Antidiabetic Properties and Mechanism of Action of Gynura procumbens Water Extract in Streptozotocin-Induced Diabetic Rats
Abstract
:1. Introduction
2. Results and Discussion
2.1. Results
2.1.1. Effects of G. procumbens water extract on body weight of streptozotocin-induced diabetic rats after 14-day treatment
| Experimental groups | Dose (mg/kg) | Body weight (g) | |
|---|---|---|---|
| Day 0 | Day 14 | ||
| Control | 240.4 ± 16.5 | 202.6 ± 16.4* | |
| Metformin | 500 | 223.5 ± 13.1 | 189.0 ± 9.4*** |
| G. procumbens water extract | 500 | 204.4 ± 12.8 | 162.4 ± 11.5*** |
| G. procumbens water extract | 1000 | 206.0 ± 5.4 | 167.4 ± 5.2*** |
2.1.2. Effects of G. procumbens water extract on fasting blood glucose levels in streptozotocin-induced diabetic rats after 14-day treatment
2.1.3. Effects of G. procumbens water extract on the plasma insulin levels in diabetic rats after 14 days of treatment

| Experimental groups | Dose (mg/kg) | Insulin concentration (ng/mL) | |
|---|---|---|---|
| Day 0 | Day 14 | ||
| Control | 2.49 ± 0.11 | 2.31 ± 0.03 | |
| Metformin | 500 | 2.17 ± 0.06 | 2.11 ± 0.05 |
| G. procumbens water extract | 500 | 2.26 ± 0.06 | 2.09 ± 0.06* |
| G. procumbens water extract | 1000 | 2.21 ± 0.06 | 2.15 ± 0.03 |
2.1.4. Effects of G. procumbens water extract on IPGTT in streptozotocin-induced diabetic rats after 14 days of treatment
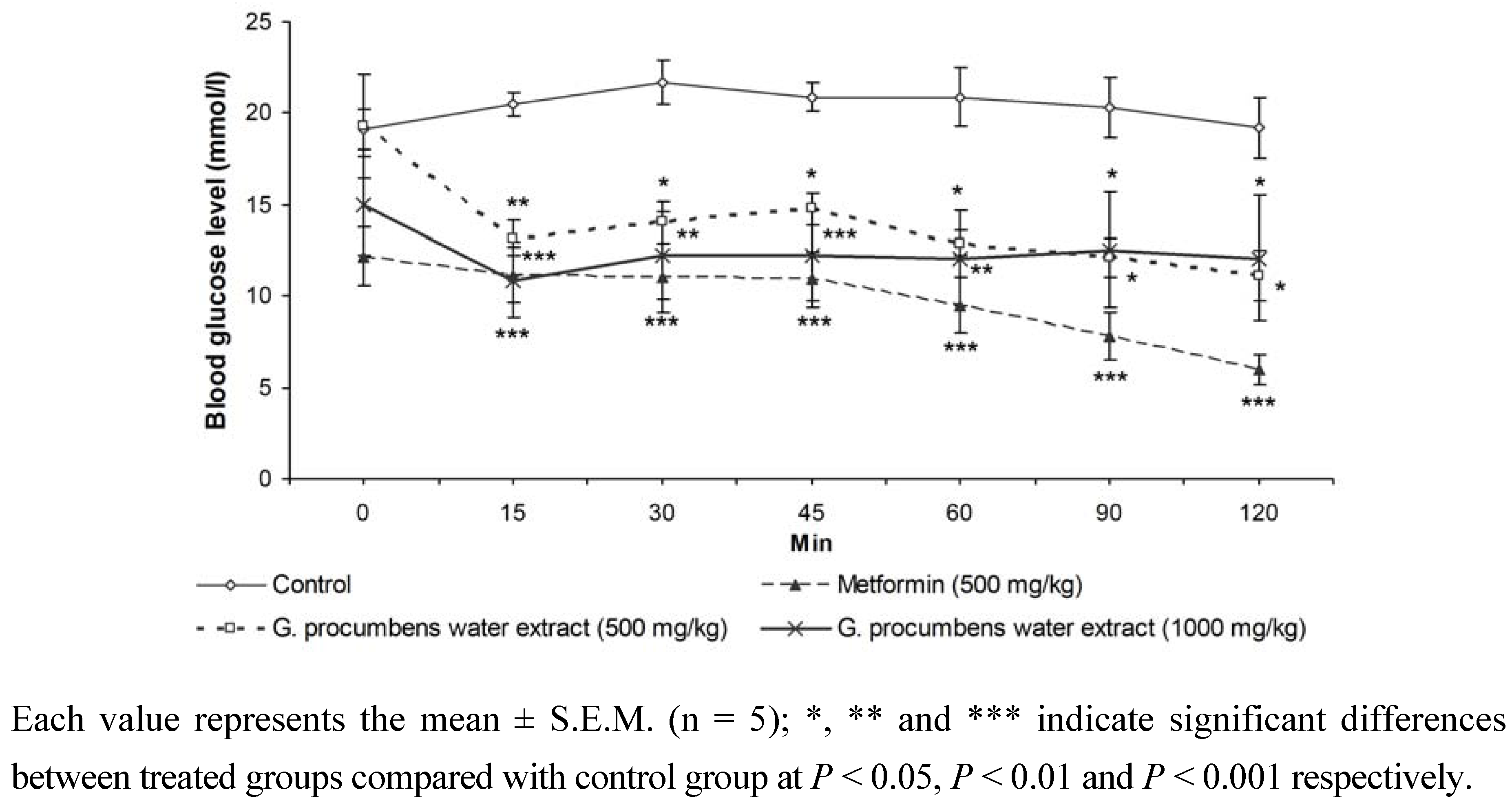
2.1.5. Effects of G. procumbens water extract on glucose absorption from the intestine

2.1.6. Effects of G. procumbens water extract on glucose uptake by isolated rat abdominal muscle

2.1.7. Effects of G. procumbens water extract on regeneration of β-cells in streptozotocin-induced rats
| Experimental group | Day of treatment | Percentage of insulin-positive cells per islet |
|---|---|---|
| Normal | -- | 82.52 ± 2.38### |
| 72 hours after STZ injection | -- | 2.93 ± 0.51*** |
| Normal saline+STZ injection | 14 | 8.43 ± 0.61***### |
| Metformin (500 mg/kg)+STZ injection | 14 | 20.93 ± 3.46***### |
| G. procumbens water extract (1000 mg/kg)+STZ injection | 14 | 7.02 ± 1.14***## |

2.1.8. Effects of G. procumbens water extract on insulin secretion by RIN-5F cells

2.1.9. Effects of G. procumbens water extract on RIN-5F cell viability
2.2. Discussion
3. Experimental
3.1. Preparation of G. procumbens extract
3.2. Animals
3.3. Streptozotocin-induced diabetic rats
3.4. 14 day treatment with the extracts
3.5. Intraperitoneal glucose tolerance test (IPGTT)
- Group I Diabetic rats given oral saline (10 mL/kg)
- Group II Diabetic rats given G. procumbens water extract (500 mg/kg)
- Group III Diabetic rats given G. procumbens water extract (1,000 mg/kg)
- Group IV Diabetic rats given metformin (500 mg/kg)
3.6. Preparation of plasma insulin
3.7. Measurement of glucose absorption from the intestine

3.8. Measurement of glucose uptake by isolated rat abdominal muscle
3.9. Studies on regeneration of β-cells in streptozotocin-induced rats
3.9.1. Preparation of the parafinized tissues
3.9.2. Immunohistochemical staining for insulin
3.10. In vitro studies
3.11. Cytotoxicity assay
3.12. Statistical analyses
4. Conclusions
Acknowledgements
References and Notes
- The Action to Control Cardiovascular Risk in Diabetes Study Group. Effects of intensive glucose lowering in type 2 diabetes. N. Engl. J. Med. 2008, 358, 2545–2559. [Google Scholar] [CrossRef]
- Rang, H.P.; Dale, M.M. The Endocrine System Pharmacology, 2nd ed; Longman Group Ltd.: London, U.K., 1999. [Google Scholar]
- Perry, L.M. Medicinal Plants of East and South East Asia; MIT Press: Cambridge, MA, USA, 1980. [Google Scholar]
- Akhtar, M.S.; Iqbal, J. Evaluation of the hypoglycaemic effect of Achyrantes aspera in normal and alloxan-diabetic rabbits. J. Ethnopharmacol. 1991, 31, 49–57. [Google Scholar] [CrossRef]
- Frati-Munari, A.C.; Gordilla, B.E.; Altamirano, P.; Ariza, C.R. Hypoglycaemic effect of Opuntia streptacantha Lemaire in NIDDM. Diabetes Care 1988, 11, 63–66. [Google Scholar]
- Merat, A.; Sahmani, M. Effect of acarbose on in vitro intestinal absorption of monosaccharides in diabetic rats. Arch. Iranian Med. 2003, 6, 40–43. [Google Scholar]
- Hirsh, A.J.; Yao, S.Y.; Young, J.D. Inhibition of glucose absorption in the rat jejunum: a novel action of alpha-D-glucosidase inhibitor. Gastroenterology 1997, 113, 205–211. [Google Scholar] [CrossRef]
- Luo, H.; Wang, L.F.; Imoto, T.; Hiji, Y. Inhibitory effect and mechanism of acarbose combined with gymnemic acid on maltose absorption in rat intestine. World J. Gastroenterol. 2001, 7, 9–15. [Google Scholar]
- DeFronzo, R.A.; Jacot, E.; Jequier, E. The effect of insulin on the disposal of intravenous glucose. Results from indirect calorimeter and hepatic and femoral venous catheterisation. Diabetes 1981, 30, 1000–1007. [Google Scholar]
- Risbud, M.V.; Bhonde, R.R. Models of pancreatic regeneration in diabetes. Diabetes Res. Clin. Pract. 2002, 58, 155–165. [Google Scholar] [CrossRef]
- Akowuah, A.G.; Amirin, S.; Mariam, A.; Aminah, I. Blood sugar lowering activity of Gynura procumbens leaf extracts. J. Trop. Med. Plants 2001, 2, 5–10. [Google Scholar]
- Chattopadhay, R.R. Possible mechanism of antihyperglycaemic effect of Azadiracta indica leaf extract. J. Ethnopharmacol. 1999, 67, 373–376. [Google Scholar] [CrossRef]
- Rosidah; Yam, M.F.; Sadikun, A.; Ahmad, M.; Akowuah, G.A.; Asmawi, M.Z. Toxicology evaluation of standardized methanol extract of Gynura procumbens. J. Ethnopharmacol. 2009, 123, 244–249. [Google Scholar] [CrossRef]
- Ahmad, M.; Razak, A.; Akowuah, G.A.; Asmawi, Z.; Zhari, I. HPLC profile and antihyperglycemic effect of ethanol extracts of Andrographis paniculata in normal and streptozotocin-induceddiabetic rats. J. Nat. Med. 2007, 61, 422–429. [Google Scholar]
- Bank, H.L. A quantitative enzyme-linked immunosorbent assay for rat insulin. J. Immunoassay 1988, 9, 135–158. [Google Scholar] [CrossRef]
- MacDonald, M.J.; Gapinski, J.P. A rapid ELISA for measuring insulin in a large number of research samples. Metabolism 1989, 38, 450–452. [Google Scholar] [CrossRef]
- Wilson, T.H.; Wiseman, G. The use of sacs of everted small intestine for the study of the transference of substances from the mucosal to the serosal surface. J. Physiol. 1954, 123, 116–125. [Google Scholar]
- Gray, A.M.; Flatt, P.R. Antihyperglycemic Actions of Eucalyptus globulus (eucalyptus) are associated with pancreatic and extra-pancreatic effects in mice. J. Nutr. 1998, 128, 2319–2323. [Google Scholar]
- Perez, C.; Dominguez, E.; Canal, J.R.; Campillo, J.E.; Torres, M.D. Hypoglycaemic activity of an aqueous extract from Ficus Carica (Fig Tree) leaves in streptozotocin diabetic rats. Pharm. Biol. 2000, 38, 181–186. [Google Scholar]
- Gray, A.M.; Flatt, P.R. Insulin-secreting activity of the traditional antidiabetic plant Viscum album (mistletoe). J. Endocrinol. 1999, 160, 409–414. [Google Scholar] [CrossRef]
- Plumb, J.A.; Milroy, R.; Kaye, S.B. Effects of the pH dependence of 3-(4,5-dimethylthiazole-2-yl)-2,5-diphenyl-tetrazoliumbromide-formazan) absorption on chemosensitivity determined by a novel tetrazolium-based assay. Cancer Res. 1989, 49, 4435–4440. [Google Scholar]
© 2010 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Hassan, Z.; Yam, M.F.; Ahmad, M.; Yusof, A.P.M. Antidiabetic Properties and Mechanism of Action of Gynura procumbens Water Extract in Streptozotocin-Induced Diabetic Rats. Molecules 2010, 15, 9008-9023. https://doi.org/10.3390/molecules15129008
Hassan Z, Yam MF, Ahmad M, Yusof APM. Antidiabetic Properties and Mechanism of Action of Gynura procumbens Water Extract in Streptozotocin-Induced Diabetic Rats. Molecules. 2010; 15(12):9008-9023. https://doi.org/10.3390/molecules15129008
Chicago/Turabian StyleHassan, Zurina, Mun Fei Yam, Mariam Ahmad, and Ahmad Pauzi M. Yusof. 2010. "Antidiabetic Properties and Mechanism of Action of Gynura procumbens Water Extract in Streptozotocin-Induced Diabetic Rats" Molecules 15, no. 12: 9008-9023. https://doi.org/10.3390/molecules15129008
APA StyleHassan, Z., Yam, M. F., Ahmad, M., & Yusof, A. P. M. (2010). Antidiabetic Properties and Mechanism of Action of Gynura procumbens Water Extract in Streptozotocin-Induced Diabetic Rats. Molecules, 15(12), 9008-9023. https://doi.org/10.3390/molecules15129008




