Antimicrobial Activity of Some Thiourea Derivatives and Their Nickel and Copper Complexes
Abstract
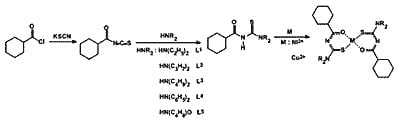
:Introduction
Results and Discussion

| Compound | S. aureus (ATCC 25923) | S. aureus (ATCC 29213) | S. epidermidis (ATCC 12228) | E. faecalis (ATCC 29212) | S. pyogenes (Clinical isolate) | B. cereus (Clinical isolate) |
|---|---|---|---|---|---|---|
| L1 | 200 | 200 | 100 | 400 | 200 | 200 |
| L2 | 400 | 200 | 200 | 200 | 200 | 100 |
| L3 | 200 | 400 | 200 | 200 | 400 | 200 |
| L4 | 100 | 100 | 100 | 100 | 200 | 100 |
| L5 | 400 | 200 | 100 | 100 | 200 | 200 |
| NiL12 | 200 | 200 | 200 | 200 | 200 | 200 |
| NiL22 | 200 | 200 | 200 | 200 | 100 | 200 |
| NiL32 | 200 | 400 | 100 | 200 | 100 | 200 |
| NiL42 | 400 | 400 | 100 | 200 | 100 | 100 |
| NiL52 | 200 | 200 | 200 | 400 | 200 | 100 |
| CuL12 | 100 | 200 | 100 | 200 | 400 | 100 |
| CuL22 | 100 | 200 | 200 | 200 | 400 | 100 |
| CuL32 | 200 | 400 | 200 | 200 | 400 | 200 |
| CuL42 | 200 | 200 | 200 | 400 | 400 | 200 |
| CuL52 | 50 | 100 | 100 | 200 | 200 | 100 |
| Amikacin | 2 | 2 | 0.5 | 4 | 1 | 2 |
| Compound | E. coli (ATCC 25922) | P. aeruginosa (ATCC 27853) | E. cloacae (ATCC 13047) | P. vulgaris (ATCC 13315) | E. aerogenes (Clinical isolate) |
|---|---|---|---|---|---|
| L1 | 400 | 400 | 400 | 200 | 200 |
| L2 | 200 | 400 | 200 | 200 | 400 |
| L3 | 200 | 400 | 200 | 200 | 200 |
| L4 | 200 | 400 | 200 | 200 | 400 |
| L5 | 200 | 400 | 400 | 200 | 400 |
| NiL12 | 200 | 400 | 200 | 200 | 400 |
| NiL22 | 200 | 400 | 200 | 200 | 200 |
| NiL32 | 400 | 400 | 400 | 200 | 400 |
| NiL42 | 400 | 400 | 400 | 400 | 200 |
| NiL52 | 200 | 400 | 400 | 400 | 400 |
| CuL12 | 200 | 400 | 400 | 400 | 200 |
| CuL22 | 400 | 400 | 200 | 200 | 200 |
| CuL32 | 400 | 400 | 200 | 100 | 400 |
| CuL42 | 400 | 400 | 400 | 200 | 400 |
| CuL52 | 200 | 400 | 400 | 400 | 200 |
| Gentamycin | 0.5 | 1 | 2 | 2 | 0.5 |
| Compound | C. albicans (ATCC 90028) | C. krusei (ATCC 6258) | C. glabrata (ATCC 32554) | C. tropicalis (ATCC 20336) | C. parapsilosis (Clinical isolate) |
|---|---|---|---|---|---|
| L1 | 50 | 50 | 50 | 50 | 50 |
| L2 | 50 | 50 | 25 | 25 | 100 |
| L3 | 100 | 100 | 25 | 25 | 50 |
| L4 | 25 | 50 | 50 | 50 | 50 |
| L5 | 50 | 50 | 50 | 50 | 50 |
| NiL12 | 25 | 50 | 50 | 100 | 25 |
| NiL22 | 25 | 50 | 25 | 50 | 50 |
| NiL32 | 100 | 100 | 50 | 100 | 50 |
| NiL42 | 50 | 25 | 25 | 50 | 100 |
| NiL52 | 50 | 50 | 50 | 50 | 50 |
| CuL12 | 25 | 50 | 25 | 50 | 50 |
| CuL22 | 25 | 50 | 25 | 25 | 25 |
| CuL32 | 25 | 50 | 50 | 50 | 50 |
| CuL42 | 50 | 25 | 25 | 25 | 50 |
| CuL52 | 25 | 25 | 25 | 25 | 25 |
| Nystatin | 1 | 0.5 | 2 | 4 | 4 |
Experimental
Synthesis
Antimicrobial activity
Acknowledgements
References
- Arslan, H.; Kulcu, N.; Florke, U. Synthesis and characterization of copper(II), nickel(II) and cobalt(II) complexes with novel thiourea derivatives. Transit. Metal Chem. 2003, 28, 816–819. [Google Scholar] [CrossRef]
- Mansuroglu, D.S.; Arslan, H.; Florke, U.; Kulcu, N. Synthesis and characterization of nickel and copper complexes with 2,2-diphenyl-N-(alkyl(aryl)carbamothioyl)acetamide: The crystal structures of HL1 and cis-[Ni(L-1)(2)]. J. Coord. Chem. 2008, 61, 3134–3146. [Google Scholar] [CrossRef]
- Ozer, C.K.; Arslan, H.; VanDerveer, D.; Binzet, G. Synthesis and characterization of N-(alky(aryl)carbamothioyl)cyclohexanecarboxamide derivatives and their Ni(II) and Cu(II) complexes. J. Coord. Chem. 2009, 62, 266–276. [Google Scholar] [CrossRef]
- Binzet, G.; Arslan, H.; Florke, U.; Kulcu, N.; Duran, N. Synthesis, characterization and antimicrobial activities of transition metal complexes of N,N-dialkyl-N'-(2-chloro-benzoyl)thiourea derivatives. J. Coord. Chem. 2006, 59, 1395–1406. [Google Scholar] [CrossRef]
- Ugur, D.; Arslan, H.; Kulcu, N. Synthesis, characterization and thermal behavior of 1,1-dialkyl-3-(4-(3,3-dialkylthioureidocarbonyl)benzoyl)thiourea and its Cu(II), Ni(II), and Co(II) complexes. Russ. J. Coord. Chem. 2006, 32, 669–675. [Google Scholar] [CrossRef]
- Emen, M.F.; Arslan, H.; Kulcu, N.; Florke, U.; Duran, N. Synthesis, characterization and antimicrobial activities of some metal complexes with N '-(2-chloro-benzoyl)thiourea ligands: The crystal structure of fac-[CoL3] and cis-[PdL2]. Pol. J. Chem. 2005, 79, 1615–1626. [Google Scholar]
- Arslan, H.; Florke, U.; Kulcu, N.; Emen, M.F. Crystal structure and thermal behaviour of copper(II) and zinc(II) complexes with N-pyrrolidine-N'-(2-chloro-benzoyl)thiourea. J. Coord. Chem. 2006, 59, 223–228. [Google Scholar]
- Henderson, W.; Nicholson, B.K.; Dinger, M.B.; Bennett, R.L. Thiourea monoanion and dianion complexes of rhodium(III) and ruthenium(II). Inorg. Chim. Acta 2002, 338, 210–218. [Google Scholar] [CrossRef]
- Sacht, C.; Datt, M.S.; Otto, S.; Roodt, A. Synthesis, characterisation and coordination chemistry of novel chiral N,N-dialkyl-N-menthyloxycarbonylthioureas. Crystal and molecular structures of N,N-diethyl-N-(-)-(3R)-menthyloxycarbonylthiourea and cis-(S,S)-[Pt(L)Cl(DMSO)] [where HL = N-(+)-(3R)-menthyloxycarbonyl-N'-morpholinothiourea or N-benzoyl-N',N'-diethylthiourea]. J. Chem. Soc., Dalton Trans. 2000, 24, 4579–4586. [Google Scholar] [CrossRef]
- Lipowska, M.; Hayes, B.L.; Hansen, L.; Taylor, A.; Marzilli, L.G. Rhenium(V) oxo complexes of novel N2S2 dithiourea (DTU) chelate ligands: Synthesis and structural characterization. Inorg. Chem. 1996, 35, 4227–4231. [Google Scholar] [CrossRef]
- Zuckerman, R.L.; Bergman, R.G. Structural factors that influence the course of overall [2+2] cycloaddition reactions between imidozirconocene complexes and heterocumulenes. Organometallics 2000, 19, 4795–4809. [Google Scholar] [CrossRef]
- Henderson, W.; Kemmitt, R.D.W.; Mason, S.; Moore, M.R.; Fawcett, J.; Russell, D.R. Thia-diazatrimethylenemethane and N,N',P-Triphenylphosphonothioic Diamide Complexes of Platinum(Ii). J. Chem. Soc., Dalton Trans. 1992, 1, 59–66. [Google Scholar]
- Yuan, Y.F.; Wang, J.T.; Gimeno, M.C.; Laguna, A.; Jones, P.G. Synthesis and characterisation of copper complexes with N-ferrocenoyl-N '(alkyl)thioureas. Inorg. Chim. Acta 2001, 324, 309–317. [Google Scholar] [CrossRef]
- Zhang, Y.M.; Wei, T.B.; Xian, L.; Gao, L. M. An efficient synthesis of polymethylene-bis-aroyl thiourea derivatives under the condition of phase-transfer catalysis. Phosphorus Sulfur Silicon Relat. Elem. 2004, 179, 2007–2013. [Google Scholar] [CrossRef]
- Zhang, Y.M.; Wei, T.B.; Wang, X.C.; Yang, S.Y. Synthesis and biological activity of N-aroyl-N '-carboxyalkyl thiourea derivatives. Indian J. Chem. Sect B 1998, 37, 604–606. [Google Scholar]
- Zhou, W. Q.; Li, B. L.; Zhu, L. M.; Ding, J. G.; Yong, Z.; Lu, L.; Yang, X. J. Structural and spectral studies on N-(4-chloro)benzoyl-N'-(4-tolyl)thiourea. J. Mol. Struct. 2004, 690, 145–150. [Google Scholar] [CrossRef]
- Eweis, M.; Elkholy, S.S.; Elsabee, M.Z. Antifungal efficacy of chitosan and its thiourea derivatives upon the growth of some sugar-beet pathogens. Int. J. Biol. Macromol. 2006, 38, 1–8. [Google Scholar] [CrossRef]
- Arslan, H.; Florke, U.; Kulcu, N. N'-(4-Chlorobenzoyl)-N,N-diphenylthiourea. Acta Cryst. E 2003, 59, O641–O642. [Google Scholar] [CrossRef]
- Arslan, H.; Florke, U.; Kulcu, N. Synthesis, characterization, and crystal structure of 1-(4-chloro-benzoyl)-3-naphthalen-1-yl-thiourea. J. Chem. Crystallogr. 2003, 33, 919–924. [Google Scholar] [CrossRef]
- Arslan, H.; Florke, U.; Kulcu, N. The crystal and molecular structure of 1-(biphenyl-4-carbonyl)-3-p-tolyl-thiourea. Acta Chim. Slov. 2004, 51, 787–792. [Google Scholar]
- Arslan, H.; Kulcu, N.; Florke, U. Normal coordinate analysis and crystal structure of N,N-dimethyl-N'-(2-chloro-benzoyl)thiourea. Spectrochim. Acta, Part A 2006, 64, 1065–1071. [Google Scholar] [CrossRef]
- Arslan, H.; Florke, U.; Kulcu, N. Theoretical studies of molecular structure and vibrational spectra of O-ethyl benzoylthiocarbamate. Spectrochim. Acta, Part A 2007, 67, 936–943. [Google Scholar] [CrossRef]
- Arslan, H.; Ozpozan, N.; Ozpozan, T. Thermal studies of p-toluidino-p-chlorophenylglyoxime and of some corresponding Ni(II), Cu(II) and Co(II) complexes. Thermochim. Acta 1999, 329, 57–65. [Google Scholar] [CrossRef]
- Avsar, G.; Kulcu, N.; Arslan, H. Thermal behaviour of copper(II), nickel(II), cobalt(II) and palladium(II) complexes of N,N-dimethyl-N '-benzoylthiourea. Turk. J. Chem. 2002, 26, 607–615. [Google Scholar]
- Ugur, D.; Florke, U.; Kulcu, N.; Arslan, H. 3-[4-(3,3-diethylthioureidocarbonoyl)-benzoyl]-1,1-diethylthiourea. Acta Cryst. E 2003, 59, O1345–O1346. [Google Scholar] [CrossRef]
- Fleet, G.H. Composition and Structure of Yeast Cell Walls: Current Topics in Medical Mycology; Springer-Verlag: New York, USA, 1985; Vol. 1. [Google Scholar]
- Hoey, A.J.; Jackson, C.M.; Pegg, G.G.; Sillence, M.N. Characteristics of cyanopindolol analogues active at the beta(3)-adrenoceptor in rat ileum. Br. J. Pharmacol. 1996, 119, 564–568. [Google Scholar] [CrossRef]
- National Committee for Clinical Laboratory Standards. Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically, Approved Standard M7-A4; NCCLS: Viallanova, PA, USA, 1997. [Google Scholar]
- National Committee for Clinical Laboratory Standards. Reference method for broth dilution antifungal susceptibility testing of yeasts, Approved Standard M27-A2; NCCLS: Wayne, PA, USA, 2002. [Google Scholar]
- Sample Availability: Samples of the compounds are available from the authors.
© 2009 by the authors; licensee Molecular Diversity Preservation International, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Arslan, H.; Duran, N.; Borekci, G.; Koray Ozer, C.; Akbay, C. Antimicrobial Activity of Some Thiourea Derivatives and Their Nickel and Copper Complexes. Molecules 2009, 14, 519-527. https://doi.org/10.3390/molecules14010519
Arslan H, Duran N, Borekci G, Koray Ozer C, Akbay C. Antimicrobial Activity of Some Thiourea Derivatives and Their Nickel and Copper Complexes. Molecules. 2009; 14(1):519-527. https://doi.org/10.3390/molecules14010519
Chicago/Turabian StyleArslan, Hakan, Nizami Duran, Gulay Borekci, Cemal Koray Ozer, and Cevdet Akbay. 2009. "Antimicrobial Activity of Some Thiourea Derivatives and Their Nickel and Copper Complexes" Molecules 14, no. 1: 519-527. https://doi.org/10.3390/molecules14010519
APA StyleArslan, H., Duran, N., Borekci, G., Koray Ozer, C., & Akbay, C. (2009). Antimicrobial Activity of Some Thiourea Derivatives and Their Nickel and Copper Complexes. Molecules, 14(1), 519-527. https://doi.org/10.3390/molecules14010519