Experimental
General
All of the synthesized compounds were chemically characterized by thin layer chromatography (TLC), infrared (IR), proton nuclear magnetic resonance (1H-NMR), mass spectra (MS) and elemental microanalyses (CHN). Alugram SIL G/UV254 (Layer: 0.2 mm) (Macherey-Nagel GmbH & Co. KG., Düren, Germany) was used for TLC and Silica gel 60 (0.040-0.063 mm, Merck) was used for Flash Column Chromatography. The 1H-NMR spectra were recorded on a Bruker 400 Ultrashield instrument (400 MHz), using TMS as internal standard and with DMSO-d6 as solvent; the chemical shifts are reported in ppm (δ) and coupling constants (J) values are given in Hertz (Hz). Signal multiplicities are represented by: s (singlet), bs (broad singlet), d (doublet), t (triplet), q (quadruplet), dd (double doublet) and m (multiplet). The IR spectra were recorded on a Nicolet Nexus FTIR (Thermo, Madison, USA) in KBr pellets. Elemental microanalyses were obtained on a CHN-900 Elemental Analyzer (Leco, Tres Cantos, Spain) from vacuum-dried samples. The analytical results for C, H and N, were within ± 0.5 of the theoretical values. Chemicals were purchased from Panreac Química S.A. (Barcelona, Spain), Sigma-Aldrich Química, S.A. (Alcobendas, Spain), Acros Organics (Janssen Pharmaceutical, Geel, Belgium) and Lancaster (Bischheim-Strasbourg, France).
General procedure for the synthesis of cyanoamines II
Malononitrile (18.0 mmol) was added to a solution of the appropriate benzofuroxane (I, 15.0 mmol) in DMF (10 mL). The mixture was allowed to stand at 0 °C. Triethylamine was added dropwise (1.5 mL), and the reaction mixture was stirred at room temperature in darkness for 1-3 days. The precipitate was filtered off and washed by adding diethyl ether affording the target compound. The obtained red solid was used in the next step without further purification.
General procedure for the synthesis of furan-2-carboxylic acid (3-cyano-1,4-di-N-oxide-quinoxalin-2-yl)-amide derivatives and thiophene-2-carboxylic acid (3-cyano-1,4-N-oxide-quinoxalin-2-yl)-amide derivatives 5-24
The corresponding 3-amino-1,4-di-N-oxide-quinoxaline-2-carbonitrile II (2 mmol) is dissolved in acetonitrile (100 mL) and triethylamine (0.4 mL) was added at room temperature under stirring and anhydrous conditions. After cooling the reaction mixture with an ice bath, 2-furoyl chloride or 2-tiophenecarbonyl chloride (2.2 mmol) are added. The reaction mixture is stirred for 9 h at room temperature. The obtained solid is filtered and EtOAc (400 mL) added to the filtrate. The organic phase is extracted, first with HCl 10% and then with water. The organic phase is dried with anhydrous Na2SO4 and filtered. The solvent is removed in vacuo and the resulting residue is precipitated with diethyl ether, and then filtered in order to obtain a yellow-orange solid.
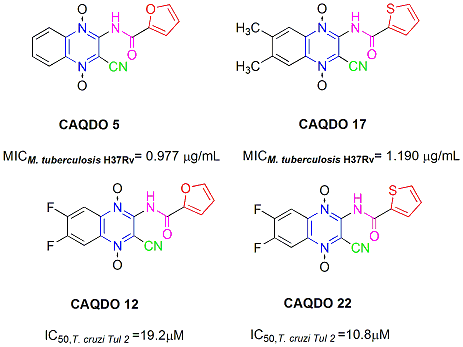
Furan-2-carboxylic acid (3-cyano-1,4-di-N-oxidequinoxalin-2-yl)amide (5). Yield 64.4 %; 1H-NMR δ ppm: 11.43 (bs, 1H, NH); 8.53-8.48 (m, 2H, H8+H5); 8.13-8.01 (m, 3H, H6+H7+H5’); 7.60 (d, 1H, H3´, J3´-4´ = 3.5 Hz); 6.81 (dd, 1H, H4´, J4´-5 ´ = 1.7 Hz); IR ν cm-1: 3,237 (m, NH); 2,236 (w, C≡N); 1,686 (s, C=O); 1,335 (s, N+O-); Anal. Calc. for C14H8N4O4: C: 56.76%; H: 2.72%; N: 18.91%. Found: C: 56.73%; H: 3.13%; N: 18.93%.
Furan-2-carboxylic acid (3-cyano-1,4-di-N-oxide-6-methylquinoxalin-2-yl)amide (6). Yield 63.0 %; 1H-NMR δ ppm: 11.37 (bs, 1H, NH); 8.42 (d, 1H, H8, J8-7 = 8.8 Hz); 8.31 (d, 1H, H5, J5-7 = 1.3 Hz); 8.10 (d, 1H, H5´, J5´-4´ = 1.7 Hz); 7.95 (dd, 1H, H 7); 7.69 (d, 1H, H3´, J3´-4´ = 3.6 Hz), 6.81 (dd, 1H, H4´), 2.61 (s, 3H, CH3); IR ν cm-1: 3,114 (m, NH); 2,238 (w, C≡N); 1,692 (s, C=O); 1,325 (s, N+O‑); Anal. Calc. for C15H10N4O4: C: 58.07%; H: 3.25%; N: 18.06%. Found: C: 57.95%; H: 3.27%; N: 18.43%.
Furan-2-carboxylic acid (3-cyano-1,4-di-N-oxide-6,7-dimethylquinoxalin-2-yl)amide (7). Yield 75.3 %; 1H-NMR δ ppm: 11.33 (bs, 1H, NH); 8.31 (s, 1H, H8); 8.28 (s, 1H, H5 ); 8.09 (d, 1H, H5´, J5´-4´ = 1.5 Hz); 7.68 (d, 1H, H3´, J3´-4´ = 3.5 Hz); 6.80 (dd, 1H; H4´); 2.54-2.52 (m, 6H, 2xCH3); IR ν cm-1: 3,285 (w, NH); 2,236 (w, C≡N); 1,709 (s, C=O); 1,324 (s, N+O-); Anal. Calc. for C16H12N4O4: C: 59.26%; H: 3.73%; N: 17.28%. Found: C: 59.33%; H: 3.77%; N: 17.30%.
Furan-2-carboxylic acid (3-cyano-1,4-di-N-oxide-6-methoxyquinoxalin-2-yl)amide (8). Yield 50.5 %; 1H-NMR δ ppm: 11.31 (bs, 1H, NH); 8.44 (d, 1H, H8, J8-7 = 9.4 Hz); 8.09 (s, 1H, H5); 7.78 (d, 1H, H5´, J5´-4´ = 2.4 Hz); 7.73 (d, 1H, H7); 7.67 (d, 1H, H3´, J3´-4’ = 3.10 Hz), 6.80 (dd, 1H, H4’); 4.03 (s, 3H, OCH3); IR ν cm-1: 3,298 (m, NH); 2,241 (w, C≡N); 1,698 (s, C=O); 1,321 (s, N+O-); Anal. Calc. for C15H10N4O5: C: 55.22%; H: 3.09%; N: 17.17%. Found: C: 54.87%; H: 3.09%; N: 16.79%.
Furan-2-carboxylic acid (3-cyano-6-chloro-1,4-di-N-oxidequinoxalin-2-yl)amide (9). Yield 6.1 %; 1H-NMR δ ppm: 11.47 (bs, 1H, NH); 8.51-8.50 (m, 2H, H8+H5); 8.14 (dd, 1H, H7, J7-8 = 9.1 Hz, J7-5 = 2.3 Hz); 8.10 (d, 1H, H5´, J5´-4 ´ = 1.7 Hz); 7.70 (d, 1H, H3´, J3´-4´ = 3.6 Hz); 6.81 (dd, 1H, H4´, J4´-3’ = 3.6 Hz, J4´-5’ = 1.7 Hz); IR ν cm-1: 3,288 (m, NH); 2,241 (w, C≡N); 1,692 (s, C=O); 1,320 (s, N+O-); Anal Calc. for C14H7ClN4O4: C: 50.58%; H: 2.13%; N: 16.94%. Found: 50.91%; H: 2.23%; N: 16.69%.
Furan-2-carboxylic acid (3-cyano-6,7-dichloro-1,4-di-N-oxidequinoxalin-2-yl)amide (10). Yield 51.0 %; 1H-NMR δ ppm: 11.56 (bs, 1H, NH); 8.72 (s, 2H, H8+H5); 8.11 (dd, 1H, H5´, J5´-4´ = 1.7 Hz, J5´-3´ = 0.7 Hz); 7.72 (d, 1H, H3´, J3´-4´ = 3.6 Hz); 6.81 (dd, 1H, H4); IR ν cm-1: 3,275 (m, NH); 2,232 (w, C≡N); 1,702 (s, C=O); 1,336 (s, N+O-); Anal. Calc. for C14H6Cl2N4O4: C: 45.04%; H: 1.61%; N: 15.01%. Found: C: 45.34%; H: 1.75%; N: 15.18%.
Furan-2-carboxylic acid (3-cyano-1,4-di-N-oxide-6-fluoroquinoxalin-2-yl)amide (11). Yield 57.8 %; 1H-NMR δ ppm: 11.44 (bs, 1H, NH); 8.59 (dd, 1H, H8, J8-F = 5.0 Hz); 8.30 (dd, 1H, H5, J5‑F = 8.6 Hz, J5-7 = 2.7 Hz); 8.10 (d, 1H, H5´, J5´-4´ = 1.5 Hz); 8.03 (ddd, 1H, H7, J7-F = 7.9 Hz); 7.70 (d, 1H, H3´, J3´-4´ = 3.6 Hz); 6.81 (dd, 1H, H4’); IR ν cm-1: 3,263 (m, NH); 2,239 (w, C≡N); 1,681 (s, C=O); 1,328 (s, N+O-); 1,242 and 1,171 (m, Ar-F); Anal. Calc. for C14H7FN4O4: C: 53.51%; H: 2.25%; N: 17.83%. Found: C: 53.06%; H: 2.27%; N: 17.86%.
Furan-2-carboxylic acid (3-cyano-6,7-difluoro-1,4-di-N-oxidequinoxalin-2-yl)amide (12). Yield 66.1 %; 1H-NMR δ ppm: 11.50 (bs, 1H, NH); 8.65-8.58 (m, 2H, H8+H5); 8.10 (dd, 1H, H5´, J5´‑4´ = 1.6 Hz, J5´-3´ = 0.6 Hz); 7.71 (dd, 1H, H3´, J3´-4´ = 3.7 Hz); 6.81 (dd, 1H, H4´); IR ν cm-1: 3,215 (m, NH); 2,236 (w, C≡N); 1,689 (s, C=O); 1,340 (s, N+O-); 1,270 and 1,181 (m, Ar-F); Anal. Calc. for C14H6F2N4O4: C: 50.61%; H:1.82%; N: 16.86%. Found: C: 50.37%; H: 1.98%; N: 16.67%.
Furan-2-carboxylic acid (3-cyano-1,4-di-N-oxide-7-trifluoromethylquinoxalin-2-yl)amide (13). Yield 78.1 %; 1H-NMR δ ppm: 12.06 (bs, 1H, NH); 8.69 (s, 1H, H8 ); 8.64 (d, 1H, H5, J5-6 = 9.0 Hz); 8.37 (d, 1H, H6 ); 8.07 (d, 1H, H5´, J5´-4´ = 1.7 Hz); 7.66 (d, 1H, H3´); 6.79 (d, 1H, H4´, J3´-4´ = 3.5 Hz); IR ν cm-1: 3,279 (m, NH); 2,244 (w, C≡N); 1,688 (s, C=O); 1,325 (s, N+O-); 1,137 (s, CF3); Anal. Calc. for C15H7F3N4O4: C: 49.46%; H: 1.96%; N: 15.38%. Found: C: 49.18%; H: 2.02%; N: 15.31%.
Furan-2-carboxylic acid (3-cyano-1,4-di-N-oxide-6-trifluoromethylquinoxalin-2-yl)amide (14). Yield 31.1 %; 1H-NMR δ ppm: 12.06 (bs, 1H, NH); 8.72 (d, 1H, H5, J5-7 = 1.7 Hz); 8.66 (d, 1H, H8, J8-7 = 9.0 Hz); 8.30 (dd, 1H, H7, J7-8 = 9.0 Hz, J7-5 = 1.7 Hz); 8.09 (dd, 1H, H5´, J5´-4´ = 1.7 Hz, J5´-3´ = 0.7 Hz); 7.69 (dd, 1H, H3´, J3´-4´ = 3.6 Hz); 6.80 (dd, 1H, H4´); IR ν cm-1: 3,263 (m, NH); 2,232 (w, C≡N); 1,701 (s, C=O); 1,341 (s, N+O-); 1,132 (s, CF3); Anal. Calc. for C15H7F3N4O4: C: 49.46%; H: 1.96%; N: 15.38%. Found: C: 49.59%; H: 1.89%; N: 15.09%.
Thiophene-2-carboxylic acid (3-cyano-1,4-di-N-oxidequinoxalin-2-yl)amide (15). Yield 33.3 %; 1H- NMR δ ppm: 11.65 (bs, 1H, NH); 8.54 (dd, 1H, H8, J8-7 = 8.6 Hz, J8-6 = 0.7 Hz); 8.50 (dd, 1H, H5, J5-6 = 8.6 Hz, J5-7 = 0.8 Hz); 8.30 (dd, 1H, H5’, J5’-4´ = 3.7 Hz, J5´-3’ = 0.9 Hz); 8.12 (td, 1H, J6-7 = 7.1 Hz, H6); 8.10-8.00 (m, 2H, H7 , H3’); 7.32 (dd, 1H, H4´, J4´-3’ = 4.9 Hz, J4’-5’ = 3.9 Hz); IR ν cm-1: 3,231 (m, NH); 2,232 (w, C≡N); 1,681 (s, C=O); 1,326 (s, N+O-); Anal. Calc. for C14H8N4O3S: C: 53.85%; H: 2.56%; N: 17.95%. Found: C: 53.88%; H: 2.82%; N: 18.35%.
Thiophene-2-carboxylic acid (3-cyano-1,4-di-N-oxide-6-methylquinoxalin-2-yl)amide (16). Yield 1.2 %; 1H-NMR δ ppm: 11.62 (bs, 1H, NH); 8.39 (d, H8, 1H, J8-7 = 8.8 Hz); 8.35 (s, 1H, H5); 8.30 (m, 1H, H5’); 8.05 (d, 1H, H3’, J3’-4 ’ = 4.9 Hz); 7.88 (d, 1H, H7); 7.33-7.31 (m, 1H, H4’); 2.66 (s, 3H, CH3); IR ν cm-1: 3,276 (w, NH); 2,225 (w, C≡N); 1,664 (s, C=O); 1,329 (s, N+O-); Anal. Calc. for C15H10N4O3S: C: 55.22%; H: 3.07%; N: 17.18%. Found: C: 55.01%; H: 3.17%; N: 17.39%.
Thiophene-2-carboxylic acid (3-cyano-6,7-dimethyl-1,4-di-N-oxidequinoxalin-2-yl)amide (17). Yield 1.4 %; 1H-NMR δ ppm: 11.59 (bs, 1H, NH); 8.32 (s, 1H, H5’); 8.28 (s, 2H, H8+H5); 8.03 (s, 1H, H3’); 7.31 (s, 1H, H4’); 2.6-2.4 (m, 6H, 2xCH3); IR ν cm-1: 3,206 (m, NH); 2,225 (w, C≡N); 1,667 (s, C=O); 1,327 (s, N+O-); Anal. Calc. for C16H12N4O3S: C: 56.47%; H: 3.53%; N: 16.47%. Found: C: 56.72%; H: 3.64%; N: 16.72%.
Thiophene-2-carboxylic acid (3-cyano-1,4-di-N-oxide-6-methoxyquinoxalin-2-yl)amide (18). Yield 41.1 %; 1H-NMR δ ppm: 11.53 (bs, 1H, NH); 8.46 (d, 1H, H8, J8-7 = 9.4 Hz); 8.28 (dd, 1H, H5’, J5’-4’ = 3.8 Hz, J5’-3’ = 0.9 Hz); 8.05 (dd, 1H, H3’, J3’-4’ = 3.8 Hz); 7.79 (d, 1H, H5, J5-7 = 2.7 Hz); 7.73 (dd, 1H, H7); 7.32 (dd, 1H, H4’); 4.03 (s, 3H, OCH3); IR ν cm-1: 3,276 (m, NH); 2,232 (w, C≡N); 1,673 (s, C=O); 1,320 (s, N+O-); Anal. Calc. for C15H10N4O4S: C: 52.63%; H: 2.93%; N: 16.37%. Found: C: 52.49%; H: 3.06%; N: 16.35%.
Thiophene-2-carboxylic acid (6-chloro-3-cyano-1,4-di-N-oxidequinoxalin-2-yl)amide (19). Yield 33.2 %; 1H-NMR δ ppm: 11.66 (bs, 1H, NH); 8.54-8.48 (m, 2H, H8 +H5); 8.30 (dd, 1H, H5’, J5’‑4’ = 3.8 Hz, J5’-3’ = 0.8 Hz); 8.15 (dd, 1H, H7, J7-8 = 9.30 Hz, J7-5 = 2.2 Hz,); 8.06 (dd, 1H, H3’, J3’-4’ = 5.0 Hz); 7.32 (t, 1H, H4’); IR ν cm-1: 3,295 (m, NH); 2,251 (w, C≡N); 1,671 (s, C=O); 1,323 (s, N+O-); Anal. Calc. for C14H7ClN4O3S: C: 48.48%; H: 2.02%; N: 16.16%. Found: C: 48.58%; H: 2.16%; N: 16.15%.
Thiophene-2-carboxylic acid (3-cyano-6,7-dichloro-1,4-di-N-oxidequinoxalin-2-yl)amide (20). Yield 19.4 %; 1H-NMR δ ppm: 8.66 (s, 1H, H8 ); 8.64 (s, 1H, H5); 8.13 (s, 1H, H5’); 7.96 (d, 1H, H3’, J3’-4’ = 4.7 Hz) 7.32 (dd, 1H, H4’, J4’-5’ = 3.9 Hz,); IR KBr (trans): 3,263 (m, NH); 2,238 (w, C≡N); 1,668 (s, C=O); 1,335 (s, N+O-); Anal. Calc. for C14H6Cl2N4O3S: C: 44.09%; H: 1.57%; N: 14.70%. Found: C: 43.83%; H: 1.67%; N: 14.70%.
Thiophene-2-carboxylic acid (3-cyano-1,4-di-N-oxide-6-fluoroquinoxalin-2-yl)amide (21). Yield 46.0 %; 1H-NMR δ ppm: 11.63 (bs, 1H, NH); 8.60 (dd, 1H, H8, J8-7 = 9.5 Hz, J8-F = 4.9 Hz,); 8.31-8.27 (m, 2H, H5+H5´); 8.06-8.01 (m, 2H, H7 +H3´); 7.32 (t, 1H, H4’); IR ν cm-1: 3,263 (m, NH); 2,226 (w, C≡N); 1,665 (s, C=O); 1,327 (s, N+O-); Anal. Calc. for C14H7FN4O3S: C: 50.91%; H: 2.12%; N: 16.97%. Found: C: 51.18%; H: 2.24%; N: 17.06%.
Thiophene-2-carboxylic acid (3-cyano-6,7-difluoro-1,4-di-N-oxidequinoxalin-2-yl)amide (22). Yield 69.1 %; 1H-NMR δ ppm: 11.74 (bs, 1H, NH); 8.70-8.50 (m, 2H, H8 +H5); 8.25-8.35 (m, 1H, H5’); 8.07 (d, 1H, H3’, J3´-4´ = 4.9 Hz); 7.34-7.31(m, 1H, H4’); IR ν cm-1: 3,238 (m, NH); 2,238 (w, C≡N); 1,673 (s, C=O); 1,341 (s, N+O-); Anal. Calc. for C14H6F2N4O3S: C: 48.27%; H: 1.72%; N: 16.01%. Found: C: 48.47%; H: 1.80%; N: 16.18%.
Thiophene-2-carboxylic acid (3-cyano-7-trifluoromethyl-1,4-di-N-oxidequinoxalin-2-yl)amide (23). Yield 11.7 %; 1H-NMR δ ppm: 11.85 (bs, 1H, NH); 8.75 (d, 1H, H8, J8-6 = 0.75 Hz); 8.65 (d, 1H, H5, J5-6 = 9.0 Hz); 8.39 (dd, 1H, H6, J6-5 = 9.1 Hz, J6-8 = 1.8 Hz); 8.27 (dd, 1H, H5’, J5’-4’ = 3.8 Hz, J5’-3’ = 1.1 Hz); 8.05 (dd, 1H, H3’, J3’-4’ = 5.0 Hz); 7.31 (dd, 1H, H4’); IR KBr (trans): 3,263 (w, NH); 2,238 (w, C≡N); 1,668 (s, C=O); 1,335 (s, N+O-); Anal. Calc. for C15H7F3N4O3S: C: 47.37%; H: 1.84%; N: 14.74%. Found: C: 47.67%; H: 1.77%; N: 14.80%.
Thiophene-2-carboxylic acid (3-cyano-6-trifluoromethyl-1,4-di-N-oxidequinoxalin-2-yl)amide (24). Yield 12.8 %; 1H-NMR δ ppm: 11.83 (bs, 1H, NH); 8.74 (s, 1H, H5); 8.68 (d, 1H, H8, J8‑7 = 9.24 Hz); 8.35-8.28 (m, 2H, H7, H5’); 8.06 (dd, 1H, H3’, J3’-4’ = 5.0 Hz, J3’- 5’ = 1.0 Hz); 7.33-7.31 (m, 1H, H4’); IR KBr (trans): 3,270 (w, NH); 2,226 (w, C≡N); 1,679 (s, C=O); 1,339 (s, N+O-); Anal. Calc. for C15H7F3N4O3S: C: 47.37%; H: 1.84%; N: 14.74%. Found: C: 47.11%; H: 1.74%; N: 14.64%.
General procedure for the synthesis of 5-nitrofuran-2-carboxylic acid (3-cyano-1,4-di-N-oxide-quinoxalin-2-yl)amide derivatives and 5-nitrothiophene-2-carboxylic acid (3-cyano-1,4-N-oxide-quinoxalin-2-yl)amide derivatives 25-44
To a solution of the corresponding 3-amino-1,4-di-N-oxide-quinoxaline-2-carbonitrile II (2 mmol) in DMF (5 mL) are added 5-nitrothiophene-2-carboxylic acid or 5-nitrofuran-2-carboxylic acid (3 mmol). The reaction mixture is then gently stirred at room temperature under anhydrous conditions. Continuing with the synthesis, N-(3-dimethylaminopropyl)-N’-ethylcarbodiimide hydrochloride (EDCI, 6 mmol) are added, and the color changes to dark-red. When the dissolution is completed, 4-dimethyl-aminopyridine (DMAP, 6 or 7 mmol) are added. The reaction mixture is stirred between 17 h-72 h. After that, EtOAc (200 mL) is added and the organic phase is extracted, first with 10% HCl and then with saturated NaHCO3. The basic phase is treated with HCl 37% until pH 2, usually shown by the color changing to yellow. This phase is extracted with dichloromethane (3 × 75 mL), dried with anhydrous Na2SO4 and filtered. The solvent is removed in vacuo. The resulting residue is precipitated with diethyl ether and filtered in order to obtain a yellow-orange solid. Sometimes, when DMAP is added, the compound precipitates due to its acidity. In those cases, HCl (300 mL) is added and the mixture is stirred gently. The 3-amino-1,4-di-N-oxide-quinoxaline-2-carbonitrile derivatives are dissolved, and the precipitated compound is filtered. The compound is first washed with 10% HCl and then with diethyl ether.
5-Nitrofuran-2-carboxylic acid (3-cyano-1,4-di-N-oxide-quinoxalin-2-yl)-amide (25). Yield 27.8 %; 1H-NMR δ ppm: 8.52-8.48 (m, 2H, H8+H5); 8.14-8.10 (m, 1H, H7 ); 8.06-8.02 (m,1H, H6); 7.91 (d, 1H, H4’, J4´-3´ = 3.9 Hz); 7.85 (d, 1H, H3’); IR ν cm-1: 3,263 (m, NH); 2,232 (w, C°N); 1,694 (s, C=O); 1,533 (s, NO2); 1,357 (s, NO2); 1,333 (s, N+O-); Anal. Calc. for C14H7N5O6: C:49.28%; H:2.07%; N:20.52%. Found: C: 48.88%; H: 2.24%; N: 20.70%.
5-Nitrofuran-2-carboxylic acid (3-cyano-6-methyl-1,4-di-N-oxide-quinoxalin-2-yl)amide (26). Yield 23.5 %; 1H-NMR δ ppm: 8.41 (d, 1H, H8, J8-7 = 8.9 Hz); 8.31 (s, 1H, H5); 7.95 (d, 1H, H7); 7.89 (d, 1H, H4’, J4’-3’ = 3.8 Hz); 7.84 (d, 1H, H3´); 2.61 (s, 3H, CH3); IR ν cm-1: 3,250 (w, NH); 2,236 (w, C°N); 1,701 (s, C=O); 1,527 (s, NO2); 1,355 (s, NO2); 1,332 (s, N+O-); Anal. Calc. for C15H9N5O6: C: 50.71%; H: 2.55%; N: 19.71%. Found: C: 50.78%; H: 2.61%; N: 19.83%.
5-Nitrofuran-2-carboxylic acid (3-cyano-6,7-dimethyl-1,4-di-N-oxide-quinoxalin-2-yl)amide (27). Yield 11.3 %; 1H-NMR δ ppm: 8.31 (s, 1H, H8 ); 8.28 (s, 1H, H5); 7.91 (d, 1H, H4’, J4’‑3’ = 3.9 Hz); 7.85 (d, 1H, H3’); 2.54 (s, 3H, CH3); 2.53 (s, 3H, CH3); IR ν cm-1: 3,173 (m, NH); 2,942 (w, CH3); 2,232 (w, C°N); 1,700 (s, C=O); 1,542 (s, NO2); 1,349 (s, NO2); 1,317 (s, N+O-); Anal. Calc. for C16H11N5O6: C: 52.04%; H: 3.00%; N: 18.96%. Found: C: 52.35%; H: 3.25%; N: 18.61%.
5-Nitrofuran-2-carboxylic acid (3-cyano-6-methoxy-1,4-di-N-oxide-quinoxalin-2-yl)amide (28). Yield 30.1 %; 1H-NMR δ ppm: 8.45 (d, 1H, H8, J8-7 = 9.0 Hz); 7.90 (d, 1H, H4´, J4´-3´ = 3.9 Hz); 7.85 (d, 1H, H3´); 7.79 (d, 1H, H5, J5-7 = 2.5Hz); 7.74 (dd, 1H, H 7); 4.04 (s, 3H, OCH3); IR ν cm-1: 3,129 (m, NH); 2,239 (w, C°N); 1,693 (s, C=O); 1,538 (s, NO2); 1,388 (s, NO2); 1,326 (s, N+O-); Anal. Calc. for C15H9N5O7: C: 48.53 %; H: 2.44 %; N: 18.86%. Found: C: 48.23%; H: 2.41%; N: 18.66%.
5-Nitrofuran-2-carboxylic acid (6-chloro-3-cyano-1,4-di-N-oxide-quinoxalin-2-yl)amide (29). Yield 16.5 %; 1H-NMR δ ppm: 8.49-8.45 (m, 2H, H8+H5); 8.13 (dd, 1H, H7, J7-8 = 9.2 Hz, J7‑5 = 2.1 Hz); 7.87 (d, 1H, H4’, J4´-3´ = 3.8 Hz); 7.83 (d, 1H, H3’); 5.75 (s, 1H, NH); IR ν cm-1: 3,273 (m, NH); 2,238 (w, C°N); 1,711 (s, C=O); 1,537 (s, NO2); 1,351 (s, NO2); 1,327 (s, N+O-); 1,077 (m, Ar-Cl); Anal. Calc. for C14H6ClN5O6: C: 44.76 %; H: 1.61%; N: 18.64%. Found: C: 44.72%; H: 1.80%; N: 18.53%.
5-Nitrofuran-2-carboxylic acid (6,7-dichloro-3-cyano-1,4-di-N-oxide-quinoxalin-2-yl)amide (30). Yield 12.4 %; 1H-NMR δ ppm: 8.70 (s, 1H, H8 ); 8.66 (s, 1H, H5); 7.87 (d, 1H, H4’, J4’‑3’ = 3.9 Hz); 7.84 (d, 1H, H3’); IR ν cm-1: 3,263 (m, NH); 2,244 (w, C°N); 1,711 (s, C=O); 1,539 (s, NO2); 1,355 (s, NO2); 1,330 (s, N+O-); 1,073 (m, Ar-Cl); Anal. Calc. for C14H5Cl2N5O6: C: 41.00%; H: 1.23%; N: 17.08%. Found: C: 41.15%; H: 1.30%; N: 17.25%.
5-Nitrofuran-2-carboxylic acid (6-fluoro-3-cyano-1,4-di-N-oxide-quinoxalin-2-yl)amide (31). Yield 9.9 %; 1H-NMR δ ppm: 8.58 (dd, 1H, H8, J8-7 = 9.4 Hz, J8-F = 5.0 Hz); 8.30 (dd, 1H, H5, J5‑F = 8.7 Hz, J5-7 = 2.5 Hz); 8.06-8.01 (m, 1H, H7); 7.90 (d, 1H, H4’, J4’-3’ = 3.9 Hz); 7.84 (d, 1H, H3’); IR ν cm-1: 3,237 (m, NH); 2,238 (w, C°N); 1,708 (s, C=O); 1,536 (s, NO2); 1,357 (s, NO2); 1,331 (s, N+O-); 1,114 (m, Ar-F); Anal. Calc. for C14H6FN5O6: C: 46.81%; H: 1.68%; N: 19.50% Found: C: 46.53%; H: 1.74%; N: 19.55%.
5-Nitrofuran-2-carboxylic acid (6,7-difluoro-3-cyano-1,4-di-N-oxide-quinoxalin-2-yl)amide (32). Yield 38.6 %; 1H-NMR δ ppm: 8.63 (dd, 1H, H8, J8-F7 = 9.9 Hz, J8-F6 = 7.2 Hz); 8.58 (dd, 1H, H5, J5-F6 = 9.9 Hz, J5-F7 = 7.3Hz); 7.89 (d, 1H, H4’, J4’-3’ = 3.9 Hz); 7.84 (d, 1H, H3’); 5.76 (s, 1H, NH); IR ν cm-1: 3,268 (m, NH); 2,244 (w, C°N); 1,711 (s, C=O); 1,534 (s, NO2); 1,394 (s, NO2); 1,350 (s, N+O-); 1,106 (m, Ar-F); Anal. Calc. for C14H5F2N5O6: C: 44.58%; H: 1.34%; N: 18.57%. Found: C: 44.04%; H: 1.34%; N: 18.33%.
5-Nitrofuran-2-carboxylic acid (7-trifluoromethyl-3-cyano-1,4-di-N-oxide-quinoxalin-2-yl)amide (33). Yield 39.8 %; 1H-NMR δ ppm: 8.67 (s, 1H, H8); 8.54 (d, 1H, H5, J5-6 = 8.9 Hz); 8.37 (dd, 1H, H6, J6-8 = 1.6 Hz); 7.84 (s, 2H, H4´+H3´); IR ν cm-1: 3,275 (m, NH); 2,244 (w, C°N); 1,707 (s, C=O); 1,536 (s, NO2); 1,391 (s, NO2); 1,350 (s, N+O-); 1,173 (m, Ar-CF3); Anal. Calc. for C15H6F3N5O6: C: 44.02%; H: 1.48%; N: 17.11%. Found: C: 44.08%; H: 1.47%; N: 16.71%.
5-Nitrofuran-2-carboxylic acid (6-trifluoromethyl-3-cyano-1,4-di-N-oxide-quinoxalin-2-yl)amide (34). Yield 51.8 %; 1H-NMR δ ppm: 8.68 (s, 1H, H5); 8.66 (d, 1H, H8, J8-7 = 9.1Hz); 8.30 (d, 1H, H7 ); 7.89 (d, 1H, H4’, J4´-3´ = 3.9Hz); 7.84 (d, 1H, H3’). IR ν cm-1: 3277 (m, NH); 2238 (w, C°N); 1709 (s, C=O); 1538 (s, NO2); 1391 (s, NO2); 1350 (s, N+O-); 1173 (m, Ar-CF3 ). Calculated analysis for C15H6F3N5O6: C:44.02%; H:1.48%; N:17.11%. Found: C:44.26%; H:1.61%; N:16.43%.
5-Nitrothiophene-2-carboxylic acid (3-cyano-1,4-di-N-oxide-quinoxalin-2-yl)amide (35). Yield 36.2 %; 1H-NMR δ ppm: 8.49 (s, 1H, H8 ), 8.47 (s, 1H, H5), 8.26-8.23 (m, 2H, H4’+H3´) 8.11 (dd, 1H, H6, J6-5 = 8.3 Hz, J6-7 = 7.4 Hz), 8.00 (dd, 1H, H7, J7-8 = 8.1 Hz); IR ν cm-1: 3,278 (w, NH); 2,232 (w, C°N); 1,673 (s, C=O); 1,538 (s, NO2); 1,356 (m, NO2); 1,333 (s, N+O-); Anal. Calc. for C14H7N5O5S: C: 47.06%; H: 1.97%; N: 19.60%. Found: C: 47,16 %; H: 2.31%; N: 19.31%.
5-Nitrothiophene-carboxylic acid (3-cyano-6-methyl-1,4-di-N-oxide-quinoxalin-2-yl)amide (36). Yield 41.3 % 1H-NMR δ ppm: 8.41 (d, 1H, H8, J8-7 = 8.8 Hz); 8.31 (s, 1H, H5); 8.25 (s, 2H, H3’+H4’); 7.96 (dd, 1H, H7, J7-8 = 8.8 Hz, J7-5 = 1.7 Hz); 2.61 (s, 3H, CH3); IR ν cm-1: 3,231 (w, NH); 2,238 (w, C°N); 1,677 (s, C=O); 1,528 (m, NO2); 1,348 (m, NO2); 1,325 (s, N+O-); Anal. Calc. for C15H9N5O5S: C: 48.52%; H: 2.44%; N: 18.86%. Found: C: 48.26%; H: 2.48%; N: 19.05%.
5-Nitrothiophene-2-carboxylic acid (3-cyano-6,7-dimethyl-1,4-di-N-oxide-quinoxalin-2-yl)amide (37). Yield 8.6 %; 1H-NMR δ ppm: 8.29 (s, 1H, H8 ); 8.28 (s, 1H, H5); 8.24 (s, 2H, H3´+H4´); 5.76 (s, 1H, NH); 2.54 (s, 3H, CH3); 2.52 (s, 3H, CH3). IR ν cm-1: 3,244 (w, NH); 2,238 (w, C°N); 1,676 (m, C=O); 1,528 (s, NO2); 1,372 (m, NO2); 1,332 (s, N+O-). Anal. Calc. for C16H11N5O5S: C: 49.87%; H: 2.88%; N:18.17%. Found: C: 49.82%; H: 2.98%; N: 18.00%.
5-Nitrothiophene-2-carboxylic acid (3-cyano-6-methoxy-1,4-di-N-oxide-quinoxalin-2-yl)amide (38). Yield 5.6 %; 1H-NMR δ ppm: 8.43 (d, 1H, H8, J8-7 = 8.8 Hz); 8.22 (d, 1H, H4´, J4´-3´ = 4.0 Hz); 8.16 (d, 1H, H3´); 7.77 (s, 1H, H5); 7.72 (d, 1H, H7 ); 4.03 (s, 3H, OCH3); IR ν cm-1: 3,270 (w, NH); 2,238 (w, C°N); 1,681 (w, C=O); 1,515 (s, NO2); 1,348 (m, NO2); 1,333 (s, N+O-); Anal. Calc. for C15H9N5O6S: C: 46.51 %; H: 2.34 %; N: 18.08%. Found: C: 46.64%; H: 2.52%; N: 18.28%.
5-Nitrothiophene-2-carboxylic acid (6-chloro-3-cyano-1,4-di-N-oxide-quinoxalin-2-yl)amide (39). Yield 36.7 %; 1H-NMR δ ppm: 8.50 (d, 1H, H5, J5-7 = 2.2 Hz); 8.48 (d, 1H, H8, J8-7 = 9.2 Hz); 8.24 (d, 1H, H4´, J4´-3´ = 4.4 Hz); 8.23 (d, 1H, H3´); 8.14 (dd, 1H, H7, J7-8 = 9.2 Hz, J7-5 = 2.2 Hz); IR ν cm-1: 3,275 (w, NH); 2,239 (w, C°N); 1,677 (s, C=O); 1,519 (s, NO2); 1,357 (m, NO2); 1,323 (s, N+O-); 1,090 (m, Ar-Cl); Anal. Calc. for C14H6ClN5O5S: C: 42.92 %; H: 1.54%; N: 17.88%. Found: C: 43.01%; H: 1.71%; N: 18.02%.
5-Nitrothiophene-2-carboxylic acid (6,7-dichloro-3-cyano-1,4-di-N-oxide-quinoxalin-2-yl)amide (40). Yield 4.4 %; 1H-NMR δ ppm: 8.66 (s, 1H, H8); 8.60 (s, 1H, H5 ); 8.19 (s, 1H, H4’); 8.05 (s, 1H, H3’); IR ν cm-1: 3,256 (w, NH); 2,238 (w, C°N); 1,675 (m, C=O); 1,505 (s, NO2); 1,354 (m, NO2); 1,330 (s, N+O-); Anal. Calc. for C14H5Cl2N5O5S: C: 39.46%; H: 1.18%; N: 16.43%. Found: C: 39.00%; H: 1.35%; N: 16.07%.
5-Nitrothiophene-2-carboxylic acid (6-fluoro-3-cyano-1,4-di-N-oxide-quinoxalin-2-yl)amide (41). Yield 24.0 %; 1H-NMR δ ppm: 8.60-8.56 (m, 1H, H8); 8.32-8.29 (m, 1H, H5); 8.25 (s, 2H, H4´+H3´); 8.07-8.02 (m, 1H, H6); 5.76 (s, 1H, NH); IR ν cm-1: 3,293 (m, NH); 2,238 (w, C°N); 1,671 (s, C=O); 1,514 (s, NO2); 1,349 (m, NO2); 1,332 (s, N+O-); 1,114 (m, Ar-F); Anal. Calc. for C14H6FN5O5S: C: 44.81%; H: 1.61%; N: 18.66%. Found: C: 44.31%; H: 1.69%; N: 18.44%.
5-Nitrothiophene-2-carboxylic acid (6,7-difluoro-3-cyano-1,4-di-N-oxide-quinoxalin-2-yl)amide (42). Yield 33.4 %; 1H-NMR δ ppm: 8.65-8.55 (m, 2H, H8+H5); 8.24 (d, 1H, H4´, J4´-3´ = 4.5 Hz); 8.23 (d, 1H, H3´); IR ν cm-1: 3,256 (w, NH); 2,232 (w, C°N); 1,685 (s, C=O); 1,517 (s, NO2); 1,359 (s, NO2); 1,341 (s, N+O-); Anal. Calc. for C14H5F2N5O5S: C: 42.76%; H: 1.28%; N: 17.81%. Found: C: 42.78%; H: 1.30%; N: 17.89%.
5-Nitrothiophene-2-carboxylic acid (7-trifluoromethyl-3-cyano-1,4-di-N-oxide-quinoxalin-2-yl)amide (43). Yield 51.8 %; 1H-NMR δ ppm: 8.65 (d, 1H, H5, J5-6 = 8.8 Hz); 8.65 (s, 1H, H8 ); 8.27 (dd, 1H, H6, J6-8 = 1.5Hz); 8.23 (d, 1H, H4’, J4´-3´ = 4.4 Hz); 8.18 (d, 1H, H3’); IR ν cm-1: 3,244 (w, NH); 2,238 (w, C°N); 1,672 (s, C=O); 1,506 (s, NO2); 1,399 (s, NO2); 1,338 (s, N+O-); 1,130 (m, Ar‑CF3); Anal. Calc. for C15H6F3N5O5S: C: 42.36%; H: 1.42%; N: 16.47%. Found: C: 42.58%; H: 1.60%; N: 16.49%.
5-Nitrothiophene-2-carboxylic acid (6-trifluoromethyl-3-cyano-1,4-di-N-oxide-quinoxalin-2-yl)amide (44). Yield 57.9 %; 1H-NMR δ ppm: 8.64 (s, 1H, H5); 8.47 (d, 1H, H8, J8-7 = 8.9 Hz); 8.35 (d, 1H, H7); 8.20 (d, 1H, H4’, J4´-3´ = 4.3 Hz); 8.08 (d, 1H, H3’); IR ν cm-1: 3,247 (w, NH); 2,238 (w, C°N); 1,675 (s, C=O); 1,520 (s, NO2); 1,399 (m, NO2); 1,333 (s, N+O-); 1,139 (m, Ar-CF3); Anal. Calc. for C15H6F3N5O5S: C: 42.36%; H: 1.42%; N: 16.47%. Found: C: 42.62%; H: 1.72%; N: 16.57%.
In vitro evaluation of antituberculosis activity
In vitro evaluation of the antituberculosis activity was carried out at the GWL Hansen’s Disease Center within the Tuberculosis Antimicrobial Acquisition & Coordinating Facility (TAACF) screening program for the discovery of novel drugs for the treatment of tuberculosis. The Southern Research Institute coordinates the overall program under the direction of the U.S. National Institute of Allergy and Infectious Disease (NIAID). The purpose of the screening program is to provide a resource whereby new experimental compounds can be tested for their capability to inhibit the growth of virulent
M. tuberculosis [
26].
Determination of growth inhibition percentage via MABA: The initial screen is conducted against
Mycobacterium tuberculosis H37Rv (ATCC 27294) in BACTEC 12B medium using the Microplate Alamar Blue Assay (MABA) [
27]. The fluorescence changes due to the reduction of Alamar blue dye during the growth of
Mycobacterium were monitored by the BACTEC 460-radiometric system. Compounds effecting <90% inhibition in the primary screen (MIC >6.25 μg/mL) were not further evaluated.
Determination of minimum inhibitory concentration (MIC) via MABA: Compounds demonstrating at least 90% inhibition in the primary screen were re-tested against M. tuberculosis H37Rv at lower concentrations in order to determine the actual minimum inhibitory concentration (MIC) in the MABA. The MIC was defined as the lowest concentration effecting a reduction in fluorescence of 90% relative to controls. RIF was used as the reference compound (RIF MIC = 0.015*20130.125 μg/mL).
In vitro evaluation of trypanocidal activity
Trypanosoma cruzi epimastigotes (Tulahuen 2 strain) were grown at 28 ºC in an axenic medium (BHI-Tryptose) as previously described [
28,
29,
30], supplemented with 5% fetal bovine serum (FBS). Cells from a 10-day-old culture (stationary phase) were inoculated into 50 mL of fresh culture medium in order to give an initial concentration of 1 × 10
6 cells/mL. Cell growth was followed by measuring the absorbance of the culture at 600 nm daily. Before inoculation, the medium was supplemented with the indicated amount of the drug from a stock solution in DMSO. The final concentration of DMSO in the culture medium never exceeded 0.4%, and the control was run in the presence of 0.4% DMSO and in the absence of any drug. No effect on epimastigote growth was observed by the presence of up to 1% DMSO in the culture medium. The percentage of growth inhibition (PGI) was calculated as follows: PGI (%) = {1 - [(Ap - A0p)/(Ac - A0c)]} × 100, where Ap = A
600 of the culture containing the drug at day 5; A0p = A
600 of the culture containing the drug just after addition of the inocula (day 0);Ac = A
600 of the culture in the absence of the drug (control) at day 5; A0c = A
600 in the absence of the drug at day 0. In order to determine IC
50 values, 50% inhibitory concentrations, parasite growth was observed in the absence (control) and presence of increasing concentrations of the corresponding drug. At day 5, the absorbance of the culture was measured and related to the control. The IC
50 value was considered to be the concentration of drug needed for reducing the absorbance ratio to 50%.