2.1. Iodine-alcohol–mediated aromatization of cyclohexenone derivatives
The use of molecular iodine as an oxidant to promote aromatization of cyclohexenone derivatives was first reported in 1980 by Tamura and Yoshimoto [
20]. These authors subjected series of cyclohexenones to iodine in refluxing methanol to afford variously substituted anisole derivatives. Their methodology was later applied by Kotnis on Hagemann’s esters
3 to afford substituted
p-methoxybenzoates
4, which are building blocks for several marine natural products (
Scheme 1) [
21].
In another development involving the use of iodine-methanol reaction mixture, Kotnis transformed a series of cyclohexane-1,3-dione derivatives
5 (for
5e; R=Ac) to substituted resorcinols
6 (
Scheme 2) [
9]. The only mechanistic suggestion was that 1,4-addition–elimination of methanol to the enol form of the cyclohexadione system takes place as a first step of the reaction. The author also used some of the prepared resorcinols as precursors for the synthesis of olivetol
2a and the antifungal antibiotic DB2073
2b [
9].
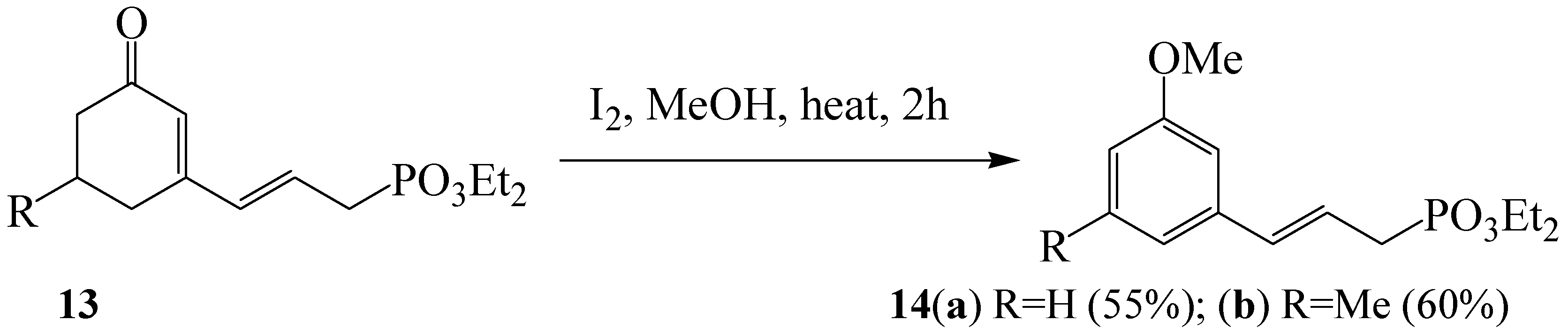
Similar reaction conditions to those previously employed by Kotnis were later applied to 3-(phosphonoalkyl)cyclohexenones
7 to afford a series of novel anisole derivatives
8 substituted at the 3-position with alkylphosphonate group (
Scheme 3) [
22]. The observed results were interpreted as a consequence of an initial 1,2- (independent of 1,4-) addition of methanol, followed by dehydration and iodine-promoted oxidative aromatization. The same products were also obtained from the corresponding 3-chlorocyclohexenols
9, presumably
via the acid catalyzed S
N2’ displacement of the allylic alcohol by methanol. Elimination of HCl would lead to the same cyclohexadiene derivative as obtained from the cylohexenone derivative and is followed by the iodine–promoted aromatization. Under similar reaction conditions applied to
7, diethyl 1-(1-hydroxy-3-methylcyclohex-2-enyl)ethylphosphonates
10 afforded the expected 3-methylbenzylphosphonates
11, as well as their 6-methoxy derivatives
12 albeit in low yields (
Scheme 4) [
22]. The reaction was also found to work well with cyclohexenone derivatives
13 bearing allylphosphonate moiety at the 3-position to afford novel 3-substituted anisole derivatives
14 in moderate yields (
Scheme 5) [
23].
Dihydrofuran
15 was previously subjected to iodine in refluxing methanol to afford the aromatized derivative
16, which is an analogue of the naturally occurring rocaglamide (
Scheme 6) [
24]. A one-pot iodine-methanol–mediated aromatization of cyclic diones
17 and subsequent fragmentation to anisole derivatives
18 has also been reported before (
Scheme 7) [
25].
The oxidative properties of iodine were recently exploited to effect aromatization of 2-bromomethyl-3,5,6,7-tetrahydrobenzofurans
19 to afford the corresponding 2-bromomethyl-4-methoxy-2,3-dihydrobenzofurans
20 (
Scheme 8) [
26]. Under similar reaction conditions, the analogous 2-bromo-2,3,4,6,7,8-hexahydro-1-benzopyran-5-ones
21 (R=H, Me) afforded the corresponding 3-bromo-5-methoxy-3,4-dihydrobenzopyran derivatives
22a (R=H) and
22b (R=Me) in 85% and 89% yields, respectively (
Scheme 9). Moreover, the combined electrophilic and oxidative properties of iodine were also exploited to effect direct one-pot iodocyclization of 2-allylcyclohexane-1,3-diones and subsequent
in situ oxidative aromatization of the resulting 2-iodomethyl-2,4,5,6-tetrahydrobenzofuran-4-ones to afford 2-iodomethyl-4-methoxy-2,3-dihydrobenzofurans [
26].
Iodine in refluxing alcohols was also employed before by Kim and co-workers on 2-acyl- and 2-propionylcyclohexane-1,3-diones
23 (
Scheme 10) [
27]. The 3-alkoxy-1-hydroxyacetophenone derivatives
24a-d were formed exclusively from the acetyl- and propionyl substituted starting materials
23a and
23d. The absence of dimethoxy derivatives in the case of acetyl- and propionyl substituted starting materials
23a and
23d was attributed to strong intramolecular hydrogen bonding that would prevent conversion of the hydroxyl group into methoxy group. Mixtures of mono-
24 and dimethoxy derivatives
25 were isolated when the benzoyl or carbomethoxycyclohexane-1,3-diones
23e,f,
h were used as substrates. In the latter instance, the authors attributed the formation of both monomethoxy and dimethoxy derivatives to be a consequence of the weakly hydrogen bonding 2-benzoyl and 2-carbomethoxy groups. However, these authors could not account for the formation of both monomethoxy and dimethoxy derivatives when the 5-methyl substituted 2-acetylcyclohexane-1,3-dione
23g was used as a substrate.
Treatment of benzo[
b]indeno[2.1-
d]furanone derivatives
26 with iodine (2 equiv.) in refluxing methanol afforded anisole derivatives
27 (minor) and
28 (major), respectively (
Scheme 11) [
28]. The formation of methyl derivatives
28 was rationalized as a consequence of initial acid-catalyzed dehydration of
27 to form a cyclic oxonium intermediate, which then undergoes addition of methanol [
25]. The proposed mechanism was proven in a follow up study involving selective methylation of systems
27 to
28 using iodine-methanol mixture and the methylated derivatives were found to be formed selectively under prolonged heating conditions (13–36 h) [
29].
Kim and coworkers also employed iodine in refluxing methanol to effect oxidative aromatization of 4-alkylidene-2-cyclohexen-1-ones
29 to afford the corresponding anisole derivatives (
Scheme 12) [
30]. The mechanism of this reaction which was also confirmed using iodine (1.1 equiv.) on
29b in deuterated methanol to afford deuterated analogue of
30b (OCD
3 in place of OMe) in 32% yield is believed to involve initial conjugate addition of methanol to the exo-methylene moiety followed by attack of the carbonyl carbon by methanol to generate a hemiketal derivative. Dehydration of the latter then occurs followed by iodine-promoted oxidative aromatization to yield the anisole derivatives. The fully conjugated systems
31f and
31g formed as minor products from substrates
29f and
29g are presumably the consequence of slow expulsion of methanol from the dimethoxy products. Kim’s group also subjected 2-methylene-2-cyclohexenones to iodine in alcohol (methanol or ethanol) to afford series of novel anisole derivatives [
31].
2-Cyclohexen-1-one and its 3-methyl derivative with iodine-cerium(IV) ammonium nitrate mixture in alcohol (methanol, ethanol, 1-propanol, 2-propanol) previously afforded the corresponding alkyl phenyl ethers in moderate to high yields [
32]. Cerium(IV) ion is believed to coordinate with the carbonyl oxygen to facilitate attack by alcohol leading to enolization, which in turn facilitates iodine-mediated oxidative aromatization to afford alkyl phenylether derivatives. 3,5,5-Trimethyl-2-cyclohexen-1-one
32 was found to undergo methyl shift upon treatment with iodine-CAN in ethanol or n-butanol to afford the corresponding 3,4,5-trimethyl substituted phenylether derivatives
33a and
b, in 89% and 90% yields, respectively (
Scheme 13) [
32].
Iodine represents a relatively less expensive reagent for oxidative aromatization of cyclohexenone moiety than metal–catalyzed aromatization of substituted cyclohexenones to the corresponding phenol ethers or phenols [
1,
2,
3,
4,
5,
6,
7,
8]. It is also superior to the use of 2,3-dichloro-5,6-dicyano-1,4-benzoquinone (DDQ) in dioxane, which was previously employed to dehydrogenate 5-acetyl-4-oxo-4,5,6,7-tetrahydrobenzofuran and methyl-4-oxo-4,5,6,7-tetrahydrobenzofuran-5-carboxylate [
33].