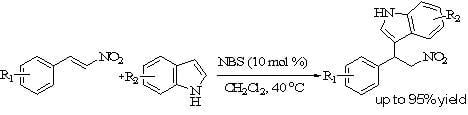An Efficient Method for the N-Bromosuccinimide Catalyzed Synthesis of Indolyl-Nitroalkanes
Abstract
:Introduction

Results and Discussion

 |

 |

 |

Conclusion
Experimental
General
General procedure for the synthesis of aryl-nitroalkanes
Acknowledgements
- Samples Availability: Samples of the compounds are available from the authors.
References
- Lim, K.H.; Hiraku, O.; Komiyama, K.; Koyano, T.; Hayashi, M.; Kam, T.S. Biologically active indole alkaloids from Kopsia arborea. J. Nat. Prod. 2007, 70, 1302–1307. [Google Scholar]
- Sundberg, R.J. Indoles; Academic Press: London, UK, 1996. [Google Scholar]
- Faulkner, D.J. Marine natural products. Nat. Prod. Rep. 2001, 18, 1–49. [Google Scholar]
- Fukuyama, T.; Chen, X. Stereocontrolled synthesis of (-)-Hapalindole G. J. Am. Chem. Soc. 1994, 116, 3125–3126. [Google Scholar] [CrossRef]
- Moore, R.E.; Cheuk, C.; Yang, X.Q.G.; Patterson, G.M.L.; Bonjouklian, R.; Smitka, T.A.; Mynderse, J.S.; Foster, R.S.; Jones, N.D. Hapalindoles, antibacterial and antimycotic alkaloids from the cyanophyte Hapalosiphon fontinalis. J. Org. Chem. 1987, 52, 1036–1043. [Google Scholar]
- Moore, R.E.; Cheuk, C.; Patterson, G.M.L. Hapalindoles: New alkaloids from the blue-green alga Hapalosiphon fontinalis. J. Am. Chem. Soc. 1984, 106, 6456–6457. [Google Scholar]
- Wang, S.Y.; Ji, S.J.; Loh, T.P. The Michael addition of indole to α,β-unsaturated ketones catalyzed by iodine at room temperature. Synlett 2003, 2377–2379. [Google Scholar]
- Olah, G.A.; Krishnamurthy, R.; Prakash, G.K.S. Comprehensive Organic Synthesis,, 1st; Trost, B.M., Fleming, I., Eds.; Pergamon: Oxford, UK, 1991; Volume 3, pp. 293–339. [Google Scholar]
- Harrington, P.E.; Kerr, M.A. Reaction of indoles with electron deficient olefins catalyzed by Yb(OTf)3·3H2O. Synlett 1996, 1047–1048. [Google Scholar]
- Kumar, V.P.; Sridhar, R.; Srinivas, B.; Narender, M.; Rao, K.R. Friedel-Crafts alkylation of indoles with nitroolefins in the presence of beta-cyclodextrin in water under neutral conditions. Can. J. Chem. 2008, 86, 907–911. [Google Scholar] [CrossRef]
- Yadav, J.S.; Abraham, S.; Reddy, B.V.S.; Sabitha, G. InCl3-catalysed conjugate addition of indoles with electron-deficient olefins. Synthesis 2001, 2165–2169. [Google Scholar]
- Bandini, M.; Melchiorre, P.; Melloni, A.; Umani-Ronchi, A. A practical indium tribromide catalysed addition of indoles to nitroalkenes in aqueous media. Synthesis 2002, 1110–1114. [Google Scholar]
- Zhan, Z.P.; Yanga, R.F.; Langa, K. Samarium triiodide-catalyzed conjugate addition of indoles with electron-deficient olefins. Tetrahedron Lett. 2005, 46, 3859–3862. [Google Scholar]
- Bartoli, G.; Bosco, M.; Giuli, S.; Giuliani, A.; Lucarelli, L.; Marcantoni, E.; Sambri, L.; Torregiani, E. Efficient preparation of 2-indolyl-1-nitroalkane derivatives employing nitroalkenes as versatile Michael acceptors: New practical linear approach to alkyl 9H-β-carboline-4- carboxylate. J. Org. Chem. 2005, 70, 1941–1944. [Google Scholar]
- Lin, C.; Hsu, J.; Sastry, M.N.V.; Fang, H.; Tu, Z.; Liu, J.T.; Yao, C.F. I2-catalyzed Michael addition of indole and pyrrole to nitroolefins. Tetrahedron 2005, 61, 11751–11757. [Google Scholar] [CrossRef]
- Firouzabadi, H.; Iranpoor, N.; Nowrouzi, F. The facile and efficient Michael addition of indoles and pyrrole to α,β-unsaturated electron-deficient compounds catalyzed by aluminium dodecyl sulfate trihydrate [Al(DS)3]·3H2O in water. Chem. Commun. 2005, 789–791. [Google Scholar]
- Azizi, N.; Arynasab, F.; Saidi, M.R. Efficient Friedel-Crafts alkylation of indoles and pyrrole with enones and nitroalkene in water. Org. Biomol. Chem. 2006, 4275–4277. [Google Scholar]
- Ballini, R.; Clemente, R.R.; Palmieri, A.; Petrini, M. Conjugate addition of indoles to nitroalkenes promoted by basic alumina in solventless conditions. Adv. Synth. Catal. 2006, 348, 191–196. [Google Scholar]
- An, L.T.; Zou, J.P.; Zhang, L.L.; Zhang, Y. Sulfamic acid-catalyzed Michael addition of indoles and pyrrole to electron-deficient nitroolefins under solvent-free condition. Tetrahedron Lett. 2007, 48, 4297–4300. [Google Scholar] [CrossRef]
- Wu, P.; Wan, Y.; Cai, J. Carbohydrate-Based Tolylsulfonyl Hydrazines: Effective catalysts for Michael addition of indoles to electron-deficient olefins in water. Synlett 2008, 1193–1198. [Google Scholar]
- Chakrabarty, M.; Basak, R.; Ghosh, N.; Harigaya, Y. Michael reaction of indoles with 3-(2’- nitrovinyl)indole under solvent-free conditions and in solution. An efficient synthesis of 2,2- bis(indolyl)nitroethanes and studies on their reduction. Tetrahedron 2004, 60, 1941–1949. [Google Scholar]
- Kusurkar, R.S.; Alkobati, N.A.H.; Gokule, A.S.; Chaudhari, P.M.; Waghchaure, P.B. Thermal and microwave-assisted conjugate additions of indole on electron-deficient nitro-olefins. Synth. Commun. 2006, 36, 1075–1081. [Google Scholar] [CrossRef]
- Itoh, J.; Fuchibe, K.; Akiyama, T. Chiral phosphoric acid catalyzed enantioselective Friedel-Crafts alkylation of indoles with nitroalkenes: Cooperative effect of 3 Å molecular sieves. Angew. Chem. Int. Ed. 2008, 47, 4016–4018. [Google Scholar]
- Ganesh, M.; Seidel, D. Catalytic enantioselective additions of indoles to nitroalkenes. J. Am. Chem. Soc. 2008, 130, 16464–16465. [Google Scholar] [CrossRef]
- Zhang, P.; Liu, R.; Cook, J.M. Regiospecific bromination of 3-methylindoles with N- bromosuccinimide. Tetrahedron Lett. 1995, 36, 3103–3106. [Google Scholar] [CrossRef]
- Fukase, K.; Hasuoka, A.; Kinoshita, I.; Aoki, Y.; Kasumoto, S. Regiospecific bromination of 3- methylindoles with N-bromosuccinimide. Tetrahedron 1995, 51, 4923–4932. [Google Scholar] [CrossRef]
- Karimi, B.; Hazarkhani, H.; Maleki, J. N-Bromosuccinimide (NBS) catalyzed highly chemoselective acetalization of carbonyl compounds using silylated diols and pentaerythritol under neutral aprotic conditions. Synthesis 2005, 279–285. [Google Scholar]
- Iranpoor, N.; Firouzabadi, H.; Shaterian, H.R. Catalytic and chemoselective deprotection of S,S- and S,O-acetals and ketals in the presence of their O,O-analogs with electrophilic halogens under neutral conditions. Tetrahedron Lett. 2003, 44, 4769–4773. [Google Scholar]
- Narender, M.; Reddy, M.S.; Rao, K.R. A mild and efficient oxidative deprotection of THP ethers with NBS in the presence of β-cyclodextrin in water. Synthesis 2004, 1741–1743. [Google Scholar]
- Koshima, H.; Matsusaka, W. N-Bromosuccinimide catalyzed condensations of indoles with carbonyl compounds under solvent-free conditions. J. Heterocycl. Chem. 2002, 39, 1089–1092. [Google Scholar] [CrossRef]
- Surendra, K.; Krishnaveni, N.S.; Kumar, V.P.; Sridhar, R.; Rao, K.R. Selective and efficient oxidation of sulfides to sulfoxides with N-bromosuccinimide in the presence of β-cyclodextrin in water. Tetrahedron Lett. 2005, 46, 4581–4583. [Google Scholar]
- Ohno, M.; Spande, T.F.; Witkop, B. Cyclization of tryptophan and tryptamine derivatives to pyrrolo[2,3-b]indoles. J. Am. Chem. Soc. 1968, 90, 6521–6522. [Google Scholar]
- Sakurai, O.; Takashahi, M.; Ogiku, T.; Hayashi, M.; Horikawa, H.; Iwasaki, T. A new synthesis of 1β-methylcarbapenems using NBS-promoted cyclization as a key step. Tetrahedron Lett. 1994, 35, 6317–6320. [Google Scholar] [CrossRef]
- Kuo, C.W.; More, S.V.; Yao, C.F. NBS as an efficient catalyst for the synthesis of 1,5- benzodiazepine derivatives under mild conditions. Tetrahedron Lett. 2006, 47, 8523–8528. [Google Scholar]
- Tu, Z.; Raju, B.R.; Liou, T.R.; Kavala, V.; Kuo, C.W.; Jang, Y.; Shih, Y.H.; Wang, C.C.; Yao, C.F. An efficient method for the synthesis of α-arylated nitroalkanes and α-arylated hydroximoyl chlorides mediated by AlCl3. Tetrahedron 2009, 65, 2436–2442. [Google Scholar] [CrossRef]
- Habib, P.M.; Kavala, V.; Kuo, C.W.; Yao, C.F. Catalyst-free aqueous-mediated conjugative addition of indoles to β-nitrostyrenes. Tetrahedron Lett. 2008, 49, 7005–7007. [Google Scholar] [CrossRef]
- Tu, Z.; Jang, Y.; Lin, C.; Liu, J.T.; Hsu, J.; Sastry, M.N.V.; Yao, C.F. The study of reaction mechanism for the transformation of nitronate into nitrile by phosphorus trichloride. Tetrahedron 2005, 61, 10541–10551. [Google Scholar]
- Yan, M.C.; Jang, Y.J.; Wu, J.; Lin, Y.F.; Yao, C.F. Radical reactions in esters with alkoxy functionality chemistry an unusual alcohol moiety hydrogen abstraction. Tetrahedron Lett. 2004, 45, 3685–3688. [Google Scholar]
- Yao, C.F.; Jang, Y.J.; Yan, M.C. An easy and efficient synthesis of 3-nitrochromans. Tetrahedron Lett. 2003, 44, 3813–3816. [Google Scholar]
- Yan, M.C.; Tu, Z.; Lin, C.; Yao, C.F. An easy and efficient method for the synthesis of hydroximoyl chloride from nitro olefin and silyl enol ether. Tetrahedron Lett. 2002, 43, 7991–7994. [Google Scholar]
- Liu, J.T.; Yao, C.F. One-pot synthesis of trans-β-alkylstyrenes. Tetrahedron Lett. 2001, 42, 6147–6150. [Google Scholar]
- Yan, M.C.; Jang, Y.J.; Yao, C.F. An easy and efficient method for the synthesis of 2,2-dialkyl-3- nitrochromene. Tetrahedron Lett. 2001, 42, 2717–2721. [Google Scholar]
- Liu, J.Y.; Liu, J.T.; Yao, C.F. Novel synthesis of alkenes via triethylaluminum-induced free radical reactions of alkyl iodides and β-nitrostyrenes. Tetrahedron Lett. 2001, 42, 3613–3615. [Google Scholar] [CrossRef]
- Yamamoto, H. Lewis Acid Reagents; Oxford University Press: New York, NY, USA, 1999. [Google Scholar]
- Itoh, K.; Kishimoto, S. The reaction of β-nitrostyrenes with 2-methoxyfuran: a novel formation of isoxazoline N-oxide together with Michael adducts. New J. Chem. 2000, 24, 347–349. [Google Scholar]
- Mahboobi, S.; Popp, A.; Burgemeister, T.; Schollmeyer, D. Diastereoselective synthesis of (−)-1- methyl-(3S,4R)-3,4-bis((2S)-N-(tert-butyloxycarbonyl)pyrrolidin-2-yl)-2-pyrrolidinone by an asymmetric Michael reaction. TetrahedronAsymmetry 1998, 9, 2369–2376. [Google Scholar]
© 2009 by the authors; licensee Molecular Diversity Preservation International, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Kuo, C.-W.; Wang, C.-C.; Fang, H.-L.; Raju, B.R.; Kavala, V.; Habib, P.M.; Yao, C.-F. An Efficient Method for the N-Bromosuccinimide Catalyzed Synthesis of Indolyl-Nitroalkanes. Molecules 2009, 14, 3952-3963. https://doi.org/10.3390/molecules14103952
Kuo C-W, Wang C-C, Fang H-L, Raju BR, Kavala V, Habib PM, Yao C-F. An Efficient Method for the N-Bromosuccinimide Catalyzed Synthesis of Indolyl-Nitroalkanes. Molecules. 2009; 14(10):3952-3963. https://doi.org/10.3390/molecules14103952
Chicago/Turabian StyleKuo, Chun-Wei, Chun-Chao Wang, Hu-Lin Fang, B. Rama Raju, Veerababurao Kavala, Pateliya Mujjamil Habib, and Ching-Fa Yao. 2009. "An Efficient Method for the N-Bromosuccinimide Catalyzed Synthesis of Indolyl-Nitroalkanes" Molecules 14, no. 10: 3952-3963. https://doi.org/10.3390/molecules14103952