Chemical Analysis of the Resinous Exudate Isolated from Heliotropium taltalense and Evaluation of the Antioxidant Activity of the Phenolics Components and the Resin in Homogeneous and Heterogeneous Systems
Abstract
:1. Introduction
2. Results and Discussion
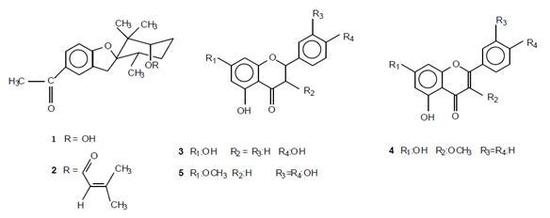
2.1. Chemical composition of the resin
2.2. Antioxidant activity

| Compounds | Concentration(mM) | Vi DPPH/EtOH (mM/s) | Vi DPPH/SDS (mM/s) |
|---|---|---|---|
| Naringenin | 15.0 | - 0.042220 | - 0.06009 |
| 30.0 | - 0.028222 | - 0.03791 | |
| 60.0 | - 0.009550 | - 0.01460 | |
| 3-O-methylgalangin | 3.00 | - 0.001690 | - 0.00245 |
| 6.00 | - 0.001790 | - 0.00270 | |
| 12.00 | - 0.002200 | - 0.00302 | |
| 7-O-methyleriodictyol | 0.014 | - 0.026210 | - 0.03390 |
| 0.028 | - 0.038780 | - 0.06061 | |
| 0.041 | - 0.042500 | - 0.10391 |

| Concentration of resin | Vi EtOH (mM/s) | Vi SDS (mM/s) |
|---|---|---|
| 100 mg/mL | -0.012398 | -0.114356 |
| 10 mg/mL | -0.011796 | -0.035083 |
| 2 mg/mL | -0.006048 | -0.009052 |
3. Experimental
3.1. General
3.2. Plant material
3.3. Chemicals
3.4. Isolation and characterization of constituent of the resin
3.5. Antioxidant activity determination
3.6. Statistical analyses
4. Conclusions
Acknowledgements
References
- Villarroel, L.; Torres, R.; Urzúa, A. Compuestos fenólicos en el exudado resinoso de Heliotropium stenophyllum. Determinación estructural y efecto antialimentario y antioxidante. Bol. Soc. Chil. Quím. 1991, 36, 169–174. [Google Scholar]
- Torres, R.; Villarroel, L.; Urzúa, A.; Delle-Monache, F.; Delle-Monache, G.; Gacs-Baitz, E. Filifolinol, a rearranged geranyl aromatic derivative from the resinous exudate of Heliotropium filifolium. Phytochemistry 1994, 36, 249–256. [Google Scholar] [CrossRef]
- Torres, R.; Modak, B.; Urzúa, A.; Villarroel, L.; Delle-Monache, F.; Sánchez-Ferrando, F. Flavonoides del exudado resinoso de Heliotropium sinuatum. Bol. Soc. Chil. Quím. 1996, 41, 195–197. [Google Scholar]
- Urzua, A.; Modak, B.; Villarroel, L.; Torres, R.; Andrade, L. Comparative flavonoid composition of the resinous exudates from Heliotropium chenopodiaceum var. chenopodiaceum and H. chenopodiaceum var. ericoideum. Biochem. Syst. Ecol. 1998, 26, 127–130. [Google Scholar] [CrossRef]
- Urzua, A.; Modak, B.; Villarroel, L.; Torres, R.; Andrade, L.; Mendoza, L.; Wilkens, M. External flavonoids from Heliotropium megalanthum and H. huascoense (Boraginaceae). Chemotaxonomic considerations. Bol. Soc. Chil. Quím. 2000, 45, 23–29. [Google Scholar]
- Urzua, A.; Modak, B.; Torres, R. Identification of a new aromatic geranyl derivative in the resinous exudate of Heliotropium filifolium (Boraginaceae). Bol. Soc. Chil. Quím. 2001, 46, 175–178. [Google Scholar]
- Modak, B.; Torres, R.; Lissi, E.; Delle-Monache, F. Antioxidant capacity of flavonoids and a new arylphenol of the resinous exudate from Heliotropium sinuatum. Nat. Prod. Res. 2003, 17, 403–407. [Google Scholar] [CrossRef]
- Modak, B.; Rojas, M.; Torres, R.; Rodilla, J.; Luebert, F. Antioxidant activity of a new aromatic geranyl derivative of the resinous exudates from Heliotropium glutinosum Phil. Molecules 2007, 12, 1057–1063. [Google Scholar] [CrossRef]
- Downum, K.; Dole, J.; Rodriguez, E. Nordihydroguaiaretic acid: inter- and intra population variation in the Sonoran Desert Creasote bush (Larrea tridentata, Zygophyllaceae). Biochem. Syst. Ecol. 1998, 16, 551–555. [Google Scholar] [CrossRef]
- Valkama, E.; Salminen, J.; Koricheva, J.; Pihlaja, K. Changes in Leaf Trichomes and Epicuticular Flavonoids during Leaf Development in Three Birch Taxa. Ann. Bot. 2004, 94, 233–242. [Google Scholar] [CrossRef]
- Urzua, A.; Mendoza, L. Composición química del exudado resinoso de Haplopappus velutinus. Bol. Soc. Chil. Quím. 1993, 38, 89–90. [Google Scholar]
- Tarahovsky, Y.; Muzafarov, E.; Kim, Y. Rafts making and rafts braking: how plant flavonoids may control membrane heterogeneity. Mol. Cell. Biochem. 2008, 314, 65–71. [Google Scholar] [CrossRef]
- Pannala, A.; Chan, T.; O’Brien, P.; Rice-Evans, C. Flavonoids B-ring chemistry and antioxidant activity: fast reaction kinetics. Biochem. Biophys. Res. Commun. 2001, 282, 1161–1168. [Google Scholar] [CrossRef]
- Rice-Evans, C.; Miller, N.; Paganga, G. Structure - antioxidant activity relationships of flavonoids and phenolic acids. Free Radical Biol. Med. 1996, 20, 933–956. [Google Scholar] [CrossRef]
- Cao, G.; Sofic, E.; Prior, R. Antioxidant and prooxidant behaviour of flavonoids: structure-activity. Free Radical Biol. Med. 1997, 22, 749–760. [Google Scholar] [CrossRef]
- Modak, B.; Contreras, M.; González-Nilo, F.; Torres, R. Structure-antioxidant activity relationships of flavonoids isolated from the resinous exudate of Heliotropium sinuatum. Bioorg. Med. Chem. Lett. 2005, 15, 309–312. [Google Scholar] [CrossRef]
- Niki, E.; Noguchi, N. Evaluation of antioxidant capacity. What capacity is being measured by which method? IUBMB Life 2000, 50, 323–329. [Google Scholar] [CrossRef]
- Naseem, B.; Sabri, A.; Hasan, A.; Shah, S. Interaction of flavonoids within organized molecular assemblies of anionic surfactant. Colloids Surf. B Biointerfac. 2004, 35, 7–13. [Google Scholar] [CrossRef]
- Liu, W.; Guo, R. Interaction between flavonoid, quercetin and surfactant aggregates with different charges. J. Colloid Interface Sci. 2006, 302, 625–632. [Google Scholar] [CrossRef]
- Abuin, E.; Lissi, E.; Ortiz, P.; Henriquez, C. Uric acid reaction with DPPH radicals at the micelar interface. Bol. Soc. Chil. Quím. 2002, 47, 145–149. [Google Scholar]
- Lissi, E.; Modak, B.; Torres, R.; Escobar, J.; Urzúa, A. Total antioxidant potential of resinous exudates from Heliotropium species, and a comparison of the ABTS and DPPH methods. Free Radic. Res. 1999, 30, 471–477. [Google Scholar] [CrossRef]
- Mendoza, L.; Modak, B.; Torres, R.; Cotoras, M. In Vitro sensitivity of Botrytis cinerea to resinous exudates of Heliotropium filifolium and geranyl derivatives compounds. J. Chil. Chem. Soc. 2008, 53, 1436–1438. [Google Scholar]
- Torres, R.; Galeno, H.; Modak, B. Effect on Hantavirus replication of resins from Heliotropium species and other selected compounds. Nat. Prod. Commun. 2008, 3, 525–528. [Google Scholar]
- Torres, R.; Modak, B.; Urzúa, A.; Delle-Monache, F.; Damonte, E.; Pujol, C. Propiedades antivirales de compuestos naturales y semi-sintéticos de la resina de Heliotropium filifolium. Bol. Soc. Chil. Quím. 2002, 47, 259–263. [Google Scholar]
- Brand-Williams, W.; Cuvelier, M.; Berset, C. Use of a free-radical method to evaluate antioxidant activity. Food Sci. Technol.-Lebensm. Wiss. 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Sample Availability: Samples of compounds 1-5 are available from the authors.
© 2009 by the authors; licensee Molecular Diversity Preservation International, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Modak, B.; Rojas, M.; Torres, R. Chemical Analysis of the Resinous Exudate Isolated from Heliotropium taltalense and Evaluation of the Antioxidant Activity of the Phenolics Components and the Resin in Homogeneous and Heterogeneous Systems. Molecules 2009, 14, 1980-1989. https://doi.org/10.3390/molecules14061980
Modak B, Rojas M, Torres R. Chemical Analysis of the Resinous Exudate Isolated from Heliotropium taltalense and Evaluation of the Antioxidant Activity of the Phenolics Components and the Resin in Homogeneous and Heterogeneous Systems. Molecules. 2009; 14(6):1980-1989. https://doi.org/10.3390/molecules14061980
Chicago/Turabian StyleModak, Brenda, Macarena Rojas, and René Torres. 2009. "Chemical Analysis of the Resinous Exudate Isolated from Heliotropium taltalense and Evaluation of the Antioxidant Activity of the Phenolics Components and the Resin in Homogeneous and Heterogeneous Systems" Molecules 14, no. 6: 1980-1989. https://doi.org/10.3390/molecules14061980
APA StyleModak, B., Rojas, M., & Torres, R. (2009). Chemical Analysis of the Resinous Exudate Isolated from Heliotropium taltalense and Evaluation of the Antioxidant Activity of the Phenolics Components and the Resin in Homogeneous and Heterogeneous Systems. Molecules, 14(6), 1980-1989. https://doi.org/10.3390/molecules14061980