Transition-Metal-Free Highly Efficient Aerobic Oxidation of Sulfides to Sulfoxides under Mild Conditions
Abstract
:1. Introduction
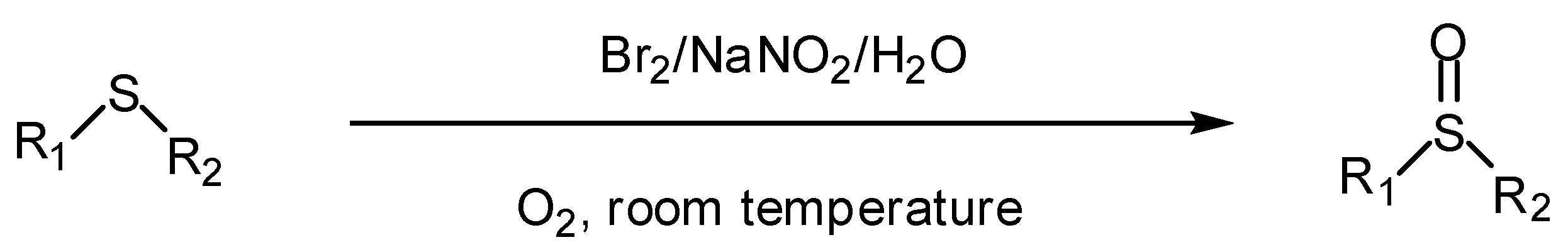
2. Results and Discussion
| Entry | NaNO2 (mol %)b | Br2 (mol %) b | Conversion (%) c |
|---|---|---|---|
| 1 | 0 | 3 | 21 |
| 2 | 5 | 0 | 10 |
| 3 | 5 | 3 | 100 |
2.1. Aerobic oxidation of sulfides to sulfoxides
| Entry | Substrate | Product | Time (h) | Br2 (mol %) | Conversion (%)b | Yield (%)c | |
|---|---|---|---|---|---|---|---|
| 1 | 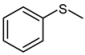 | 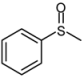 | 1 | 3 | 100 | 96 | |
| 2 | 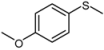 | 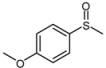 | 1 | 3 | 100 | 92 | |
| 3 | 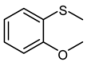 |  | 1 | 3 | 100 | 95 | |
| 4 |  | 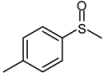 | 1 | 3 | 100 | 94 | |
| 5 | 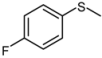 |  | 1 | 3 | 100 | 95 | |
| 6 | 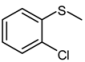 | 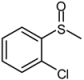 | 1 | 3 | 100 | 94 | |
| 7 |  | 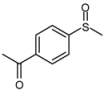 | 1 | 3 | 100 | 93 | |
| 8 |  | 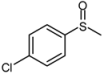 | 2 | 3 | 100 | 92 | |
| 9 | 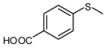 | 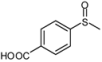 | 5 | 5 | 100 | 96 | |
| 10 | 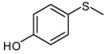 |  | 5 | 5 | 25 | 21d | |
| 11 | 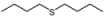 | 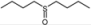 | 1 | 3 | 100 | 92 | |

2.2. Mechanism of aerobic sulfide oxidation

3. Experimental
3.1. Materials
3.2. Instrumentation
3.3. General procedure for catalytic aerobic sulfides oxidation
3.4. Spectroscopic data for products
4. Conclusions
Acknowledgements
- Sample Availability: Samples of the compounds are available from the authors.
References and Notes
- Hudlicky, M. Oxidations in Organic Chemistry; American Chemical Society: Washington, DC, USA, 1990. [Google Scholar]
- Carreno, M.C. Applications of Sulfoxides to asymmetric synthesis of biologically active compounds. Chem. Rev. 1995, 95, 1717–1760. [Google Scholar] [CrossRef]
- Solladie, G. Comprehensive Organic Synthesis; Trost, B.M., Fleming, I., Eds.; Pergamon: Oxford, UK, 1991; Volume 6, pp. 148–170. [Google Scholar]
- Holland, H.L. Chiral sulfoxidation by biotransformation of organic sulfides. Chem. Rev. 1988, 88, 473–485. [Google Scholar] [CrossRef]
- Block, E. The organosulfur chemistry of the genus allium-implications for the organic chemistry of sulfur. Angew. Chem. Int. Ed. Engl. 1992, 31, 1135–1178. [Google Scholar] [CrossRef]
- Compagnini, A.; Santagati, M.; Marziano, N.; Passerini, R. Reaction of nitric acid with aromatic sulfur compounds. VI. Scission of diphenyl sulfides with bromine and nitric acid. Ann. Chim-Rome 1970, 60, 537–542. [Google Scholar]
- Oae, S.; Kawai, T.; Furukawa, N. Ligand coupling through σ-sulfurane-complete retention of configuration of 1-phenylethyl group in the reaction of 1-phenylethyl 2-pyridyl sulfoxide with grignard reagent1. Tetrahedron Lett. 1984, 35, 69–72. [Google Scholar]
- Xiong, Z.X.; Huang, N.P.; Zhong, P. A selective and convenient oxidation of sulfides to sulfoxides with trichloroisocyanuric acid. Syn.Commun. 2001, 31, 245–248. [Google Scholar] [CrossRef]
- Novitskaya, N.N.; Chernikova, S.I. Comparative study of the oxidation of sulfides by hydrogen peroxide. Neftekhimiya 1970, 10, 429–436. [Google Scholar]
- Kirihara, M.; Yamamoto, J.; Noguchi, T.; Hirai, Y. Selective synthesis of sulfoxides and sulfones by tantalum (V) catalyzed oxidation of sulfides with 30% hydrogen peroxide. Tetrahedron Lett. 2009, 50, 1180–1183. [Google Scholar] [CrossRef]
- Boring, E.; Geletii, Y.V.; Hill, C.L. Catalysts for Selective Aerobic Oxidation under Ambient Conditions, in Catalysis by Metal Complexes; Simándi, L.I., Ed.; Springer: New York, NY, USA, 2003; Volume 26, pp. 227–264. [Google Scholar]
- Ishii, Y.; Sakaguchi, S. Modern Oxidation Methods; Wiley-VCH: Weinheim, Gemany, 2004; pp. 119–163. [Google Scholar]
- Bortolini, O.; Di Furia, F.; Modena, G. Metal catalysis in oxidation by peroxides. Part 16. Kinetics and mechanism of titanium-catalyzed oxidation of sulfides with hydrogen peroxide. J. Mol. Catal. 1982, 16, 69–80. [Google Scholar] [CrossRef]
- Linden, A.A.; Kruger, L.; Backvall, J.E. Highly Selective sulfoxidation of allylic and vinylic sulfides by hydrogen peroxide using a flavin as catalyst. J. Org. Chem. 2003, 68, 5890–5896. [Google Scholar] [CrossRef]
- Yao, H.; Richardson, D.E. Bicarbonate surfoxidants: Micellar oxidations of aryl sulfides with bicarbonate-activated hydrogen peroxide. J. Am. Chem. Soc. 2003, 125, 6211–6221. [Google Scholar]
- Trevisan, V.; Signoretto, M.; Colonna, S.; Pironti, V.; Strukul, G. Microencapsulated chloroperoxidase as a recyclable catalyst for the enantioselective oxidation of sulfides with hydrogen peroxide. Angew. Chem. Int. Ed. 2004, 43, 4097–4099. [Google Scholar]
- Mba, M.; Prins, L.J.; Licini, G. C3-Symmetric Ti(IV) triphenolate amino complexes as sulfoxidation catalysts with aqueous hydrogen peroxide. Org. Lett. 2007, 9, 21–24. [Google Scholar]
- Golchoubian, H.; Hosseinpoor, F. Effective oxidation of sulfides to sulfoxides with hydrogen peroxide under transition-metal-free conditions. Molecules 2007, 12, 304–311. [Google Scholar] [CrossRef]
- Mashkina, A.V. Catalytic synthesis of sulfoxides and sulfones via oxidation of sulfides by molecular oxygen. Cat. Rev. Sci. Eng. 1990, 32, 105–161. [Google Scholar] [CrossRef]
- Komatsu, M.; Uda, M.; Suzuki, H. Air oxidation of sulfides to sulfoxides using BiBr3-Bi(NO3)3 as a binary catalyst. Chem. Lett. 1997, 1229–1230. [Google Scholar]
- Iwahama, T.; Sakaguchi, S.; Ishii, Y. Selective oxidation of sulfides to sulfoxides with molecular oxygen catalyzed by N-hydroxyphthalimide (NHPI) in the presence of alcohols. Tetrahedron lett. 1998, 39, 9059–9062. [Google Scholar]
- Shii, Y.; Sakaguchi, S.; Iwahama, T. Innovation of hydrocarbon oxidation with molecular oxygen and related reactions. Adv. Synth. Catal. 2001, 343, 393–427. [Google Scholar] [CrossRef]
- Huang, R.L.; Espenson, J.H. Molecular oxygen reactions catalyzed by an oxorhenium(V) Compound. J. Mol. Catal. A Chem. 2001, 168, 39–46. [Google Scholar] [CrossRef]
- Aldea, R.; Alper, H. Selective aerobic oxidation of sulfides using a novel palladium complex as the catalyst precursor. J.Org. Chem. 1995, 60, 8365–8366. [Google Scholar] [CrossRef]
- Zhou, X.T.; Ji, H.B.; Cheng, Z.; Xu, J.C.; Pei, L.X.; Wang, L.F. Selective oxidation of sulfides to sulfoxides catalyzed by ruthenium (III) meso-tetraphenylporphyrin chloride in the presence of molecular oxygen. Bioorg. Med. Chem. Lett. 2007, 17, 4650–4653. [Google Scholar] [CrossRef]
- Song, G.Q.; Wang, F.; Zhang, H.; Lu, X.L.; Wang, C. Efficient oxidation of sulfides catalyzed by transition metal salts with molecular oxygen in the presence of aldehydes. Syn. Commun. 1998, 28, 2783–2787. [Google Scholar]
- Firouzabadi, H.; Iranpour, N.; Zolfigol, M.A. Selective and effcient transformation of thioethers to their sulfoxides and catalytic conversion of thiols to the disulfides with hydrate iron(III) and copper(II) nitrates in aprotic organic solvents or under solvent free conditions. Syn. Commun. 1998, 28, 1179–1187. [Google Scholar] [CrossRef]
- Martin, S.E.; Rossi, L.I. An efficient and selective aerobic oxidation of sulfides to sulfoxides catalyzed by Fe(NO3)3-FeBr3. Tetrahedron Lett. 2001, 42, 7147–7151. [Google Scholar] [CrossRef]
- Okun, N.M.; Tarr, J.C.; Hilleshiem, D.A.; Zhang, L.; Hardcastle, K.I.; Hill, C.L. Highly reactive catalysts for aerobic thioether oxidation. The Fe-substituted polyoxometalate/hydrogen dinitrate system. J. Mol. Catal. A Chem. 2006, 246, 11–17. [Google Scholar] [CrossRef]
- Dell'Anna, M.M.; Mastrorilli, P.; Nobile, C.F. Aerobic oxidation of sulfides catalyzed by cobalt(II) complexes under homogeneous and heterogeneous conditions. J. Mol. Catal. A Chem. 1996, 108, 57–62. [Google Scholar] [CrossRef]
- Boring, E; Geletii, Y.V; Hill, C.L. A homogeneous catalyst for selective O(2) oxidation at ambient temperature diversity-based discovery and mechanistic investigation of thioether oxidation by the Au(III)Cl(2)NO(3)(thioether)/O(2) system. J. Am. Chem. Soc. 2001, 123, 1625–1635. [Google Scholar] [CrossRef]
- Bosch, E; Kochi, J.K. Selective catalysis of thioether oxidations with dioxygen. Critical role of nitrosonium EDA Complexes in the thermal and photochemical transfer of oxygen atom from nitrogen oxides to sulfur centers. J. Org. Chem. 1995, 60, 3172–3183. [Google Scholar] [CrossRef]
- Stamier, J.S.; Singel, D.J.; Loscalzo, J. Biochemistry of nitric oxide and its redox-activated forms. Science 1992, 258, 1898–1902. [Google Scholar]
- Wang, P.G.; Xian, M.; Tang, X.; Wu, X.; Wen, Z.; Cai, T.; Janczuk, A.J. Nitric oxide donors: Chemical activities and biological applications. Chem. Rev. 2002, 102, 1091–1134. [Google Scholar] [CrossRef]
- Zhang, H.; Chen, C.Y.; Liu, R.H.; Rui, Y. DBDMH/NaNO2 catalyst: Transition-metal-free approach to highly efficient aerobic oxidation of sulfides to sulfoxides. Syn. Commun. 2008, 38, 4445–4451. [Google Scholar]
- Chen, C.Y.; Zhang, H.; Zhang, L.X.; Li, L.D.; Yan, Y.L. HBr/t-BuONO catalysts for activation of oxygen to selective aerobic sulfide oxidation. Chin. J. Org. Chem. 2008, 28, 1978–1981. [Google Scholar]
- Kowalski, P.; Mitka, K.; Ossowska, K.; Kolarska, Z. Oxidation of sulfides to sulfoxides. Part 1: Oxidation using halogen derivatives. Tetrahedron 2005, 61, 1933–1953. [Google Scholar] [CrossRef]
- Bravo, A.; Dordi, B.; Fontana, F.; Minisci, F. Oxidation of organic Sulfides by Br2 and H2O2 electrophilic and free-radical processes. J. Org. Chem. 2001, 66, 3232–3234. [Google Scholar]
- Liu, R.H.; Liang, X.M.; Dong, C.Y.; Hu, X.Q. Transition-metal-free: A highly efficient catalytic aerobic alcohol oxidation process. J. Am. Chem. Soc. 2004, 126, 4112–4113. [Google Scholar] [CrossRef]
© 2010 by the authors; licensee Molecular Diversity Preservation International, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Zhang, H.; Chen, C.; Liu, R.; Xu, Q.; Zhao, W. Transition-Metal-Free Highly Efficient Aerobic Oxidation of Sulfides to Sulfoxides under Mild Conditions. Molecules 2010, 15, 83-92. https://doi.org/10.3390/molecules15010083
Zhang H, Chen C, Liu R, Xu Q, Zhao W. Transition-Metal-Free Highly Efficient Aerobic Oxidation of Sulfides to Sulfoxides under Mild Conditions. Molecules. 2010; 15(1):83-92. https://doi.org/10.3390/molecules15010083
Chicago/Turabian StyleZhang, Hua, Chunyu Chen, Renhua Liu, Qiang Xu, and Weiqie Zhao. 2010. "Transition-Metal-Free Highly Efficient Aerobic Oxidation of Sulfides to Sulfoxides under Mild Conditions" Molecules 15, no. 1: 83-92. https://doi.org/10.3390/molecules15010083
APA StyleZhang, H., Chen, C., Liu, R., Xu, Q., & Zhao, W. (2010). Transition-Metal-Free Highly Efficient Aerobic Oxidation of Sulfides to Sulfoxides under Mild Conditions. Molecules, 15(1), 83-92. https://doi.org/10.3390/molecules15010083





