Free Radical Scavenging Activity and Characterization of Sesquiterpenoids in Four Species of Curcuma Using a TLC Bioautography Assay and GC-MS Analysis
Abstract
:1. Introduction
2. Results and Discussion
2.1. Optimization of method
2.2. Validation of the method
2.2.1. Calibration curve for antioxidant capacity of vitamin C
2.2.2. Stability
2.2.3. Repeatability
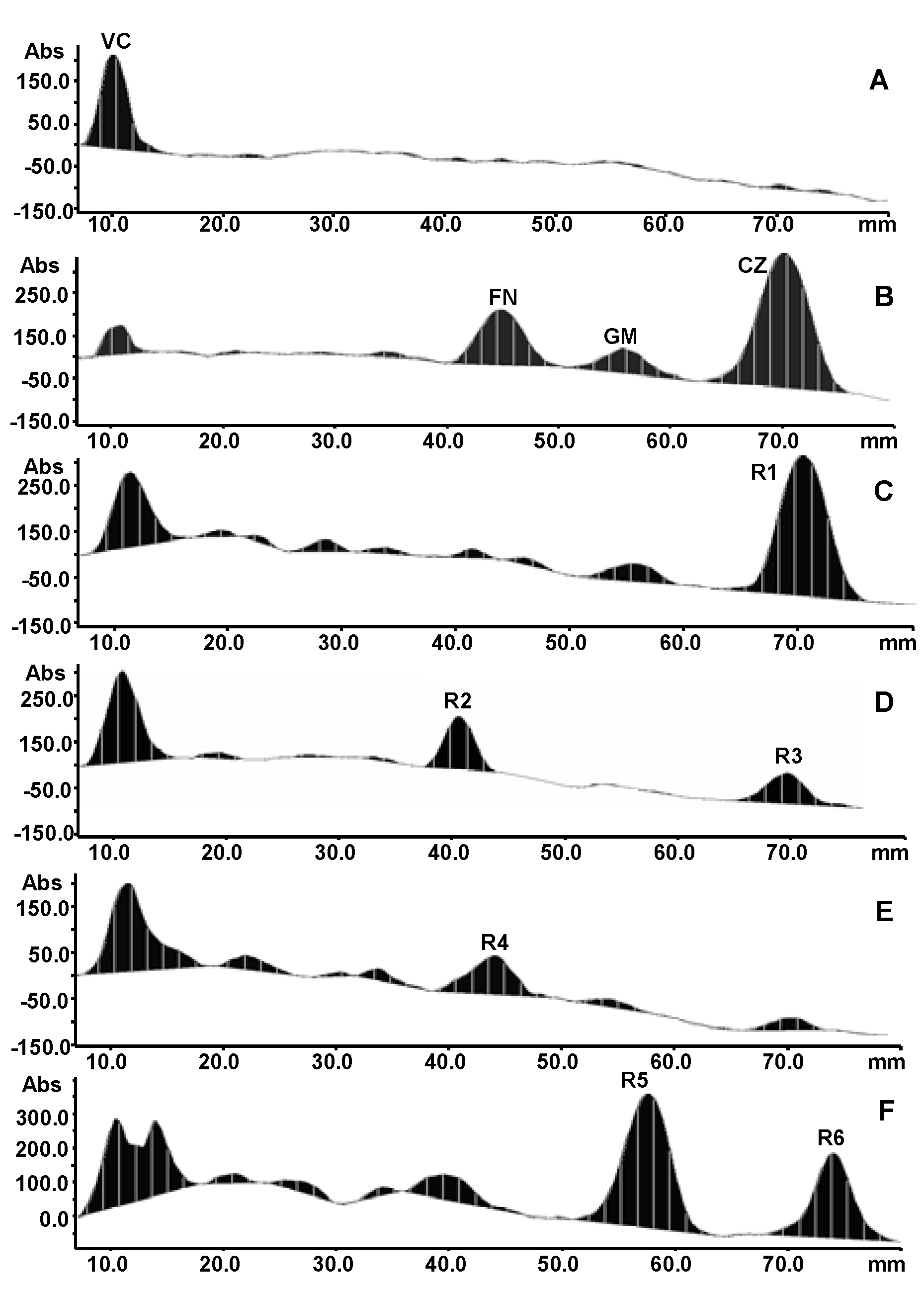
2.3. TLC bioautography assay
2.4. Identification of compounds in active bands
2.5. Comparison of antioxidant capacities of four species of Curcuma
3. Experimental
3.1. Materials
3.2. Chemicals
3.3. Standard and sample preparation
3.4. TLC analysis
3.5. Characterization of antioxidants
4. Conclusions
Acknowledgements
References
- Pharmacopoeia Commission of PRC (Ed.) Pharmacopoeia of the People’s Republic of China; China Medical Science and Technology Press: Beijing, China, 2010; Volume I, pp. 247–248. [Google Scholar]
- Sacchetti, G.; Maietti, S.; Muzzoli, M.; Scaglianti, M.; Manfredini, S.; Radice, M.; Bruni, R. Comparative evaluation of 11 essential oils of different origin as functional antioxidants, antiradicals and antimicrobials in food. Food Chem. 2005, 91, 621–632. [Google Scholar] [CrossRef]
- Li, S.Y.; Li, S.P. Antioxidant activities of essential oil of Curcuma longa and Curcuma wenyujin. Int. J. Essent. Oil Ther. 2009, 3, 31–34. [Google Scholar]
- Yang, F.Q.; Li, S.P.; Chen, Y.; Lao, S.C.; Wang, Y.T.; Dong, T.T.; Tsim, K.W. Identification and quantitation of eleven sesquiterpenes in three species of Curcuma rhizomes by pressurized liquid extraction and gas chromatography–mass spectrometry. J. Pharm. Biomed. Anal. 2005, 39, 552–558. [Google Scholar] [CrossRef] [PubMed]
- Yang, F.Q.; Wang, Y.T.; Li, S.P. Simultaneous determination of 11 characteristic components in three species of Curcuma rhizomes using pressurized liquid extraction and high-performance liquid chromatography. J. Chromatogr. A 2006, 1134, 226–231. [Google Scholar] [CrossRef] [PubMed]
- Diallo, D.; Marston, A.; Terreaux, C.; Touré, Y.; Smestad Paulsen, B.; Hostettmann, K. Screening of Malian medicinal plants for antifungal, larvicidal, molluscicidal, antioxidant and radical scavenging activities. Phytother. Res. 2001, 15, 401–406. [Google Scholar] [CrossRef] [PubMed]
- Chatterjee, S.; Niaz, Z.; Gautam, S.; Adhikari, S.; Variyar, P.S.; Sharma, A. Antioxidant activity of some phenolic constituents from green pepper (Piper nigrum L.) and fresh nutmeg mace (Myristica fragrans). Food Chem. 2007, 101, 515–523. [Google Scholar] [CrossRef]
- Lee, S.E.; Hwang, H.J.; Ha, J.S.; Jeong, H.S.; Kim, J.H. Screening of medicinal plant extracts for antioxidant activity. Life Sci. 2003, 73, 167–179. [Google Scholar] [CrossRef]
- Zhao, G.R.; Zhang, H.M.; Ye, T.X.; Xiang, Z.J.; Yuan, Y.J.; Guo, Z.X.; Zhao, L.B. Characterization of the radical scavenging and antioxidant activities of danshensu and salvianolic acid B. Food Chem. Toxicol. 2008, 46, 73–81. [Google Scholar] [CrossRef] [PubMed]
- Koleva, I.I.; Niederländer, H.A.; van Beek, T.A. An on-line HPLC method for detection of radical scavenging compounds in complex mixtures. Anal. Chem. 2000, 72, 2323–2328. [Google Scholar] [CrossRef] [PubMed]
- Dapkevicius, A.; van Beek, T.A.; Niederländer, H.A. Evaluation and comparison of two improved techniques for the on-line detection of antioxidants in liquid chromatography eluates. J. Chromatogr. A 2001, 912, 73–82. [Google Scholar] [CrossRef]
- Koleva, I.I.; Niederländer, H.A.; van Beek, T.A. Application of ABTS radical cation for selective on-line detection of radical scavengers in HPLC eluates. Anal. Chem. 2001, 73, 3373–3381. [Google Scholar] [CrossRef] [PubMed]
- Li, S.P; Zhao, J.; Qian, Z.M.; Li, J. Advanced development of chromatography in screening and identification of effective compounds in Chinese materia medica. Sci. Sinica Chim. 2010, 40, 651–667. [Google Scholar]
- Teijo, Y.; Li, P.W.; Jari, S.; Anu, H.; Heikki, V. Free radical-scavening activity of phenolics by reversed-phase TLC. J. Amer. Oil Chem. Soc. 2003, 80, 9–14. [Google Scholar]
- Bhattarai, H.D.; Paudel, B.; Hong, S.G.; Lee, H.K.; Yim, J.H. Thin layer chromatography analysis of antioxidant constituents of lichens from Antarctica. Nat. Med. 2008, 62, 481–484. [Google Scholar] [CrossRef] [PubMed]
- Pozharitskaya, O.N.; Ivanova, S.A.; Shikov, A.N.; Makarov, V.G. Separation and evaluation of free radical-scavenging activity of phenol components of Emblica officinalis extract by using an HPTLC-DPPH* method. J. Sep. Sci. 2007, 30, 1250–1254. [Google Scholar] [CrossRef] [PubMed]
- Pozharitskaya, O.N.; Ivanova, S.A.; Shikov, A.N.; Makarov, V.G.; Galambosi, B. Separation and evaluation of free radical-scavenging activity of phenol components of green, brown, and black leaves of Bergenia crassifolia by using HPTLC-DPPH* method. J. Sep. Sci. 2007, 30, 2447–2451. [Google Scholar] [CrossRef] [PubMed]
- Jaime, L.; Mendiola, J.A.; Herrero, M.; Soler-Rivas, C.; Santoyo, S.; Señorans, F.J.; Cifuentes, A.; Ibáñez, E. Separation and characterization of antioxidants from Spirulina platensis microalga combining pressurized liquid extraction, TLC, and HPLC-DAD. J. Sep. Sci. 2005, 28, 2111–2119. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.S.; Guan, J.; Yang, F.Q.; Liu, H.G.; Cheng, X.J.; Li, S.P. Qualitative and quantitative analysis of four species of Curcuma rhizomes using twice development thin layer chromatography. J. Pharm. Biomed. Anal. 2008, 48, 1024–1028. [Google Scholar] [CrossRef] [PubMed]
- Yang, F.Q.; Li, S.P.; Zhao, J.; Lao, S.C.; Wang, Y.T. Optimization of GC-MS conditions based on resolution and stability of analytes for simultaneous determination of nine sesquiterpenoids in three species of Curcuma rhizomes. J. Pharm. Biomed. Anal. 2007, 43, 73–82. [Google Scholar] [CrossRef] [PubMed]
- Qin, N.Y.; Yang, F.Q.; Wang, Y.T.; Li, S.P. Quantitative determination of eight components in rhizome (Jianghuang) and tuberous root (Yujin) of Curcuma longa using pressurized liquid extraction and gas chromatography-mass spectrometry. J. Pharm. Biomed. Anal. 2007, 43, 486–492. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the compounds and materials are available from the authors. |


| Bands a | Samples | Mass Data | Compound |
|---|---|---|---|
| R1, R3, R7 | C. wenyujin EOCW | 216 (M+, 19), 201 (12), 148 (38), 108 (100), 93 (12), 91 (18), 79 (17), 77 (16) | curzerene |
| R1, R3, R7 | C. wenyujin EOCW | 216 (M+, 47), 201 (16), 159 (30), 145 (30), 108 (100), 91 (38), 77 (26), 65 (12), 53 (13), 41 (17) | furanodiene |
| R2 | C. kwangsciensis | 232 (M+, 100), 203 (27), 162 (60), 147 (63), 135 (68), 134 (36), 119 (32), 91 (38), 79 (36), 77 (29) | unknown |
| R5, R8 | C. longa EOCL | 218 (M+, 4), 120 (44), 119 (37), 111 (24), 105 (89), 93 (18), 91 (35), 85 (11), 83 (100), 77 (29), 55 (27) | α-turmerone |
| R5, R8 | C. longa EOCL | 218 (M+, 33), 121 (14), 120 (100), 105 (18), 93 (5), 92 (13), 91 (21), 83 (36), 79 (5), 77 (14), 55 (11) | β-turmerone |
| R6, R9 | C. longa EOCL | 204 (M+, 25), 161 (55), 133 (42), 120 (31), 93 (57), 91 (74), 77 (47), 69 (100) | β-sesquiphellandrene |
| Samples a | Bands b | Contribution to antioxidant capacityof the sample c (%) | Antioxidant capacity of the sample(correspond to VC, mg/g) |
|---|---|---|---|
| W1 | R1 | 78 | 12.1 |
| W2 | R1 | 100 | 7.5 |
| W3 | R1 | 96 | 8.6 |
| W4 | R1 | 87 | 15.8 |
| K1 | R2 | 68 | 6.6 |
| R3 | 32 | ||
| K2 | R2 | 57 | 6.5 |
| R3 | 36 | ||
| K3 | R2 | 47 | 10.2 |
| R3 | 53 | ||
| K4 | R2 | 60 | 8.8 |
| R3 | 35 | ||
| P1 | R4 | 53 | 2.1 |
| P2 | R4 | 49 | 3.5 |
| P3 | R4 | 45 | 5.2 |
| P4 | R4 | 41 | 4.2 |
| L1 | R5 | 59 | 12.4 |
| R6 | 28 | ||
| L2 | R5 | 61 | 10.9 |
| R6 | 25 | ||
| L3 | R5 | 62 | 13.1 |
| R6 | 27 | ||
| L4 | R5 | 65 | 10.6 |
| R6 | 29 | ||
| EOCW | R7 | 100 | 759.3 |
| EOCL | R8 | 44 | 373.1 |
| R9 | 56 |
| Species | Code | Location |
|---|---|---|
| C. wenyujin | W1, W2 | Yueqing, Zhejiang Province, China |
| W3 | Rui’an, Zhejiang Province, China | |
| W4 | Yongjia, Zhejiang Province, China | |
| C. kwangsiensis | K1 | Qingzhou, Guangxi Province, China |
| K2, K3, K4 | Wuming, Guangxi Province, China | |
| C. phaeocaulis | P1, P2 | Chongzhou, Sichuan Province, China |
| P3 | Zhoudu, Sichuan Province, China | |
| P4 | Wangdan, Sichuan Province, China | |
| C. longa | L1, L2, L3 | Chongzhou, Sichuan Province, China |
| L4 | Shuangliu, Sichuan Province, China |
© 2010 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Zhao, J.; Zhang, J.-s.; Yang, B.; Lv, G.-P.; Li, S.-P. Free Radical Scavenging Activity and Characterization of Sesquiterpenoids in Four Species of Curcuma Using a TLC Bioautography Assay and GC-MS Analysis. Molecules 2010, 15, 7547-7557. https://doi.org/10.3390/molecules15117547
Zhao J, Zhang J-s, Yang B, Lv G-P, Li S-P. Free Radical Scavenging Activity and Characterization of Sesquiterpenoids in Four Species of Curcuma Using a TLC Bioautography Assay and GC-MS Analysis. Molecules. 2010; 15(11):7547-7557. https://doi.org/10.3390/molecules15117547
Chicago/Turabian StyleZhao, Jing, Jiang-sheng Zhang, Bin Yang, Guang-Ping Lv, and Shao-Ping Li. 2010. "Free Radical Scavenging Activity and Characterization of Sesquiterpenoids in Four Species of Curcuma Using a TLC Bioautography Assay and GC-MS Analysis" Molecules 15, no. 11: 7547-7557. https://doi.org/10.3390/molecules15117547
APA StyleZhao, J., Zhang, J.-s., Yang, B., Lv, G.-P., & Li, S.-P. (2010). Free Radical Scavenging Activity and Characterization of Sesquiterpenoids in Four Species of Curcuma Using a TLC Bioautography Assay and GC-MS Analysis. Molecules, 15(11), 7547-7557. https://doi.org/10.3390/molecules15117547




