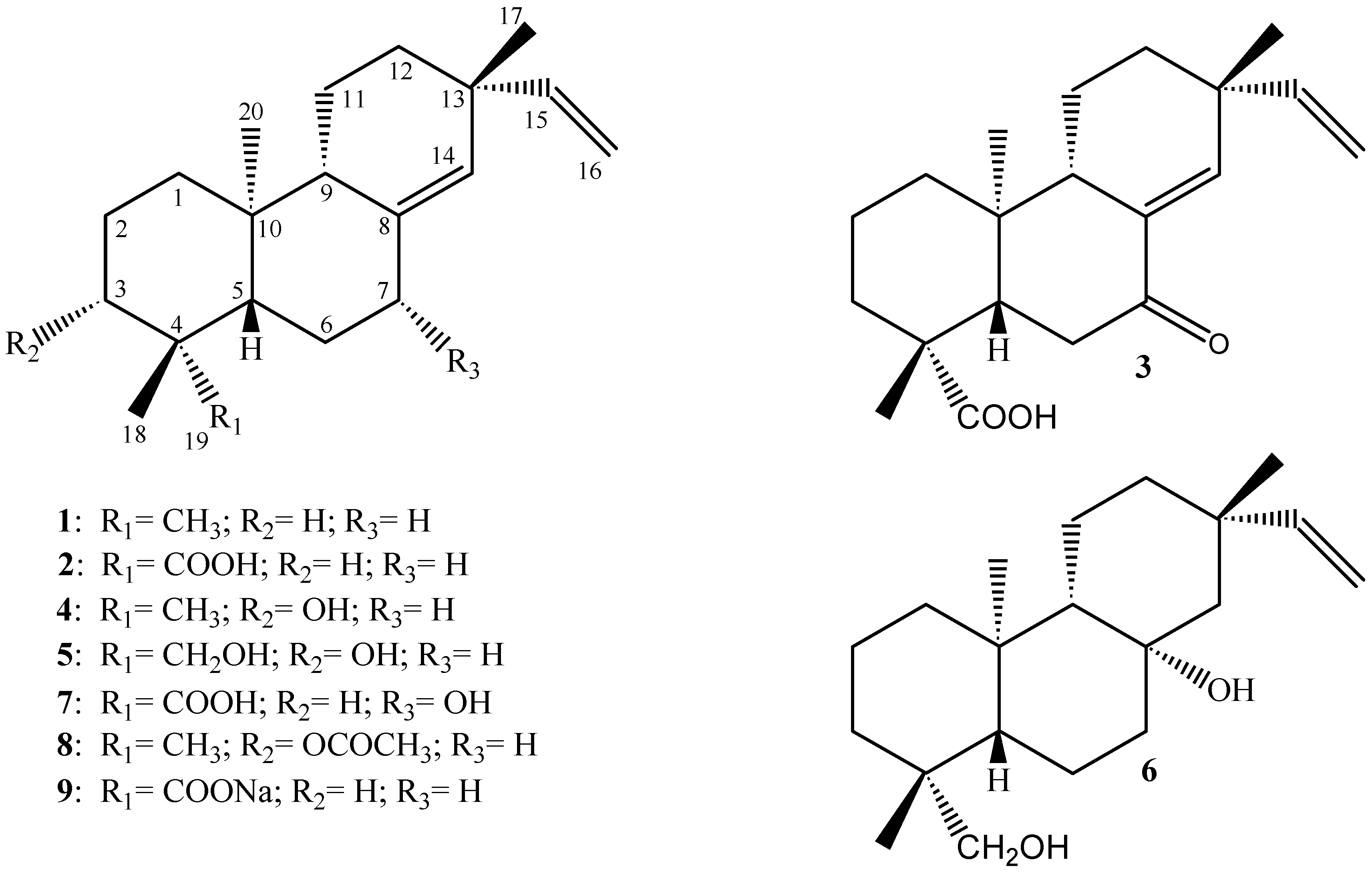
Results and Discussion
| Compound | Minimum inhibitory concentration – μg∙mL-1 (μM) | |||||
|---|---|---|---|---|---|---|
| Microorganism | ||||||
| L. casei | S. mitis | S. mutans | S. sanguinis | S. sobrinus | S. salivarius | |
| VaDRE | 12.0 | 10.0 | 12.0 | 10.0 | 10.0 | 10.0 |
| 1 | * | * | * | * | * | * |
| 2 | 3.0 (9.9) | 4.0 (13.2) | 4.5 (14.9) | 2.5 (8.3) | 4.0 (13.2) | 5.0 (16.5) |
| 3 | * | * | * | * | * | * |
| 4 | 2.5 (8.7) | 4.0 (13.9) | 2.5 (8.7) | 4.5 (15.6) | 6.0 (20.8) | 4.0 (13.9) |
| 5 | * | * | * | * | * | * |
| 6 | 6.0 (19.6) | 4.0 (13.1) | 6.0 (19.6) | 6.0 (19.6) | 4.0 (13.1) | 3.0 (7.8) |
| 7 | * | 16.0 (50.2) | 20.0 (62.8) | * | 16.0 (50.2) | * |
| 8 | 6.0 (18.2) | 8.0 (24.2) | 6.0 (18.2) | 6.0 (18.2) | 6.0 (18.2) | 8.0 (24.2) |
| 9 | 2.0 (6.2) | 3.0 (9.3) | 2.5 (7.7) | 2.5 (7.7) | 4.0 (12.3) | 3.5 (10.8) |
| PC | 0.0922 (0.16) | 0.3688 (0.64) | 0.0922 (0.16) | 0.7375 (1.27) | 0.0922 (0.16) | 0.0922 (0.16) |

Conclusions
Experimental
General
Plant material
Extraction and isolation
Semi-synthetic derivatives
Purity of the evaluated diterpenes
Antimicrobial assays
Acknowledgements
References
- Allaker, R.P.; Douglas, C.W.I. Novel anti-microbial therapies for dental plaque-related diseases. Int. J. Antimicrob. Agents 2008. [Google Scholar]
- Ambrosio, S.R.; Furtado, N.A.J.C; De Oliveira, D.C.R.; Da Costa, F. B.; Martins, C.H.G.; De Carvalho, T.C.; Porto, T.S.; Veneziani, R.C.S. Antimicrobial activity of kaurane diterpenes against oral pathogens. Z. Naturforsch. 2008, 63c, 326–330. [Google Scholar]
- Chung, J.Y.; Choo, J.H.; Lee, M.H.; Hwang, J.K. Anticariogenic activity of macelignan isolated from Myristica fragrans (nutmeg) against Streptococcus mutans. Phytomedicine 2006, 13, 261–266. [Google Scholar] [CrossRef]
- Hirasawa, M.; Takada, K. Susceptibility of Streptococcus mutans and Streptococcus sobrinus to cell wall inhibitors and development of a novel selective medium for S. sobrinus. Caries Res. 2002, 36, 155–160. [Google Scholar] [CrossRef]
- Haffajee, A.D.; Yaskell, T.; Socransky, S.S. Antimicrobial effectiveness of an herbal mouthrinse compared with an essential oil and a chlorhexidine mouthrinse. J. Am. Dent. Assoc. 2008, 139, 606–611. [Google Scholar]
- More, G.; Tshikalange, T.E.; Lall, N.; Botha, F.; Meyer, J.J.M. Antimicrobial activity of medicinal plants against oral microorganisms. J. Ethnopharmacol. 2008, in press. [Google Scholar]
- Cai, L.; Wu, C.D. Compounds from Syzygium aromaticum possessing growth inhibitory activity against oral pathogens. J. Nat. Prod. 1996, 59, 987–990. [Google Scholar] [CrossRef]
- Tsui, V.W.K.; Wong, R.W.K.; Rabie, A.B.M. The inhibitory effects of narigin on the growth of periodontal pathogens in vitro. Phytother. Res. 2008, 22, 401–406. [Google Scholar] [CrossRef]
- Fabricant, D.S.; Farnsworth, N.R. The value of plants used in traditional medicine for drug discovery. Environ. Health. Persp. 2001, 109, 69–75. [Google Scholar]
- Balunas, M.J.; Kinghorn, A.D. Drug discovery from medicinal plants. Life Sci. 2005, 78, 431–441. [Google Scholar] [CrossRef]
- Katsura, H.; Tsukiyama, R.I.; Suzuki, A.; Kobayashi, M. In vitro antimicrobial activities of bakuchiol against oral microorganisms. Antimicrob. Agents Chemother. 2001, 45, 3009–3013. [Google Scholar] [CrossRef]
- Mihashi, S. Further study on the diterpenes of Aralia spp. Tetrahedron Lett. 1969, 21, 1683–1686. [Google Scholar] [CrossRef]
- Matsuo, A.; Uto, S.; Nakayama, M.; Hayashi, S.; Yamasaki, K.; Kasai, R.; Tanaka, H. Thermarol, a new ent-pimarane-class diterpene diol from Jungermannia thermarum (Liverwort). Tetrahedron Lett. 1976, 28, 2451–2454. [Google Scholar]
- Ansell, S.M.; Pegel, K.H.; Taylor, D.A.H. Diterpenes from the timber of 20 Erythroxylum species. Phytochemistry 1993, 32, 953–959. [Google Scholar] [CrossRef]
- Garcia, E.E.; Guerreiro, E.; Joseph-Nathan, P. Ent-pimaradiene diterpenes from Gochnatia glutinosa. Phytochemistry 1985, 24, 3059–3060. [Google Scholar] [CrossRef]
- Ríos, J.L.; Recio, M.C. Medicinal plants and antimicrobial activity. J. Ethnopharmacol. 2005, 100, 80–84. [Google Scholar] [CrossRef]
- Eley, B.M. Antibacterial agents in the control of supragingival plaque – a review. Br. Dent. J. 1999, 186, 286–296. [Google Scholar]
- Mascarenhas, A.K.; Allen, C.M.; Loudon, J. The association between Viadent use and oral leukoplakia. Epidemiology 2001, 12, 741–743. [Google Scholar] [CrossRef]
- Urzúa, A.; Rezende, M.C.; Mascayano, C.; Vásquez, L. A structure-activity study of antibacterial diterpenoids. Molecules 2008, 13, 882–891. [Google Scholar] [CrossRef]
- Tsuchiya, H.; Sato, M.; Iinuma, M.; Yokoyama, J.; Tanaka, T.; Takase, I.; Namikawa, I. Inhibition of the growth of cariogenic bacteria in vitro by plant flavanones. Experientia 1994, 50, 846–849. [Google Scholar] [CrossRef]
- Cunha, S.L.C.; Silva, M.L.A.; Furtado, N.A.J.C.; Vinhólis, A.H.C.; Martins, C.H.G; Silva-Filho, A.A.; Cunha, W.R. Antibacterial activity of triterpene acids and semi-synthetic derivatives against oral pathogens. Z. Naturforsch. 2007, 62c, 668–672. [Google Scholar]
- Ambrosio, S.R.; Schorr, K.; Da Costa, F. B. Terpenoids of Viguiera arenaria (Asteraceae). Biochem. Syst. Ecol. 2004, 32, 221–224. [Google Scholar] [CrossRef]
- Ambrosio, S.R.; Arakawa, N.S.; Esperandim, V.R.; de Albuquerque, S.; Da Costa, F.B. Trypanocidal activity of pimarane diterpenes from Viguiera arenaria (Asteraceae). Phytother. Res. 2008, 22, 1413–1415. [Google Scholar] [CrossRef]
- Da Costa, F.B.; Vichnewiski, W.; Herz, W. Diterpenes and synthetic derivatives from Viguiera aspillioides with trypanomicidal activity. Planta Med. 1996, 62, 557–559. [Google Scholar] [CrossRef]
- Daló, N.L.; Sosa-Sequera, M.C.; Usubillaga, A. On the anticonvulsant activity of kaurenic acid. Invest. Clin. 2007, 48, 349–358. [Google Scholar]
- National Committee for Clinical Laboratory Standards. NCCLS document M7-A6 - Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically; NCCLS: Wayne, PA, 2003. [Google Scholar]
- Palomino, J.C.; Martin, A.; Camacho, M.; Guerra, H.; Swings, J.; Portaels, S. Resazurin microtiter assay plate: simple and inexpensive method for detection of drug resistence in Mycobacterium tuberculosis. Antimicrob. Agents Chem. 2002, 46, 2720–2722. [Google Scholar] [CrossRef]
- Sample Availability: Samples are not available.
© 2009 by the authors; licensee Molecular Diversity Preservation International, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Porto, T.S.; Rangel, R.; Furtado, N.A.J.C.; De Carvalho, T.C.; Martins, C.H.G.; Veneziani, R.C.S.; Da Costa, F.B.; Vinholis, A.H.C.; Cunha, W.R.; Heleno, V.C.G.; et al. Pimarane-type Diterpenes: Antimicrobial Activity against Oral Pathogens. Molecules 2009, 14, 191-199. https://doi.org/10.3390/molecules14010191
Porto TS, Rangel R, Furtado NAJC, De Carvalho TC, Martins CHG, Veneziani RCS, Da Costa FB, Vinholis AHC, Cunha WR, Heleno VCG, et al. Pimarane-type Diterpenes: Antimicrobial Activity against Oral Pathogens. Molecules. 2009; 14(1):191-199. https://doi.org/10.3390/molecules14010191
Chicago/Turabian StylePorto, Thiago S., Rander Rangel, Niege A. J. C. Furtado, Tatiane C. De Carvalho, Carlos H. G. Martins, Rodrigo C. S. Veneziani, Fernando B. Da Costa, Adriana H. C. Vinholis, Wilson R. Cunha, Vladimir C. G. Heleno, and et al. 2009. "Pimarane-type Diterpenes: Antimicrobial Activity against Oral Pathogens" Molecules 14, no. 1: 191-199. https://doi.org/10.3390/molecules14010191
APA StylePorto, T. S., Rangel, R., Furtado, N. A. J. C., De Carvalho, T. C., Martins, C. H. G., Veneziani, R. C. S., Da Costa, F. B., Vinholis, A. H. C., Cunha, W. R., Heleno, V. C. G., & Ambrosio, S. R. (2009). Pimarane-type Diterpenes: Antimicrobial Activity against Oral Pathogens. Molecules, 14(1), 191-199. https://doi.org/10.3390/molecules14010191




