The Biosynthesis of Artemisinin (Qinghaosu) and the Phytochemistry of Artemisia annua L. (Qinghao)
Abstract
:- Introduction
- 1.1
- Malaria
- 1.2
- Artemisia annua (Qinghao)
- 1.3
- Artemisinin (Qinghaosu)
- The Phytochemistry of Artemisia annua L. (Qinghao)
- 2.1
- Aliphatic hydrocarbons, alcohols, aldehydes and acids
- 2.2
- Aromatic alcohols, ketones and acids
- 2.3
- Phenylpropanoids
- 2.4
- Flavonoids
- 2.5
- Monoterpenoids
- 2.5.1
- Regular acyclic monoterpenes
- 2.5.2
- Irregular acyclic monoterpenes
- 2.5.3
- Monocyclic monoterpenes
- 2.5.4
- Bicyclic monoterpenes
- 2.6
- Sesquiterpenoids
- 2.6.1
- Farnesane sesquiterpenes
- 2.6.2
- Monocyclic sesquiterpenes
- 2.6.3
- Bicyclic sesquiterpenes
- 2.6.4
- Tricyclic sesquiterpenes
- 2.7
- Higher terpenoids
- 2.7.1
- Diterpenes
- 2.7.2
- Triterpenes and sterols
- 2.8
- Nitrogen-containing natural products
- The Biosynthesis of artemisinin (Qinghaosu)
- 3.1
- Phase 1 (isopentenyl pyrophosphate to amorpha-4,11-diene)
- 3.2
- Phase 2 (amorpha-4,11-diene to dihydroartemisinic acid)
- 3.3
- Phase 3 (dihydroartemisinic acid to artemisinin)
- Strategies for the production of artemisinin from A. annua and derived systems
- 4.1
- Plant breeding programmes
- 4.2
- Plant tissue culture
- 4.3
- Endophytic fungi
- 4.4
- Genetic engineering
- Acknowledgements
- References
1. Introduction
1.1. Malaria
1.2. Artemisia annua (Qinghao)
1.3. Artemisinin (Qinghaosu)

2. The Phytochemistry of Artemisia annua L. (Qinghao)
2.1. Aliphatic Hydrocarbons, Alcohols, Aldehydes and Acids
| Structure | Name | CAS Number | References |
|---|---|---|---|
| CH3(CH2)3CH3 | Pentane (1) | [109-66-0] | [58] |
| CH3(CH2)4CH3 | Hexane (2) | [110-54-3] | [22] |
| CH3(CH2)10CH3 | Dodecane (3) | [112-40-3] | [24] |
| CH3(CH2)11CH3 | Tridecane (4) | [629-50-5] | [24] |
| CH3(CH2)14CH3 | Hexadecane (5) | [544-76-3] | [24] |
| CH3(CH2)15CH3 | Heptadecane (6) | [629-78-7] | [27] |
| CH3(CH2)16CH3 | Octadecane (7) | [593-45-3] | [32,43] |
| CH3(CH2)17CH3 | Nonadecane (8) | [629-92-5] | [27,32,43] |
| CH3(CH2)18CH3 | Eicosane (9) | [112-95-8] | [32,43] |
| CH3(CH2)19CH3 | Heneicosane (10) | [629-94-7] | [32,34] |
| CH3(CH2)21CH3 | Tricosane (11) | [638-67-5] | [32] |
| CH3(CH2)22CH3 | Tetracosane (12) | [646-31-1] | [32] |
| CH3(CH2)23CH3 | Pentacosane (13) | [629-99-2] | [32] |
| CH3(CH2)24CH3 | Hexacosane (14) | [630-01-3] | [32] |
| CH3(CH2)27CH3 | Nonocosane (15) | [630-03-5] | [58] |
| CH3(CH2)32CH3 | Tetratriacontane (16) | [14167-59-0] | [59] |
| Structure | Name | Alternative Name(s) | CAS Number | References |
|---|---|---|---|---|
| Alcohols | ||||
| CH3(CH2)3CH2OH | 1-Pentanol (17) | Pentyl alcohol | [71-41-0] | [24] |
| CH3(CH2)4CH2OH | n-Hexanol (18) | [111-27-3] | [22, 32, 43] | |
| CH3(CH2)4CH2O-(C=O)CH2CH(CH3)2 | n-Hexyl isovalerate (19) | 3-Methylbutyric acid hexyl ester | [10032-13-0] | [32, 43] |
| CH3(CH2)4CH2O-(C=O)C(CH3)=CHCH3 | n-Hexyl tiglate (20) | (2E)- 2-Butenoic acid, 2-methyl-, hexyl ester | [16930-96-4] | [32, 43] |
| CH3(CH2)6CH2OH | 1-Octanol (21) | Caprylic alcohol | [111-87-5] | [24] |
| CH3(CH2)7CH2OH | n-Nonyl alcohol (22) | 1-Nonanol | [143-08-8] | [24] |
| CH3(CH2)26CH2OH | Octacosanol (23) | [557-61-9] | [59, 60, 61] | |
| CH3(CH2)27CH2OH | Nonacosanol (24) | [6624-76-6] | [59, 62] | |
| Aldehydes and Ketones | ||||
| CH3COCH3 | Acetone (25) | 2-Propanone | [67-64-1] | [24] |
| CH3(CH2)2CHO | Butanal (26) | Butyraldehyde | [50] | |
| CH3(CH2)4CHO | Hexanal (27) | Caproic aldehyde | [66-25-1] | [23] |
| CH3CO(CH2)4CH3 | 2-Heptanone (28) | Methyl pentyl ketone | [110-43-0] | [45] |
| CH3(CH2)6CHO | Octanal (29) | Capric aldehyde | [124-13-0] | [32] |
| CH3(CH2)11CHO | Tridecanal (30) | Tridecyl aldehyde | [10486-19-8] | [24] |
| Structure | Name | Alternative Name(s) | CAS Number | References |
|---|---|---|---|---|
| CH3CH2OCHO | Ethyl formate (31) | [109-94-4] | [50] | |
| CH3CH2CO2CH2CH3 | Propanoic acid, ethyl ester (32) | Ethyl propionate | [105-37-3] | [50] |
| CH3(CH2)3CO2H | Pentanoic acid (33) | Valeric acid | [109-52-4] | [24] |
| CH3(CH2)3CO2C(CH3)3 | Pentanoic acid, tert-butyl ester (34) | Pentanoic acid, 1,1-dimethylethyl ester | [23361-78-6] | [50] |
| CH3(CH2)8CO2H | Decanoic acid (35) | Capric acid | [334-48-5] | [63, 64] |
| CH3(CH2)10CO2H | Dodecanoic acid (36) | Lauric acid | [143-07-7] | [63, 64] |
| CH3(CH2)10CO2CH2CH3 | Dodecanoic acid, ethyl ester (37) | Ethyl laurate | [106-33-2] | [24] |
| CH3(CH2)11CO2H | Tridecanoic acid (38) | [638-53-9] | [63] | |
| CH3(CH2)12CO2H | Tetradecanoic acid (39) | Myristic acid | [544-63-8] | [23, 63, 64] |
| CH3(CH2)13CO2H | Pentadecanoic acid (40) | [1002-84-2] | [63] | |
| CH3(CH2)14CO2H | Hexadecanoic acid (41) | Palmitic acid | [57-10-3] | [20, 23, 24, 27, 32, 43, 63, 64, 65] |
| CH3(CH2)14CO2CH3 | Hexadecanoic acid, methyl ester (42) | Methyl hexadecanoate Methyl palmitate | [112-39-0] | [27] |
| CH3(CH2)14CO2CH2CH3 | Hexadecanoic acid, ethyl ester (43) | Ethyl palmitate | [628-97-7] | [24] |
| CH3(CH2)15CO2H | Heptadecanoic acid (44) | Margaric acid | [506-12-7] | [63] |
| CH3(CH2)16CO2H | Octadecanoic acid (45) | Stearic acid | [57-11-4] | [27, 43, 63, 64] |
| CH3(CH2)16CO2CH3 | Octadecanoic acid, methyl ester (46) | Methyl octadecanoate Methyl stearate | [112-61-8] | [27] |
| CH3(CH2)17CO2H | Nonadecanoic acid (47) | [646-30-0] | [20] | |
| CH3(CH2)18CO2H | Eicosanoic acid (48) | Arachidic acid | [506-30-9] | [64] |
| CH3(CH2)20CO2H | Docosanoic acid (49) | Behenic acid | [112-85-6] | [64] |
| CH3(CH2)22CO2H | Tetracosanoic acid (50) | Lignoceric acid | [557-59-5] | [64] |
| CH3(CH2)28CO2(CH2)30CH3 | Hentriacontanyl triacontanoate (51) | Triacontanoic acid hentriacontyl ester | [135729-36-1] | [59, 62] |
| Structure | Name | Alternative Name(s) | CAS Number | References |
|---|---|---|---|---|
| Hydrocarbons | ||||
| CH2=CHCH=CHCH3 | 1,3-Pentadiene (52) | 1-Methyl-1,3-butadiene | [504-60-9] | [50] |
| CH3CH=CHCH=CHCH3 | trans,trans-2,4-Hexadiene (53) | [5194-51-4] | [50] | |
| H2C=CHCH=CHCH=CHCH3 | trans,trans-1,3,5-Heptatriene (54) | [17679-93-5] | [50] | |
| H2C=CH(CH2)4CH=CH2 | 1,7-Octadiene (55) | [3710-30-3] | [50] | |
| H2C=CH(CH2)2CH=CH(CH2)2CH=CH2 | trans-1,5,9-Decatriene (56) | [39139-91-8] | [50] | |
| Alcohols | ||||
| CH2=CHCH2CH(OH)CH3 | 4-Pentene-2-ol (57) | 1-Penten-4-ol | [625-31-0] | [25] |
| CH2=CH(CH2)3O-(C=O)CH2CH3 | 4-Penten-1-ol, propionate (58) | 4-Pentenyl propionate | [30563-30-5] | [43] |
| CH3(CH2)2CH=CHCH2OH | (E)-2-Hexenol (59) | 2-Hexen-1-ol | [928-95-0] | [45] |
| CH3CH2CH=CHCH2CH2OH | (E)-3-Hexen-1-ol (60) | [928-97-2] | [19] | |
| CH3CH2CH=CHCH2CH2OH | (Z)-3-Hexen-1-ol (61) | Phyllol | [928-96-1] | [22, 32, 43] |
| CH3CH2CH=CHCH2CH2O-(C=O)CH3 | (E)-3-Hexen-1-ol, acetate (62) | [3681-82-1] | [19] | |
| CH3CH2CH=CHCH2CH2O-(C=O)CH2CH3 | (Z)-3-Hexenyl propanoate (63) | [33467-74-2] | [20] | |
| CH3CH2CH=CHCH2CH2O-(C=O)CH2CH2CH3 | 3-Hexenyl butanoate (64) | [2142-93-0] | [23] | |
| CH3CH2CH=CHCH2CH2O-(C=O)(CH2)4CH3 | 3-Hexenyl hexanoate (65) | [84434-19-5] | [24] | |
| CH3CH2CH=CHCH2CH2O-(C=O)CH2CH(CH3)2 | (Z)-3-Hexenyl isovalerate (66) | [35154-45-1] | [32] | |
| CH3CH2CH=CHCH2CH2O-(C=O)C(CH3)=CHCH3 | (Z)-3-Hexenyl tiglate (67) | [67883-79-8] | [43] | |
| H2C=CHCH(OH)(CH2)3CH3 | 1-Hepten-3-ol (68) | [4938-52-7] | [24] | |
| H2C=CHCH(OH)(CH2)4CH3 | 1-Octen-3-ol (69) | [3391-86-4] | [31,32,43] | |
| H2C=CH(CH2)7CH2OH | 9-Decen-1-ol (70) | [13019-22-2] | [32, 43] | |
| Structure | Name | Alternative ame(s) | CAS Number | References |
|---|---|---|---|---|
| Ketones and aldehydes | ||||
| H2C=CHCH2CH2CHO | 4-Pentenal (71) | [2100-17-6] | [50] | |
| CH3(CH2)2CH=CHCHO | 2-Hexenal (72) | Leaf aldehyde | [505-57-7] [6728-26-3] | [24, 31, 43] |
| CH3(CH2)3CH=CHCHO | 2-Heptenal (73) | [2463-63-0] | [24] | |
| CH3(CH2)5CH=CHCHO | (Z)-2-Nonenal (74) | [60784-31-8] | [50] | |
| CH3(CH2)3CH=CHCH=CHCHO | (2E,4E)- Nonadienal (75) | [5910-87-2] | [19] | |
| CH3(CH2)4CH=CHCOCH3 | 3-Nonen-2-one (76) | [14309-57-0] | [24] | |
| CH3(CH2)6CH=CHCHO | 2-Decenal (77) | [3913-71-1] | [24] | |
| CH3(CH2)4CH=CHCH=CHCHO | 2,4-Decadienal (78) | [2363-88-4] | [24] | |
| H2C=CH(CH2)8CHO | 10-Undecenal (79) | [112-45-8] | [24] | |
| Carboxylic acids and esters | ||||
| CH3(CH2)7CH=CH(CH2)7CO2H | Oleic acid (80) | (Z)-9-Octadecanoic acid | [112-80-1] [27104-13-8] | [28, 63, 64] |
| CH3(CH2)7CH=CH(CH2)7CO2CH3 | Methyl 9-octadecenoate (81) | Methyl elaidate | [2462-84-2] | [24] |
| CH3(CH2)7CH=CH(CH2)7CO2CH2CH(OH)CH2OH | 9-Octadecenoic acid, 2,3-dihydroxypropyl ester (82) | [251983-54-7] | [24] | |
| CH3(CH2)4CH=CHCH2CH=CH(CH2)7CO2H | Linoleic acid (83) | (Z,Z)-9,12-Octadecadienoic acid | [60-33-3] [27213-43-0] [28984-77-2] | [20, 63] |
| CH3CH2CH=CHCH2CH=CHCH2CH=CH(CH2)7CO2H | α-Linolenic acid (84) | (Z,Z,Z)-9,12,15-Octadecatrien-oic acid | [463-40-1] | [20, 63] |
| Structure | Name | Alternative Name(s) | CAS Number | References |
|---|---|---|---|---|
| Hydrocarbons | ||||
| CH3(CH2)8CH(CH3)CH2CH2CH(CH3)2 | Tetradecane, 2,5-dimethyl- (85) | [56292-69-4] | [24] | |
| (CH3)2CH(CH2)26CH(CH3)2 | Triacontane, 2,29-dimethyl- (86) | 2,29-Dimethyltriacontane | [135729-37-2] | [62] |
| (CH3)2CHC(CH3)3 | 2,2,3-Trimethylbutane (87) | [464-06-2] | [50] | |
| CH3(CH2)3CH(CH3)(CH2)7CH3 | Tridecane, 5-methyl- (88) | 5-Methyltridecane | [25117-31-1] | [24] |
| Alcohols | ||||
| (CH3)2CHCH2CH2O-(C=O)CH3 | 3-Methyl-1-butanol, acetate (89) | Isoamyl acetate | [123-92-2] | [43] |
| (CH3)2CHCH2O-(C=O)CH2CH3 | 2-methylpropylpropionate (90) | Isobutyl propionate | [540-42-1] | [50] |
| (CH3)2C(OH)(CH2)2CH3 | 2-Methyl-2-pentanol (91) | [590-36-3] | [25] | |
| (CH3)2C(OH)(CH2)3CH3 | 2-Methyl-2-hexanol (92) | [625-23-0] | [24] | |
| (CH3)2CH(CH2)3CH2OH | 5-Methyl-1-hexanol (93) | 1-Hexanol, 5-methyl- | [627-98-5] | [24] |
| Aldehydes and Ketones | ||||
| (CH3)2CHCH2CHO | 3-Methylbutanal (94) | Isovaleraldehyde | [590-86-3] | [24] |
| CH3CH2CH(CH3)CH2CHO | 3-Methylpentanal (95) | [15877-57-3] | [20] | |
| (CH3)2CHCH2COCH3 | 4-Methyl-2-pentanone (96) | Isobutyl methyl ketone | [108-10-1] | [24] |
| (C6H5)CH2CH(CHO)(CH2)5CH3 | 2-Benzyloctanal (97) | Benzenepropanal, α-hexyl- | [161403-65-2] | [24] |
| (CH3)2CH(CH2)5C=O(CH2)14CH2OH | 8-Tricosanone, 23-hydroxy-2-methyl- (98) | [135729-35-0] | [59, 62] | |
| Structure | Name | Alternative Name(s) | CAS Number | References |
|---|---|---|---|---|
| CH3CH2CH(CH3)CO2H | 2-Methylbutanoic acid (99) | [868-57-5] | [22] | |
| CH3CH2CH(CH3)CO2CH2CH3 | 2-Methyl butanoic acid, ethyl ester (100) | Ethyl 2-methylbutyrate | [7452-79-1] | [22, 32] |
| (CH3CH2CH(CH3)CO2)O | 2-Methylbutanoic acid anhydride (101) | 2-Methylbutyryl anhydride | [1519-23-9] | [50] |
| CH3CH2CH(CH3)CO2(CH2)4CH3 | Amyl 2-methylbutyrate (102) | Pentyl 2-methylbutanoate | [68039-26-9] | [43] |
| CH3CH2CH(CH3)CO2CH2CH(CH3)CH2CH3 | 2-Methyl-butanoic acid, 2-methylbutyl ester (103) | 2-Methylbutyl 2-methylbutyrate | [2445-78-5] | [31] |
| (CH3)2CHCH2CO2CH2CH3 | 3-Methylbutanoic acid, ethyl ester (104) | Ethyl 3-methylbutanoate Ethyl isovalerate | [108-64-5] | [23] |
| (CH3)2CHCH2CO2(CH2)3CH3 | 3-Methylbutanoic acid, butyl ester (105) | Butyl-3-methylbutanoate | [109-19-3] | [25] |
| (CH3)2CHCH2CO2CH2CH2C(=CH2)CH3 | 3-Methylbutanoic acid, 3-methyl-3-butenyl ester (106) | 3-Methyl-3-butenyl 3-methylbutyrate | [54410-94-5] | [20] |
| CH3CH2CH(CH2CH3)CO2CH3 | 2-Ethylbutanoic acid, methyl ester (107) | 2-Methyl-ethylbutanoate | [816-11-5] | [25] |
| Structure | Name | Alternative Name(s) | CAS Number | References |
|---|---|---|---|---|
| Hydrocarbons | ||||
| H2C=CHC(CH3)3 | 3,3-Dimethyl-1-butene (108) | tert-Butylethylene | [558-37-2] | [20] |
| H2C=CHCH(CH3)2 | 2,4-Dimethyl-2-pentene (109) | [625-65-0] | [24] | |
| CH3CH=CHCH(CH3)CH2CH3 | trans-4-Methyl-2-hexene (110) | [3683-22-5] | [20] | |
| Aldehydes | ||||
| CH3(CH2)4CH=CH(CHO)(CH2)3CH3 | 2-Butyl-2-octenal (111) | [13019-16-4] | [24] | |
| (CH3)2C=CHCH=CH(C=O)CH3 | 6-Methyl-3,5-heptadien-2-one (112) | [16647-04-4] | [66] | |
| Structure | Name | Alternative Name(s) | CAS Number | References |
|---|---|---|---|---|
| CH3C≡CCH2OMe | 1-Methoxy-2-butyne (113) | [2768-41-4] | [50] | |
| H2C=CH(CH2)3C≡CH | 1-Hepten-6-yne (114) | [65939-59-5] | [50] | |
| CH3O(C=O)C≡C(CH2)5CH3 | 2-Nonynoic cid, methyl ester (115) | Methyl 2-nonynoate | [111-80-8] | [50] |
| CH3(CH2)9C≡CH | 1-Dodecyne (116) | Decylacetylene | [765-03-7] | [23] |
| CH3C≡C(CH2)8CH2OH | 10-Dodecyn-1-ol (117) | [69221-99-4] | [23] | |
| (CH3)2CHC≡CCH=CHCH(CH3)2 | 3-Octen-5-yne, 2,7-dimethyl- (118) | [91400-77-0] | [50] | |
| CH3(CH2)6CH=CHC≡CH3 | 3-Undecen-1-yne (119) | [74744-32-4] [91250-91-8] | [23] | |
| (C6H5)CH2C≡C-C≡C-CH3 | Capillene (120) | 2,4-Hexadiynylbenzene | [520-74-1] | [30] |
| Ponticaepoxide (121) | 2-Ethenyl-3-(1-nonen-3,5,7-triynyl)oxirane 2-(1-Nonen-3,5,7-triynyl)-3-vinyloxirane 3,4-Epoxy-1,5-tridecadiene-7,9,11-triyne | [3562-36-5] | [9, 67] | |
| Annuadiepoxide (122) | 1,3,5-Tridecatriene-7,9,11-triyne (E,E), 3,4:5,6-diepoxide 3,4:5,6-Diepoxy-1-tridecene-7,9,11-triyne | [139122-80-8] | [9, 67] |

| Name | Alternative Name(s) | CAS Number | References |
|---|---|---|---|
| 1,1’-Bicyclopropyl, 2,2’-dimethyl (123) | [1975-84-6] | [23] | |
| Bicyclo[2.2.2]octa-2,5-diene, 1,2,3,6-tetramethyl- (124) | [62338-43-6] | [20] | |
| 3,5-Cycloheptadienone (125) | [1121-65-9] | [28] | |
| Cyclooctane, 1,4-dipropyl- (126) | [251983-53-6] | [24] | |
| Cyclopropane, (1-methyl-1,2-propadien-1-yl)- (127) | 3-Cyclopropyl-1,2-butadiene | [51549-86-1] | [50] |
| Cyclopropene, 3-ethenyl-3-methyl-(128) | 3-Methyl-3-vinylcyclopropene | [71153-30-5] | |
| 1,1-Dicyclopropylethylene (129) | Cyclopropane, 1,1’-ethenylidenebis- | [822-93-5] | [50] |
| Hexylcyclohexane (130) | [4292-75-5] | [20] | |
| Jasmone (131) | 3-Methyl-2-(2-pentenyl)-2-cyclopenten-1-one | [488-10-8] | [22, 32, 43] |
| Methyl cyclopentane (132) | [96-37-7] | [22] |

| Name | Alternative Name(s) | CAS Number | References |
|---|---|---|---|
| 2,5-Dihydro-3-methylfuran (133) | [1708-31-2] | [50] | |
| 2-Ethylfuran (134) | [3208-16-0] | [50] | |
| 4-Methyl-2,3-dihydrofuranfuran (135) | [34314-83-5] | [34] | |
| 3-Methylfuran (136) | [930-27-8] | [34] | |
| 5-Methyl-2-furancarboxyaldehyde (137) | 5-Methylfurfural | [620-02-0] | [24] |

2.2. Aromatic Alcohols, Ketones and Acids
| Name | Alternative Name(s) | CAS Number | References |
|---|---|---|---|
| Anisole (138) | Phenyl methyl ether | [100-66-3] | [48] |
| Benzyl isovalerate (139) | 3-Methylbutanoic acid, benzyl ester | [103-38-8] | [22, 23, 25, 32, 43] |
| Benzyl 2-methyl butyrate (140) | 2-Methylbutanoic acid, benzyl ester | [56423-40-6] | [41] |
| Benzyl phenylacetate (141) | Benzeneacetic acid, phenylmethyl ester | [102-16-9] | [50] |
| Benzyl valerate (142) | Benzyl pentanoate | [10361-39-4] | [19, 23] |
| 5-Nonadecylresorcinol-3-O-methyl ether (143) | Phenol 3-methoxy-5-nonadecyl | [68] |

| Name | Alternative Name(s) | CAS number | References |
|---|---|---|---|
| 2’,4’,6’-Trihydroxyacetophenone 2’,4’-dimethyl ether (144) | 2-Hydroxy-4,6-dimethoxyacetophenone | [68] | |
| 2’,4’,6’-Trihydroxyacetophenone 2’-methyl ether (145) | 2’,4’-dihydroxy-6’-methoxyacetophenone | [3602-54-8] | [68, 69] |
| 2’,4’,6’-Trihydroxyacetophenone 2’-methyl ether 4’-O-β-D-glucopyranosde (146) | Annphenone | [61775-18-6] | [70] |
| 2’,4’,6’-Trihydroxyacetophenone 4’-methyl ether 2-O-β-D-glucopyranoside (147) | Domesticoside | [24587-97-1] | [15] |

| Name | Alternative Name(s) | CAS Number | References |
|---|---|---|---|
| Benzoic acid (148) | [65-85-0] | [9, 24] | |
| Salicylic acid (149) | 2-Hydroxybenzoic acid | [69-72-7] | [15] |
| Methyl salicylate (150) | Methyl-2-hydroxybenzoate | [119-36-8] | [27, 32] |
| 2-Hydroxybenzoic acid, 3-methylbutyl ester (151) | Isoamyl salicylate | [87-20-7] | [24] |
| 3,4-Dihydroxybenzoic acid (152) | Benzoic acid, 3,4-dihydroxy- Protocatechuic acid | [99-50-3] | [71] |
| Protocatechuic acid 4-glucoside (153) | Benzoic acid, 4-(β-D-glucosyloxy)-3-hydroxy- | [7361-59-3] | [71] |
| Phenylacetic acid (154) | [103-82-2] | [24] | |
| Phenylpropanoic acid (155) | [9] | ||
| Benzenepropanoic acid, 3-cyanophenyl ester (156) | [40123-39-5] | [50] |

2.3. Phenylpropanoids
| Name | Alternative Name(s) | CAS Number | References |
|---|---|---|---|
| p-Allylanisole (157) | 1-Methoxy-4-(2-propenyl)benzene | [140-67-0] | [29, 72, 73] |
| Anethole (158) | 1-Methoxy-4-(1-propenyl)benzene | [4180-23-8] | [32, 43, 50] |
| 3-Allyl-6-methoxyphenol (159) | 4-Allyl-2-hydroxyl-1-methoxybenzene | [501-19-9] | [48] |
| Eugenol (160) | 2-Methoxy-4-(2-propenyl)phenol | [97-53-0] | [19, 32, 41, 43, 74] |
| Methyl eugenol (161) | 1,2-Dimethoxy-4-(2-propenyl)benzene | [93-15-2] | [43, 48] |
| Eugenyl isovalerate (162) | 2-Methoxy-4-(2-propenyl)phenol 3-methylbutanoyl | [61114-24-7] | [34] |
| 2-Methoxy-3-(2-propenyl)phenol (163) | [1941-12-4] | [23] |

| Name | Alternative Name(s) | CAS Number | Refs |
|---|---|---|---|
| Methyl cinnamate (164) | 3-phenyl-2-propenoic acid methyl ester | [103-26-4] | [24] |
| Benzyl cinnamate (165) | 3-Phenyl-2-propenoic acid benzyl ester | [103-41-3] | [24] |
| Chlorogenic acid (166) | 3-(3,4-Dihydroxycinnamoyl)quinic acid | [327-97-9] | [71] |
| Cyclohexanecarboxylic acid, 1,3,4-trihydroxy-5-[[3-(4-hydroxy-3-methoxyphenyl)-1-oxo-2-propenyl]oxy]-, (167) | [53905-80-9] | [71] | |
| Cyclohexanecarboxylic acid, 3-[[3-(3,4-dihydroxyphenyl)-1-oxo-2-propenyl]oxy]-1,4,5-trihydroxy-, (168) | [342811-68-1] | [71] | |
| Isochlorogenic acid B (169) | 3,4-Di-O-caffeoylquinic acid | [4534-61-3] | [71] |
| 3-Caffeoyl-4-feruloylquinic acid (170) | 4-O-Feruloyl-5-O-caffeoylquinic acid | [125132-81-2] | [71] |
| 3,4-Diferuoylquinic acid (171) | [342811-70-5] | [71] | |
| Isochlorogenic acid A (172) | 3,5-bis-(3,4-Dihydroxycinnamoyl)quinic acid) | [2450-53-5] | [71] |
| 3-Caffeoyl-5-feruloylquinic acid (173) | [478156-24-0] | [71] | |
| 3-Feruloyl-5-caffeoylquinic acid (174) | [1039007-73-2] | [71] | |
| 3,5-Diferuoylquinic acid (175) | [333753-65-4] | [71] | |
| Isochlorogenic acid C (176) | 4,5-Di-O-caffeoylquinic acid | [57378-72-0] | [71] |
| 4-Caffeoyl-5-feruloylquinic acid (177) | [478156-25-1] | [71] | |
| 4-Feruloyl-5-caffeoylquinic acid (178) | [882535-14-0] | [71] | |
| 4,5-Diferuoylquinic acid (179) | [342811-69-2] | [71] | |
| Cyclohexanecarboxylic acid, 3-[[3-(3,4-dihydroxyphenyl)-1-oxo-2-propenyl]oxy]-4,5-dihydroxy-1-[[3-(4-hydroxy-3-methoxyphenyl)-1-oxo-2-propenyl]oxy]-, (180) | [865095-58-5] | [71] | |
| 1-Caffeoyl-5-feruoylquinic acid (181) | [865095-57-4] | [71] | |
| Cyclohexanecarboxylic acid, 3,4,5-tris[[3-(3,4-dihydroxy phenyl)-1-oxo-2-propenyl]oxy]-1-hydroxy-(182) | [437611-66-0] | [71] |

| Name | Alternative Name(s) | CAS Number | References |
|---|---|---|---|
| Coumarin (183) | 2H-1-Benzopyran-2-one | [91-64-5] | [56, 65, 68, 75, 76, 77, 79, 80] |
| Scopoletin (184) | 7-Hydroxy-6-methoxycoumarin 7-Hydroxy-6-methoxy-2H-1-benzopyran-2-one | [92-61-5] | [77, 79, 80, 79, 80, 81, 82, 83, 84, 85, 86, 87] |
| Scoparone (185) | 6,7-Dimethoxycoumarin 6,7-Dimethoxy-2H-1-benzopyran-2-one | [120-08-1] | [69, 88, 89] |
| Scopolin (186) | Scopoletin-O-β-D-glucopyranoside 7-Hydroxy-6-methoxycoumarin-O-β-D-glucopyranoside | [531-44-2] | [15, 71, 79, 89, 90] |
| Isofraxidin (187) | 6,8-Dimethoxy-7-hydroxy coumarin | [486-21-5] | [79, 86, 87, 89] |
| Tomentin (188) | 5,6,7-Trihydroxy-2H-1-benzopyran-2-one 6,7-dimethyl ether | [28449-62-9] | [89] |
| 6,7-Dimethoxydihydrocoumarin (189) | 2H-1-Benzopyran-2-one,3,4 3,4-Dihydro-6,7-dimethoxy-coumarin | [56680-28-5] | [88] |
| 2,2,6-Trihydroxychromene (190) | 2H-1-Benzopyran-2,2,6-triol | [161585-88-2] | [89] |
| 2,2-Dihydroxy-6-methoxy-2H-1-benzopyran (191) | 6-Methoxy-2H-1-benzopyran-2,2-diol 2H-1-Benzopyran-2,2,6-triol 6-methyl ether | [161585-87-1] | [89] |

2.4. Flavonoids
| Name | Alternative Name(s) | CAS Number | References |
|---|---|---|---|
| Apigenin (192) | 4’,5,7-Trihydroxyflavone 5,7-Dihydroxy-2-(4-hydroxyphenyl)-4H-1-benzopyran-4-one | [520-36-5] | [71, 89] |
| Luteolin tetrahydroxyflavones | |||
| Luteolin (193) | 3’,4’,5,7-Tetrahydroxyflavone 2-(3,4-Dihydroxyphenyl)-5,7,-dihydroxy-4H-1-benzopyran-4-one | [491-70-3] | [84, 89] |
| Luteolin-7-methyl ether (194) | 3’,4’,5-Trihydroxy-7-methoxyflavone 2-(3,4-Dihydroxyphenyl)-5-hydroxy-7-methoxy-4H-1-benzopyran-4-one | [20243-59-8] | [89] |
| Glucoluteolin (195) | 3,4’,5,7-Tetrahydroxyflavone-7-O-β-D-glucopyranoside Luteolin 7-glucoside | [5373-11-5] | [84, 89] |
| Chrysoeriol (196) | 4’,5,7-Trihydroxy-3’-methoxyflavone 5,7-Dihydroxy-2-(4-hydroxy-3-methoxyphenyl)-4H-1-benzopyran-4-one | [491-71-4] | [76, 94] |
| Other tetrahydroxyflavones | |||
| Cirsimaritin (197) | 4’,5-Dihydroxy-6,7-dimethoxyflavone 5-Hydroxy-2-(4-hydroxyphenyl)-6,7-dimethoxy-4H-1-benzopyran-4-one | [6601-62-3] | [76, 94] |
| Pentahydroxyflavones | |||
| Cirsiliol (198) | 3’,4’,5,6,7-Pentahydroxyflavone 6,7-dimethyl ether 3’,4’,5-Trihydroxy-6,7-dimethoxyflavone | [34334-69-5] | [76, 94] |
| Eupatorin (199) | 6-Methoxy luteolin 7,4’-dimethyl ether 3’,5-Dihydroxy-4’,6,7-trimethoxyflavone 5-Hydroxy-2-(3-hydroxy-4-methoxyphenyl)-6,7-dimethoxy-4H-1-benzopyran-4-one | [855-96-9] | [76, 94, 95] |
| 5-Hydroxy-3’,4’,6,7-tetramethoxyflavone (200) | 3’,4’,5,6,7-Pentahydroxyflavone 3’,4’,6,7-tetra methyl ether | [21763-80-4] | [96] |
| 4H-1-Benzopyran-4-one, 2-(2,4-dihydroxyphenyl)-5-hydroxy-6,7-dimethoxy- (201) | [101909-51-7] | [76] | |
| Hexahydroxyflavones | |||
| 2,4’,5’-Trihydroxy-5’6,7-trimethoxyflavone (202) | [94] | ||

| Name | Alternative Name(s) | CAS Number | References |
|---|---|---|---|
| Kaempferols | |||
| Kaempferol (203) | 3,4’,5,7-Tetrahydroxyflavone 3,5,7-Trihydroxy-2-(4-hydroxyphenyl)-4H-1-benzopyran-4-one 4’,5,7-Trihydroxyflavonol | [520-18-3] | [84, 89] |
| Kaempferol-3-O-glucoside (204) | Astragalin 3-O-β-D-Glucopyranosyloxy-4’,5,7-trihydroxyflavone | [480-10-4] | [84, 89] |
| Rhamnocitrin (205) | 3,4’,5-Trihydroxy-7-methoxyflavone 3,5-Dihydroxy-2-(4-hydroxyphenyl)-7-methoxy-4H-1-benzopyran-4-one 4’,5-Dihydroxy-7-methoxyflavonol | [569-92-6] | [76, 94] |
| Other Tetrahydroxyflavonols | |||
| 4H-1-Benzopyran-4-one, 3-hydroxy-6,7-dimethoxy-2-(4-methoxyphenyl)- (206) | [77184-81-7] | [71] | |

| Name | Alternative Name(s) | CAS Number | Refs |
|---|---|---|---|
| Quercetin | |||
| Quercetin (207) | 3,3’,4’,5,7-Pentahydroxyflavone 2-(3,4-Dihydroxyphenyl)-3,5,7-trihydroxy-4H-1-benzopyran-4-one 3’,4’,5,7-Tetrahydroxyflavonol | [117-39-5] | [84] |
| Quercetin 3-methyl ether (208) | 3’,4’,5,7-Tetrahydroxy-3-methoxyflavone 2-(3,4-Dihydroxyphenyl)-5,7-dihydroxy-3-methoxy-4H-1-benzopyran-4-one | [1486-70-0] | [76, 94] |
| Isoquercitrin (209) | Quercetin-3-glucofuranoside 2-(3,4-Dihydroxyphenyl)-3-(β-D-glucopyranosyloxy)-5,7-dihydroxy-4H-1-benzopyran-4-one 3-Glucopyranosyloxy-3’,4’,5,7-tetrahydroxyflavoneQuercetin-3-glucopyranoside | [21637-25-2] [482-35-9] | [71, 89] |
| Quercetin 3-rutinoside (210) | [153-18-4] | [84] | |
| Isorhamnetin 3-glucoside (211) | 3-Glucopyranosyloxy-4’,5,7-trihydroxy-3’-methoxyflavone | [5041-82-7] | [71] |
| Rhamnetin (212) | 3,3’,4’,5-Tetrahydroxy-7-methoxyflavone 2-(3,4-Dihydroxyphenyl)-3,5-dihydroxy-7-methoxy-4H-1-benzopyran-4-one 3’,4’5-Trihydroxy-7-methoxyflavonol | [90-19-7] | [76, 94] |
| Quercimeritrin (213) | 7-O-β-D-glucopyranosyloxy-3,3’,4’,5-tetrahydroxyflavone Quercetin 7-glucoside | [491-50-9] | [89] |
| Isorhamnetin (214) | 3,4’,5,7-Tetrahydroxy-3’-methoxyflavone 3,5,7-Trihydroxy-2-(4-hydroxy-3-methoxyphenyl)-4H-1-benzopyran-4-one 4’,5,7-Trihydroxy-3’-methoxyflavonol Quercetin 3’-methyl ether | [480-19-3] | [89] |
| Quercetin 3’-glucoside (215) | 3,3,’4’,5,7-Pentahydroxyflavone 3’-O-β-D-glucopyranoside | [19254-30-9] | [84, 89] |
| Tamarixetin (216) | 3,3’,5,7-Tetrahydroxy-4’-methoxyflavone 3,5,7-Trihydroxy-2-(3-hydroxy-4-methoxyphenyl)-4H-1-benzopyran-4-one 3’,5,7-Trihydroxy-4’-methoxyflavonol | [603-61-2] | [76, 94] |
| Other pentahydroxyflavonols | |||
| Eupalitin (217) | 3,4’,5-Trihydroxy-6,7-dimethoxyflavone 3,5-Dihydroxy-2-(4-hydroxyphenyl)-6,7-dimethoxy-4H-1-benzopyran-4-one 4’,5-Dihydroxy-6,7-dimethoxyflavonol | [29536-41-2] | [96] |
| Penduletin (218) | 3,4’,5,6,7-Pentahydroxyflavone 3,6,7-trimethyl ether 4’,5-Dihydroxy-3,6,7-trimethoxyflavone | [569-80-2] | [76, 94, 97] |
| 3,4’,5,6,7-Pentahydroxyflavone 3,4’,6,7-tetramethyl ether (219) | 5-Hydroxy-3,4’,6,7-tetramethoxyflavone | [14787-34-9] | [61, 75, 78, 97] |
| Mikanin (220) | 3,4’,5,6,7-Pentahydroxyflavone 4’,6,7-trimethyl ether 3,5-Dihydroxy-4’,6,7-trimethoxyflavone | [4324-53-2] | [71] |

| Name | Alternative Name(s) | CAS Number | References |
|---|---|---|---|
| Quercetagetin 3-methyl ether (221) | 3’,4’,5,6,7-Pentahydroxy-3-methoxyflavone 2-(3,4-Dihydroxyphenyl)-5,6,7-trihydroxy-3-methoxy-4H-1-benzopyran-4-one | [64190-88-1] | [89] |
| Axillarin (222) | Quercetagetin 3,6-dimethyl ether 3’,4’,5,7-Tetrahydroxy-3,6-dimethoxyflavone 2-(3,4-Dihydroxyphenyl)-5,7-dihydroxy-3,6-dimethoxy-4H-1-benzopyran-4-one | [5188-73-8] | [76, 84] |
| Quercetagetin-3,4’-dimethyl ether (223) | 3’,5,6,7-Tetrahydroxy-3,4’-dimethoxyflavone 3,3’,4’,5,6,7-Hexahydroflavone 3,4’-di-methyl ether | [59171-34-5] | [76] |
| Bonanzin (224) | 5,7-Dihydroxy-3,3’,4’,6-tetramethoxyflavone 2-(3,4-Dimethoxyphenyl)-5,7-dihydroxy-3,6-dimethoxy-4H-1-benzopran-4-one | [35688-42-7] | [96] |
| Chrysosplenol D (225) | 2-(3,4-Dihydroxyphenyl)-5-hydroxy-3,6,7-trimethoxy-4H-1-benzopyran-4-one | [14965-20-9] | [15, 76, 84, 92, 94, 95, 98] |
| Chrysosplenetin (226) | Chrysosplenol B 5,4’-Dihydroxy-3,6,7,3’-tetramethoxyflavone 5-Hydroxy-2-(4-hydroxy-3-methoxyphenyl)-3,6,7-trimethoxy-4H-1-benzopyran-4-one | [603-56-5] | [15, 76, 80, 84, 91, 92, 95, 96, 99, 100, 101] |
| Casticin (227) | Quercetagetin 6,3,7,4’-tetramethyl ether 3’,5-Dihydroxy-3,4’,6,7-tetramethoxyflavone 5-Hydroxy-2-(3-hydroxy-4-methoxyphenyl)-3,6,7-trimethoxy-4H-benzopyran-4-one | [479-91-4] | [76, 84, 91, 94, 95, 98, 99, 100, 103] |
| Artemetin (228) | 5-Hydroxy-3,6,7,3’,4’-Pentamethoxyflavone 2-(3,4,-Dimethoxyphenyl)-5-hydroxy-3,6,7-trimethoxy-4H-1-benzopyran-4-one | [479-90-3] | [15, 75, 77, 78, 80, 94, 95, 96, 98, 100, 105, 106] |
| Patuletin-3-O-glucoside (229) | Quercetagetin 6-methyl ether 3-O-glucoside 6-Methoxykaempferol -3-O-glucoside | [19833-27-3] | [84] |
| Patuletin (230) | 3,3’,4’,5,7-Pentahydroxy-6-methoxyflavone 2-(3,4-Dihydroxyphenyl)-3,5,7-trihydroxy-6-methoxy-4H-1-benzopyran-4-one 3’,4’,5,7-Tetrahydroxy-6-methoxyflavonol | [519-96-0] | [84] |
| Cirsilineol (231) | 3’,4’,5,6,7-Pentahydroxyflavone 3’,6,7-tri methyl ether 4’,5-Dihydroxy-3’,6,7-trimethoxyflavone | [41365-32-6] | [76, 94, 95, 98] |
| Eupatin (232) | 3,3’,5-Trihydroxy-4’,6,7-trimethoxyflavone 3,5-Dihydroxy-2-(3-hydroxy-4-methoxyphenyl)-6,7-dimethoxy-4H-1-benzopyran-4-one 3’,5-Dihyhdroxy-4’,6,7-trimethoxyflavonol Quercetagetin 4’,6,7-trimethyl ether | [19587-65-6] | [61, 77, 98] |
| Quercetagetin-6,7,3’,4’-tetramethylether (233) | 3,5-Dihydroxy-3’,4’,6,7-tetramethoxyflavone 3,3’,4’,5,6,7-Hexahydroflavone 3’,4’,6,7-tetra methyl ether | [57296-14-7] | [61, 71, 77, 105, 107] |
| Quercetagetin 4’-methyl ether (234) | 3,3’,4’,5,6,7-Hexahydroxyflavone 4’-methyl ether 3,3’,5,6,7-Pentahydroxy-4’-methoxyflavone 3’,5,6,7-Tetrahydroxy-4’-methoxyflavonol | [161585-86-0] | [89] |

| Name | Alternative Name(s) | CAS Number | References |
|---|---|---|---|
| 4H-1-Benzopyran-4-one 5-hydroxy-2-(2-hydroxy-3,4-di-methoxyphenyl)-3,7-dimethoxy (235) | [1186306-45-5] | [15] | |
| 4H-1-Benzopyran-4-one, 2-(3,5-dihydroxy-4-methoxy-phenyl)-3-(β-D-glucopyranosyloxy)-5,7-dihydroxy- (236) | [230283-37-1] | [71] | |
| Mearnsetin (237) | 3,3’,5,5’,7-Pentahydroxy-4’-methoxy-flavone 2-(3,5-Dihydroxyphenyl-4-methoxy-phenol)-3,5,7-trihydroxy-4H-1-benzopyran-4-one 3’,5’,5’,7-Tetrahydroxy-4’-methoxy-flavonol | [16805-10-0] | [71] |
| Chrysosplenol E (238) | 2’,3,4’,5,5’,7-Hexahydroxyflavone 3,4’,5’,7-tetramethyl ether 2’,5-Dihydroxy-3,4’,5’,7-tetramethoxy-flavone | [23289-81-8] | [80] |
| 5,3’-Dihydroxy, 3,6,7,5’-tetramethoxyflavone (239) | [99] | ||
| 3’,5,7,8-Tetrahydroxy-3,4’-dimethoxyflavone (240) | 3,3’,4’,5,7,8-Hexahydroxyflavone 3,4’-di-ethyl ether | [123563-74-6] | [76, 94] |


2.5. Monoterpenoids

2.5.1. Regular Acyclic Monoterpenes
| Name | Alternative Name(s) | CAS number | References |
|---|---|---|---|
| Citronellol (242) | 3,7-Dimethyl-6-octen-1-ol | [106-22-9] | [24] |
| Citronellal (243) | 3,7-Dimethyl-6-octenal | [106-23-0] | [28] |
| Geraniol (244) | (E)-3,7-Dimethyl-2,6-octadien-1-ol | [106-24-1] | [22] |
| Geranyl acetate (245) | (E)-3,7-Dimethyl-2,6-octadien-1-ol acetate | [105-87-3] | [23, 51] |
| 3,7-Dimethyl-2,6-octadienyl, isobutyric acid, ester (246) | (E)-Isobutyric acid, 3,7-dimethyl-2,6-octadienyl ester | [1188-06-3] | [50] |
| 2,6-Octadien-1-ol, 2,6-dimethyl-8-[(tetrahydro-2H-pyran-2-yl)oxy]- (247) | [80444-67-3] | [23] | |
| Nerol (248) | (Z)-3,7-Dimethyl-2,6-octadien-1-ol | [106-25-2] | [23, 58] |
| Neryl acetate (249) | (Z)-3,7-Dimethyl-2,6-octadien-1-ol acetate | [141-12-8] | [45] |
| Linalool (250) | 3,7-Dimethyl-1,6-octadien-3-ol | [78-70-6] | [19, 28, 31, 32, 45, 51, 74, 111] |
| Linalyl acetate (251) | 3,7-Dimethyl-1,6-octadien-3-ol acetate | [115-95-7] | [32, 43] |
| 3,7-Octadien-2-ol, 2,6-dimethyl- (252) | [62911-76-6] | [23] | |
| Myrcenol (253) | 2-Methyl-6-methylene-7-octen-2-ol | [543-39-5] | [30] |
| Myrcene (254) | 7-Methyl-3-methylene-1,6-octadiene | [123-35-3] | [19, 23, 29, 32, 33, 34, 43, 56, 50, 51, 73, 112, 113] |
| Ipsdienol (255) | 2-Methyl-6-methylene-2,7-octadien-4-ol | [35628-00-3] | [28] |
| allo-Ocimene (256) | 2,6-Dimethyl-2,4,6-octatriene | [673-84-7] | [22] |
| trans-α-Ocimene (257) | 3,7-Dimethyl-1,3,7-Octatriene | [27400-72-2] [3779-61-1] | [22, 32, 111] |
| (E)- 3,7-Dimethyl-1,3,6-octatriene (258) | [3779-61-1] | [43] | |
| (Z)-3,7-Dimethyl-1,3,6-octatriene (259) | [3338-55-4] | [32, 43] | |
| 2,6-Dimethyl-3,5,7-octatrien-2-ol (260) | [103272-78-2] | [23] | |
| 3,7-Dimethyl-1,5,7-octatrien-3-ol (261) | [29957-43-5] | [48] | |
| 2,6-Dimethyl-1,5,7-octatrien-3-ol (262) | [29414-56-0] | [23] | |
| 3,7-Octadien-2-ol, 2-methyl-6-methylene (263) | [22459-09-2] | [31] | |
| β-myrcene hydroperoxide (264) | 2-Methyl-6-methylene-3,7-octadiene-2-ol (E), 2-hydroperoxide | [9, 114] | |
| α- myrcene hydroperoxide (265) | 2-Methyl-6-methylene-1,7-octadiene, 3-hydro-peroxide | [9, 114] | |
| 1,6-Octadien-4-one, 7-methyl-3-methylene- (266) | [1079223-79-2] | [43] | |
| 1,7-Octadien-3-one, 2-methyl-6-methylene- (267) | 2-Methyl-6-methylene-1,7-octadien-3-one | [41702-60-7] | [20] |
| cis-Epoxyocimene (268) | 3,7-Dimethyl-1,3,6-Octatriene 6R,7-epoxide | [255832-06-5] | |
| 2,6-Dimethyl-1,3,5,7-octatetraene (269) | [90973-78-7] | [50] | |
| Perillene (270) | 3-(4-Methyl-3-pentenyl)furan | [539-52-6] | [23, 43] |
| 1,10-Oxy-α-myrcene hydroxide (271) | [9] | ||
| 1,10-Oxy-β-myrcene hydroxide (272) | [9] |


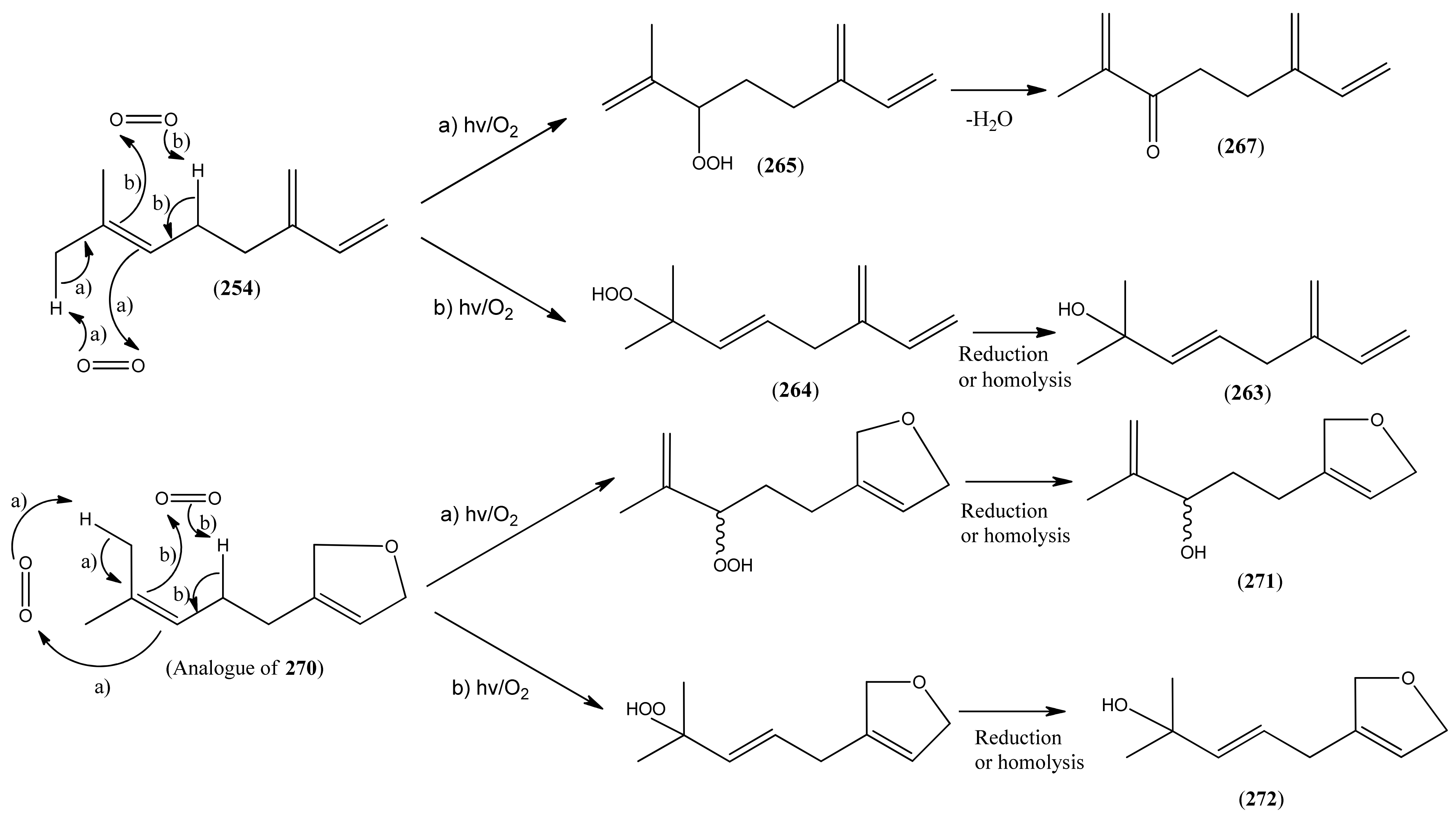
2.5.2. Irregular Acyclic Monoterpenes

| Name | Alternative Name(s) | CAS Number | References |
|---|---|---|---|
| Artemisanes | |||
| Artemisia alcohol (273) | 3,3,6-Trimethyl-1,5-heptadien-4-ol | [29887-38-5] [27644-04-8] [77363-66-7] | [19, 23, 29, 33, 38, 40, 43, 45, 48, 51, 66, 73, 121] |
| Artemisyl acetate (274) | Artemisia alcohol acetate 3,3,6-Trimethyl-1,5-heptadien-4-ol acetate | [3465-88-1] [29887-38-5] | [25, 40, 43, 45, 72, 112, 113] |
| (E)-2-Butenoic acid, 2-methyl-, 2,2-dimethyl-1-(2-methyl-1-propenyl)-3-butenyl ester (275) | [62594-30-3] | [30] | |
| Artemisia ketone (276) | 3,3,6-Trimethyl-1,5-heptadien-4-one | [546-49-6] | [19, 21, 22, 23, 24, 26, 29, 31, 33, 36, 38, 40, 41, 42, 43, 44, 45, 49, 53, 66, 72, 73, 112, 113, 121] |
| Yomogi alcohol (277) | 2,5,5-Trimethyl-3,6-heptadien-2-ol | [26127-98-0] | [19, 32, 38, 45] |
| Artemisiatriene (278) | 2,5,5-Trimethyl-1,3,6-heptatriene | [29548-02-5] | [23] |
| Lavandulanes | |||
| trans-5-Hydroxy-2-isopropenyl-5-methylhex-3-en-1-ol (279) | 3-Hexene-1,5-diol, 5-methyl-2-(1-methyl-ethenyl)- | [403797-33-1] | [129] |
| 4-Hydroxy-2-isopropenyl-5-methylene-hexan-1-ol (280) | [9] | ||
| Lavandulyl acetate (281) | 5-Methyl-2-(1-methylethenyl)-4-hex-en-1-ol | [20777-39-3] | [19] |
| Santolinanes | |||
| Santolina alcohol (282) | 3-Ethenyl-2,5-dimethyl-4-hexen-2-ol | [35671-15-9] | [19, 32, 43] |
| Santolinatriene (283) | 3-Ethenyl-2,5-dimethyl-1,4-hexadiene | [70005-95-7] [2153-66-4] | [23, 25, 43, 45, 50] |



2.5.3. Monocyclic Monoterpenes

| Name | Alternative Name(s) | CAS number | References |
|---|---|---|---|
| p-Menth-3-ene (287) | [500-00-5] | [50] | |
| p-Mentha-2,4-diene (288) | [586-68-5] | [25] | |
| α-Phellandrene (289) | p-Mentha-1,5-diene | [99-83-2] | [32] |
| β-Phellandrene (290) | p-Mentha-1(7),2-diene | [555-10-2] | [22, 25, 45] |
| α-Terpinene (291) | p-Menthan-1,3-diene | [99-86-5] | [19, 32, 40, 41, 45, 66, 74] |
| γ-Terpinene (292) | p-Menthan-1,4,diene | [99-85-4] | [19, 28, 32, 40, 41, 43, 66] |
| Terpinolene (293) | p-Mentha-1,4(8)-diene | [586-62-9] | [32, 43] |
| Limonene (294) | p-Mentha-1,8-diene | [138-86-3] [5989-27-5] | [24, 31, 34, 43, 45, 50, 66, 111] |
| p-Cymene (295) | 1-Methyl-4-isopropyl benzene | [99-87-6] | [19, 24, 25, 28, 29, 32, 34, 38, 40, 43, 45, 48, 73, 74] |
| Cuminic alcohol (296) | p-Mentha-1,3,5-trien-7-ol 4-Isopropenylbenzyl alcohol | [536-60-7] | [51] |
| Cuminal (297) | Cuminaldehyde p-Menthan-1,3,5-trien-1-al 4-Isopropylbenzaldehyde | [122-03-2] | [23, 25, 32, 43, 45] |
| Carvacrol (298) | p-Mentha-1,3,5-trien-2-ol | [499-75-2] | [31, 32, 43] |
| Thymol (299) | p-Cymen-3-ol p-Mentha-1,3,5-trien-3-ol | [89-83-8] | [19, 32, 43] |
| p-Cymen-8-ol (300) | 2-(4-Methylphenyl)-2-propanol | [1197-01-9] | [19, 25, 32] |
| Menthol (301) | p-Menthan-3-ol | [89-78-1] | [23, 25, 30, 60, 111] |
| β-Terpineol (302) | p-Menth-8-en-1-ol | [7299-41-4] | [43] |
| cis-p-Menth-2-en-1-ol (303) | [29803-81-4] | [19, 45] | |
| trans-p-Menth-2-en-1-ol (304) | [29803-82-5] | [19, 45] | |
| p-Menth-2,8-dien-1-ol (305) | 1-Methyl-4-(1-methylethyl)-2-cyclohexen-1-ol | [3886-78-0] | [32] |
| trans-Carveol (306) | p-Mentha-1,8-dien-6-ol, trans- | [1197-07-5] | [19, 25, 41, 43, 45] |
| cis-Carveol (307) | p-Mentha-1,8-dien-6-ol, cis- | [1197-06-4] | [19, 41, 43] |
| trans-Carvyl acetate (308) | p-Mentha-6,8-dien-2-ol, acetate, trans- | [1134-95-8] | [19, 31] |
| cis-Carvyl acetate (309) | p-Mentha-6,8-dien-2-ol, acetate, cis- | [1205-42-1] | [19] |
| Carvone (310) | p-Mentha-1,8-dien-6-one | [99-49-0] | [19, 24, 25, 2832, 43] |
| p-Mentha-1(7),5-dien-2-ol (311) | [30681-15-3] | [43] | |
| p-Mentha-1(7),8-dien-2-ol (312) | [35907-10-9] | [51] | |
| p-Menth-1-en-5-ol (313) | [55708-42-4] | [22] | |
| p-Mentha-1,4(8)-dien-3-ol (314) | [6753-08-8] | [32] | |
| 3-Cyclohexene-1-methanol 2-hydroxy-α,α,4-trimethyl-, 1-acetate (315) | [138913-54-9] | [25] | |
| Iso-menthone (316) | p-Menthan-3-one | [491-07-6] | [45] |
| Piperitone (317) | p-Menth-1-en-3-one | [89-81-6] | [24] |
| Terpinen-4-ol (318) | p-Menth-1-en-4-ol | [562-74-3] | [19, 30, 31, 32, 34, 41, 43, 45, 51, 66] |
| 4-Terpinyl acetate (319) | p-Menth-1-en-4-ol acetate | [4821-04-9] | [34] |
| Phellandral (320) | p-Menth-1-en-7-al | [21391-98-0] | [23] |
| Perillaldehyde (321) | p-Mentha-1,8-dien-7-al | [2111-75-3] | [45] |
| α-Terpineol (322) | l-α-Terpineol p-Menth-1-en-8-ol | [98-55-5] [10482-56-1] | [19, 22, 23, 28, 32, 40, 43, 45, 74] |
| δ-Terpineol (323) | p-Menthen-1(7)-en-8-ol | [7299-42-5] | [19] |
| Limonene-1,2-epoxide (324) | Limonene oxide 1,2-Epoxy-p-menth-8-ene | [1195-92-2] | [23, 51] |
| 1,4-Cineole (325) | 1,4-Epoxy-p-menthane | [470-67-7] | [112] |
| 1,8-Cineole (326) | Eucalyptol 1,8-Epoxy-p-menthane | [470-82-6] | [19, 22, 23, 24, 25, 28, 29, 32, 33, 34, 36, 37, 38, 40, 41, 43, 45, 48, 49, 50, 51, 53, 66, 72, 73, 74, 112, 113] |
| 2,3-Dihydro-1,8-cineole (327) | 1,8-Epoxy-p-menth-2-ene | [92760-25-3] | [19, 32, 41, 43] |
| 2-α-Hydroxy-1,8-cineole (328) | [60761-00-4] | [113] | |
| Ascaridole (329) | 1,4-Epidioxy-p-menth-2-ene | [512-85-6] | [28] |
| 2-Cyclohexen-1-one, 2-methyl-5-(1-methylcyclopropyl)- (330) | [26541-44-6] | [43] |



2.5.4. Bicyclic Monoterpenes
| Name | Alternative Name(s) | CAS Number | References |
|---|---|---|---|
| Borneol (334) | [464-43-7] [507-70-0] [124-76-5] | [19, 25, 30, 31, 34, 40, 41, 43, 45, 72, 73, 111, 112] | |
| Bornyl acetate (335) | [76-49-3] [92618-89-8] | [25, 31, 40, 45] | |
| Borneol isobutyrate (336) | [24717-86-0] | [45] | |
| Bornyl valerate (337) | Bornyl pentanoate | [7549-41-9] | [31] |
| 2-Butenoic acid, 3-methyl-(1S,2R,4S)-1,7,7-trimethylbicyclo[2.2.1]hept-2-yl ester (338) | [91404-82-9] | [31] | |
| Cyclopentanecarboxylic acid, 3-methylene-, 1,7,7-trimethylbicyclo-[2.2.1]hept-2-yl ester (339) | [74793-59-2] | [31] | |
| Isobornyl acetate (340) | 2-Bornanol acetate | [125-12-2] | [19] |
| Camphor (341) | 1,7,7-Trimethylbicyclo[2.2.1] heptan-2-one | [76-22-2] [464-48-2] | [19, 23, 25, 26, 29, 32, 34, 36, 37, 38, 40, 41, 42, 43, 44, 45, 48, 49, 51, 53, 66, 72, 73, 74, 112, 113] |
| endo-Dehydronorborneol (342) | Bicyclo[2.2.1]hept-5-en-2-ol | [694-97-3] | [23] |


| Name | Alternative Name(s) | CAS Number | References |
|---|---|---|---|
| α-Pinene (347) | 2,6,6-Trimethylbicyclo[3.1.1]hept-2-ene | [80-56-8] [7785-26-4] [7785-70-8] | [19, 22, 25, 28, 29, 32, 33, 34, 36, 37, 38, 40, 41, 42, 43, 44, 45, 50, 51, 66, 73, 74, 112, 113, 120, 132] |
| Verbenyl acetate (348) | Verbenol acetate 2-Pinen-4-ol, acetate | [33522-69-9] | [23] |
| trans-Chrysanthenol (349) | [38043-83-3] | [43] | |
| cis-Chrysanthenyl acetate (350) | [67999-48-8] | [19] | |
| Myrtenol (351) | 2-Pinen-10-ol | [515-00-4] [564-94-3] | [19, 23, 32, 41, 43, 66] |
| (-)-Myrtenyl acetate (352) | (-)-O-Acetylmyrtenol | [36203-31-3] | [23] |
| Verbenone (353) | 2-Pinen-4-one | [80-57-9] | [19, 25, 34, 43, 45] |
| Chrysanthenone (354) | 2-Pinen-6-one | [473-06-3] | [32, 43, 66] |
| Myrtenal (355) | 2-Pinen-10-al | [23727-16-4] | [66] |
| β-Pinene (356) | 6,6-Dimethyl-2-methylenebicyclo[3.1.1]-heptane | [127-91-3] [1330-16-1] | [19, 22, 23, 25, 26, 27, 28, 32, 33, 34, 36, 38, 40, 43, 45, 51, 66, 73, 74, 112, 113, 120, 132] |
| (-)-trans-Pinocarveol (357) | 2(10)-Pinen-3-ol | [547-61-5] [3917-59-7] | [19, 23, 25, 32, 33, 40, 43] |
| cis-Pinocarveol (358) | Isopinocarveol 2(10)-Pinen-3-ol, cis- | [6712-79-4] [5947-36-4] | [30, 43] |
| Pinocarvyl acetate (359) | 2(10)-Pinen-3-ol, acetate | [1078-95-1] | [30] |
| Pinocarvone (360) | 2(10)-Pinen-3-one | [30460-92-5] [19890-00-7] | [19, 23, 25, 28, 30, 32, 33, 42, 43, 45, 66] |
| 3-Pinanol (361) | 2,6,6-Trimethylbicyclo[3.1.1]heptan-3-ol | [25465-95-6] | [30] |
| β-Pinene oxide (362) | 2,10-Epoxypinane | [6931-54-0] | [19] |
| Bicyclo[3.1.1]heptan-3-one, 2,6,6-trimethyl-4-methylene- (363) | [62594-31-4] | [30] |


| Name | Alternative Name(s) | CAS Number | References |
|---|---|---|---|
| α-Thujene (366) | 3-Thujene 2-Methyl-5-(1-methylethyl)bicyclo[3.1.0]hex-2-ene | [2867-05-2] | [28, 34, 41, 43, 45, 66] |
| 3-Thujen-2-ol (367) | Bicyclo[3.1.0]hex-3-en-2-ol, 4-methyl-1-(1-methylethyl) | [3310-03-0] | [25] |
| 3-Thujen-10-al (368) | [57129-54-1] | [50] | |
| (-)-α-Thujone (369) | [546-80-5] | [45, 66, 111] | |
| Sabinene (370) | 4(10)-Thujene Bicyclo[3.1.0]hexane,4-methylene-1-(1-methylethyl)- | [2009-00-9] [3387-41-5] [204524-73-2] | [19, 23, 31, 32, 34, 40, 41, 43, 45, 51, 66, 74, 113] |
| Sabinol (371) | 4(10)-Thujen-3-ol | [471-16-9] | [32, 43] |
| trans-Sabinyl acetate (372) | Bicyclo[3.1.0]hexan-3-ol, 4-methylene-1-(1-methylethyl)-, 3-acetate | [139757-62-3] [3536-54-7] | [43] |
| β-Sabinene hydrate (373) | 4-Thujanol | [546-79-2] [15537-55-0] [17699-16-0] | [19, 25, 32, 34,41, 43, 45] |
| Sabina ketone (374) | Didehydrosabina ketone Bicyclo[3.1.0]hexan-2-one, 5-(1-methylethyl)-, 5-Isopropyl- bicyclo[3.1.0]hexan-2-one | [513-20-2] [110716-99-9] [147043-52-5] | [19, 43] |


2.6. Sesquiterpenoids
2.6.1. Farnesane Sesquiterpenes
| Name | Alternative Name(s) | CAS Number | References |
|---|---|---|---|
| Farnesol (376) | 2,6,10-Farnesatrien-1-ol 3,7,11-Trimethyl-2,6,10-dodecatrien-1-ol | [4602-84-0] [106-28-5] | [23, 27, 135] |
| 2,6,10-Farnesatrien-1-ol acetate (377) | [4128-17-0] | [27] | |
| Farnesyl pyrophosphate (378) | Farnesyl diphosphate 2,6,10-Dodecatrien-1-ol, 3,7,11-trimethyl-, trihydrogen pyrophosphate Diphosphoric acid, mono (3,7,11-trimethyl-2,6,10-dodecatrienyl) ester | [13058-04-3] | [135, 136] |
| Farnesal (379) | 2,6,10-Farnesatrien-1-al 3,7,11-Trimethyl-2,6,10-dodecatrienal | [19317-11-4] | [27] |
| trans-Nerolidol (380) | 3,6,10-Farnesatrien-3-ol 3,7,11-Trimethyl-1,6,10-dodecatrien-3-ol | [7212-44-4] [3790-78-1] | [19, 23, 27, 32, 43, 45, 134] |
| (E)-Nerolidyl acetate (381) | (E)-Nerolidol acetate 1,6,10-Dodecatrien-3-ol, 3,7,11-trimethyl-, 3-acetate | [85611-33-2] | [27] |
| trans-β-Farnesene (382) | (E)-1,3(15),6,10-Farnesatetraene (E)-7,11-Dimethyl-3-methylene-1,6,10-dodecatriene | [77129-48-7] [18794-84-8] | [19, 22, 27, 30, 32, 43, 48, 50, 51, 66, 134, 135] |
| (Z)-1,3(15),6,10-Farnesatetraene (383) | [28973-97-9] | [23, 31, 32] | |
| α-Farnesene (384) | 1,3,6,10-Farnesatetraene 3,7,11-Trimethyl-1,3,6,10-dodecatetraene | [502-61-4] [125037-13-0] | [27, 41, 48, 50, 74, 111] |

2.6.2. Monocyclic Sesquiterpenes
2.6.2.1. Bisabolanes
| Name | Alternative Name(s) | CAS Number | References |
|---|---|---|---|
| α-Bisabolol (385) | [515-69-5] | [31, 32, 43, 45, 134] | |
| cis-Lanceol (386) | 2,7(14),10-Bisabolatrien-12-ol | [147129-37-1] | [46] |
| 2,7,10-Bisabolatriene (387) | [58845-44-6] | [28] | |
| 2,3-Epoxy-7,10-bisaboladiene (388) | [111536-37-9] | [23] | |
| 7-Oxabicyclo[4.1.0]heptane, 4-(1,5-dimethyl-4-hexen-1-ylidene)-1-methyl-, (1R,4Z,6S)- (389) | [94347-02-1] | [31] |

2.6.2.2. Germacranes and Elemanes
| Name | Alternative Name(s) | CAS Number | References |
|---|---|---|---|
| (+)-Germacrene A (390) | Germacrene (1(10)E,4(E))-Germacra-1(10),4,11-triene | [28028-64-0] | [74] |
| Germacrene B (391) | (1(10)E,4(E)-1(10),4,7(11)-Germacratriene | [15423-57-1] | [23, 28, 46] |
| Germacrene D (392) | 1(10),4(15),5-Germacratiene | [23986-74-5] | [19, 23, 31, 32, 33, 36, 37, 42, 43, 45, 48, 66, 135] |
| 1β-Hydroxy-4(15),5(E),10(14)-germacratriene (393) | [9] | ||
| Pregeijerene (394) | 11,12,13-tri-nor-1(10),4,6,-Germacratriene 1,5-Dimethyl-1,5,7-cyclodecatriene | [20082-17-1] | [41] |


| Name | Alternative Name(s) | CAS Number | References |
|---|---|---|---|
| β-Elemene (396) | 1,3,11-Elematriene | [33880-83-0] [515-13-9] | [27, 31, 34, 40, 43, 45] |
| γ-Elemene (397) | 1,3,7(11)-Elematriene | [29873-99-2] [3242-08-8] | [23, 28, 31] |
| δ-Elemene (398) | 1,3,6-Elemantriene | [20307-84-0] | [32, 48] |
| Elemol (399) | 1,3-Elemadien-11-ol | [639-99-6] | [27] |
| Elemyl acetate (400) | Elemol acetate | [60031-93-8] | [19] |

2.6.2.3. Humulanes and Caryophyllanes
| Name | Alternative Name(s) | CAS Number | References |
|---|---|---|---|
| Humulanes | |||
| α-Humulene (401) | 2,6,9-Humulatriene | [6753-98-6] | [19, 27, 28, 32, 40, 43, 66] |
| 14-Hydroxy-α-humulene (402) | [108043-85-2] | [27] | |
| Humulene epoxide I (403) | 2,3-Epoxy-6,9-humuladiene | [19888-33-6] | [45] |
| Humulene epoxide II (404) | 6,7-Epoxy-2,9-humuladiene | [19888-34-7] | [27, 45] |
| Caryophyllanes | |||
| β-Caryophyllene (405) | (E)-3(15),6-Caryophylladiene | [87-44-5] | [19, 22, 23, 24, 26, 27, 29, 32, 36, 37, 40, 41, 42, 43, 45, 48, 49, 51, 66, 72, 73, 74, 112, 140] |
| γ-Caryophyllene (406) | Isocaryophyllene (Z)-3(15),6-Caryophylladiene | [118-65-0] | [31] |
| (1R,3Z,9S)-Bicyclo[7.2.0]undec-3-ene, 4,11,11-trimethyl-8-methylene- (407) | [136296-35-0] | [50] | |
| Caryophylladienol I (408) | Caryophyllenol Bicyclo[7.2.0]undecan-5-ol, 10,10-dimethyl-2,6-bis(methylene)-, (1S,5S,9R)-Caryophylla-4(12),8(13)-dien-5β-ol | [19431-80-2] [38284-26-3] | [28, 32, 43] |
| Caryophylladienol II (409) | Caryophyllenol Bicyclo[7.2.0]undecan-5-ol, 10,10-dimethyl-2,6-bis(methylene)-, (1S,5R,9R)-Caryophylla-4(12),8(13)-dien-5α-ol | [19431-79-9] [38284-26-3] | [28, 32, 43] |
| Caryophyllene oxide (410) | Isocaryophyllene oxide 6,7-Epoxy-3(15)-caryophyllene | [1139-30-6] [113877-94-6] [17627-43-9] | [9, 19, 23, 25, 27, 30, 32, 34, 43, 45, 48, 49, 50, 66] |
| cis-Caryophyllene oxide (411) | 5-Oxatricyclo[8.2.0.04,6]dodecane, 4,12,12-trimethyl-9-methylene-, (1R,4S,6R,10S)- | [60594-23-2] | [32] |

2.6.3. Bicyclic Sesquiterpenes
2.6.3.1. Eudesmanes and Eremophilanes
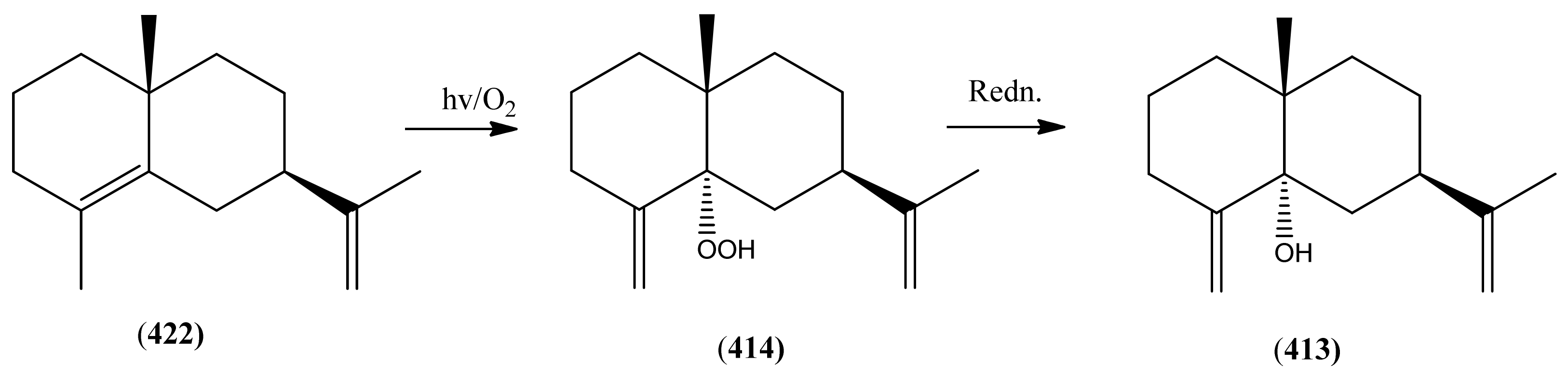
| Name | Alternative Name(s) | CAS Number | References |
|---|---|---|---|
| β-Selinene (412) | 4(15),11-Eudesmadiene | [17066-67-0] | [19, 23, 28, 30, 31, 32, 34, 40, 42, 43, 48, 113] |
| 5α-Hydroxy-eudesma-4(15),11-diene (413) | 4(15),11-Eudesmadien-5α-ol | [9, 97] | |
| 5α-Hydroperoxy-eudesma-4(15),11-diene (414) | [9] | ||
| 1β,6α-Dihydroxy-4(15)-eudesmane (415) | [9] | ||
| 1β-Hydroxy-4(15),5-eudesmadiene (416) | [9] | ||
| 1β-Hydroxy-4(15),7-eudesmadiene (417) | [9] | ||
| γ-Selinene (418) | 4(15),7(11)-Eudesmadiene 4(15),7(11)-Selinadiene | [515-17-3] | [45, 48] |
| β-Eudesmol (419) | 4(15)-Eudesmen-11-ol | [473-15-4] | [45] |
| α-Selinene (420) | (5α,7β,10β)-α-Eudesmane 3,11-Eudesmadiene Selina-3,11-diene | [473-13-2] | [34, 111] |
| Kongol (421) | 11-Eudesmen-4-ol(4α,5α,7β,10β) Selin-11-en-4α-ol | [16641-47-7] | [19, 27] |
| Selina-4,11-diene (422) | Eudesma-4,11-diene | [17627-30-4] | [135] |
| γ-Eudesmol (423) | 4-Eudesmen-11-ol | [1209-71-8] | [27] |
| 10-epi-γ-Eudesmol (424) | 4-Eudemen-11-ol (7β,10α) | [15051-81-7] | [27, 45] |
| Occidentalol (425) | 1,3-Eudesmadien-11-ol | [473-17-6] | [27] |
| Occidentalol acetate (426) | [346608-97-7] | [27] | |
| Occidol (427) | 1,2,3,4-Tetrahydro-α,α-5,8-tetramethyl-2-naphthalenemethanol | [5986-36-7] | [27] |
| Artemisin (428) | [141] | ||
| α-Hydroxysantonin (429) | [142] |


2.6.3.2. Cadinanes, Muurolanes and Amorphanes
| Name | Alternative Name(s) | CAS number | References |
|---|---|---|---|
| Cadinanes | |||
| Artemisinol (433) | 12-Cadinanol | [82890-78-6] | [143] |
| δ-Cadinene (434) | 1(10),4-Cadinadiene | [483-76-1] | [19, 23, 30, 31, 32, 40, 43, 48, 51] |
| 14-Hydroxy-δ-cadinene (435) | [153408-92-5] | [27] | |
| 4(15),5,11-Cadinatriene (436) | 1-epi-Bicyclosesquiphellandrene | [54274-73-6] | [48] |
| α-Cadinene (437) | 4,9-Cadinadiene | [24406-05-1] | [111, 130] |
| β-Cadinene (438) | 3,9-Cadinadiene | [523-47-7] | [22, 66] |
| γ-Cadinene (439) | 4(10),15-Cadinadiene | [39029-41-9] | [32, 34, 40, 43] |
| α-Cadinol (440) | 4-Cadinen-10-ol | [481-34-5] | [19, 43, 45] |
| γ-Cadinol (441) | 2-Naphthalenol, 1,2,3,4,4a,7,8,8a-octahydro-2,5-dimethyl-8-(1-methylethyl)- | [50895-55-1] | [45] |
| cis-Calamenene (442) | [72937-55-4] | [32, 43] | |
| Cubenol (443) | 4-Cadinen-1-ol | [21284-22-0] | [19, 43] |
| epi-Cubenol (444) | 4-Muurolen-1-ol | [19912-67-5] | [41] |
| Muurolanes | |||
| γ-Muurolene (445) | 4,10(14)-Muuroladiene | [30021-74-0] | [43] |
| δ-Muurolene (446) | 4(15),10(14)-Muuroladiene | [1136-29-4] | [23] |
| 4-Muurolen-10-ol (447) | Cedrelanol | [5937-11-1] | [25] |
| t-Muurolol (448) | 4-Muurolen-10-ol (1β, 6β, 7β,10β) | [19912-62-0] | [19] |


| Name | Alternative Name(s) | CAS Number | References |
|---|---|---|---|
| 4,7(11)-Amorphadien-12-al (449) | Cadin-4,7(11)-dien-12-al (name ascribed by original authors) | [67604-12-0] | [66] |
| 4(15),11-Amorphadien-9-one (450) | Cadin-4(15),11-dien-9-one (name ascribed by original authors) | [159662-31-4] | [66] |
| (-)-Amorpha-4,11-diene (451) | Naphthalene, 1,2,3,4,4a,5,6,8a-octahydro-4,7-dimethyl-1-(1-methylethenyl)-, (1R,4R,4aS,8aR)- | [92692-39-2] | [135, 136, 196] |
| 4-Amorphene-3,7-diol (3α,7α) (452) | [97] | ||
| 4-Amorphene-3,7-diol (3α,7α), acetate- (453) | 7α-Dihydroxyamorph-4-ene 3-acetate | [9] | |
| 4-Amorphen,3,11-diol (454) | 1-Naphthalenemethanol, 1,2,3,4,4a,5,6,8a-octahydro-6-hydroxy-α,α,4,7-tetramethyl- | [159662-32-5] | [66] |
| 4-Amorphen,3,11-diol 3-(2-methylpropanoyl) (455) | 3-Isobutylcadin-4-en-11-ol | [159662-30-3] | [66] |
| Amorph-4-en-7-ol (456) | 1-Naphthalenol, 1,2,3,4,4a,5,6,8a-octahydro-4,7-dimethyl-1-(1-methylethyl)-, (1R,4R,4aS,8aR)- | [140385-39-3] | [134] |
| Annulide (457) | Naphtho[1,8-bc]pyran-2(3H)-one, decahydro-6-methyl-3,9-bis(methylene)-[3aR-3aα,6α,6aα,9aβ,9bα)- | [103739-95-3] | [97, 128, 129, 182, 197] |
| trans-Arteannuic alcohol (458) | Artemisinic alcohol Amorpha-4,11-dien-12-ol 1-Naphthaleneethanol, 1,2,3,4,4a,5,6,8a-octahydro-4,7-dimethyl-β-methylene-, (1R,4R,4aS,8aR) | [125184-95-4] | [27, 32, 43, 135, 196] |
| cis-Arteannuic alcohol (459) | 4,11(13)-Cadinadien-12-ol | [147648-62-2] | [27, 32, 43] |
| Artemisinic aldehyde (460) | 1-Naphthaleneacetaldehyde, 1,2,3,4,4a,5,6,8a-octahydro-4,7-dimethyl-α-methylene-, (1R,4R,4aS,8aR)- | [125276-60-0] | [135, 196] |
| Arteannuin A (461) | Artemisinin I Qinghaosu I | [82442-48-6] | [56,171,172, 198, 199] |
| Arteannuin B (462) | Qing Hau Sau II Arteannuin C | [50906-56-4] | [15, 20, 25, 55, 59, 69 96, 105, 147,149, 153] |
| Arteannuin E (463) | Qinghaosu V 4-Hydroxy-11(13)-amorphen-12,5-olide; 4β,5α | [82003-84-7] | [56, 132, 147, 150, 176, 180, 182, 199, 205] |
| Arteannuin F (464) | Artemisilactone 4-Hydroxy-11(13)-amorphen-12,5-olide 4α,5α | [92691-97-9] | [56, 99, 132, 147, 150,165, 176, 180, 199,205] |
| Arteannuin H (465) | Naphtho[1,8-cd]-1,2-dioxepin-3(4H)-one, decahydro-4,7-dimethyl-10-methylene- (4R,4aR,7R,7aS,10aS,10bS)- | [207446-83-1] | [166, 183] |
| Arteannuin I (466) | Naphtho[1,8-bc]pyran-2(3H)-one, decahydro-3,6-dimethyl-9-methylene- (3R,3aR,6R,6aS,9aS,9bS)- | [207446-85-3] | [129, 166] |
| Arteannuin J (467) | Naphtho[1,8-bc]pyran-2(3H)-one, 3a,4,5,6,6a,7,9a,9b-octahydro-3,6,9-trimethyl (3R,3aR,6R,6aS.9aS,9bS)- | [207446-87-5] | [129, 166, 201] |
| Arteannuin K (468) | 2H-Naphtho[8a,1-b]furan-2-one, 3,3a,4,5,6,6a,7,10-octahydro-10-hydroxy-3,6,9-trimethyl-, (3R,3aS,6R,6aS,10R,10aS)- | [207446-88-6] | [166, 206] |
| Arteannuin L (469) | 2H-Naphtho[8a,1-b]furan-2-one, decahydro-10-hydroxy-3,6-dimethyl-9-methylene-, (3R,3aS,6R,6aS,10R,10aS)- | [207446-89-7] | [166, 206] |
| Arteannuin M (470) | 2H-Naphtho[8a,1-b]furan-2-one, decahydro-9,10-dihydroxy-3,6,9-trimethyl-, (3R,3aS,6R,6aS,9R,10R,10aS)- | [207446-90-0] | [166, 186, 187] |
| Arteannuin N (472) | 5-Oxo-3-amorphen-12-oic acid | [207446-92-2] | [166] |
| Arteannuin O (471) | 2H-Naphtho[8a,1-b]furan-2-one, decahydro-9,10-dihydroxy-3,6,9-trimethyl-, (3R,3aS,6R,6aS,9S,10R,10aS)- | [382600-19-3] | [184] |
| Artemisinic acid (473) | Arteannuic acid 4,11(13)-Amorphadien-12-oic acid Qing Hau acid | [80286-58-4] | [15, 20, 31, 55, 56, 59 61, 65, 69, 105, 143, 147, 148, 153, 160, 163, 164, 184, 198 200, 204, 207, 208, 209, 210, 211] |
| Artemisinic acid, methyl ester (474) | Methyl artemisinate 4,11(13)-Amorphadien-12-oic acid methyl ester | [82869-24-7] | [97, 143, 166, 212] |
| Artemisinin B (475) | 1-Naphthaleneacetic acid, 1,2,3,4,4a,5,8,8a-octahydro-8,8a-dihydroxy-4,7-dimethyl-α-methylene-, (1S,4R,4aS,8R,8aR)- | [145941-07-7] | [65] |
| 6,7-Dehydroartemisinic acid (476) | 4,11(13)-Amorphadien-12-oic acid 6,7-didhydro 4,6,11(13)-Cadinatrien-12-oic acid | [120193-24-0] | [160, 163, 213] |
| Deoxyarteannuin B (477) | Deoxyisoartemisinin C | [128301-55-3] | [97, 129, 163, 175, 201, 214, 215] |
| epi-Deoxyarteannuin B (478) | Deoxyisoartemisinin B | [84237-06-9] | [68, 96, 97, 129, 158, 160, 163, 164, 197] |
| Dihydroarteannuin B (479) | 3H-Oxireno[7,8]naphtho[8a,1-b]furan-3-one, decahydro-4,7,9a-trimethyl- [1aR-(1aα,1bR,4β,4aβ,7β,7aβ,9aα)]- | [64390-16-5] | [166] |
| 11R-(-)-Dihydroartemisinic acid (480) | 4,11(13)-Amorphadien-12-oic acid (11R,13-dihydro) | [85031-59-0] | [20, 32, 43, 135, 166, 167, 216] |
| Dihydroartemisinic acid hydroperoxide (481) | 4-Hydroxyperoxy-5-amorphen-12-oic acid, 4α, 11R | [85031-60-3] | [167, 170, 185] |
| Dihydroartemisinic alcohol (482) | 1-Naphthaleneethanol, 1,2,3,4,4a,5,6,8a-octahydro-α,4,7-trimethyl-, (1R,4R,4aS,8aS)- | [855425-50-2] | [135] |
| Dihydroartemisinic aldehyde (483) | 1-Naphthaleneacetaldehyde, 1,2,3,4,4a,5,6,8a-octahydro-α,4,7-trimethyl-, (1R,4R,4aS,8aS)- | [855425-51-3] | [135] |
| Dihydro-deoxyarteannuin B (484) | 2H-Naphtho[8a,1-b]furan-2-one, 3,3a,4,5,6,6a,7,8-octahydro-3,6,9-trimethyl- [3R-(3α,3aβ,6β,6aβ,9aR)]- | [89956-69-4] | [129] |
| Dihydro-epi-deoxyarteannuin B (485) | 4-Cadinen-12,6-olide (6β,10βH,12αH) | [104196-16-9] | [60, 68, 129, 166] |
| Dihydroxycadinanolide (486) | [217] | ||
| α-Epoxyartemisinic acid (487) | α-Epoxy-arteannuic acid | [129, 160, 194] | |
| α-Epoxy-dihydroartemisinic (488) | [9] | ||
| 4α,5α-Epoxy-6α-hydroxy amorphan-12-oic acid (489) | [9] | ||
| Isoannulide (490) | Naphtho[1,8-bc]pyran-2(3H)-one, 3a,4,5,6,6a,7,9a,9b-octahydro-6,9-dimethyl-3-methylene-, [3aR-(3aα,6α,6aα,9aβ,9bα)]- | [103739-94-2] | [97, 128, 129, 182, 197] |
| 2-Naphthalenol, decahydro-1-methyl-6-methylene-4-(1-methylethenyl)- (491) | [159662-33-6] | [66] | |
| Verboccidentene (492) | Amorpha-4,7(11)-diene | [79982-58-4] | [134] |

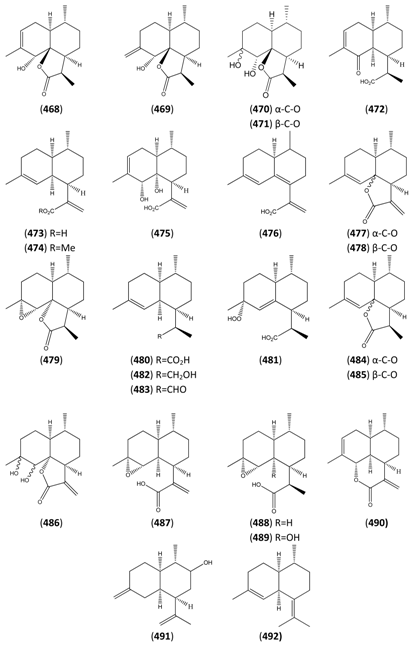
| 11,13-Dihydro form | 11,13-Dehydro form |
|---|---|
| Dihydroartemisinic acid (480) | Artemisinic acid (473) |
| Dihydroarteannuin B(479) | Arteannuin B (462) |
| Dihydro-epi-deoxyarteannuin B(485) | epi-Deoxyarteannuin B (478) |
| Dihydro-deoxyarteannuin B (484) | Deoxyarteannuin B (477) |
| α-Epoxy-dihydroartemisinic acid (488) | α-Epoxy-artemisinic acid (487) |
| Dihydro-seco-cadinane (493) | seco-Cadinane (494) |
| Arteannuin I (466) | Annulide (457) |
| Arteannuin J (467) | Isoannulide (490) |
| Artemisinin (495) | Artemisitene (497) |


2.6.3.3. Seco-Cadinanes
| Name | Alternative Name(s) | CAS Number | References |
|---|---|---|---|
| 1α-Aldehyde-2β-[3-butanone]-3α-methyl-6β-[2-propanoic acid]-cyclohexane (493) | [9] | ||
| 1α-Aldehyde-2β-[3-butanone]-3α-methyl-6β-[2-propenoic acid]-cyclohexane (494) | 4,5-Dioxo-4,5-seco-11(13)cadinen-12-oic acid | [9, 217] | |
| Artemisinin (495) | Arteannuin Qinghaosu Octahydro-3,6,9-trimethyl-3,12-epoxy-12H-pyrano[4,3-j]-1,2,benzodioxepin-10(3H)-one | [63968-64-9] | [4, 5, 15, 20, 34, 55, 56, 59, 69, 82, 91, 95, 96, 98, 105, 147, 150, 156, 171, 198, 200, 203, 204, 211, 213, 214, 215, 220, 230, 231, 234, 236, 239, 245, 246, 247, 248, 249, 250, 251, 252] |
| Arteannuin G (496) | [9, 56, 132, 147, 150, 176, 180, 182, 199, 205, 253] | ||
| Artemisitene (497) | Artemisinin, 11,13-didehydro | [101020-89-7] | [25, 55, 149, 200, 213, 250] |
| Arteannuin D (498) | 3α-Hydroxy-deoxyartemisinin Qinghaosu IV Artemisinin IV | [82003-85-8] | [15, 56] |
| Deoxyartemisinin (499) | Deoxyarteannuin Qing Hau Sau III Artemisinin III Octahydro-3,6,9-trimethyl-10αH-9,10b-epoxy-pyrano[4,32-jk][2]benzoxepin-2(3H)-one | [72826-63-2] | [56,59,60,105,132,145,147,169,229,254] |
| 3α-Hydroxy-4α,5α-epoxy-7-oxo-(8[7→6]-abeo-amorphane (500) | [9] | ||
| Norannuic acid (501) | [152135-59-6] | [199] | |
| Norannuic acid formyl ester (502) | [9] | ||
| 15-nor-10-Hydroxy-oplopan-4-oic acid (503) | [9] | ||
| 1-Oxo-2β-[3-butanone]-3α-methyl-6β-[2-propanoic acid]-cyclohexane (504) | [9] | ||
| 1-Oxo-2β-[3-butanone]-3α-methyl-6β-[2-propanol formyl ester]-cyclohexane (505) | [9, 218] |

2.6.3.4. Guaianes
| Name | Alternative Name(s) | CAS Number | References |
|---|---|---|---|
| α-Guaiene (506) | 1(5),11-Guaiadiene | [3691-12-1] | [28, 36] |
| β-Guaiene (507) | 1(5),7(11)-Guaiadiene | [88-84-6] | [32] |
| γ-Gurjunene (508) | 5,11-Guaiadiene | [22567-17-5] | [31, 34] |
| Guaiazulene (509) | 2,4-Dimethyl-7-(1-methylethyl)azulene | [492-45-5] | [24] |

| Name | Alternative Name(s) | CAS Number | References |
|---|---|---|---|
| α-Aromadendrene (510) | allo-Aromadendrene 10(14)-Aromadendrene | [25246-27-9] [14682-34-9] | [23, 40, 43] |
| α-Gurjunene (511) | 4-Aromadendrene | [489-40-7] | [48] |
| Globulol (512) | (1α,4α,5β,6α,7α,10α)-10-Aromadendranol | [489-41-8] | [19, 28, 34] |
| epi-Globulol (513) | 1H-Cycloprop[e]azulen-4-ol, decahydro-1,1,4,7-tetramethyl-, (1aR,4S,4aR,7R,7aS,7bS)- | [88728-58-9] | [34] |
| Ledol (514) | 10-Aromadendrol (1β,4α,5β,6β,7β,10α) | [577-27-5] | [28] |
| (-)-Spathulenol (515) | 10(14)-Aromadendren-4-ol | [77171-55-2] [6750-60-3] | [19, 27, 31, 32, 41, 43, 45] |
| Cycloprop[7,8]azuleno[3a,4-b]oxirene, decahydro-1,4a,7,7-tetramethyl-, (1R,6aR,7aR,7bS)- (516) | [199983-75-0] | [34] | |
| Aromadendrene epoxide (517) | Isoaromadendrene epoxide 10(14)-Aromadendrene 10β,14-epoxide | [85710-39-0] [499134-59-7] | [23, 34] |
| Cyclocolorenone (518) | 4-Aromadendren-3-one | [489-45-2] | [45] |

2.6.4. Tricyclic Sesquiterpenes


| Name | Alternative Name(s) | CAS Number | References |
|---|---|---|---|
| Cedrol (528) | 3-Cedranol Cedran-8-ol 6-Isocedrol epi-Cedrol | [77-53-2] [19903-73-2] | [19, 27, 32, 43, 45, 48] |
| Cedryl acetate (529) | 3-Cedranol acetate | [77-54-3] | [27] |
| Cedra-8(15)-en-9α-ol (530) | β-Cedren-9-ol Cedrenol | [13567-41-4] [28231-03-0] | [27, 43] |
| Cedra-8(15)-en-9α-ol acetate (531) | [65082-66-8] | [27, 32, 43] | |
| 3-Cedren-12-ol (532) | [18319-35-2] | [27] | |
| Cedra-8-en-13-ol, acetate (533) | [18319-34-1] | [27] | |
| 3α,15-Dihydroxy cedrane (534) | [9] |



2.7. Higher Terpenoids
2.7.1. Diterpenes

| Name | Alternative Name(s) | CAS Number | References |
|---|---|---|---|
| Phytol (550) | 2-Phyten-1-ol (2E, 7R,11R) | [150-86-7] | [27, 31, 43, 45] |
| Isophytol (551) | 1-Phyten-3-ol 3,7,11,15-Tetramethyl-1-hexadecen-3-ol | [505-32-8] | [27] |
| Phytene-1,2-diol (552) | 3(20)-Phytene-1,2-diol (7R,11R) | [9, 260, 261] | |
| Phytene-1-ol-2-hydroperoxide (553) | [9, 260, 261] | ||
| (2E)-Hexadecene (554) | 3,7,11,15-Tetramethylhexadec-2-ene | [2437-936] [532426-78-1] | [20] |
| Phytone (555) | Hexahydrofarnesyl acetone 6,10,14-Trimethyl-2-pentadecanone | [502-69-2] | [23] |


2.7.2. Triterpenes and Sterols
| Name | Alternative Name(s) | CAS Number | References |
|---|---|---|---|
| Oleananes | |||
| β-Amyrin (559) | 12-Oleanen-3-ol | [559-70-6] | [58, 63, 66] |
| β-Amyrin 3-acetate (560) | 12-Oleanen-3-ol acetate | [1616-93-9] | [58, 66, 198] |
| Oleanolic acid (561) | 3β-Hydroxy-12-oleanen-28-oic acid | [508-02-1] | [58, 66, 96] |
| Friedalanes | |||
| Friedelan-3-β-ol (562) | Epifriedelanol | [5085-72-3] [16844-71-6] | [105] |
| Friedelin (563) | [559-74-0] | [105] | |
| Ursanes | |||
| α-Amyrin (564) | 12-Ursen-3-ol | [638-95-9] | [58, 63, 66, 198] |
| α-Amyrenone (565) | α-Amyrone 12-Urs-en-3-one | [638-96-0] | [58, 66] |
| Taraxastanes | |||
| Taraxasterone (566) | 20(30)-Taraxasten-3-one | [6786-16-9] | [58, 66] |
| Baurenol (567) | 7-Bauren-3-ol | [6466-49-0] | [58, 66] |
| Sterols | |||
| β-Sitosterol (568) | Stigmast-5-en-3-ol | [83-46-5] | [15, 59, 61, 63, 69, 75, 78, 96, 198] |
| Daucosterol (569) | Stigmast-5-en-3-ol O-beta-D-glucopyranoside | [474-58-8] | [15] |
| Stigmasterol (570) | Stigmast-5,22-dien-3-ol 3β (22E,24S) | [83-48-7] | [56, 59, 61, 63, 65, 69, 75, 78, 96, 105, 198] |


2.8. Nitrogen-Containing Natural Products

3. The Biosynthesis of Artemisinin (Qinghaosu)

3.1. Phase 1 (Isopentenyl Pyrophosphte to Amorpha-4,11-diene)


3.2. Phase 2 (Amorpha-4,11-diene to Dihydroartemisinic Acid)
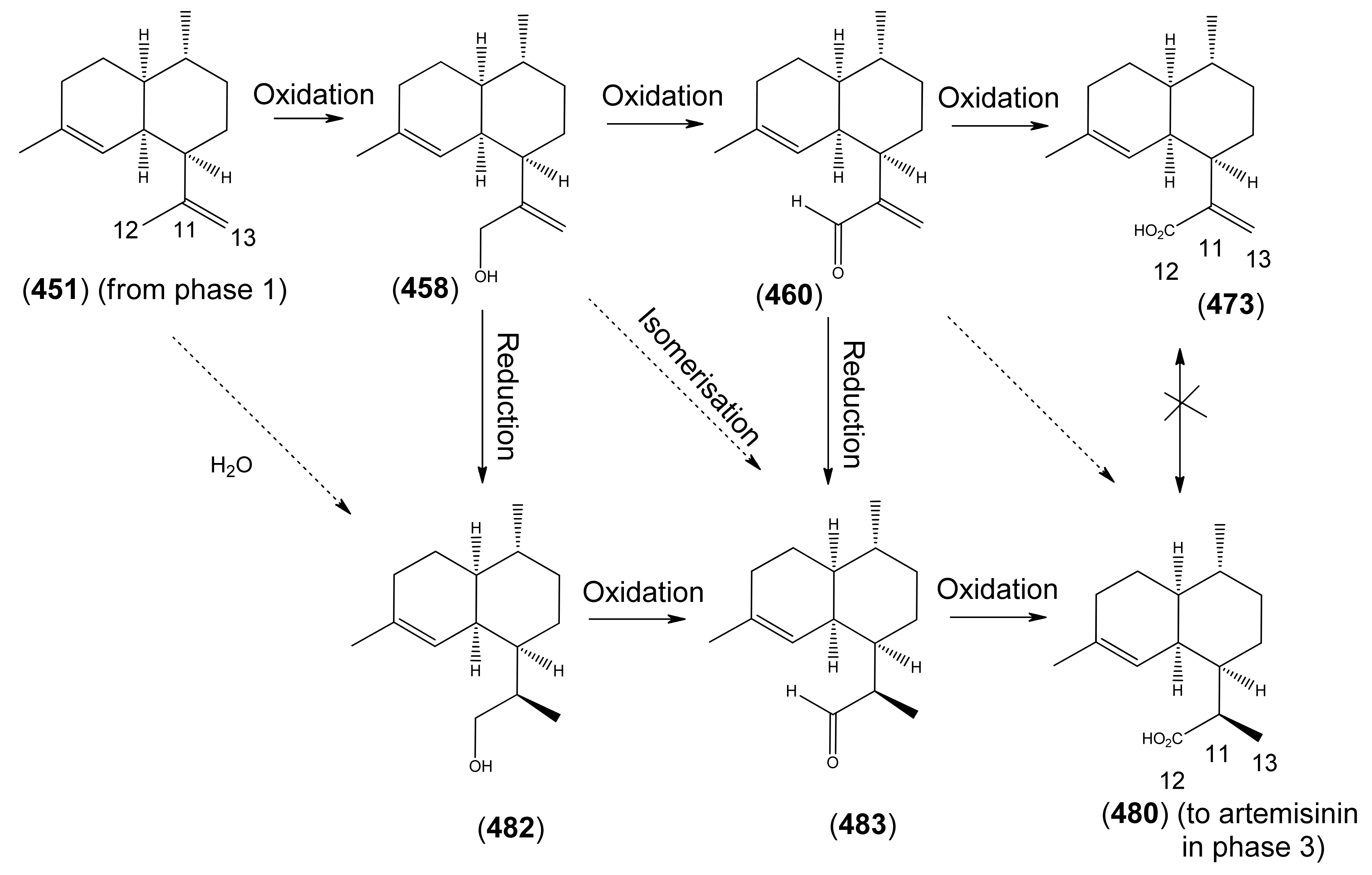
3.3. Phase 3 (Dihydroartemisinic acid to Artemisinin)
3.3.1. Dihydroartemisinic Acid as a Late-Stage Precursor to Artemisinin

3.3.2. Artemisinic Acid (473) as a Late-Stage Precursor to Artemisinin
3.3.3. Arteannuin B (462) and Dihydroarteannuin B (479) as Late-Stage Precursors to Artemisinin
3.3.4. epi-Deoxyarteannuin B (478) and Dihydro-epi-deoxyarteannuin B (485) as Late-Stage Precursors to Artemisinin
3.3.5. The seco-Cadinane (494) and Artemisitene (497) as Late-Stage Precursosr to Artemisinin
4. Strategies for the Production of Artemisinin from A. annua and Derived Systems
4.1. Plant Breeding Programmes
4.2. Plant Tissue Culture
4.3. Endophytic fungi
| Endophyte | Compound | Reference |
|---|---|---|
| Myrothecium roridum (IFB-E009; IFB-E012) | Myrothecine A (585) | [359] |
| Myrothecine B (586) | [359] | |
| Myrothecine C (587) | [359] | |
| Hypoxylon truncatum (IFB-18) | Daldinone C (588) | [360] |
| Daldinone D (589) | [360] | |
| Altechromone A (590) | [360] | |
| (4S)-5,8-Dihydroxy-4-methoxy-α-tetralone (591) | [360] | |
| Leptosphaeria sp. (strain number IV403) | Leptosphaeric acid (592) | [361] |
| Leptosphaerone (593) | [362] | |
| Colletotrichum sp. | 3β-Hydroxy-ergosta-5-ene (594) | [363] |
| Ergosterol (595) | [363] | |
| 3β,5α,6β-Trihydroxyergosta-7,22-diene (596) | [363] | |
| 3β,5α-Dihydroxy-6β-acetoxy-ergosta-7,22-diene (597) | [363] | |
| 3β,5α-Dihydroxy-6β-phenylacetyloxy-ergosta-7,22-diene (598) | [363] | |
| 3β-Hydroxy-5α,8α-epidioxy-ergosta-6,22-diene (599) | [363] | |
| 3β-Hydroxy-5α,8α-epidioxy-ergosta-6,9(11),22-triene (600) | [363] | |
| 3-Oxo-ergosta-4-ene (601) | [363] | |
| 3-Oxo-ergosta-4,6,8(14),22-tetraene (602) | [363] | |
| Indole-3-acetic acid (603) | [363] | |
| 6-Isoprenylindole-3-carboxylic acid (604) | [363] |

4.4. Genetic Engineering

Acknowledgements
References
- Hsu, E. The history of qing hao in the Chinese materia medica. Trans. R. Soc. Trop. Med. Hyg. 2006, 100, 505–508. [Google Scholar] [CrossRef] [PubMed]
- Yeung, H.C. Handbook of Chinese Herbs and Formulas; Institute of Chinese Medicine: Los Angeles, CA, USA, 1985; Volume 1, p. 430. [Google Scholar]
- Anonymous. Pharmacopoeia of the People’s Republic of China, English edition ed.; Guangdong Science and Technology Press: Guangzhou, China, 1992; p. 91. [Google Scholar]
- Klayman, D.L. Qinghaosu (artemisinin): an antimalarial drug from China. Science 1985, 228, 1049–1055. [Google Scholar] [CrossRef] [PubMed]
- Luo, X.D.; Shen, C.C. The chemistry, pharmacology, and clinical applications of Qinghaosu (artemisinin) and its derivatives. Med. Res. Rev. 1987, 7, 29–52. [Google Scholar] [CrossRef] [PubMed]
- Anonymous. Kang nue xin yao qin hao su de yan jiu (in Chinese). Chin. Pharm. Bull. 1979, 2, 49–53. [Google Scholar]
- Sriram, D.; Rao, V.S.; Chandrasekhar, K.V.G.; Yogeeswari, P. Progress in the research of artemisinin and its analogues as antimalarials: an update. Nat. Prod. Res. 2004, 18, 503–527. [Google Scholar] [CrossRef] [PubMed]
- Begue, J.P.; Bonnet-Delpon, D. The future of antimalarials: Artemisinins and synthetic endoperoxides. Drugs Future 2005, 30, 509–518. [Google Scholar] [CrossRef]
- Brown, G.D.; Liang, G.-Y.; Sy, L.-K. Terpenoids from the seeds of Artemisia annua. Phytochemistry 2003, 64, 303–323. [Google Scholar] [CrossRef]
- Klayman, D.L.; Lin, A.J.; Acton, N.; Scovili, J.P.; Hoch, J.M.; Milhous, W.K.; Theoharides, A.D. Isolation of artemisinin (Qinghaosu) from Artemisia annua growing in the US. J. Nat. Prod. 1984, 47, 715–717. [Google Scholar] [CrossRef] [PubMed]
- Balachandran, S.; Vishwakarma, R.A.; Popli, S.P. Chemical investigation of some Artemisia species: Search for artemisinin or other related sesquiterpene lactones with a peroxide bridge. Indian J. Pharm. Sci. 1987, 49, 152–154. [Google Scholar]
- Bhakuni, R.S.; Jain, D.C.; Sharma, R.P. Phytochemistry of Artemisia annua and the Development of Artemisinin-Derived Antimalarial Agents. In Artemisia; Wright, C., Ed.; Taylor and Francis: Oxford, UK, 2002; Chapter 12; pp. 211–249. [Google Scholar]
- Bhakuni, R.S.; Jain, D.C.; Sharma, R.P.; Kumar, S. Secondary metabolites of Artemisia annua and their biological activity. Curr. Sci. 2001, 80, 35–48. [Google Scholar]
- Bhakuni, R.S.; Jain, D.C.; Sharma, R.P. Phytochemistry of Artemisia annua and the development of artemisinin-derived antimalarial agents. Med. Aromat. Plants-Ind. Profiles 2002, 18, 211–248. [Google Scholar]
- Chen, J.; Zhou, Y.-B.; Zhang, X.; Huang, L.; Sun, W.; Wang, J.-H. Chemical constituents of Artemisia annua L. Shenyang Yaoke Daxue Xuebao 2008, 25, 866–870. [Google Scholar]
- Buckingham, J. (Ed.) Introduction to the Type of Compound Index. In Dictionary of Natural Products; Chapman and Hall: London, UK, 2004; Volume 1, pp. xi–lxii. [Google Scholar]
- Asahina, Y.; Yoshitomi, E. Essential oil of Artemisia annua L. I. Yakugaku Zasshi 1917, 489. [Google Scholar]
- Imada, Y. Volatile Oil of Artemisia annua L. Yakugaku Zasshi 1917, 119–135. [Google Scholar]
- Billa, A.R.; Flamini, G.; Morgenni, F.; Isacchi, B.; Franco, F. GC-MS analysis of the volatile constituents of essential oil and aromatic waters of Artemisia annua L. at different developmental stages. Nat. Prod. Comm. 2008, 3, 2075–2078. [Google Scholar]
- Ma, C.; Wang, H.; Lu, X.; Xu, G.; Liu, B. Metabolic fingerprinting investigation of Artemisia annua L. in different stages of development by gas chromatography and gas chromatography-mass spectrometry. J. Chromatogr. A. 2008, 1186, 412–419. [Google Scholar] [CrossRef] [PubMed]
- Woerdenbag, H. J.; Bos, R.; Salomons, M. C.; Hendriks, H.; Pras, N.; Malingre, T. M. Volatile constituents of Artemisia annua L. (Asteraceae). Flavour Frag. J. 1993, 8, 131–137. [Google Scholar] [CrossRef]
- Liu, Q.; Yang, Z.-Y.; Deng, Z.-B.; Sa, G.; Wang, X. Preliminary analysis on chemical constituents of essential oil from inflorescence of of Artemisia annua L. Acta Botanica Sinica (Zhiwu Xuebao) 1988, 30, 223–225. [Google Scholar]
- Tian, J.; Feng, W.; He, B. Study on volatile constituents of herba Artemisia annua and its preparation by GC-MS. Shizen Guoyi Guoyao 2007, 18, 1840–1842. [Google Scholar]
- Zou, Y.; Shi, J.; Shi, H. Analysis of volatile components from Artemisia annua Linn. Fenxi Ceshi Xuebao 1999, 18, 55–57. [Google Scholar]
- Chalchat, J.-C.; Raymond, P.G.; Michet, A.; Gorunovic, M.; Stosic, D. A contribution to chemotaxonomy of Artemisia annua L., Asteraceae. Acta Pharm. Jugosl. 1991, 41, 233–236. [Google Scholar]
- Zhong, Y.; Cui, S. Studies of volatile constituents from Artemisia annua. Zhongyao Tongbao 1983, 8, 31–32. [Google Scholar] [PubMed]
- Goel, D.; Goel, R.; Singh, V.; Ali, M.; Mallavarapu, G.R.; Kumar, S. Composition of the essential oil from the root of Artemisia annua. J. Nat. Med. 2007, 61, 458–461. [Google Scholar] [CrossRef]
- Mukhtar, H.M.; Ansari, S.H.; Ali, M.; Mir, S.R.; Abdin, M.Z.; Singh, P. GC-MS analysis of volatile oil of aerial parts of Artemisia annua L. J. Essent. Oil-Bearing Plants 2007, 10, 168–171. [Google Scholar] [CrossRef]
- Genov, N.; Georgiev, E. Gas-Chromatographic study of the essential oil of Artemisia annua L. Naunchi Trudove - Vissh Institut po Khranitelna i Vkusova Promishlenost, Plodiv 1983, 30, 141–148. [Google Scholar]
- Tsankova, E.; Ognyanov, I. On the composition of the essential oil from Artemisia annua Linnaeus. Italiana Essenze, Profumi, Piante Officinale, Aromi, Saponi, Cosmetici, Aerosol 1976, 58, 502–504. [Google Scholar]
- Ma, C.; Wang, H.; Lu, X.; Li, H.; Liu, B.; Xu, G. Analysis of Artemisia annua L. volatile oil by comprehensive two-dimensional gas chromatography time-of-flight mass spectrometry. J. Chromatogr. A. 2007, 1150, 50–53. [Google Scholar] [CrossRef] [PubMed]
- Goel, D.; Singh, V.; Ali, M.; Mallavarapu, G.R.; Kumar, S. Essential oils of petal, leaf and stem of the antimalarial plant Artemisia annua. J. Nat. Med. 2007, 61, 187–191. [Google Scholar] [CrossRef]
- Libbey, L.M. Unusual essential oils grown on Oregon II. Artemisia annua L. J. Essent. Oil Res. 1989, 1, 201–202. [Google Scholar] [CrossRef]
- Ali, M.; Siddiqui, N.A. Volatile oil constituents of Artemisia annua leaves. J. Med. Aromat. Plant Sci. 2000, 22, 568–571. [Google Scholar]
- Nakajima, V.T. Essential Oils of Artemisia annua. Yakugaku Zasshi 1962, 82, 1323–1326. [Google Scholar] [CrossRef] [PubMed]
- Biougne, J.; Chalchat, J.-C.; Garry, R.P.; Lamy, J. Essential oil of Artemisia annua: Seasonal variations in chemical composition. Rivista Italiania EPPOS 1993, 4, 622–629. [Google Scholar]
- Holm, Y.; Laasko, I.; Hitunen, R.; Galambosi, B. Variation in the essential oil composition of Artemisia annua L. of different origin cutivated in Finland. Flavour Frag. J. 1997, 12, 241–246. [Google Scholar] [CrossRef]
- Hethelyl, E.B.; Cseko, I.B.; Grosz, M.; Mark, G.; Palinkos, J.J. Chemical composition of the Artemisia annua essential oils from Hungary. J. Essent. Oil Res. 1995, 7, 45–48. [Google Scholar] [CrossRef]
- Popescu, H.; Tamas, M.L.; Tibori, A.G. Artemisia annua: An indigenous source of volatile oil. Clujul Med. 1980, 53, 331–337. [Google Scholar]
- Dembitskii, A.D.; Krotova, G.I.; Kuchukhidze, N.M.; Yakobashvili, N.Z. Essential Oil of Artemisia annua L. Maslozhirovaya Promyshlennost 1983, 31–34. [Google Scholar]
- Verdian-Rizi, M.R.; Sadat-Ebrahimi, E.; Hadjakhoondi, A.; Fazeli, M.R.; Pirali, H.M. Chemical composition and antimicrobial activity of Artemisia annua L. essential oil from Iran. Fasinmah-i Giyahan-i-Daruyi 2008, 7, 58–62. [Google Scholar]
- Lari, Y.H.; Khavarinejad, R.A.; Roustalan, A.H. The composition of essential oil from Artemisia annua L. growing wild in Iran. Falsnamah-i-Giyahan-i Daruyi 2002, 1, 41–48. [Google Scholar]
- Goel, D.; Mallavarupu, G.R.; Kumar, S.; Singh, V.; Ali, M. Volatile metabolite composition of the essential oil from aerial parts of ornamanetal and artemisinin-rich cultivars of Artemisia annua. J. Essent. Oil Res. 2007, 20, 147–152. [Google Scholar] [CrossRef]
- Bagchi, G.D.; Haider, F.; Dwivedi, P.D.; Singh, A.; Naqvi, A.A. Essential oil constituents of Artemisia annua during different growth periods at monsoon conditions of subtropical North Indian plants. J. Essent. Oil Res. 2003, 15, 59–62. [Google Scholar]
- Jain, N.; Srivastava, S.K.; Aggarawal, K.K.; Kumar, S.; Syamasundar, K.V. Essential oil composition of Artemisia annua L. "Asha" from the plains of Northern India. J. Essent. Oil Res. 2002, 14, 305–307. [Google Scholar] [CrossRef]
- Yang, Z.; Yu, Z.; Luo, S.; Peng, Q. Investigation of essential oil from leaves of Artemisia annua L. in Guizhou. Shizen Guoyi Guoyao 2008, 19, 255–257. [Google Scholar]
- He, B.; Feng, W.-Y.; Tian, J.; Li, C.-H.; Ai, H.-B. Analysis of chemical composition of voatile oil in Youyang Artemisia annua by GC-MS. Huaxi Yaoxue Zazhi 2008, 23, 30–31. [Google Scholar]
- Li, R.; Wang, D.; Liao, H. Chemical constituents of essential oil from the fruits of Artemisia annua L. Zhongnan Yaoxue 2007, 5, 230–232. [Google Scholar]
- Sun, Y.; Chen, X.; Zhang, H.; Xu, X.; Li, P. GC determination of eucalyptol, artemisia ketone, camphor, caryophyllene and caryophyllene oxide in Artemisia annua L. produced in Sichuan. Yaowu Fenxi Zazhi 2006, 26, 239–241. [Google Scholar]
- Zhang, Y.; Zhang, J.; Yao, J.; Wang, L.; Huang, A.-L.; Dong, L.-N. Studies on the chemical composition of the essential oil of Artemisia annua L. from Xinjiang. Xibei Shifan Daxue Xuebao, Ziran Kexueban 2004, 40, 67–69. [Google Scholar]
- Nguyen, X.D.; Leclercq, P.A.; Dinh, H.K.; Nguyen, M.T. Chemical Composition of essential oil of Vietnamese Artemisia annua L. Tap Chi Duoc Hoc 1990, 11–13. [Google Scholar]
- Pham, G.D. Chemical components of the essential oil of Artemisia annua L. in Vietnam and Bulgaria. Tap Chi Duoc Hoc 2003, 11–12. [Google Scholar]
- Charles, D.J.; Cebert, E.; Simon, J.E. Characterization of the essential oil of Artemisia annua L. J. Essent. Oil Res. 1991, 3, 33–39. [Google Scholar] [CrossRef]
- Tellez, M.R.; Canel, C.; Duke, S.O.; Rimando, A. Comparison of the essential oil of Artemisia annua L. and a chemotype of A. annua L. without glandular trichomes. Book of Abstracts; In proceedings of 216th ACS National Meeting, Boston, MA, USA, August 23-27, 1998. [Google Scholar]
- Woerdenbag, H.J.; Pras, N.; Bos, R.; Visser, J.F.; Hendriks, H.; Malingre, T.M. Analysis of artemisinin and related sesquiterpenes from Artemisia annua L. by combined gas chromatography/mass spectrometry. Phytochem. Analysis 1991, 2, 215–219. [Google Scholar] [CrossRef]
- Tian, Y.; Wei, Z.; Wu, Z. Studies on the chemical constituents of Qinghao (Artemisia annua), a traditional chinese herb. Zhongcaoyao 1982, 13, 9–11. [Google Scholar]
- Szeto, Y.-T.; Benzie, I. F.-F. Is the yin-yang nature of Chinese herbal medicine equivalent to antioxidation-oxidation? J. Ethnopharmacol. 2006, 108, 361–366. [Google Scholar] [CrossRef] [PubMed]
- Ululeben, A.; Halfon, B. Phytochemical investigation of the herb of Artemisia annua. Planta Med. 1976, 29, 258–260. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.M.A.A.; Jain, D.C.; Bhakuni, R.S.; Zaim, M.; Thakur, R.S. Occurrence of some antiviral sterols in Artemisia annua. Plant Sci. 1991, 75, 161–166. [Google Scholar] [CrossRef]
- Singh, T.; Bhakuni, R.S. A new sesquiterpene lactone from Artemisia annua leaves. Indian J. Chem. Sect. B: Organic Chemistry including Medicinal Chemistry 2004, 43B, 2734–2736. [Google Scholar] [CrossRef]
- Tu, Y.; Zhu, Q.; Shen, X. Constituents of young Artemisia annua. Zhongyao Tongbao 1985, 10, 419–20. [Google Scholar]
- Bhakuni, R.S.; Jain, D.C.; Shukla, Y.N.; Thakur, R.S. Lipid constituents from Artemisia annua. J. Indian Chem. Soc. 1990, 67, 1004–1006. [Google Scholar]
- Ul’chenko, N.T.; Khushbaktova, Z.A.; Bekker, N.P.; Kidisyuk, E.N.; Syrov, V.N.; Glushenkov, A.I. Lipids from flowers and leaves of Artemisia annua and their biological activity. Chem. Nat. Comp. 2005, 41, 280–284. [Google Scholar] [CrossRef]
- Zhang, J.; Zhang, Y.; Zhang, H.-B.; Wang, Y.-F.; Yao, J. Studies on the composition of the fatty acids of five species of Artemisia in Xinjiang. Xibei Shifan Daxue Xuebao, Ziran Kexueban 2004, 40, 64–67. [Google Scholar]
- Deng, S.-J.; Li, C.-Y.; Chen, S.; He, X.-X.; Gu, W.-X.; Gao, Z.-M. Alleochemicals isolation and structure identification of Artemisia annua. Huanan Nongye Daxue Xuebao 2008, 29, 42–46. [Google Scholar]
- Ahmad, A.; Misra, L.N. Terpenoids from Artemisia annua and constituents of its essential oil. Phytochemistry 1994, 37, 183–186. [Google Scholar] [CrossRef]
- Manns, D.; Hartmann, R. Annuadiepoxide, a new polyacetylene from the aerial parts of Artemisia annua. J. Nat. Prod. 1992, 55, 29–32. [Google Scholar] [CrossRef]
- Brown, G. D. Two new compounds from Artemisia annua. J. Nat. Prod. 1992, 55, 1756–1760. [Google Scholar] [CrossRef]
- Chen, Y.; You, B.; Dong, L.; Zhou, L. Isolation and identification of arteannuin and its precursor. Zhongcaoyao 2001, 32, 302–303. [Google Scholar]
- Singh, A.K.; Pathak, V.; Agrawal, P.K. Annphenone, a phenolic acetophenone from Artemisia annua. Phytochemistry 1997, 44, 555–557. [Google Scholar] [CrossRef]
- Han, J.; Ye, M.; Qiao, X.; Xu, M.; Wang, B.-R.; Guo, D.-A. Characterization of phenolic compounds in the Chinese herbal drug Artemisia annua by liquid chromatography coupled to electrospray ionization mass spectrometry. J. Pharm. Biomed. Anal. 2008, 47, 516–525. [Google Scholar] [CrossRef] [PubMed]
- Georgiev, E.; Genov, N. Changes in the essential oil of Artemisia annua L. during storage. Nauchni Trudove - Vissh Institut po Khranitelna i Vkusova Promishlenost 1983, 30, 149–160. [Google Scholar]
- Georgiev, E.; Genov, N.; Lazarova, R.; Ganchev, G. On the distillation of annual wormwood (Artemisia annua Linnaeus). Rivista Italiana Essenze, Profumi, Piante Officinali, Arromatizanti, Syndets, Saponi, Cosmaetici, Aerosols 1978, 60, 302–306. [Google Scholar]
- Perazzo, F.F.; Carvalho, J.C.T.; Carvalho, J.E.; Rehder, V.L.G. Central properties of the essential oil and the crude ethanol extract from aerial parts of Artemisia annua L. Pharmacol. Res. 2003, 48, 497–502. [Google Scholar] [CrossRef]
- Tu, Y.-Y.; Yin, J.-P.; Ji, L.; Huang, M.-M; Liang, X.-T. Chemical constituents of sweet wormwood (Artemisia annua) (III). Zhongcaoyao 1985, 16, 200–201. [Google Scholar]
- Shilin, Y.; Roberts, M.F.; Phillipson, J.D. Methoxylated flavones and coumarins from Artemisia annua. Phytochemistry 1989, 28, 1509–1512. [Google Scholar] [CrossRef]
- Liu, H.-M.; Li, K.-L.; Wo, W.-C. Studies on the constituents of Artemisia annua. Yaoxue Tongbao 1980, 15, 39. [Google Scholar]
- Htut, Z.W. Artemisinin resistance in Plasmodium falciparum malaria. New Engl. J. Med. 2009, 361, 1807–1808. [Google Scholar] [PubMed]
- Saitibaeva, I.M. Coumarins from Artemisia annua. Khim. Prir. Soedin. 1970, 6, 758. [Google Scholar] [CrossRef]
- Pham, G.D. Coumarin and its derivatives in Artemisia annua L. in Vietnam. Tap Chi Duoc Hoc 2002, 11–13. [Google Scholar]
- Djermanovic, M.; Stepanovic, M.; Djermanovic, V.; Milovanovic, M. Some new constituents from domestic plant species of Artemisia monogyna W and K and Artemisia annua L. J. Serb. Chem. Soc. 1997, 62, 113–116. [Google Scholar]
- Tzeng, T.-C.; Lin, Y.-L.; Jong, T.-T.; Chang, C.-M.J. Ethanol modified supercritical fluid extraction of scopoletin and artemisinin from Artemisia annua L. Sep. Purif. Technol. 2007, 56, 18–24. [Google Scholar] [CrossRef]
- Yang, L.; Wang, M.-Y.; Zhang, D.; Tu, Y. Determination of scopoletin in Artemisia annua by HPLC. Zhongguo Shiyan Fangjixue Zazhi 2006, 12, 10–11. [Google Scholar]
- Marco, J.A.; Sanz, J.F.; Bea, J.F.; Barbera, O. Phenolic constituents of Artemisia annua. Pharmazie 1990, 45, 382–383. [Google Scholar]
- Nguyen, V.B.; Do, D.R.; Nguyen, G.C.; Bui, T.B.; Chu, D.K. Research on the extraction of a coumarin derivative from the leaves of Artemisia annua L. growing in Vietnam. Tap Chi Duoc Hoc 1998, 36, 58–61. [Google Scholar]
- Brown, G.D. Secondary metabolism in tissue culture of Artemisia annua. J. Nat. Prod. 1994, 57, 975–977. [Google Scholar] [CrossRef]
- Sy, L.-K.; Brown, G.D. Coniferaldehyde derivatives from tissue culture of Artemisia annua and Tanacetum parthenium. Phytochemistry 1999, 50, 781–785. [Google Scholar] [CrossRef]
- Aleskerova, A.N. Detection of esculetin in Artemisia annua L. Izvestiya Akademii Nauk Azerbaidzhanskoi SSR Seriya Bioligicheskikh Nauk 1985, 25–26. [Google Scholar]
- Yang, S.L.; Roberts, M.F.; O’Neill, M.J.; Bucar, F.; Phillipson, J.D. Flavonoids and chromenes from Artemisia annua. Phytochemistry 1995, 38, 255–257. [Google Scholar] [CrossRef]
- Agarwal, P.K.; Singh, A.K.; Bhakuni, R.S.; Jain, D.C. Studies on medicinal plants. 43. Characterization of a coumarin from Artemisia annua: Correlation of assigned chemical shifts with its hydrated form. Curr. Res. Med. Aromat. Plants 1995, 17, 321–325. [Google Scholar]
- Elford, B.C.; Roberts, M.F.; Philipson, J.D.; Wilson, R.J.M. Potentiation of the antimalarial activity of qinghaosu by methoxylated flavones. Trans. R. Soc. Trop. Med. Hyg. 1987, 81, 434–436. [Google Scholar] [CrossRef]
- Stermitz, F.R.; Scriven, L.N.; Tegos, G.; Lewis, K. Two flavanols from Artemisia annua which potentiate the activity of berberine and norfloxacin against a resistant strain of Staphylococcus aureus. Planta Med. 2002, 68, 1140–1141. [Google Scholar] [CrossRef] [PubMed]
- Baraldi, R.; Isacchi, B.; Predieri, S.; Marconi, G.; Vincieri, F.F.; Anna, R. Distribution of artemisinin and bioactive flavonoids from Artemisia annua L. during plant growth. Biochemical Syst. Ecol. 2008, 36, 340–348. [Google Scholar] [CrossRef]
- Yang, S.; Roberts, M.F.; Philipson, J.D. Methoxylated flavones and coumarins from Artemisia annua. Phytochemistry 1989, 28, 1509–1511. [Google Scholar]
- Liu, K.C.S.C.; Yang, S.L.; Roberts, M.F.; Elford, B.C.; Phillipson, J.D. Antimalarial activity of Artemisia annua flavonoids from whole plants and cell cultures. Plant Cell Rep. 1992, 11, 637–640. [Google Scholar] [CrossRef] [PubMed]
- Tang, H.-Q.; Hu, J.; Yang, L.; Tan, R.-X. Terpenoids and flavonoids from Artemisia species. Planta Med. 2000, 66, 391–393. [Google Scholar] [CrossRef] [PubMed]
- Sy, L.-K.; Brown, G.D. Three sesquiterpenes from Artemisia annua. Phytochemistry 1998, 48, 1207–1211. [Google Scholar]
- Bilia, A.R.; de Malgalhaes, P.M.; Bergonzi, M.C.; Vincieri, F.F. Simultaneous analysis of artemisinin and flavonoids of several extracts of Artemisia annua L. obtained from a commercial sample and a selected cultivar. Phytomedicine 2006, 13, 487–493. [Google Scholar] [CrossRef] [PubMed]
- Jeremic, D.; Stefanovic, M.; Dokovic, D.; Milosavljevic, S. Flavonols from Artemisia annua. Glas. Khem. Drush. Beograd. 1979, 44, 615–618. [Google Scholar]
- Baeva, R.T.; Nabi-Zade, L.I.; Zapesochnaya, G.G.; Karryev, M.O. Flavonoids of Artemisia annua. Khim. Prir. Soedin. 1988, 298–299. [Google Scholar] [CrossRef]
- Bhardwaj, D.K.; Jain, R.K.; Jain, S.C.; Manchanda, C.K. Constituents of Artemisia annua. Proc. Indian Nat. Sci. Acad. 1985, 51A, 741–745. [Google Scholar]
- Schramek, N.; Wang, H.; Roemisch-Margl, W.; Keil, B.; Radykewicz, T.; Winzenhoerlein, B.; Beerhuse, L.; Bacher, A.; Rodich, F.; Gershenzon, J.; Liu, B.; Eisenreich, W. Artemisinin biosynthesis in growing plants of Artemisia annua. A 13CO2 study. Phytochemistry. 2010, 71, 179–187. [Google Scholar] [CrossRef] [PubMed]
- Han, X.; Ma, X.; Zhang, T.; Zheng, Y.; Liu, Q.; Ito, Y. Isolation of high-purity casticin from Artemisia annua L. by high-speed counter-current chromatography. J. Chromatogr. A. 2007, 1151, 180–182. [Google Scholar] [CrossRef] [PubMed]
- Olsson, M.E.; Olofsson, L.M.; Lindahl, A.-L.; Lundgren, A.; Brodelius, M.; Brodelius, P.E. Localization of enzymes of artemisinin biosynthesis to the apical cells of glandular secretory trichomes of Artemisia annua L. Phytochemistry 2009, 70, 1123–1128. [Google Scholar] [CrossRef] [PubMed]
- Zheng, G.-Q. Cytotoxic terpenoids and flavonoids from Artemisia annua. Planta Med. 1994, 60, 54–57. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.-M.; Li, G.-L.; Wu, H.-Z. Studies on the chemical constituents of Artemisia annua L. Yaoxue Xuebao (Acta Pharmaceutica Sinica) 1981, 16, 65–67. [Google Scholar]
- Djermanovic, M.; Jokic, A.; Mladenovic, S.; Stefanovic, M. Quercetagetin 6,7,3’,4’-tetramethyl ether, a new flavonol from Artemisia annua. Phytochemistry 1975, 14, 1873. [Google Scholar] [CrossRef]
- Tellez, M.R.; Canel, C.; Rimando, A.M.; Duke, S.O. Differential accumulation of isoprenoids in glanded and glandless Artemisia annua L. Phytochemistry 1999, 52, 1035–1040. [Google Scholar] [CrossRef]
- Banthorpe, D.V.; Christou, P.N.; Pink, C.R.; Watson, D.G. Metabolism of linaloyl, neryl and geranyl pyrophosphates in Artemisia annua. Phytochemistry 1983, 22, 2465–2468. [Google Scholar] [CrossRef]
- Jia, J.-W.; Crock, J.; Lu, S.; Croteau, R.; Chen, X.-Y. (3R)-Linalool synthase from Artemisia annua L: cDNA isoation, characterization and wound induction. Arch. Biochem. Biophys. 1999, 372, 143–149. [Google Scholar] [CrossRef] [PubMed]
- Lawrence, B. M. Progress in Essential Oils. Perfum. Flavor. 1982, 7, 43–44. [Google Scholar]
- Georgiev, E.; Genov, N.; Khristova, N. Changes in yield and quality of essential oil in annual wormwood (Artemisia annua L.) during growth. Rastenievundi Nauki 1981, 18, 95–102. [Google Scholar]
- Yakobashvili, N.Z.; Kuchukhidze, N.M.; Chikvanya, V.E. Fractionation of sweet wormwood oil. Maslozhirovaya Promyshlennost 1981, 28–29. [Google Scholar]
- Ruecker, G.; Mayer, R.; Manns, D. α- and β-Myrcene hydroperoxide from Artemisia annua. J. Nat. Prod. 1987, 50, 287–289. [Google Scholar] [CrossRef]
- Asahina, Y.; Takagi, S. Report on the essential oil of Artemisia annua L. II. Constitution of artemisia ketone. Yakugaku Zasshi 1920, 837–864. [Google Scholar]
- Ruzicka, L.; Reichstein, T.; Pulver, R. Synthesis of tetrahydroartemisia ketone. Helv. Chim. Acta 1936, 19, 646–649. [Google Scholar] [CrossRef]
- Takemoto, T.; Nakajima, T. Essential oil of Artemisia annua. I. Isolation of a new ester compound. Yakugaku Zasshi 1957, 77, 1307–1309. [Google Scholar] [CrossRef]
- Takemoto, T.; Nakajima, T. Essential oil of Artemisia annua. II. Structure of 1-β-artemisia alcohol. Yakugaku Zasshi 1957, 77, 1310–1313. [Google Scholar] [CrossRef]
- Takemoto, T.; Nakajima, T. Essential oil of Artemisia annua. III. Structure of artemisia ketone. Yakugaku Zasshi 1957, 77, 1339–1344. [Google Scholar] [CrossRef]
- Takemoto, T.; Nakajima, T. Essential oil of Artemisia annua. IV. Dihydroartemisia ketone. Yakugaku Zasshi 1957, 77, 1344–1347. [Google Scholar] [CrossRef]
- Hethelyl, I.; Ceseko, I.; Grosz, M.; Mark, G.; Palinkas, J. Capillary gas chromatographic investigation of Artemisia annua essential oils. Olaj, Szappan, Kozmetika 1994, 43, 103–106. [Google Scholar]
- Suga, T.; Shishibori, T.; Kotera, K.; Fujii, R. Biosynthesis of a non-head-to-tail monoterpene, artemisia ketone. Chem. Lett. 1972, 7, 533–534. [Google Scholar] [CrossRef]
- Simpson, J.B. On the Biosynthesis of Artemisia Ketone in Artemisia annua; Louisiana State Univ.: Baton Rouge, LA, USA, 1979; p. 135. [Google Scholar]
- Banthorpe, D.V.; Charlwood, B.V.; Greaves, G.M.; Voller, C.M. Terpene biosynthesis. Part 20. Role of geraniol and nerol in the biosynthesis of artemisia ketone. Phytochemistry 1977, 16, 1387–1392. [Google Scholar] [CrossRef]
- Risinger, G.E.; Karimian, K.; Jungk, S.; Simpson, J.B. On the biosynthesis of artemisia ketone and bakuchiol. Experientia 1978, 34, 1121–1122. [Google Scholar] [CrossRef]
- Allen, K.G.; Banthorpe, D.V.; Charlwood, B.V.; Voller, C.M. Terpene biosynthesis. Part 17. Biosynthesis of irregular monoterpenes in extracts from higher plants. Phytochemistry 1977, 16, 85–92. [Google Scholar]
- Banthorpe, D.V.; Charlwood, B.V. Biosynthesis of artemisia ketone. Nature (London), New Biol. 1971, 231, 285–286. [Google Scholar] [CrossRef]
- Boulton, K.; Shirley, I.; Smith, I.H.; Whiting, D.A. Mechanism of formation of natural cyclopropanes: Synthesis of postulated intermediates in presqualene and chrysanthemyl alcohol biosynthesis. J. Chem. Soc. Perkin Trans. I 1986, 1817–1824. [Google Scholar] [CrossRef]
- Sy, L.-K.; Brown, G.D. Deoxyarteannuin B, dihydro-deoxyarteannuin B and trans-5-hydroxy-2-isopropenyl-5-methylhex-3-en-1-ol from Artemisia annua. Phytochemistry 2001, 58, 1159–1166. [Google Scholar] [CrossRef]
- Youann, T. Essential oil of Artemisia annua. Perfumery and Essential Oil Rec. 1955, 46, 75–78. [Google Scholar]
- Lu, S.; Xu, R.; Jia, J.-W.; Pang, J.; Matsuda, S.P.T.; Chen, X.-Y. Cloning and functional characterization of a β-pinene synthase from Artemisia annua that shows a circadian pattern of expression. Plant Physiol. 2002, 130, 477–486. [Google Scholar] [CrossRef] [PubMed]
- Tu, Y.-Y.; Liang, X.-T. Studies on the constituents of Artemisia annua. Yaoxue Xuebao (Acta Pharmaceutica Sinica) 1981, 16, 366–369. [Google Scholar]
- Picaud, S.; Brodelius, M.; Brodelius, P.E. Expression, purification and characterization of recombinant (E)-β-farnesene synthase from Artemisia annua. Phytochemistry 2005, 66, 961–967. [Google Scholar] [CrossRef] [PubMed]
- Picaud, S.; Olofsson, L.; Brodelius, M.; Brodelius, P.E. Expression, purification and characterization of recombinant amorpha-4,11-diene synthase from Artemisia annua L. Arch. Biochem. Biophys. 2005, 436, 215–226. [Google Scholar] [CrossRef] [PubMed]
- Bertea, C.M.; Freije, J.R.; van der Woude, H.; Verstappen, F.W.A.; Perk, L.; Marquez, V.; De Kraker, J.-W.; Posthumus, M.A.; Jansen, B.J.M.; de Groot, A.; Franssen, M.C.R.; Bouwmeester, H.J. Identification of intermediates and enzymes involved in early steps of artemisinin biosynthesis in Artemisia annua. Planta Med. 2005, 71, 40–47. [Google Scholar] [CrossRef] [PubMed]
- Picaud, S.; Mercke, P.; He, X.; Sterner, O.; Brodelius, M.; Cane, D.E.; Brodelius, P.E. Amorpha-4,11-diene synthase: mechanism and stereochemistry of the enzymatic cycization of farnesyl diphosphate. Arch. Biochem. Biophys. 2006, 448, 150–155. [Google Scholar] [CrossRef] [PubMed]
- Bertea, C.M.; Voster, A.; Verstappen, F.W.A.; Maffei, M.; Beekwilder, J.; Bouwmeester, H.J. Isoprenoid biosynthesis in Artemisia annua: cloning and heterologous expression of a germacrene A synthase from a glandular trichome cDNA library. Arch. Biochem. Biophys. 2006, 448, 3–12. [Google Scholar] [CrossRef] [PubMed]
- Kong, J. Q.; Huang, Y.; Shen, J.; Wang, W.; Cheng, K.; Zhu, P. Recent advances in the study of amorpha-4,11-diene synthase and its metabolic engineering. Yaoxue Xuebao 2009, 44, 1320–1327. [Google Scholar]
- Cai, Y.; Jia, J.-W.; Crock, J.; Lin, Z.-X.; Chen, X.-Y.; Croteau, R. A cDNA clone for β-caryophyllene synthase from Artermisia annua. Phytochemistry 2002, 61, 523–529. [Google Scholar] [CrossRef]
- Liu, Y.-M.; Pu, X.-F.; Zhang, L. Determination of camphor and caryophyllene in Artemisia annua L. and Herba artemisiae annuae oil by capillary GC. Huaxue Yaoxue Zashi 2007, 22, 218–220. [Google Scholar]
- Bolt, A.J.N.; Cocker, W.; McMurray, T.B.H. The stereochemistry of artemisin. J. Chem. Soc. 1963, 5235–5238. [Google Scholar] [CrossRef]
- Pinhey, J.T.; Sternhell, S. Structure of α-hydroxysantonin and some aspects of the stereochemistry of related eudesmanolides and guaianolides. Aust. J. Chem. 1995, 18, 543–547. [Google Scholar] [CrossRef]
- Zhu, D.; Zhang, S.; Liu, B.; Fan, G.; Liu, J.; Xu, R. Study on antibacterial constituents of Qing Hao (Artemisia annua L.). Zhongcaoyao 1982, 13, 6. [Google Scholar]
- Zhou, W.; Xu, X. Synthesis of arteannuin and related compounds. Yiyao Gongye 1987, 18, 470–477. [Google Scholar]
- Agrawal, P.K.; Bishnoi, V. NMR spectral investigations. Part 41. Sesquiterpenoids from Artemisia annua: 13C NMR shielding behavior. J. Sci. Ind. Res. 1996, 55, 17–26. [Google Scholar]
- Marsaioli, A.J.; Fujiwara, F.Y.; Foglio, M.A.; Sharapin, N.; Zhang, J.-S. Proton and carbon-13 NMR and conformation in solution of some amorphanes (Qinghaosu derivatives). Mag. Res. Chem. 1994, 32, 583–90. [Google Scholar] [CrossRef]
- Tu, Y.-Y.; Ni, M.-Y.; Zhong, Y.-R.; Li, L.-N.; Cui, S.-L.; Zhang, M.-Q.; Wang, X.-Z.; Ji, Z.; Liang, X.-T. Studies on the constituents of Artemisia annua: 2. Planta Med. 1982, 44, 143–145. [Google Scholar]
- Misra, L.N.; Ahmad, A.; Thakur, R.S.; Lotter, H.; Wagner, H. Crystal structure of artemisinic acid: A possible biogenetic precursor of antimalarial artemisinin from Artemisia annua. J. Nat. Prod. 1993, 56, 215–219. [Google Scholar] [CrossRef] [PubMed]
- Jeremic, D.; Jokic, A.; Behbud, A.; Stefanovic, M. New type of sesquiterpene lactone isolated from Artemisia annua. Arteannuin B. Tetrahedron Lett. 1973, 3039–42. [Google Scholar] [CrossRef]
- Liu, J.-M.; Ni, M.-Y.; Fan, J.-F.; Tu, Y.-Y.; Wu, Z.-H.; Wu, Y.-L.; Chou, W.-S. Structure and reaction of arteannuin. Huaxue Xuebao 1979, 37, 129–143. [Google Scholar]
- Tu, Y.-Y.; Ni, M.-Y.; Chung, Y.-Y.; Li, L.-N. Chemical constituents in Artemisia annua L. and the derivatives of artemisinin. Chung Yao Tong Pao 1981, 6, 31. [Google Scholar]
- Pang, G.; Kenzo, H.; Yoichi, I.; Seiichi, I. Study on the absolute configuration of artemisinic acid isolated from chinese antimalaria herb Artemisia annua L. Shenyang Yaoke Daxue Xuebao 1997, 14, 189–195. [Google Scholar]
- Kim, S.-U.; Han, J.; Lim, Y.-H. Revised assignment of 1H-NMR signals of artemisinic acid. Planta Med. 1996, 62, 480–481. [Google Scholar] [CrossRef] [PubMed]
- Zhou, W.-S. Studies on the structure and synthesis of arteannuin and related compounds. Huaxue Xuebao 1989, 47, 710–715. [Google Scholar]
- Brown, G.D.; Sy, L.-K. In vivo transformations of artemisinic acid in Artemisia annua plants. Tetrahedron 2007, 63, 9548–9566. [Google Scholar] [CrossRef]
- Roth, R.J.; Acton, N. A simple conversion of artemisinic acid into artemisinin. J. Nat. Prod. 1989, 52, 1183–1185. [Google Scholar] [CrossRef] [PubMed]
- Haynes, R.K.; Vonwiller, S.C. The Development of new peroxide antimalarials. Chem. Aust. 1991, 58, 64–67. [Google Scholar]
- Jung, M.; Yoo, Y.; Elsohly, H.N.; McChesney, J.D. One-step stereospecific synthesis of (-)-arteannuin B. J. Nat. Prod. 1987, 50, 972–973. [Google Scholar] [CrossRef]
- Xu, X.; Zhu, J.; Zhou, W. Stereoselective synthesis of arteannuin B. Kexue Tongbao 1982, 27, 1022. [Google Scholar]
- El-Feraly, F.S.; Al-Meshal, I.A.; Khalifa, S.I. epi-Deoxyarteannuin B and 6,7-dehydroartemisinic acid from Artemisia annua. J. Nat. Prod. 1989, 52, 196–198. [Google Scholar] [CrossRef]
- Acton, N.; Roth, R.J. Synthesis of epi-deoxy- and deoxyarteannuin B. Phytochemistry 1989, 28, 3530–3531. [Google Scholar] [CrossRef]
- Kim, S.-U. Efficient conversion of arteannuic acid into epi-deoxyarteannuin B. Han’guk Nonghwa Hakhoechi 1990, 33, 174–176. [Google Scholar]
- Roth, R.J.; Acton, N. Isolation of epi-deoxyarteannuin B from Artemisia annua. Planta Med. 1987, 53, 576. [Google Scholar] [CrossRef] [PubMed]
- Roth, R.J.; Acton, N. Isolation of arteannuic acid from Artemisia annua. Planta Med. 1987, 53, 501–502. [Google Scholar] [CrossRef] [PubMed]
- Sy, L.-K.; Brown, G.D. Synthesis of 6,7-dehydroartemisinic acid. J. Chem. Soc. Perkin Trans. 1 2002, 2421–2429. [Google Scholar] [CrossRef]
- Sy, L.-K.; Brown, G.D.; Haynes, R. A novel endoperoxide and related sesquiterpenes from Artemisia annua which are possibly derived from allylic hydroperoxides. Tetrahedron 1998, 54, 4345–4356. [Google Scholar] [CrossRef]
- Wallaart, E.T.; van Uden, W.; Lubberink, H.G.M.; Woerdenbag, H.J.; Prass, N.; Quax, W.J. Isolation and identification of dihydroartemisinic acid from Artemisia annua and its possible role in the biosynthesis of artemisinin. J. Nat. Prod. 1999, 62, 430–433. [Google Scholar] [CrossRef] [PubMed]
- Brown, G.D.; Sy, L.-K. Synthesis of labelled dihydroartemisinic acid. Tetrahedron 2004, 60, 1125–1138. [Google Scholar] [CrossRef]
- Sy, L.-K.; Zhu, N.-Y.; Brown, G.D. Synthesis of dihydroartemisinic acid and dihydro-epi-deoxyarteannuin B incorporating a stable isotope label at the 15-position for studies into the biosynthesis of artemisinin. Tetrahedron 2001, 57, 8495–8510. [Google Scholar] [CrossRef]
- Wallaart, T.E.; Pras, N.; Quax, W.J. Isolation and identification of dihydroartemisinic acid hydroperoxide from Artemisia annua: A novel biosynthetic precursor of artemisinin. J. Nat. Prod. 1999, 62, 1160–1162. [Google Scholar] [CrossRef] [PubMed]
- Zhou, W.-S. Total synthesis of arteannuin (Quinghaosu) and related compounds. Pure Appl. Chem. 1986, 58, 817–824. [Google Scholar] [CrossRef]
- Zhou, W.; Zhang, L.; Xu, X. Studies on the structure and syntheses of arteannuin and related compounds XVIII. Synthesis of arteannuin A. Huaxue Xuebao 1986, 44, 968–970. [Google Scholar]
- Yusupova, I.M.; Tashkhodzhaev, B.; Mallabaev, A. Molecular structure of the sesquiterpene lactone arteannuin B. Khim. Prir. Soedin. 1986, 6, 788–790. [Google Scholar] [CrossRef]
- Leppard, D.G.; Rey, M.; Dreiding, A.S.; Grieb, R. The structure of arteannuin B and its acid hydrolysis product. Helv. Chim. Acta 1974, 57, 602–615. [Google Scholar] [CrossRef] [PubMed]
- Agrawal, P.K.; Vishwakarma, R.A.; Jain, D.C.; Roy, R. High field NMR spectroscopic studies of arteannuin B and a reappraisal of the structure of arteannuin C. Phytochemistry 1991, 30, 3469–3471. [Google Scholar] [CrossRef]
- Stefanovic, M.; Solujic, S.; Jeremic, D.; Jokic, A.; Miljkovic, D.; Velimirovic, S. Chemical transformations of arteannuin B - cadinane sesquiterpenic lactone - isolated from Artemisia annua L. Glas. Khem. Drus., Beograd 1977, 42, 227–236. [Google Scholar]
- Lansbury, P.T.; Mojica, C.A. Total synthesis of (+/-)-arteannuin B. Tetrahedron Lett. 1986, 27, 3967–3970. [Google Scholar] [CrossRef]
- Pajarin, M.; Mojica, C.A. The total synthesis of (+/-)-arteannuin B. Diss. Abstr. Int. 1987, 48, 444. [Google Scholar]
- Goldberg, O.; Deja, I.; Rey, M.; Dreiding, A.S. The synthesis of stereoisomers of arteannuin B. Helv. Chim. Acta 1980, 63, 2455–2468. [Google Scholar] [CrossRef]
- Misra, L.N. Arteannuin C, a sesquiterpene lactone from Artemisia annua. Phytochemistry 1986, 25, 2892–2893. [Google Scholar] [CrossRef]
- Zhou, W.-S.; Zhang, L.; Xu, X.-X. Studies on the structures and synthesis of arteannuin and related compounds XVI. Synthesis of arteannuin E and epoxy fission reaction of methyl α-epoxy arteannuate. Huaxue Xuebao 1985, 43, 845–851. [Google Scholar]
- Zhu, D.; Deng, D.; Zhang, S.; Xu, R. The structure of artemisilactone. Huaxue Xuebao 1984, 42, 937–939. [Google Scholar]
- Sy, L.-K.; Ngo, K.-S.; Brown, G.D. Biomimetic synthesis of arteannuin H and the 3,2-rearrangement of allylic hydroperoxides. Tetrahedron 1999, 55, 15127–15140. [Google Scholar] [CrossRef]
- Sy, L.-K.; Cheung, K.-K.; Zhu, N.-Y.; Brown, G.D. Structure Elucidation of arteannuin O, a novel cadinane diol from Artemisia annua and the synthesis of arteannuins K, L, M and O. Tetrahedron 2001, 57, 8481–8493. [Google Scholar] [CrossRef]
- Brown, G.D.; Sy, L.-K. In vivo transformations of dihydroartemisinic acid in Artemisia annua plants. Tetrahedron 2004, 60, 1139–1159. [Google Scholar] [CrossRef]
- Barriault, L.; Deon, D.H. Recent progress towards the total synthesis of arteannuin M. Abstracts of Papers; In Proceedings of 221st ACS national Meeting, San Diego, CA, USA, April 1-5, 2001. [Google Scholar]
- Barriault, L.; Deon, D.H. Total Synthesis of (+)-arteannuin M using the tandem Oxy-Cope/ene reaction. Org. Lett. 2001, 3, 1925–1927. [Google Scholar] [CrossRef] [PubMed]
- Engel, D.A.; Lam, H.T.C.; Singletary, J.A.; Poon, K.W.C.; Dudley, G.B. Synthetic approaches to artemisinin-related natural products, dihydroartemisinic acid and arteannuin M. Abstracts of Papers; In Proceedings of 231st ACS national Meeting, Atlanta, GA, USA, March 26-30, 2006. [Google Scholar]
- Brown, G.D. 13C-2H correlation NMR spectroscopy studies of the in vivo transformations of natural products from Artemisia annua. Phytochem. Rev. 2003, 2, 45–59. [Google Scholar] [CrossRef]
- Brown, G.D.; Sy, L.-K. In vivo transformations of dihydro-epi-deoxyarteannuin B in Artemisia annua plants. Tetrahedron 2007, 63, 9548–9566. [Google Scholar] [CrossRef]
- Dudley, G.B.; Engel, D.A.; Ghiviriga, I.; Lam, H.; Poon, K.-W.C.; Singletary, J.A. Synhesis of (+)-dihydro-epi-deoxyarteannuin B. Org. Lett. 2007, 9, 2839–2842. [Google Scholar] [CrossRef] [PubMed]
- Engel, D.A.; Lam, H.T.C.; Singletary, J.A.; Poon, K.-W.C.; Dudley, G.B. Synthetic approaches to artemisinin-related natural products, didesmethylartemisinin and dihydro-epi-deoxyarteannuin B. Abstracts of Papers; In Proceedings of 235th ACS national Meeting, New Orleans, LA, USA, April 6-10, 2008. [Google Scholar]
- Sy, L.-K.; Brown, G.D. The mechanism of the spontaneous autoxidation of dihydroartemisinic acid. Tetrahedron 2002, 58, 897–908. [Google Scholar] [CrossRef]
- Wu, Z.; Wang, Y. Structure and synthesis of arteannuin and related compounds. XI, Identification of epoxyarteannuic acid. Huaxue Xuebao 1984, 42, 596–598. [Google Scholar]
- Graham, I.A.; Besser, K.; Blumer, S.; Branigan, C.A.; Czechowski, T.; Elias, L.; Guterman, I.; Harvey, D.; Issac, P.G.; Khan, A.M.; Larson, T.R.; Li, Y.; Pawson, T.; Penfield, T.; Rae, A.M.; Rathbone, D.A.; Reid, S.; Ross, J.; Smallwood, M.F.; Segura, V.; Townsend, T.; Vyas, D.; Winza, T.; Bowles, D. The genetic map of Artemisia annua L. identifies loci affecting yield of the antimalarial drug artemisinin. Science 2010, 327, 328–331. [Google Scholar] [CrossRef] [PubMed]
- Teoh, K.T.; Polichuk, D.R.; Reed, D.W.; Nowak, G.; Covello, P. Artemisia annua L. (Asteraceae) trichome-specific cDNAs reveal CYP71AV1, a cytochrome P450 with a key role in the biosynthesis of the antimalarial sesquiterpene lactone artemisinin. FEBS Lett. 2006, 580, 1411–1416. [Google Scholar] [CrossRef] [PubMed]
- Brown, G.D. Annulide, a sesquiterpene lactone from Artemisia annua. Phytochemistry 1993, 32, 391–393. [Google Scholar] [CrossRef]
- Pham, G.D. Terpenoid compounds in leaves of Artemisia annua L. in Vietnam. Tap Chi Duoc Hoc 2002, 5–7. [Google Scholar]
- Misra, L.N.; Ahmad, A.; Thakur, R.S.; Jakupovic, J. Bisnorcadinanes from Artemisia annua and definitive carbon-13 assignments of β-arteether. Phytochemistry 1993, 33, 1461–1464. [Google Scholar] [CrossRef]
- van Nieuwerburgh, F.C.W.; van de Casteele, S.R.F.; Maes, L.; Goossens, A.; Inze, D.; van Bocxlaer, J.D.; Dieter, L.D. Quantitation of artemisinin and its biosynthetic precursors in Artemisia annua L. by high performance liquid chromatography-electrospray quadrupole time-of-flight tandem mass spectrometry. J. Chromatogr. A. 2006, 1118, 180–187. [Google Scholar] [CrossRef] [PubMed]
- Uskobvich, N.R.; Williams, T.H.; Blount, J.F. The structure and absolute configuration of arteannuin B. Helv. Chim. Acta 1974, 57, 600–602. [Google Scholar]
- Wang, J.-W.; Zheng, L.-P.; Tan, R.-X. The preparation of an elicitor from a fungal endophyte to enhance artemisinin production in hairy root cultures of Artemisia annua L. Chinese J. Biotechnol. 2006, 22, 829–834. [Google Scholar]
- Akhila, A.; Thakur, R.S.; Popli, S.P. Biosynthesis of artemisinin in Artemisia annua. Phytochemistry 1987, 26, 1927–1930. [Google Scholar] [CrossRef]
- Haynes, R.K.; Vonwiller, S.C. From Qinghao, marvelous herb of antiquity, to the antimalarial trioxane qinghaosu - and some remarkable new chemistry. Acc. Chem. Res. 1997, 30, 73–79. [Google Scholar] [CrossRef]
- Kasymov, S.Z.; Ovezdurdyev, A.; Yusupov, M. I.; Sham’yanov, I. D.; Malikov, V. M. Lactones of Artemisia annua. Khim. Prir. Soedin. 1986, 5, 636. [Google Scholar]
- Kalita, B.; Nabin, C. Synthesis of a new series of 10α-nitrodeoxoartemisinin and their antimalarial activity. Indian J. Chem., Sect. B Organic Chemistry including Medicinal Chemistry 2001, 40B, 1125–1128. [Google Scholar]
- Deng, A.; Zhu, D.; Jao, Y.; Dai, J.; Xu, R. Studies on the structure of the artemisic acid. Kexue Tongbao 1981, 26, 1209–1211. [Google Scholar]
- Kim, S.-U.; Lim, H.-J. Isolation of arteannuic acid from Artemisia annua. Han’guk Nonghwa Hakhoechi 1989, 32, 178–179. [Google Scholar]
- Akhila, A.; Rani, K.; Thakur, R.S. Biosynthesis of artemisinic acid in Artemisia annua. Phytochemistry 1990, 29, 2129–2132. [Google Scholar] [CrossRef]
- Vonwiller, S.C.; Haynes, R.K.; King, G.; Wang, H.-J. An improved method for the isolation of Qinghao (artemisinic) acid from Artemisia annua. Planta Med. 1993, 59, 562–563. [Google Scholar] [CrossRef] [PubMed]
- van den Berghe, D.R.; Vergauwe, A.N.; van Montagu, M.; van den Eeckhout, E.G. Simultaneous determination of artemisinin and its bioprecursors in Artemisia annua. J. Nat. Prod. 1995, 58, 798–803. [Google Scholar] [CrossRef]
- Zhu, D. Studies on the antibacterial artemisinin. Zhongcaoyao (Chinese Traditional and Herbal Drugs) 1982, 13, 6. [Google Scholar]
- El-Feraly, F.S.; Ayalp, A.; Al-Yahya, M.A.; McPhail, D.R.; McPhail, A.T. Conversion of artemisinin to artemisitene. J. Nat. Prod. 1990, 53, 66–71. [Google Scholar] [CrossRef]
- Ranasinghe, A.; Sweatlock, J.D.; Cooks, R.G. A rapid screening method for artemisinin and its congeners using Ms/Ms: Search for new analogues in Artemisia annua. J. Nat. Prod. 1993, 56, 552–563. [Google Scholar] [CrossRef] [PubMed]
- Sangwan, R.S.; Agarwal, K.; Luthra, R.; Thakur, R.S.; Singh Sangwan, N. Biotransformation of arteannuic acid into arteannuin-B and artemisinin in Artemisia annua. Phytochemistry 1993, 34, 1301–1302. [Google Scholar] [CrossRef]
- Huang, J.; Xia, Z.; Wu, L. Constituents of Artemisia annua L. I. Isolation and identification of 11R-(-)-dihydroarteannuic acid. Huaxue Xuebao 1987, 45, 609–612. [Google Scholar]
- Brown, G.D. Cadinanes from Artemisia annua that may be intermediates in the biosynthesis of artemisinin. Phytochemistry 1994, 36, 637–641. [Google Scholar] [CrossRef]
- Sy, L.-K.; Brown, G.D. The role of the 12-carboxylic acid group in the spontaneous autoxidation of dihydroartemisinc acid. Tetrahedron 2002, 58, 909–923. [Google Scholar] [CrossRef]
- Anonymous; Anonymous. Co-ordinating group of research on the structure of Qing Hao Su. A new type of lactone - Qing Hau Sau. K’O Hsueh Tung Pao 1977, 22, 142, (CA 1977, 87, 98788). [Google Scholar]
- Blasko, G.; Cordell, G.A.; Lankin, D.C. Definitive 1H and 13C NMR assignments of artemisinin (qinghaosu). J. Nat. Prod. 1988, 51, 1273–1276. [Google Scholar] [CrossRef]
- Huang, J.; Nicholis, K.M.; Cheng, C.; Wang, Y. Two-dimensional NMR studies of arteannuin. Huaxue Xuebao 1988, 45, 305–308. [Google Scholar]
- Leban, I.; Golic, L.; Japelj, M. Crystal and molecular structure of Qinghaosu: A redetermination. Acta Pharmaceutica Jugoslavica 1988, 38, 71–78. [Google Scholar]
- Madhusudanan, K.P.; Vishwakarma, R.A.; Balachandran, S.; Popli, S.P. Mass spectral studies on artemisinin, dihydroartemisinin and artether. Indian J. Chem. Sect. B, Organic Chemistry including Medicinal Chemistry 1989, 28, 751–754. [Google Scholar]
- Mi, J. Circular dichroism of some synthetic intermeidates of qinghaosu. Yaowu Fenxi Zazhi 1987, 7, 262–266. [Google Scholar]
- Wang, Z.; Nakashima, T.T.; Kopesky, K.R.; Molina, J. Qinghaosu: proton and carbon-13 nuclear magnetic resonance spectral assignments and luminescence. Can. J. Chem. 1985, 63, 3070–3074. [Google Scholar]
- Wang, B.-D.; Yin, M.-L.; Shong, G.-Q.; Chen, Z.-L. The 13C NMR spectroscopy of arteannuin analogues. Huaxue Xuebao 1986, 44, 834–838. [Google Scholar]
- Lisgarten, J.N.; Potter, B.S.; Bantuzeko, C.; Palmer, R.A. Structure, absolute configuration and conformation of the antimalarial compound, artemisinin. J. Chem. Crystallogr. 1998, 28, 539–543. [Google Scholar] [CrossRef]
- Schmid, G.; Hofheinz, W. Total synthesis of qinghaosu. J. Am. Chem. Soc. 1983, 105, 624–625. [Google Scholar] [CrossRef]
- Xu, X.-X.; Zhu, J.; Huang, D.-Z.; Zhou, W.-S. Total synthesis of arteannuin and deoxyarteannuin. Tetrahedron 1986, 42, 819–828. [Google Scholar]
- Ravindranathan, T.; Kumar, M.A.; Menon, R.B.; Hiremath, S.V. Stereoselective synthesis of artemisinin. Tetrahedron Lett. 1990, 31, 755–758. [Google Scholar] [CrossRef]
- Avery, M.A.; Chong, W.K.M.; Jennings White, C. Stereoselective total synthesis of (dextro)-artemisinin, the antimalarial constituent of Artemisia annua L. J. Am. Chem. Soc. 1992, 114, 974–979. [Google Scholar] [CrossRef]
- Bhonsle, J.B.; Pandey, B.; Deshpande, V.H.; Ravindranathan, T. New synthetic strategies towards (dextro)-artemisinin. Tetrahedron Lett. 1994, 35, 5489–5492. [Google Scholar] [CrossRef]
- Liu, H.-J.; Yeh, W.-L.; Chew, S.-Y. A total synthesis of the antimalarial natural product (+)-qinghaosu. Tetrahedron Lett. 1993, 34, 4435–4438. [Google Scholar]
- Zhou, W.-S.; Xu, X.-X. Total synthesis of the antimalarial sesquiterpene peroxide quinghaosu and yingzhaosu A. Acc. Chem. Res. 1994, 27, 211–216. [Google Scholar] [CrossRef]
- Avery, M.A.; Jennings-White, C.; Chong, W.K.M. The total synthesis of (+)-artemisinin and (+)-9-desmethylartemisinin. Tetrahedron Lett. 1987, 28, 4629–4632. [Google Scholar] [CrossRef]
- Ye, B.; Wu, Y.-L. An efficient synthesis of qinghaosu and deoxoqinghaosu from arteannuin acid. J. Chem. Soc., Chem. Commun. 1990, 726–727. [Google Scholar] [CrossRef]
- Roth, R.J.; Acton, N. A facile semisynthesis of the antimalarial drug qinghaosu. J. Chem. Educ. 1991, 68, 612–613. [Google Scholar] [CrossRef]
- Acton, N.; Roth, R.J. On the conversion of dihydroartemisininic acid into artemisinin. J. Org. Chem. 1992, 57, 3610–3614. [Google Scholar] [CrossRef]
- Nowak, D.M.; Lansbury, P.T. Synthesis of (+)-artemisinin and (+)-deoxyartemisinin from arteannuin B and arteannuic aid. Tetrahedron 1998, 54, 319–336. [Google Scholar] [CrossRef]
- Woerdenbag, H.J.; Pras, N.; Chan, N.G.; Bang, B.T.; Bos, R.; van Uden, W.; van Boi, N.; Batterman, S.; Lugt, C.B. Artemisinin related sesquiterpenes, and essential oil in Artemisia annua during a vegetation period in Vietnam. Planta Med. 1994, 60, 272–275. [Google Scholar] [CrossRef] [PubMed]
- Ekthawatchai, S.; Lertvorachon, J.; Meepowpan, P.; Thongpanchang, T.; Thebtaranonth, Y.; Yuthavong, Y. An environmentally friendly, low cost, one-pot synthesis of artemisitene. Synth. Commun. 2003, 33, 1855–1860. [Google Scholar] [CrossRef]
- Zhang, L.; Zhou, W. Structure and synthesis of arteannuin and related compounds. XXIV. Conversion of arteannuin into artemisitene. Youji Huaxue 1988, 8, 329–330. [Google Scholar]
- Haynes, R.K.; Vonwiller, S.C. Catalyzed oxygenation of allylic hydroperoxides derived from qinghao (artemisinic) acid. Conversion of qinghao acid into dehydroqinghaosu (artemisitene) and qinghaosu (artemisinin). J. Chem. Soc., Chem. Commun. 1990, 451–453. [Google Scholar] [CrossRef]
- Duke, M.V.; Paul, R.N.; Elsohly, H.N.; Sturtz, G.; Duke, S.O. Localization of artemisinin and artemisitene in foliar tissues of glanded and glandless biotypes of Artemisia annua L. Int. J. Plant Sci. 1994, 155, 365–372. [Google Scholar] [CrossRef]
- Zaman, S.S.; Sharma, R.P. Some aspects of the chemistry and biological activity of artemisinin and related antimalarials. Heterocycles 1991, 32, 1593–1638. [Google Scholar]
- Butler, A.R. Artemisinin (Qinghaosu): a new type of antimalarial drug. Chem. Soc. Rev. 1992, 21, 85–90. [Google Scholar] [CrossRef]
- Lansbury, P.T.; Nowak, D.M. An efficient partial synthesis of (dextro)-artemisinin and (dextro)-deoxoartemisinin. Tetrahedron Lett. 1992, 33, 1029–1032. [Google Scholar] [CrossRef]
- Nair, M.S.R.; Basile, D.V. Bioconversion of arteannuin B to artemisinin. J. Nat. Prod. 1993, 56, 1559–1566. [Google Scholar] [CrossRef] [PubMed]
- Bharel, S.; Gulati, A.; Abdin, M.Z.; Srivastava, P.S.; Vishwakarma, R.A.; Jain, S.K. Enzymic synthesis of artemisinin from natural and synthetic precursors. J. Nat. Prod. 1998, 61, 633–636. [Google Scholar] [CrossRef] [PubMed]
- Acton, N.; Klayman, D.L. Artemisitene, a new sesquiterpene lactone endoperoxide from Artemisia annua. Planta Med. 1985, 441–442. [Google Scholar] [CrossRef] [PubMed]
- El-Feraly, F.S.; Al-Meshal, I.A.; Al-Yahya, M.A.; Hifnawy, M.S. On the possible role of qinghao acid in the biosynthesis of artemisinin. Phytochemistry 1986, 25, 2777–2778. [Google Scholar] [CrossRef]
- Gu, H. Pharmacological studies on artemisinin and its derivatives as antimalarial. Int. Congr. Ser. - Excerpta Med. 1987, 750, 657–660. [Google Scholar]
- Wei, Z.; Pan, J.; Li, Y. Artemisinin G: a sesquiterpene from Artemisia annua. Planta Med. 1992, 58, 300. [Google Scholar] [CrossRef] [PubMed]
- Jung, M.; ElSohly, H.N.; Croom, E.M.; McPhail, A.T.; McPhail, D.R. Practical conversion of artemisinic acid into desoxyartemisinin. J. Org. Chem. 1986, 51, 5417–5419. [Google Scholar] [CrossRef]
- Jung, M.; Li, X.; Bustos, D.A.; El-Sohly, H.N.; McChesney, J.D. A short and stereosepcific synthesis of (+)-deoxoartemisinin and (-)-deoxodesoxyartemisinin. Tetrahedron Lett. 1989, 30, 5973–5976. [Google Scholar] [CrossRef]
- Yang, R.-Y.; Zeng, X.-M.; Lu, Y.-Y.; Lu, W.-J.; Feng, L.-L.; Yang, X.-Q.; Zeng, Q.-P. Senescent leaves of Artemisia annua are one of the most active organs for overexpression of artemisinin biosynthesis responsible genes upon burst of singlet oxygen. Planta Med. 2010, 76, 734–742. [Google Scholar] [CrossRef] [PubMed]
- de Oliveira, C.M.; Ferracini, V.L.; Foglio, M.A.; de Meijire, A.; Marsaioli, A.J. Detection, synthesis and absolute configuration of (+)-nortaylorione, a new terpene from Artemisia annua. Tetrahedron Asymm. 1997, 8, 1833–1839. [Google Scholar] [CrossRef]
- Mercke, P.; Crock, J.; Croteau, R.; Brodelius, P.E. Cloning, expression and characterization of epi-cedrol synthase, a sesquiterpene cyclase from Artemisia annua L. Arch. Biochem. Biophys. 1999, 369, 213–222. [Google Scholar] [CrossRef] [PubMed]
- Hua, L.; Matsuda, S.P.T. The molecular cloning of 8-epicedrol synthase from Artemisia annua. Arch. Biochem. Biophys. 1999, 369, 208–212. [Google Scholar] [CrossRef] [PubMed]
- Wong, H.-F.; Brown, G.D. Photo-oxygenation of phytol and the structure revision of phytene-1,2-diol from Artemisia annua to phytene-1-ol-2-hydroperoxide. J. Chem. Res. 2002, 30–33. [Google Scholar] [CrossRef]
- Brown, G.D. Phytene-1,2-diol from Artemisia annua. Phytochemistry 1994, 36, 1553–1554. [Google Scholar] [CrossRef]
- Shukla, A.; Farooqi, A.H.A.; Shukla, Y.N. Growth inhibitors from Artemisia annua. Indian Drugs 1991, 28, 376–377. [Google Scholar]
- Feng, L.; Zeng, Q. Cloning and sequencing of Artemisia annua squalene synthase gene and its cDNA. Guangzhou Zhongyiyao Daxue Xuebao 2004, 21, 387–390. [Google Scholar]
- Li, Z.; Wang, H.; Wang, H.; Li, G.; Ye, H. Escherichia coli expression, purification and functional identification of a squalene synthase from Artemisia annua L. Yingyong Yu Huanjung Shengwu Xuebao 2007, 13, 309–312. [Google Scholar]
- Liu, Y.; Ye, H.; Wang, H.; Li, G. Molecuar cloning, Escherichia coli expression and genomic organization of squalene synthase gene from Artemisia annua. Acta Bot. Sin. 2003, 45, 608–613. [Google Scholar]
- Feng, L.-L.; Yang, R.-Y; Yang, X.-Q.; Zeng, Q.-P. Gene targetting of squalene synthase in Artemisia annua. Zhongcaoyao 2006, 37, 1857–1861. [Google Scholar]
- Zhang, Y.; Liu, Y.; Wang, H.; Ye, H.; Li, G. Regulation of squalene synthase gene expression in tobacco by antisense transformation with Artemisia annua squalene synthase gene. Nongye Shengwu Jishu Xuebao 2005, 13, 416–422. [Google Scholar]
- Kirby, J.; Romanini, D.W.; Paradise, E.M.; Keasling, J.D. Engineering triterpene production in Saccharomyces cerevisiae - β amyrin synthase from Artemisia annua. FEBS J. 2008, 275, 1852–1859. [Google Scholar] [CrossRef] [PubMed]
- Shukla, A.; Farooqi, A.H.A.; Shukla, Y.N. A new adenine derivative from Artemisia annua. J. Indian Chem. Soc. 1997, 74, 59. [Google Scholar]
- Lu, S.; Chen, X.-Y. Terpenoid metabolism in cotton (Gossypium spp.) and qinghao. Adv. Plant Physiol. 2005, 8, 265–292. [Google Scholar]
- Haynes, R.K. From artemisinin to new antimalarials: biosynthesis, extraction, old and new derivatives and medicinal chemistry requirements. Curr. Top. Med. Chem. 2006, 6, 509–537. [Google Scholar] [CrossRef] [PubMed]
- Ryden, A.-M.; Kayser, O. Chemistry, biosynthesis and biological activity of artemisinin and related natural peroxides. Top. Heterocycl. Chem. 2007, 9, 1–31. [Google Scholar]
- Bharel, S.; Gulati, A.; Abdin, M.Z.; Srivastava, P.S.; Jain, S.K. Structure, biosynthesis and functions of artemisinin. Fitoterapia 1996, 67, 387–402. [Google Scholar]
- Liu, C.; Wang, Y.; Quyang, F. Advance in artemisinin biosynthesis research. Tianran Chanwu Yanjiu Yu Kaifa 2000, 12, 83–86. [Google Scholar]
- Chen, D.-H.; Ye, H.-C.; Li, G.-F.; Liu, Y. Advances in molecular biology of plant isoprenoid metabolic pathway. Zhiwu Xuebao 2000, 42, 551–558. [Google Scholar]
- Wang, H.-Y.; Ye, H.; Liu, B.; Li, Z.; Li, G. Advances in molecular regulation of artemisinin biosynthesis. Shengwu Gongcheng Xuebao 2003, 19, 646–650. [Google Scholar]
- Weathers, P.J.; Elkholy, S.; Wobbe, K.K. Artemisinin: the biosynthetic pathway and its regulation in Artemisia annua, a terpenoid-rich species. In Vitro Cell. Dev. Biol.: Plant 2006, 42, 309–317. [Google Scholar] [CrossRef]
- Covello, P.; Teoh, K.; Polichuk, D.R.; Reed, D.W.; Nowak, G. How the antimalarial artemisinin is made in plants. Abstracts of Papers; In Prceedings of 234th ACS national Meeting, Boston, MA, USA, August 19-23, 2007. [Google Scholar]
- Covello, P.; Teoh, K.H.; Polichuk, D.R.; Reed, D.W.; Nowak, G. Functional genomics and the biosynthesis of artemisinin. Phytochemistry 2007, 68, 1864–1871. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Xia, Z.; Zhou, F.; Wu, Y.; Huang, J.; Wang, Z. Studies on the biosynthesis of arteannuin. III. Arteannuic acid as a key intermediate in the biosyntheses of arteannuin and arteannuin B. Huaxue Xuebao 1988, 46, 1152–1153. [Google Scholar]
- Chen, P.-K.; Lukonis, C.; Go, L.; Leather, G.R. Increasing artemisinin production through biotransformation of precursors. Proc. Plant Growth Regul. Soc. Am. 1991, 2–8. [Google Scholar]
- Li, Y.; Yang, Z.-X.; Chen, Y.-X.; Zhang, X. Synthesis of [15-14C] labeled artemisinin. Yaoxue Xuebao (Acta Pharmaceutica Sinica) 1994, 29, 713–716. [Google Scholar]
- Wang, Y.; Xia, Z.; Zhou, F.; Wu, Y.; Huang, J.; Wang, Z. Studies on the biosynthesis of arteannuin. IV. The biosynthesis of arteannuin and arteannuin B by the leaf homogenate of Artemisia annua L. Chin. J. Chem. 1993, 11, 457–463. [Google Scholar] [CrossRef]
- Nair, M.S.; Basile, D.V. Use of cell-free systems in the production of the potent antimalarial, artemisinin. Indian J. Chem., Sect. B 1992, 31B, 880–882. [Google Scholar]
- Wang, Y.; Shen, Z.; Xia, Z.; Zhou, F. Studies on the biosynthesis of arteannuin. V. The role of 6-epi-deoxyarteannuin B in arteannuin biosynthesis. Chin. J. Chem. 1993, 11, 476–478. [Google Scholar] [CrossRef]
- Towler, M.J.; Weathers, P.J. Evidence of artemisinin production from IPP stemming from both mevalonate and non-mevalonate pathways. Plant Cell Rep. 2007, 26, 2129–2136. [Google Scholar] [CrossRef] [PubMed]
- Kudaksseril, G.J.; Lam, L.; Staba, E.J. Effect of sterol inhibitors on the incorporation of carbon-14 isopentenyl pyrophosphate into artemisinin by a cell-free system from Artemisia annua tissue cultures and plants. Planta Med. 1987, 53, 280–284. [Google Scholar] [CrossRef] [PubMed]
- Marchese, J.A.; Broetto, F.; Ming, L.C.; Ducatti, C.; Rodella, R.A.; Ventrella, M.C.; Gomes, G.D.R.; de Franchesci, L. Carbon isotope composition and leaf anatomy as a tool to characterize the photosynthetic mechanism of Artemisia annua L. Braz. J. Plant Physiol. 2005, 17, 187–190. [Google Scholar] [CrossRef]
- Matsushita, Y.; Kang, W.; Charlwood, B.V. Cloning and anaysis of a cDNA encoding farnesyl diphosphate synthase from Artemisia annua. Gene 1996, 172, 207–209. [Google Scholar] [CrossRef]
- Bouwmeester, H.J.; Wallaart, T.E.; Janssen, M.H.A.; van Loo, B.; Jansen, B.J.M.; Posthumus, M.A.; Schmidt, C.O.; de Kraker, J.W.; Koenig, W.A.; Franssen, M.C.R. Amorpha-4,11-diene synthase catalyses the first probable step in artemisinin biosynthesis. Phytochemistry 1999, 52, 843–854. [Google Scholar] [CrossRef]
- Shen, H.-Y.; Li, Z.-Q.; Wang, H.; Ma, L.-Q.; Liu, B.-Y; Yan, F.; Li, G.-F.; Ye, H.-C. Advances in sesquiterpene synthase cyclases of Artemisia annua. Shengwu Gongcheng Xuebao 2007, 23, 976–981. [Google Scholar] [CrossRef]
- Jang, Y.-J.; Kim, S.-U.; Park, S.-H.; Song, S.-H. Amorpha-4,11-diene synthase of Artemisia annua, its coding gene, expression vector containing the gene and E. coli and plant transformants transformed with the vector. Repub. Korean Kongkae Taeho Kongbo 2001. [Google Scholar]
- Wallaart, T.E.; Bouwmeester, H.J.; Hille, J.; Poppinga, L.; Maijers, N.C.A. Amorpha-4,11-diene synthase: cloning and functional expression of a key enzyme in the biosynthetic pathway of the novel antimalarial drug artemisinin. Planta 2001, 212, 460–465. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.-H.; Heo, K.; Chang, Y.-J.; Park, S.-H.; Rhee, S.-K.; Kim, S.-U. Cyclization mechanism of amorpha-4,11-diene synthase, a key enzyme in artemisinin biosynthesis. J. Nat. Prod. 2006, 69, 758–762. [Google Scholar] [CrossRef] [PubMed]
- Mercke, P.; Bengtsson, M.; Bouwmeester, H.J.; Posthumus, M.A.; Brodelius, P.E. Molecular cloning, expression, and characterization of amorpha-4,11-diene synthase, a key enzyme of artemisinin biosynthesis in Artemisia annua L. Arch. Biochem. Biophys. 2000, 381, 173–180. [Google Scholar] [CrossRef] [PubMed]
- Kim, N.-C.; Kim, J.-G.; Lim, H.-J.; Hahn, T.R.; Kim, S.-U. Production of secondary metabolites by tissue culture of Artemisia annua L. J. Kor. Agric. Chem. Soc. 1992, 35, 99–105. [Google Scholar]
- Zhang, Y.; Teoh, K.H.; Reed, D.W.; Maes, L.; Goossens, A.; Olson, D.J.H.; Ross, A.R.S.; Covello, P.S. The Molecular cloning of artemisinic aldehyde Δ11(13) reductase and its role in glandular trichome-dependent biosynthesis of artemisinin in Artemisia annua. J. Biol. Chem. 2008, 283, 21501–21508. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Liu, B.; Li, Z.; Ye, H.; Wang, H.; Li, G.; Han, J. Molecular cloning of a classical plant peroxidase from Artemisia annua and its effect on the biosynthesis of artemisinin in vitro. Acta Bot. Sin. 2004, 46, 1338–1346. [Google Scholar]
- Zhang, Y.; Ye, H.; Li, G. Effect of horseradish peroxidase on the biosynthesis of artemisinin in Artemisia annua in vitro. Yingyong Yu Huanjung Shengwu Xuebao 2003, 9, 616–618. [Google Scholar]
- Lommen, W.J.M.; Bouwmeester, H.J.; Verstappen, F.W.A. Trichome dynamics and artemisinin accumulation during development and senescence of Artemisia annua leaves. Planta Med. 2006, 72, 336–345. [Google Scholar] [CrossRef] [PubMed]
- Vonwiller, S.C.; Warner, J.A.; Mann, S.T.; Haynes, R.K. Copper (II) trifluoromethanesulfonate-induced cleavage oxygenation of allylic hydroperoxides derived from qinghao acid in the synthesis of qinhaosu derivatives: evidence for the intermediacy of enols. J. Am. Chem. Soc. 1995, 117, 11098–11105. [Google Scholar] [CrossRef]
- Vonwiller, S.C.; Warner, J.A.; Mann, S.T.; Haynes, R.K. The formation of a peracetal and trioxane from an enol ether with Copper(II) triflate and oxygen: unexpected oxygenation of aldol intermediates. Tetrahedron Lett. 1997, 38, 2363–2366. [Google Scholar] [CrossRef]
- Adam, W.; Kliem, U.; Lucchini, V. Preparative UV-VIS laser photochemistry: Photocycloadditions of methylenelactones with benzophenone and p-benzoquinone: Oxygen trapping of Paterno-Buchi triplet 1,4-diradicals as model reactions for Qinghaosu-type 1,2,4-trioxanes. Liebigs Ann. Chem. 1988, 869–875. [Google Scholar] [CrossRef]
- Adam, W.; Kliem, U.; Mosandl, T.; Peters, E.M.; Peters, K.; von Schnering, H.G. Preparative visible-laser photochemistry: Qinghaosu-type 1,2,4-trioxanes by molecular oxygen trapping of Paterno-Buchi triplet 1,4-diradicals derived from 3,4-dihydro-4,4-dimethyl-2H-pyran-2-one and quinones. J. Org. Chem. 1988, 53, 4986–4992. [Google Scholar] [CrossRef]
- Cumming, J.N.; Wang, D.; Park, S.B.; Shapiro, T.A.; Posner, G.H. Design, synthesis, derivatization, and structure-activity relationships of simplified, tricyclic, 1,2,4-trioxane alcohol analogues of the antimalarial artemisinin. J. Med. Chem. 1998, 41, 952–964. [Google Scholar] [CrossRef] [PubMed]
- Kim, N.-C.; Kim, S.-U. Biosynthesis of artemisinin from 11, 12-dihydroarteannuic acid. J. Kor. Agric. Chem. Soc. (Han’guk Nonghwa Hakhoechi) 1992, 35, 106–109. [Google Scholar]
- Huang, J.; Zhou, F.; Wu, L.; Zeng, G. Biosynthesis of arteannuin. I. In vivo biosynthesis of arteannuic acid of Artemisia annua. Huaxue Xuebao 1990, 48, 275–7. [Google Scholar]
- Tatineni, R.; Doddapaneni, K.K.; Dalavayi, S.; Kulkarni, S.M.; Narasu, M.L. Microbacterium trichothecenolyticum enzyme mediated transformation of arteannuin B to artemisinin. Process Biochem. 2006, 41, 2464–2467. [Google Scholar] [CrossRef]
- Dhingra, V.; Narasu, M.L. Purification and Characterization of an enzyme involved in biochemical transformation of arteannuin B to artemisinin from Artemisia annua. Biochem. Biophys. Res. Commun. 2001, 281, 558–561. [Google Scholar] [CrossRef] [PubMed]
- Dhingra, V.; Rajoli, C.; Narasu, M.L. Partial purification of proteins involved in the bioconversion of arteannuin B to artemisinin. Bioresource Technol. 2000, 73, 279–282. [Google Scholar] [CrossRef]
- Abdin, M.Z.; Israr, M.; Kumar, P.A.; Jain, S.K. Molecular approaches to enhance artemisinin content in Artemisia annua L. Recent Prog. Med. Plants 2004, 4, 145–161. [Google Scholar]
- Brown, G.D. Production of anti-malarial and anti-migraine drugs in tissue culture of Artemisia annua and Tanacetum parthenium. Acta Hortic. 1993, 330, 269–276. [Google Scholar] [CrossRef]
- Covello, P. Making artemisinin. Phytochemistry 2008, 69, 2881–2885. [Google Scholar] [CrossRef] [PubMed]
- Abdin, M.Z.; Jain, S.K. Artemisinin, a novel antimalarial drug: biochemical and molecular approaches for enhanced production. Planta Med. 2003, 69, 289–299. [Google Scholar] [CrossRef] [PubMed]
- Dhingra, V.; Rao, K.V.; Narasu, M.L. Current status of artemisinin and its derivatives as antimalarial drugs. Life Sci. 1999, 66, 279–300. [Google Scholar] [CrossRef]
- Liu, C.; Wang, Y.; Ouyang, F.; Ye, H.; Li, G. Advances in artemisinin research. Huaxue Jinzhan 1999, 11, 41–48. [Google Scholar]
- van Geldre, E.; Vergauwe, A.; van den Eeckhout, E. State of the art of the production of the antimalarial compound artemisinin in plants. Plant Mol. Biol. 1997, 33, 199–209. [Google Scholar] [CrossRef] [PubMed]
- Gulati, A.; Bharel, S.; Srivastava, P.S.; Abdin, M.Z.; Jain, S.K. Experimental studies on Artemisia, a herbal remedy to malaria. Fitoterapia 1996, 67, 403–410. [Google Scholar]
- Wu, J.; Ding, W.; Zhang, Y.; Zhou, Y. Advances in studies on biotechnology of getting high-yield artemisinin. Zhongcaoyao 2007, 38, 305–308. [Google Scholar]
- Laughlin, J.C. Agricultural production of artemisinin - a review. Trans. R. Soc. Trop. Med. Hyg. 1994, 88, 21–22. [Google Scholar] [CrossRef]
- de Magalhaes, P.M.; Pereira, B.; Sartoratto, A.; de Oliveira, J.; Debrunner, N. New hybrid lines of the antimalarial species Artemisia annua L. Acta Hortic. 1999, 502, 377–381. [Google Scholar] [CrossRef]
- Delabays, N.; Benakis, A.; Collet, G. Selection and breeding for high artemisinin (qinghaosu) yielding strains of A. annua L. Acta Hortic. 1993, 330, 203–205. [Google Scholar] [CrossRef]
- Simmonet, X.; Carlen, C. New Artemisia annua hybrids with high artemisinin content. Acta Hortic. 2008, 769, 371–373. [Google Scholar] [CrossRef]
- Ferreira, J.F.S.; Janick, J. Floral morphology of Artemisia annua with special reference to trichomes. Int. J. Plant Sci. 1995, 156, 807–815. [Google Scholar]
- Liersch, R.; Soicke, H.; Stehr, C.; Tuellner, H.U. Formation of artemisinin in Artemisia annua during one vegetation period. Planta Med. 1986, 387–390. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.; Vishwakarma, R.A.; Husain, A. Evaluation of Artemisia annua strains for higher artemisinin production. Planta Med. 1988, 54, 475–476. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Li, G.-F. Studies on the effects of fpf1 gene on Artemisia annua flowering time and on the linkage between flowering and artemisinin biosynthesis. Planta Med. 2004, 70, 347–352. [Google Scholar] [PubMed]
- Ferreira, J.F.S.; Simon, J.E.; Janick, J. Developmental studies of Artemisia annua: Flowering and artemisinin production under greenhouse and field conditions. Planta Med. 1995, 61, 167–170. [Google Scholar] [CrossRef] [PubMed]
- Jain, D.C.; Mathur, A.K.; Gupta, M.M.; Singh, A.K.; Verma, R.K.; Gupta, A.P.; Kumar, S. Isolation of high artemisinin-yielding clones of Artemisia annua. Phytochemistry 1996, 43, 993–1001. [Google Scholar] [CrossRef]
- Chen, H.; Chen, M.; Zhong, F.; Chen, F.; Huang, J.; Zhang, M.; Huang, J. Factors affecting the content of artemisinin in Artemisia annua. Zhongyao Tongbao 1986, 11, 393–395. [Google Scholar]
- Chen, F.; Zhang, G. Studies of several physiological factors on artemisinin synthesis in Artemisia annua. Zhiwu Shenglixue Tongxun 1987, 5, 26–30. [Google Scholar]
- Wang, J.-X.; Wang, Z.-M. Effects of irradiance on growth, photosynthetic characteristics and artemisinin content of Artemisia annua L. Photosynthetica 2008, 46, 17–20. [Google Scholar] [CrossRef]
- Gupta, S.S.; Singh, P.; Bajpai, P.; Ram, G.; Singh, D.; Gupta, M.M.; Jain, D.C.; Khanuja, S.P.; Kumar, S. Morphogenetic variation for artemisinin and volatile oil in Artemisia annua. Ind. Crops Prod. 2002, 16, 217–224. [Google Scholar] [CrossRef]
- Ferreira, J.F.S. Nutrient deficiency in the production of artemisinin, dihydroartemisinic acid, and artemisinic acid in Artemisia annua L. J. Agric. Food Chem. 2007, 55, 1686–1694. [Google Scholar] [CrossRef] [PubMed]
- Kawamoto, H.; Sekine, H.; Furuya, T. Production of artemisinin and related sesquiterpenes in Japanese Artemisia annua during a vegetation period. Planta Med. 1999, 65, 88–89. [Google Scholar] [CrossRef] [PubMed]
- Laughlin, J.C. The influence of distribution of antimalarial constituents in Artemisia annua L. on time and method of harvest. Acta Hortic. 1995, 390, 67–73. [Google Scholar] [CrossRef]
- Wallaart, T.E.; Pras, N.; Beekmann, A.C.; Quax, W.J. Seasonal variation of artemisinin and its biosynthetic precursors in plants of Artemisia annua of different geographical origin. Proof for the existence of chemotypes. Planta Med. 2000, 66, 57–62. [Google Scholar] [CrossRef] [PubMed]
- Yang, R.-Y.; Zeng, Q.-P. Quantitative transcript profiling reveals down-regulation of a sterol pathway relevant gene and overexpression of artemisinin biogenetic genes in transgenic Artemisia annua plants. Planta Med. 2008, 74, 1510–1516. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Jing, F.; Fupeng, L. Development of transgenic Artemisia annua (Chinese wormwood) plants with enhanced content of artemisinin, an effective antimalarial drug, by hairpin-RNA-mediated gene silencing. Biotechnol. Appl. Biochem. 2009, 52, 199–207. [Google Scholar] [CrossRef] [PubMed]
- Han, J.-L.; Wang, H.; Ye, H.-C.; Liu, Y.; Li, Z.-Q.; Zhang, Y.; Zhang, Y.-S.; Yan, F.; Li, G.-F. High efficiency of genetic transformation and regeneration of Artemisia annua L. via Agrobacterium tumefaciens-mediated procedure. Plant Sci. 2004, 168, 73–80. [Google Scholar] [CrossRef]
- Martinez, B.C.; Staba, E.J. The production of artemisinin in Artemisia annua L. tissue cultures. Adv. Cell Cult. 1988, 6, 69–87. [Google Scholar]
- Ferreira, J.F.S.; Janick, J. Roots as an enhancing factor for the production of artemisinin in shoot cultures of Artemisia annua. Plant Cell Tissue Org. Cult. 1996, 44, 211–217. [Google Scholar] [CrossRef]
- Chen, P.-K.; Hua, H.; Yim, J.-W. Enhanced synthesis of the natural phytotoxin artemisinin in tissue culture. PGRSA Q. 1993, 21, 151–160. [Google Scholar]
- Zhang, L.; Li, G. Related factors of artemisinin biosynthesis in clone strain of Artemisia annua L. Yingyong Yu Huanjung Shengwu Xuebao 2004, 10, 277–280. [Google Scholar]
- Nair, M.S.R.; Acton, N.; Klayman, D.L.; Kendrick, K.; Basile, D.V.; Mante, S. Production of artemisinin in tissue cultures of Artemisia annua. J. Nat. Prod. 1986, 49, 504–507. [Google Scholar] [CrossRef] [PubMed]
- Elhag, H.M.; E1-Domiaty, M.M.; E1-Feraly, F.S.; Mossa, J.S.; E1-Olemy, M.M. Selection and micropropagation of high artemisinin producing clones of Artemisia annua L. Phytother. Res. 1992, 6, 20–24. [Google Scholar] [CrossRef]
- Li, H.; Yi, Z.; Yong, G.; Yao, R. Artificial regulation of artemisinin biosynthesis metabolite in cultured cells of Artemisia annua. Zhongguo Shengwu Huaxue Yu Fenzi Shengwu Xuebao 1999, 15, 479–483. [Google Scholar]
- Xie, D.; Li, G. Isolation and production of artemisinin and stigmasterol in hairy root cultures of Artemisia annua. Plant Cell Tissue Org. Cult. 2001, 63, 161–166. [Google Scholar] [CrossRef]
- Xie, D.; Li, G.; Guo, Z. Selection of hairy root clones of Artemisia annua L. for artemisinin production. Isr. J. Plant Sci. 2001, 49, 129–134. [Google Scholar] [CrossRef]
- Chen, D.-H.; Chun, Y.-H.; Li, G.-F. Expression of a chimeric farnesyl diphosphate synthase gene in Artemisia annua L. transgenic plants via Agrobacterium tumefaciens-mediated transformation. Plant Sci. 2000, 155, 179–185. [Google Scholar] [CrossRef]
- Wang, J.; Tan, R.-X. Preparation of an elicitor from a fungal endophyte to enhance artemisinin production in hairy root culture of Artemisia annua L. Shengwu Gongcheng Xuebao 2006, 22, 829–834. [Google Scholar]
- Wang, J.-W.; Xiang, R. Stimulation of artemisinin production in Artemisia annua hairy roots by the elicitor from the endophytic Colleotrichum Ssp. Biotechnol. Lett. 2001, 23, 857–860. [Google Scholar] [CrossRef]
- Wobbe, K.K.; Zhang, X.; Weathers, P. Correlations between peroxidase activity, calcium and artemisinin levels in hairy roots of Artemisia annua L. Curr. Top. Plant Physiol. 1998, 432–434. [Google Scholar]
- Woerdenbag, H.J.; Pras, N.; van Uden, W.; de Boer, A.; Batterman, S.; Visser, J.F.; Malingre, T.M. High peroxidase activity in cell cultures of Artemisia annua with minute artemisinin contents. Nat. Prod. Lett. 1992, 1, 121–128. [Google Scholar] [CrossRef]
- Steirle, A.; Strobel, G.A.; Stierle, D. Taxol and taxane production by Taxomyces andreanae, an endophytic fungus of Pacific Yew. Science 1993, 260, 214–216. [Google Scholar] [CrossRef]
- Liu, C.-H.; Zou, W.-X.; Lu, H.; Tan, R.-X. Antifungal activity of Artemisia annua endophyte cultures against phytopathenogenic fungi. J. Biotechnol. 2001, 88, 277–282. [Google Scholar] [CrossRef]
- Simanjuntak, P.; Bustanussalam, O.; Dian, M.; Rahayuningsih, M.; Said, E.G. Studies on endophytic microbes of Artemisia spp (3). Isolation and identification of artemisinin from product of endophyte microbe cultivation from Artemisia annua. Majalah Farmasi Indonesia 2004, 15, 68–74. [Google Scholar]
- Shen, L.; Jiao, R.-H.; Ye, Y.-H.; Wang, X.-T.; Xu, C.; Song, Y.-C.; Zhu, H.-L.; Tan, R.-X. Absolute configuration of new cytotoxic and other bioactive tricothecene macrolides. Chem. - Eur. J. 2006, 12, 5596–5602. [Google Scholar] [CrossRef] [PubMed]
- Gu, W.; Ge, H.M.; Song, Y.C.; Ding, H.; Zhu, H.L.; Zhao, X.A.; Tan, R.-X. Cytotoxic Benzo[j]fluoranthene metabolites from Hypoxylon truncatum IFB-18, an endophyte of Artemisia annua. J. Nat. Prod. 2007, 70, 114–117. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.-Y.; Liu, C.-H.; Zou, W.-X.; Tan, R.-X. Leptosphaeric acid, a metabolite with a novel carbon skeleton from Leptosphaeria sp. IV403, an endophytic fungus in Artemisia annua. Helv. Chim. Acta 2003, 86, 657–660. [Google Scholar] [CrossRef]
- Liu, J.-Y.; Liu, C.-H.; Zou, W.-X.; Tian, X.; Tan, R.-X. Leptosphaerone, a metabolite with a novel skeleton from Leptosphaeria sp. IV403, an endophytic fungus in Artemisia annua. Helv. Chim. Acta 2002, 85, 2664–2667. [Google Scholar] [CrossRef]
- Lu, H.; Zou, W. X.; Meng, J. C.; Hu, J.; Tan, R.X. New bioactive metabolites produced by Colletotrichum sp., an endophytic fungus in Artemisia annua. Plant Sci. 2000, 151, 67–73. [Google Scholar] [CrossRef]
- Keasling, J. D. Synthetic biology for synthetic chemistry. ACS Chem. Biol. 2008, 3, 64–76. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Zeng, Q. Genetic manipulation on biosynthesis of terpenoids. Zhongguo Shengwu 2006, 26, 60–64. [Google Scholar]
- Zeng, Q.-P.; Yuan, L. Production of artemisinin by genetically modified microbes. Biotechnol. Lett. 2008, 30, 581–592. [Google Scholar] [CrossRef] [PubMed]
- Withers, S.T.; Keasling, J.D. Biosynthesis and engineering of small isoprenoid molecules. Appl. Microbiol. Biotechnol. 2007, 73, 980–990. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.-Y.; Kim, H.-U.; Park, J.-H.; Park, J.-M.; Kim, T.-Y. Metabolic engineering of microorganisms. Drug Discov. Today 2009, 14, 78–88. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Zhao, Y.; Wang, Y. Artemisinin: current status and perspectives for biotechnological production of an antimalarial drug. Appl. Microbiol. Biotechnol. 2006, 72, 11–20. [Google Scholar] [CrossRef] [PubMed]
- Kong, J.-Q.; Cheng, K.; Wang, L.; Zheng, X.; Dai, J.; Zhu, P.; Wang, W. Increase of copy number of HMG-CoA reductase and FPP synthase genes improves the amorpha-4,11-diene production in engineered yeast. Yaoxue Xuebao 2007, 42, 1314–1319. [Google Scholar]
- Kim, O. T.; Ahn, J.C.; Hwang, S.J.; Hwang, B. Cloning and expression of a farnesyl diphosphate synthase in Centella asiatica (L.) Urban. Mol. Cells 2005, 19, 294–299. [Google Scholar] [PubMed]
- Chen, D.-H.; Liu, C.-J.; Ye, H.-C.; Li, G.-F.; Liu, B.-Y.; Men, Y.-L.; Chen, X.-Y. Ri-mediated transformation of Artemisia annua with a recombinant farnesyl diphosphate synthase gene for artemisinin production. Plant Cell Tissue Org. Cult. 1999, 57, 157–162. [Google Scholar] [CrossRef]
- Zhao, Y.; Ye, H.; Li, G.; Chen, D.; Liu, Y. Cloning and enzymology analysis of farnesyl pyrophosphate synthase gene from a superior strain of Artemisia annua L. Chin. Sci. Bull. 2003, 48, 63–67. [Google Scholar] [CrossRef]
- Han, J.-L.; Li, G. Effects of overexpression of the endogenous farnesyl diphosphate synthase on the artemisinin content in Artemisia annua L. J. Integr. Plant Biol. 2006, 48, 482–487. [Google Scholar] [CrossRef]
- Huang, Y.; Yin, L.; Feng, L.; Yang, R.; Yang, X.; Zeng, Q.-P. Cloning and expression of Artemisia annua L. amorpha-4,11-diene synthase gene. Guangzhou Zhongyiyao Daxue Xuebao 2008, 25, 68–73. [Google Scholar]
- Kong, J.-Q.; Wang, W.; Wang, L.-N.; Zhong, X.-D.; Cheng, K.-D.; Zhu, P. The improvement of amorpha-4,11-diene production by a yeast-conform variant. J. Appl. Microbiol. 2009, 106, 941–951. [Google Scholar] [PubMed]
- Chang, Y.-J.; Song, S.-H.; Park, S.-H.; Kim, S.-U. Amorpha-4,11-diene synthase of Artemisia annua: cDNA isolation and bacterial expression of a terpene synthase involved in artemisinin biosynthesis. Arch. Biochem. Biophys. 2000, 383, 178–184. [Google Scholar] [CrossRef] [PubMed]
- Chang, M.C.Y.; Eachus, R.A.; Trieu, W.; Ro, D.-K.; Keasling, J.D. Engineering Escherichia coli production of functionalized terpenoids using plant P450s. Nat. Chem. Biol. 2007, 3, 274–277. [Google Scholar] [CrossRef] [PubMed]
- Huang, B.; Guo, J.; Yi, B.; Yu, X.; Sun, L.; Chen, W. Heterologous production of secondary metabolites as pharmaceuticals in Saccharomyces cerevisiae. Biotechnol. Lett. 2008, 30, 1121–1137. [Google Scholar] [CrossRef] [PubMed]
- Lindahl, A.-L.; Olsson, M.E.; Mercke, P.; Tollbom, O.; Schelin, J.; Brodelius, M.; Brodelius, P.E. Production of the artemisinin precursor amorpha-4,11-diene by engineered Saccharomyces cerevisiae. Biotechnol. Lett. 2006, 28, 571–580. [Google Scholar] [CrossRef] [PubMed]
- Newman, J.D.; Marshall, J.; Chang, M.; Nowroozi, F.; Paradise, E.; Pitera, D.; Newman, K.L.; Keasling, J.D. High-level production of amorpha-4,11-diene in a two phase partitioning bioreactor of metabolically engineered Escherichia coli. Biotechnol. Bioeng. 2006, 95, 684–691. [Google Scholar] [CrossRef] [PubMed]
- Anthony, J.R.; Anthony, L.C.; Nowroozi, F.; Kwon, G.; Newman, J.D.; Keasling, J.D. Optimization of the mevalonate-based isoprenoid biosynthetic pathway in Escherichia coli for production of the antimalarial drug precursor amorpha-4,11-diene. Metab. Eng. 2009, 11, 13–19. [Google Scholar] [CrossRef] [PubMed]
- Picaud, S.; Olsson, M.E.; Brodelius, P.E. Improved conditions for production of recombinant plant sesquiterpene synthases in Escherichia coli. Prot. Expr. Purif. 2007, 51, 71–79. [Google Scholar] [CrossRef] [PubMed]
- Keasling, J.D. Engineering microbes for anti-malarial production. Abstracts of Papers; In Proceedings of 229th ACS National Meeting, San Diego, CA, USA, March 13-17, 2005. [Google Scholar]
- Ro, D.-K.; Paradise, E.M.; Ouellet, M.; Fisher, K.J.; Newman, K.L.; Ndungu, J.M.; Ho, K.A.; Eachus, R.A.; Ham, T.S.; Kirby, J.; Chang, M.C.Y.; Withers, S.T.; Shiba, Y.; Sarpong, R.; Keasling, J.D. Production of the antimalarial drug precursor artemisinic acid in engineered yeast. Nature 2006, 440, 940–943. [Google Scholar] [CrossRef] [PubMed]
- Ro, D.-K.; Ouellet, M.; Paradise, E.M.; Burd, H.; Eng, D.; Paddon, C.J.; Newman, J.D.; Keasling, J.D. Induction of multiple pleiotropic drug resistance genes in yeast engineered to produce an increased level of anti-malarial drug precursor, artemisinic acid. BMC Biotechnol. 2008, 8. [Google Scholar] [CrossRef] [PubMed]
- Dietrich, J.A.; Yoshikuni, Y.; Fisher, K.J.; Woolard, F.X.; Ockey, D.; McPhee, D.J.; Renninger, N.S.; Chang, M.C.Y.; Baker, D.; Keasling, J.D. A novel semi-biosynthetic route for artemisinin production using engineered substrate-promiscuous P450BM3. ACS Chem. Biol. 2009, 4, 261–267. [Google Scholar] [CrossRef] [PubMed]
- Sy, L.-K.; Brown, G.D. Novel seco-cycloartanes from Kadsura coccinea and the assisted autoxidation of a tri-substituted alkene. Tetrahedron 1999, 55, 119–132. [Google Scholar] [CrossRef]
Sample Availability: Not available. |
© 2010 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Brown, G.D. The Biosynthesis of Artemisinin (Qinghaosu) and the Phytochemistry of Artemisia annua L. (Qinghao). Molecules 2010, 15, 7603-7698. https://doi.org/10.3390/molecules15117603
Brown GD. The Biosynthesis of Artemisinin (Qinghaosu) and the Phytochemistry of Artemisia annua L. (Qinghao). Molecules. 2010; 15(11):7603-7698. https://doi.org/10.3390/molecules15117603
Chicago/Turabian StyleBrown, Geoffrey D. 2010. "The Biosynthesis of Artemisinin (Qinghaosu) and the Phytochemistry of Artemisia annua L. (Qinghao)" Molecules 15, no. 11: 7603-7698. https://doi.org/10.3390/molecules15117603
APA StyleBrown, G. D. (2010). The Biosynthesis of Artemisinin (Qinghaosu) and the Phytochemistry of Artemisia annua L. (Qinghao). Molecules, 15(11), 7603-7698. https://doi.org/10.3390/molecules15117603




