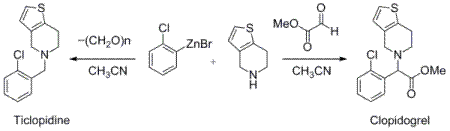
2-Chlorophenyl Zinc Bromide: A Convenient Nucleophile for the Mannich-Related Multicomponent Synthesis of Clopidogrel and Ticlopidine
Abstract
:Introduction
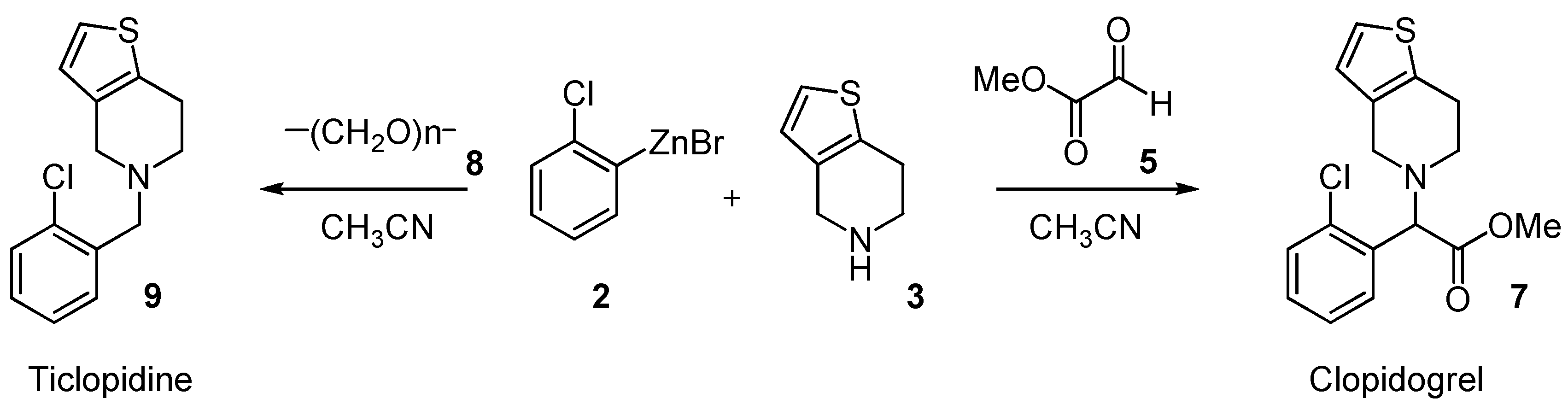
Results and Discussion
Experimental
General
Conclusions
Acknowledgements
References and Notes
- Most reported works consist of the industrial optimization of known multi-step processes. For a recent representative work, see: Wang, L.; Shen, J.; Tang, Y.; Chen, Y.; Wang, W.; Cai, Z.; Du, Z. Synthetic Improvements in the Preparation of Clopidogrel. Org. Process Res. Dev. 2007, 11, 487–489. [Google Scholar] [CrossRef]
- For a reference book, see: Zhu, J.; Bienaymé, H. Multicomponent Reactions; Wiley-VCH: Weinheim, Germany, 2005. [Google Scholar]
- Hulme, C.; Gore, V. Multi-component reactions: emerging chemistry in drug discovery from xylocain to crixivan. Curr. Med. Chem. 2003, 10, 51–80. [Google Scholar] [CrossRef] [PubMed]
- Arndtsen, B.A. Metal-Catalyzed One-Step Synthesis: Towards Direct Alternatives to Multistep Heterocycle and Amino Acid Derivative Formation. Chem. Eur. J. 2009, 15, 302–313. [Google Scholar] [CrossRef] [PubMed]
- Isambert, N.; Lavilla, R. Heterocycles as Key Substrates in Multicomponent Reactions: The Fast Lane towards Molecular Complexity. Chem. Eur. J. 2008, 14, 8444–8454. [Google Scholar] [CrossRef] [PubMed]
- D’Souza, D.M.; Müller, T.J.J. Multi-component syntheses of heterocycles by transition-metal catalysis. Chem. Soc. Rev. 2007, 36, 1095–1108. [Google Scholar] [CrossRef] [PubMed]
- Dömling, A. Recent Developments in Isocyanide-Based Multicomponent Reactions in Applied Chemistry. Chem. Rev. 2006, 106, 17–89. [Google Scholar] [CrossRef] [PubMed]
- Simon, C.; Constantieux, T.; Rodriguez, J. Utilisation of 1,3-Dicarbonyl Derivatives in Multicomponent Reactions. Eur. J. Org. Chem. 2004, 4957–4980. [Google Scholar] [CrossRef]
- Zhu, J. Recent Developments in the Isonitrile-Based Multicomponent Synthesis of Heterocycles. Eur. J. Org. Chem. 2003, 1133–1144. [Google Scholar] [CrossRef]
- Orru, R.V.A.; De Greef, M. Recent advances in solution-phase multicomponent methodology for the synthesis of heterocyclic compounds. Synthesis 2003, 1471–1499. [Google Scholar] [CrossRef]
- Balme, G.; Bossharth, E.; Monteiro, N. Palladium-Assisted Multicomponent Synthesis of Heterocycles. Eur. J. Org. Chem. 2003, 4101–4111. [Google Scholar] [CrossRef]
- Kalinski, C.; Lemoine, H.; Schmidt, J.; Burdack, C.; Kolb, J.; Umkehrer, M.; Ross, G. Multicomponent Reactions as a Powerful Tool for Generic Drug Synthesis. Synthesis 2008, 4007–4011. [Google Scholar] [CrossRef]
- Petasis, N.A.; Boral, S. One-step three-component reaction among organoboronic acids, amines and salicylaldehydes. Tetrahedron Lett. 2001, 42, 539–542. [Google Scholar] [CrossRef]
- Petasis, N.A.; Zavialov, I.A. Highly stereocontrolled one-step synthesis of anti-beta-amino alcohols from organoboronic acids, amines, and alpha-hydroxy aldehydes. J. Am. Chem. Soc. 1998, 120, 11798–11799. [Google Scholar] [CrossRef]
- Petasis, N.A.; Akritopoulou, I. The boronic acid mannich reaction: A new method for the synthesis of geometrically pure allylamines. Tetrahedron Lett. 1993, 34, 583–586. [Google Scholar] [CrossRef]
- Ugi, I.; Meyr, R.; Fetzer, U.; Steinbrückner, C. Versuche mit Isonitrilen. Angew. Chem. 1959, 71, 386–388. [Google Scholar]
- Dömling, A.; Ugi, I. Multicomponent Reactions with Isocyanides. Angew. Chem., Int. Ed. 2000, 39, 3168–3210. [Google Scholar] [CrossRef]
- Aubert, D.; Ferrand, C.; Maffrand, J.-P. Nouveaux dérivés de la thiéno (3,2-c) pyridine, leur procédé de préparation et leur application thérapeutique (in French). French Patent FR2530247 1984. [Google Scholar]
- Haurena, C.; Le Gall, E.; Sengmany, S.; Martens, T.; Troupel, M. A Straightforward Three-component Synthesis of α-Amino esters containing a Phenylalanine or a Phenylglycine Scaffold. J. Org. Chem. 2010, 75, 2645–2650. [Google Scholar] [CrossRef] [PubMed]
- Le Gall, E.; Haurena, C.; Sengmany, S.; Martens, T.; Troupel, M. Three-component synthesis of α-branched amines under Barbier-like conditions. J. Org. Chem. 2009, 74, 7970–7973. [Google Scholar] [CrossRef] [PubMed]
- A commercial ~50 % w/w solution in toluene was used.
- Fillon, H.; Gosmini, C.; Périchon, J. New Chemical Synthesis of Functionalized Arylzinc Compounds from Aromatic or Thienyl Bromides under Mild Conditions Using a Simple Cobalt Catalyst and Zinc Dust. J. Am. Chem. Soc. 2003, 125, 3867–3870. [Google Scholar] [CrossRef] [PubMed]
- Kazmierski, I.; Gosmini, C.; Paris, J.-M.; Périchon, J. New progress in the cobalt-catalysed synthesis of aromatic organozinc compounds by reduction of aromatic halides by zinc dust. Tetrahedron Lett. 2003, 44, 6417–6420. [Google Scholar] [CrossRef]
- Gosmini, C.; Amatore, M.; Claudel, S.; Périchon, J. New Efficient Preparation of Functionalized Arylzinc or Thienylzinc -Compounds from Aryl or Thienyl Chlorides Using Cobalt Catalysis. Synlett 2005, 2171–2174. [Google Scholar] [CrossRef]
- 25. As the arylzinc species can not be isolated from the reaction medium, the amount of the reagent could be modulated by the transfer of matching volumes of the acetonitrile solution. However, this relies on the knowledge of the organozinc concentration, which was estimated as follows: an aliquot of the reaction medium was exposed to the successive action of iodine crystals and sodium thiosulphate. Organic compounds were then extracted with diethyl ether. The amount of 2-chloroiodobenzene was compared to the amount of the starting 2-chlorobromobenzene via an internal standard (dodecane) by gas chromatography (GC). The concentration could be calculated through the estimation of the solution volume (approx. 8 mL for a reaction on a 6 mmol scale).
- All amounts are expressed in equivalents relative to the amine 3.
- Ethyl glyoxylate was depolymerized prior to use by a 20 min heating period at 60 °C.
- All reactions were monitored by gas chromatography.
- Products were isolated only for representative experiments.
- Similar yields were obtained, whatever the power/reaction time combination which was applied.
- Cheng, D.; Liu, D.K.; Liu, M.; Liu, Y.; Xu, W.R.; Wei, R.; Liu, C.X. Synthesis and activity evaluation of some novel derivatives of 4,5,6,7-tetrahydrothieno [3,2-c]-pyridine. Chin. Chem. Lett. 2008, 19, 689–692. [Google Scholar] [CrossRef]
- Van der Meijden, M.W.; Leeman, M.; Gelens, E.; Noorduin, W.L.; Meekes, H.; van Enckevort, W.J.P.; Kaptein, B.; Vlieg, E.; Kellogg, R.M. Attrition-Enhanced Deracemization in the Synthesis of Clopidogrel - A Practical Application of a New Discovery. Org. Process Res. Dev. 2009, 13, 1195–1198. [Google Scholar] [CrossRef]
- Yun, S.; Kim, E.S.; Kim, H.S.; Ha, T.H.; Suh, K.-H.; Lee, G.S. Method of preparing thieno [3,2-c]pyridine derivatives and intermediates. PCT Int. Appl. 2005087779 2005. [Google Scholar]
- Considering the chemical yield and inherent losses due to the transfer of the solution using a syringe, a reaction conducted on a 6 mmol scale leads to the formation of ~4 mmol of potentially usable organozinc reagent. Increased amounts of the organozinc reagent should be obtained with similar yields by using proportional amounts of the starting reagents and solvent.
Sample Availability: Samples of the compounds are available from the authors. |




| Entry | Conditions | Time (h) | Conversion (%)b | Yield (%) |
|---|---|---|---|---|
| 1 | H2SO4, cat. | 18 | <5 | - |
| 2 | H2SO4, excess | 18 | 88 | 85c |
| 3 | MeONa, excess | 0,5 | 90 | 86c (52d) |
| 4 | I2, cat. | 18 | <5 | - |
| 5 | In + I2, excess | 18 | 72 | 68c |
| Entry | Organozinc 2 (equiv.)b | Time (h) | Conversion (%)c | Yield (%) |
|---|---|---|---|---|
| 1 | 1 | 1 | 0 | - |
| 2 | 2 | 1 | 0 | - |
| 3 | 2.5 | 1 | <5 | - |
| 4 | 3 | 0.5 | 100 | 82d (>95e) |
| 5 | 3.5 | 0.5 | 100 | >95e |
| 6 | 4 | 0.5 | 100 | >95e |
| Entry | Glyoxylate 4 (equiv.)b | Time (h) | Conversion (%)c | Yield (%)d |
|---|---|---|---|---|
| 1 | 1.25 | 1.5 | 43 | - |
| 2 | 2 | 0.5 | 100 | 78 (>95e) |
| 3 | 2.5 | 0.5 | 100 | 82 (>95e) |
| 4 | 5 | 0.5 | 100 | 75 (>95e) |
| Entry | Temperature (°C) | Time (h) | Conversion (%)b | Yield (%) |
|---|---|---|---|---|
| 1 | 0 | 2 | 25 | - |
| 2 | 25 | 0.5 | 100 | 78c (>95d) |
| 3 | 60 | 0.3 | 100 | >95d |
| Entry | Amine 3 (mmol) | Time (h) | Conversion (%)b | Yield (%)c |
|---|---|---|---|---|
| 1 | 1 | 0.5 | 100 | 78 |
| 2 | 5 | 1 | 100 | 89 |
| 3 | 30 | 1.5 | 100 | 75 |

| Entry | Conditions | Time (h) | Conversion (%)b | Yield (%) |
|---|---|---|---|---|
| 1 | H2SO4, cat. | 18 | <5 | - |
| 2 | H2SO4, excess | 18 | 88 | 85c |
| 3 | MeONa, excess | 0,5 | 90 | 86c (52d) |
| 4 | I2, cat. | 18 | <5 | - |
| 5 | In + I2, excess | 18 | 72 | 68c |
© 2010 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Aillaud, I.; Haurena, C.; Gall, E.L.; Martens, T.; Ricci, G. 2-Chlorophenyl Zinc Bromide: A Convenient Nucleophile for the Mannich-Related Multicomponent Synthesis of Clopidogrel and Ticlopidine. Molecules 2010, 15, 8144-8155. https://doi.org/10.3390/molecules15118144
Aillaud I, Haurena C, Gall EL, Martens T, Ricci G. 2-Chlorophenyl Zinc Bromide: A Convenient Nucleophile for the Mannich-Related Multicomponent Synthesis of Clopidogrel and Ticlopidine. Molecules. 2010; 15(11):8144-8155. https://doi.org/10.3390/molecules15118144
Chicago/Turabian StyleAillaud, Isabelle, Caroline Haurena, Erwan Le Gall, Thierry Martens, and Gino Ricci. 2010. "2-Chlorophenyl Zinc Bromide: A Convenient Nucleophile for the Mannich-Related Multicomponent Synthesis of Clopidogrel and Ticlopidine" Molecules 15, no. 11: 8144-8155. https://doi.org/10.3390/molecules15118144
APA StyleAillaud, I., Haurena, C., Gall, E. L., Martens, T., & Ricci, G. (2010). 2-Chlorophenyl Zinc Bromide: A Convenient Nucleophile for the Mannich-Related Multicomponent Synthesis of Clopidogrel and Ticlopidine. Molecules, 15(11), 8144-8155. https://doi.org/10.3390/molecules15118144